Abstract
New imidazole ring derivatives comprising 1,3-oxazoline, Schiff's bases, thiadiazole, oxadiazole and 1,2,4-triazole moieties are reported. 3-Aminobiimidazol-4-one compounds 7a-c were synthesized by the reaction of compounds 6a-c with hydrazine hydrate. Biimidazole esters 9a-c were converted into biimidazole hydrazide esters 10a-c. Compounds 7a-c and 10a-c were converted into a variety of derivatives.
Keywords: metronidazole; biimidazole; imidazole; 1,3,4-oxadiazole; 1,2,4-triazole; 1,3,4-thiadiazole
1. Introduction
Metronidazole (MTZ, 1) is a synthetic compound used in the treatment of infections caused by Gram negative anaerobic bacteria like Helicobacter pylori and protozoa such as Giardia, Lamblia, and Entomoeba histolytica, [1] Imidazole and its derivatives are of great significance due to their important roles in biological systems, particularly in enzymes, as proton donors and/or acceptors, coordination system ligands and the base of charge–transfer processes. Unlike pyrrole (a proton donor) and pyridine (a proton acceptor), 1H-imidazole has both proton donor and acceptor properties [2,3]. Imidazole functionalities have been used for complex reactions with different molecular components such as carboxylic acids to obtain liquid crystalline assemblies [4]. The imidazole nucleus appears in a number of naturally occurring products like the amino acids histidine and purines, which comprise many of the most important bases in nucleic acids. Imidazole derivatives possess a broad spectrum of pharmacological activities such as anticonvulsant [5], anti-Parkinson [6] and mono-aminooxidase (MAO) inhibitory [7] activity. Oxadiazole, triazole and thiadiazole chemistry has been developed extensively and are still being developed presently. There are a number of drugs used clinically [8] which comprise oxadiazole, triazole and thiadiazole moieties in association with various heterocyclic rings. In view of these facts, a project was undertaken to synthesize a new series of imidazoles containing oxadiazole, triazole, thiadiazole and Schiff's bases and to evaluate the new compounds for their biological activity
2. Results and Discussion
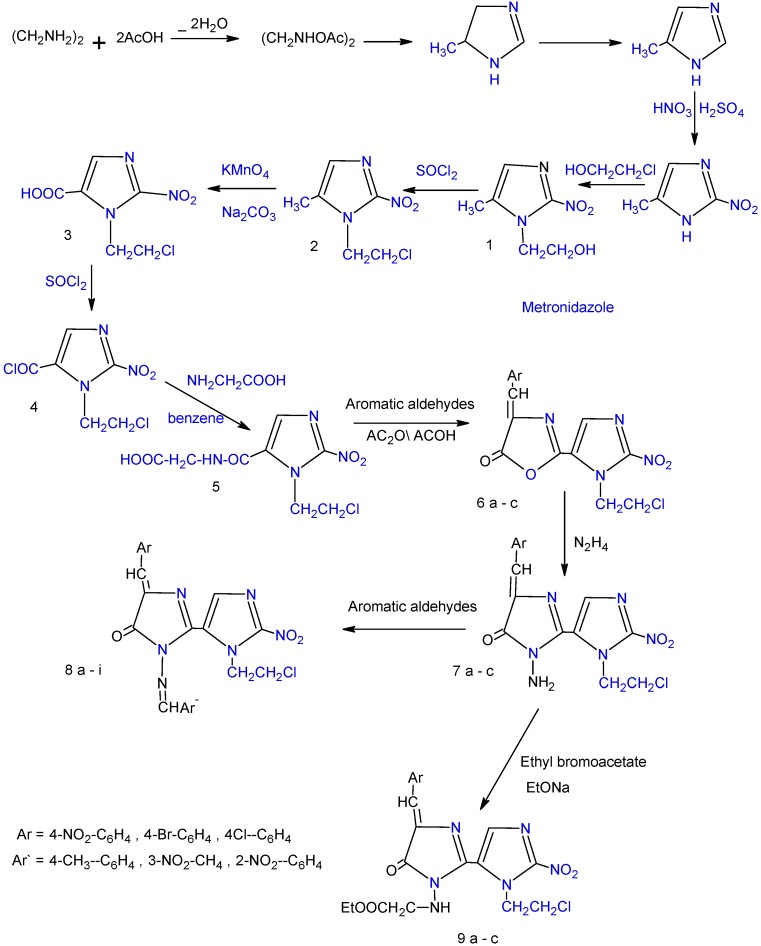
The designated compounds were synthesized according to Scheme 1 and Scheme 2. Reaction of 2-(5-methyl-2-nitro-1H-imidazole-1-yl) ethanol (metronidazole, 1) with thionyl chloride afforded 1-(2-chloroethyl)-5-methyl-2-nitro-1H-imidazole (2) [4].
Scheme 1.
The synthesis of compounds 1 - 9a-c.
Scheme 2.
The synthesis of compounds 10(a-c) – 15(a-c).
The IR spectrum of the product collected and recrystallized from ethanol indicated the absence of absorption bands due to OH and the presence of a C-Cl absorption band at (768 cm-1). 1-(2-Chloro-ethyl)-2-nitro-1H-imidazol-5-carboxylic acid (3) [9] which was readily prepared via oxidation of the CH3 group of compound 2, was converted into acid chloride 4 through reaction with thionyl chloride. The structures of compounds 3 and 4 were confirmed by 1H-NMR and IR spectral data and elemental analysis. In the IR spectrum compound 3 the presence of an OH absorption at 3,270- 2,650 cm-1 besides the C=O absorption at 1,715 cm-1 was observed. The 1H-NMR spectrum showed a triplet at 3.51-3.88 ppm integrating for protons of the CH2-Cl and a triplet at 2.95-3.21 ppm integrating for two protons of the N-CH2. The IR spectrum of compound 4 showed disappearance of the absorption band due to OH and an increase in the frequency of carbonyl moiety. Reaction of compound 4 with an amino acid (glycine) gave ({[1-(2-chloroethyl)-2-nitro-1H-imidazole-5-yl] carbonyl}amino) acetic acid (5) [10], while on the other hand, oxidative cyclization of compound 5 with aromatic aldehydes (Scheme 1) afforded 2-[1-(2-chloroethyl)-2-nitro-1H-imidazole-5-yl]-4-arylidene1,3-oxazol-5(4H)-ones 6a-c [11]. The IR spectrum of compound 5 showed two sharp absorption bands, the first appears at 1,720 cm-1 and is attributed to carbonyl function of the carboxylic acid and the other, observed at 1,690 cm-1, was assigned to a C=O stretching frequency corresponding to the amide carbonyl. In the 1H-NMR spectrum, the proton signals due to (CH2-NH) resonated at 4.42-4.67 ppm, integrating for two protons, while the proton signals due to ethyl group (N-CH2a-CH2b-Cl) were recorded between 2.82-2.93 ppm integrating for two protons (a) and at 3.40-3.64 ppm integrating for two protons (b). The structures of compounds 6a-c were indicated by the absence of the characteristic O—H stretching in addition to the absorption bands for the NH. The 1H-NMR spectra of compounds 6a-c showed new signals observed at 6.55-6.81 ppm integrating for two protons and at 7.31-7.75 ppm integrating for two protons assigned to aryl groups. The key intermediate 3-amino-3'-(2-chloroethyl)-5-arylidene-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-ones 7a-c [12] were prepared from the reaction of hydrazine hydrate with compounds 6a-c. The structures of all compounds 7a-c were proven based on the melting point (m.p), thin layer chromatography (TLC) and spectral data. The spectra of compounds 7a-c exhibited a NH2 stretching vibration at 3,360-3,210 cm-1 and C=O stretching vibrations at 1,660-1,695 cm-1. Reaction of compounds 7a-c with aromatic aldehydes produced new Schiff's bases 8a-c in high yield (Scheme1). The Schiff's bases 8a-i display in their IR carbonyl and isomethine absorptions near 1,690-1,643 cm-1 and 1640-1627 cm-1, respectively, in addition to absence of NH2stretching vibrations. Alkylation of compounds 7a-c with ethyl bromoacetate give ethyl {[3'-(2-chloroethyl)-4-arylidene-2'-nitro-5-oxo-4, 5-dihydro-1H, 3'H-2, 4'-biimidazol-1-yl] amino} acetates 9a-c [13]. The formation of compounds 9a-c was confirmed by the presence of a sharp absorption near 1,730-1,715 cm-1 for the ester C=O and at 1,250-1,300 cm-1 due to C—O stretching. In the 1H NMR spectra, the proton signals due to ethyl group of ester O-CH2c-CH3d were recorded between 1.35-1.78 ppm, integrating for three protons (d) and 3.31-3.78 ppm integrating for two protons (c). The treatment of compounds 9a-c with hydrazine hydrate, gave thiosemicarbazide compounds 10a-c [6] and compounds 11a-c [6], respectively. The spectral data of compounds 10a-c and 11a-c are given in the Experimental section. Acid hydrazides are useful intermediates leading to the formation of some heterocyclic ring such as 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. The 3'-(2-chloroethyl)-5-arylidene-3-{[5-mercapto-1,3,4-oxadiazol-2-yl-methyl]amino}-2'-nitro-3,5-dihydro-3'H,4H,2,4'-biimidazol-4-one compounds 12a-c [13] were synthesized from the reaction of compounds 10a-c with carbon disulfide in the presence of potassium hydroxide (Scheme 2).
The IR spectra of compounds 12a-c displayed the SH absorption at 2,470-2,580 cm-1 in addition to the C=S absorption at 1,210-1,280 cm-1. The NH and SH protons derived from the tautomeric equilibrium resonated between 12.55-13.19 ppm as a broad singlet integrating for one proton. Moreover, NHNH2 signals disappeared from the 1H-NMR and IR spectra. The condensation of the same intermediates 10a-c with carbon disulphide in basic media produced a potassium salt, that without isolation and purification was treated with hydrazine hydrate to give 3-{[(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)methyl]amino}-3'-(2-chloroethyl)-5-arylidene-2'-nitro-3',5-dihydro-3'H,4H-2,4'-biimidazol-4-ones 13a-c [14]. In contrast to those of 12a-c, the IR spectra of compounds 13a-c contained additional NH2 absorption bands. Moreover, proton signals due to NH2 group of compounds 13a-c resonated at 6.42-6.53 ppm, integrating for two protons. Oxidative cyclization of compounds 11a-c with aqueous sodium hydroxide (Scheme 2) afforded 3'-(2-chloroethyl)-5-arylidene-2'-nitro-3-[(4H-1,2,4-triazol-3-yl-methyl)amino]-3,5-dihydro3'H,4H-2,4'-biimidazol-4-ones 14a-c [7], while the treatment of the same compounds 11a-c with conc. H2SO4 afforded 3-{[(5-amino-1,3,4-thiadiazol-2-yl) methyl]amino}-3'-(2-chloroethyl)-5-arylidene-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-ones 15a-c [14].
The formation of the 1,2,4-triazole derivatives 14a-c as confirmed by the presence of weak absorptions near 2,570-2,630 cm-1 for SH and 1,230-1,300 cm-1 due to C=S. The structures of compounds 15a-c were confirmed by 1H-NMR, IR and elemental analysis. The data are shown the Experimental section.
2.1. Antibacterial activity
The antibacterial activity of the imidazole derivatives was tested by the agar disc-diffusion method against Staph. aureus, E. coli and Proteus mirabilis bacteria. Dimethylsulphoxide (DMSO) was used as solvent control, and the concentration of tested compounds was 10-3 M. The results of these studies are summarized in Table 1. It could be observed that all the tested compounds were active toward Proteus mirabilis, except compound 8b, and all the tested compounds were active toward E. Coli, except for compounds 7a and 8d and all the compounds were active toward Staph. aureus except 8b, 8f, 8i, 13a, 13b and 14c. On the other hand, compounds 15a-c showed high inhibition toward all kinds of bacteria tested. In addition compounds 6a, 8c and 8h, compounds 7c, 12b, 13c and 14a and compounds 7b, 12c, 13a-c, 14b and 14c showed high inhibition toward Staph. aureus, E. coli. and Proteus mirabilis, respectively.
Table 1.
Antibacterial Activity of New Compounds.
| Compound No. | Staph. aureus | E. coli | Proteus mirabilis |
|---|---|---|---|
| DMSO | - | - | - |
| 6a | +++ | ++ | ++ |
| 6b | + | ++ | ++ |
| 6c | ++ | + | + |
| 7a | ++ | - | ++ |
| 7b | ++ | ++ | +++ |
| 7c | + | +++ | ++ |
| 8a | ++ | ++ | + |
| 8b | - | + | - |
| 8c | +++ | ++ | ++ |
| 8d | ++ | - | ++ |
| 8e | ++ | + | + |
| 8f | - | ++ | ++ |
| 8g | ++ | + | + |
| 8h | +++ | ++ | ++ |
| 8i | - | ++ | ++ |
| 9a | + | + | + |
| 9b | + | + | ++ |
| 9c | ++ | + | + |
| 10a | ++ | ++ | + |
| 10b | ++ | ++ | ++ |
| 10c | + | + | + |
| 11a | + | + | ++ |
| 11b | ++ | + | ++ |
| 11c | ++ | + | ++ |
| 12a | + | ++ | + |
| 12b | + | +++ | + |
| 12c | ++ | ++ | +++ |
| 13a | - | ++ | +++ |
| 13b | - | + | +++ |
| 13c | + | +++ | +++ |
| 14a | ++ | +++ | ++ |
| 14b | ++ | ++ | +++ |
| 14c | - | + | +++ |
| 15a | +++ | +++ | +++ |
| 15b | +++ | +++ | +++ |
| 15c | +++ | ++ | +++ |
Zone diameter of growth inhibition: - = no inhibition, + = (3 – 6) mm, ++ = (7 – 10) mm and +++ = (11 – 15) mm. Conc. 10 -3 M.
3. Experimental
3.1. General
Melting points were determined in open capillary tubes on a Gallenkamp melting point apparatus and are uncorrected. The IR spectra were recorded on KBr disks, using a Perkin-Elmer 1600 series FTIR spectrometer. UV spectra were recorded on a Hitachi 2000 spectrophotometer. 1H-NMR spectra were recorded in DMSO-d6 on a Varian-Mercury 200 MHz Spectrometer. Combustion analysis was performed on a Carlo Erba 1106 elemental analyzer. Compound 1 was synthesized by a published method [15].
3.2. Synthesis of 1-(2-chloroethyl) -5-methyl-2-nitro-1H-imidazole (2)
Thionyl chloride (1.18 g , 0.01 mole) was added to a solution of compound 1 (1.71 g, 0.01 mole) in dry benzene (20 mL), then the reaction mixture was refluxed for 7 hrs. After evaporation, the product was collected and crystallized from ethanol-water. Yield: 90%; m.p. 85-87 °C; IR (ν, cm-1): 2,970-2,855 (C-Haliph.), 1,605 (C=N), 1,530-1,470 (NO2), 768 (C-Cl); 1H-NMR: δ (ppm) 1.75 (s, CH3), 2.81-3.10 (t, N-CH2), 3.71-4.10 (t, CH2-Cl), 8.48 (s, 1H, imidazole); Anal. % calc./found for C6H8N3O2Cl (m.w. 189.5) C, 38.01/39.67; H, 4.25/4.34, N 22.16/23.34.
3.3. Synthesis 1-(2-chloroethyl)-2-nitro-1H-imidazol-5-carboxylic acid (3)
Compound 2 (1.89 g, 0.01 mole) was added to a solution of sodium bicarbonate (1.06 g, 0.01 mole) and potassium permanganate (1.57 g, 0.01 mole) in water (20 mL), then the reaction mixture was refluxed for 15 hrs. The reaction mixture was cooled and acidified with conc. HCl and the product was collected and recrystallized from ethanol. Yield: 55%; m.p. 181-183°C; IR (ν, cm-1): 3,270-2,650 (OHacid), 2,985-2,890 (C-Haliph.), 1,715 (C=Oacid), 1,615 (C=N), 1,530-1,370 (NO2); 1H-NMR: δ (ppm) 2.95-3.11 (t, N-CH2), 3.51-3.88 (t, CH2-Cl), 12.32 (s, acid OH), 8.82 (s, 1H, imidazole); Anal. % calc./found for C6H6N3O4Cl (m.w. 219.5): C, 32.82/33.65; H, 2.75/3.32; N, 19.14/18.09.
3.4. Synthesis of 1-(2-chloroethyl)-2-nitro-1H-imidazole-5-carbonyl chloride (4)
This compound was synthesized following the same procedure used in synthesis of compound 2, without purification. Yield: 83%; m.p. 127-129°C; IR (ν, cm-1): 2,988-2,850 (C-Haliph.), 1,765 (C=Oacid chloride), 1620 (C=N), 1570-1390 (NO2); 1H-NMR: δ (ppm) 2.73-2.91 (t, N-CH2), 3.75-3.95 (t, CH2-Cl) 8.75 (s, 1H, imidazole); Anal. % calc./found for C6H5N3O3Cl2 (m.w. 238): C, 30.28/29.69; H, 2.12/2.79; N, 17.65/18.07.
3.5. Synthesis of ({[1-(2-chloroethyl)-2-nitro-1H-imidazole-5-yl] carbonyl}amino) acetic acid (5)
Compound 4 (2.38 g, 0.01 mole) was added to a stirring solution of glycine (0.75 g, 0.01 mole) and sodium hydroxide (10 mL, 10% solution). Then, the reaction mixture was shaken vigorously for 1 hr, and a few grams of crushed ice were added with stirring. After that, the solution was acidified with conc. HCl and the product was collected and recrystallized from ethanol. Yield: 80%; m.p. 165-167°C; IR (ν, cm-1): 3,220 (NH), 3,150 (OHacid), 2,985-2,870 (C-Haliph.), 1,720 (C=Oacid), 1,690 (C=Oamide), 1,510-1,370 (NO2); 1H-NMR: δ (ppm) 2.82-2.93 (t, N-CH2), 3.40-3.72 (t, CH2-Cl), 4.42-4.66 (s, CO-CH2-NH), 8.72 (s, 1H, imidazole), 10.57 (s, NHamide), 12.20 (s, OHacid); Anal. % calc./found for C8H9N4O5Cl (m.w. 276.5): C, 34.73/35.54; H, 3.26/3.55; N, 20.25/21.28.
3.6. Synthesis of 2-[1-(2-chloroethyl)-2-nitro-1H-imidazole-5-yl]-4-arylidene1,3-oxazol-5(4H)-ones 6a-c
Aromatic aldehyde (0.01 mole) was added to a stirring mixture of compound 5 (2.76 g, 0.01 mole) acetic acid (5 mL) and acetic anhydride (20 mL). The temperature of reaction was increased to 70 °C for 10 min., then the mixture was poured into crushed ice and stirred for 30 min. the product was collected and recrystallized from ethanol to afforded the desired compound.
2-[1-(2-Chloroethyl)-2-nitro-1H-imidazole-5-yl]-4-(4-nitrophenyl)1,3-oxazol-5(4H)-one (6a): Yield: 53%; m.p. 201-204 °C; IR (ν, cm-1): 3,050 (C-Har.), 2,983-2,868 (C-Haliph.), 1,710 (C=Ooxazole), 1,620 (C=Calkene), 1,280 (C-O); 1H-NMR: δ (ppm) 2.50-2.71 (t, N-CH2), 3.11 (s, C=CH-), 3.42-363 (t, CH2-Cl), 6.65-6.81 (d, 2H, ArH), 7.43-775 (d, 2H, ArH), 8.72 (s, 1H, imidazole); Anal. % calc./found for C15H10N5O6Cl (m.w. 389.5): C, 45.99/45.08; H, 2.57/3.11; N, 17.88/18.79.
2-[1-(2-Chloroethyl)-2-nitro-1H-imidazole-5-yl]-4-(4-bromophenyl)1,3-oxazol-5(4H)-one (6b): Yield: 57%; m.p. 230-232 °C; IR (ν, cm-1): 3,080 (C-Har.), 2,990-2,890 (C-Haliph.), 1705 (C=Ooxazole), 1,610 (C=Calkene), 1,280 (C-O); 1H-NMR: δ (ppm) 2.33-2.56 (t, N-CH2), 3.21 (s, C=CH-), 3.59-3.72 (t, CH2-Cl), 6.55-6.71 (d, 2H, ArH), 7.31-7.59 (d, 2H, ArH), 8.85 (s, 1H, imidazole); Anal. % calc./found for C15H10N4O4BrCl (m.w. 425.5): C, 42.33/43.64; H, 2.37/3.00; N, 13.16/14.23.
2-[1-(2-Chloroethyl)-2-nitro-1H-imidazole-5-yl]-4-(4-chlorophenyl1,3-oxazol-5(4H)-ones (6c): Yield: 51%; m.p. 236-239 °C; IR (ν, cm-1): 3,050 (C-Har.), 2,896-2,810 (C-Haliph.), 1,725 (C=Ooxazole), 1,610 (C=Calkene), 1,300 (C-O); 1H-NMR: δ (ppm) 2.11-2.31 (t, N-CH2), 2.92 (s, C=CH-), 3.40-3.61 (t, CH2-Cl), 6.75-6.85 (d, 2H, ArH), 7.39-7.50 (d, 2H, ArH), 8.63 (s, 1H, imidazole); Anal. % calc./found for C15H10N4O2Cl2 (m.w. 381.5): C, 47.27/47.44; H, 2.64/2.89; N, 14.70/15.06.
3.7. Synthesis of 3-amino-3'-(2-chloroethyl)-5-arylidene-2'-nitro-3,5-dihydro-3'H,4H-2,4'biimidazol-4-ones 7a-c
Hydrazine hydrate (99%, 10 mL) was added to a mixture of compound 6 (0.01 mole) in dry pyridine (5 mL). The reaction mixture was refluxed for 20 hrs. Then, the mixture was allowed to cool to room temperature and pyridine was removed. The product was recrystallized from ethanol to afford the desired compound.
3-Amino-3'-(2-chloroethyl)-5-(4-nitrophenyl)-2'-nitro-3,5-dihydro-3'H,4H-2,4'biimidazol-4-one (7a): Yield: 47%; m.p. 198-201°C; IR (ν, cm-1): 3,360-3,290 (NH2), 3,080 (C-Har.), 2,950-2,890 (C-Haliph.), 1,695 (C=O) 1,620 (C=Calkene); 1H-NMR: δ (ppm) 2.37-2.49 ( t, N-CH2), 3.13 (s, C=CH-), 3.41-3.53 (t, CH2-Cl), 6.40 (s, NH2), 6.73-6.91 (d, 2H, ArH), 7.37-7.72 (d, 2H, ArH), 8.49 (s, 1H, imidazole); Anal. % calc./found for C15H12N7O5Cl (m.w. 405.5): C, 44.40/46.53; H, 2.98/3.67; N, 24.16/24.68.
3-Amino-3'-(2-chloroethyl)-5-(4-bromophenyl)-2'-nitro-3,5-dihydro-3'H,4H-2,4'biimidazol-4-one (7b): Yield: 35%; m.p. 256-259°C; IR (ν, cm-1): 3,310-3,260 (NH2), 3,060 (C-Har.), 2,975-2,859 (C-Haliph.), 1,670 (C=O), 1,615 (C=Calkene); 1H-NMR: δ (ppm) 2.25-2.43 (t, N-CH2), 3.35 (s, C=CH-), 3.49-3.61 (t, CH2-Cl), 6.21 (s, NH2), 6.63-6.89 (d, 2H, ArH), 7.22-7.45 (d, 2H, ArH), 8.80 (s, 1H, imidazole); Anal. % calc./found for C15H12N6O3BrCl (m.w. 439.5): C, 40.98/41.79; H, 2.75/3.43; N, 19.12/20.21.
3-Amino-3'-(2-chloroethyl)-5-(4-chlorophenyl)-2'-nitro-3,5-dihydro-3'H,4H-2,4'biimidazol-4-one (7c): Yield: 38%; m.p. 283-285°C; IR (ν, cm-1): 3,345-3,250 (NH2), 3,080 (C-Har.), 2,990-2,890 (C-Haliph.), 1,660 (C=O), 1,610 (C=Calkene); 1H-NMR: δ (ppm) 2.19-2.31 (t, N-CH2), 3.31 (s, C=CH-), 3.52-3.73 (t, CH2-Cl), 6.11 (s, NH2), 6.61-6.85 (d, 2H, ArH), 7.42-7.69 (d, 2H, ArH), 8.74 (s, 1H, imidazole); Anal. % calc./found for C15H12N6O3Cl2 (m.w. 395): C, 45.59/45.03; H, 3.06/3.86; N, 21.27/22.76.
3.8. Synthesis of (5Z)-3́'-(2-chloroethyl)-5-arylidene-3-(arylideneamino)-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-ones 8a–i
The corresponding aryl aldehyde (0.01 mole) was added to a stirred solution of compound 7 (0.01 mole) in absolute ethanol (20 mL) and the mixture was refluxed for 2 hrs. After cooling, the mixture was filtered and the solid recrystallized from ethanol to afford the desired compound.
(5Z)-3́'-(2-Chloroethyl)-5-(4-nitrophenyl)-3-({4'-methylphenyl}amino)-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (8a): Yield: 70%; m.p. 260-263°C; IR: (ν, cm-1) 3,080 (C-Har), 2,970-2,880 (C-Haliph.), 1,685 (C=O), 1,640 (C=N), 1,610 (C=Calkene); 1H-NMR: δ (ppm) 1.59 (s, CH3), 2.31-2.47 (t, N-CH2), 3.25 (s, C=CH-), 3.49-3.65 (t, CH2-Cl) 6.30-6.82 (d, 4H, ArH), 7.33-7.65 (d, 4H, ArH), 8.21 (s, N=CH-), 8.81 (s, 1H, imidazole); Anal. % calc. for C23H18N7O5Cl (m.w. 507.5): C, 54.39/55.43; H, 3.57/4.98; N, 19.30/20.20.
(5Z)-3́'-(2-Chloroethyl)-5-(4-nitrophenyl)-3-({3'-nitrophenyl}amino)-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (8b): Yield: 78%; m.p. 267-269°C;IR: (ν, m-1) 3,060 (C-Har.), 2,985-2,880 (C-Haliph.), 1,690 (C=O), 1,633 (C=N), 1,612 (C=Calkene); 1H-NMR: δ (ppm) 2.22-2.49 (t, N=CH2), 3.19 (s, C=CH-), 3.40-3.57(t, CH2-Cl), 6.48-6.80 (d, 4H, ArH), 7.29-7.72 (d, 4H, ArH), 8.51 (s, N=CH-), 8.88 (s, 1H, imidazole); Anal. % calc./found for C22H15N8O7Cl (m.w. 538.5): C, 49.04/51.09; H, 2.81/3.29; N, 20.79/21.39.
(5Z)-3́'-(2-Chloroethyl)-5-(4-nitrophenyl)-3-({2'-nitrophenyl}amino)-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (8c): Yield: 83%; m.p. 294-295°C; IR: (ν, cm-1) 3,080 (C-Har.), 2,990-2,865 (C-Haliph.), 1,665 (C=O), 1,628 (C=N), 1,615 (C=Calkene); 1H-NMR: δ (ppm) 2.38-2.46 (t, N=CH2), 3.21 (s, C=CH-), 3.42-3.60 (t, CH2-Cl), 6.38-6.81 (d, 4H, ArH), 7.22-7.82 (d, 4H, ArH), 8.31 (s, N=CH-), 8.92 (s, 1H, imidazole); Anal. % calc./found for C22H15N8O7Cl (m.w. 538.5): C, 49.04/50.23; H, 2.81/3.65; N, 20.79/20.87.
(5Z)-3́'-(2-Chloroethyl)-5-(4-bromophenyl)-3-({4'-methylphenyl}amino)-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (8d): Yield: 67%; m.p. 274-277°C; IR (ν, cm-1) 3,030 (C-Har.), 2,983-2,870 (C-Haliph.), 1,677 (C=O), 1,632 (C=N), 1,608 (C=Calkene); 1H-NMR: δ (ppm) 1.67 (s, CH3), 2,32-2.52 (t, N-CH2), 3.35 (s, C=CH-), 3.53-3.79 (t, CH2-Cl), 6.22-6.75 (d, 4H, ArH), 7.31-7.73 (d, 4H, ArH), 8.18 (s, N=CH-), 8.79 (s, 1H, imidazole); Anal. % calc./found for C23H18N6O3BrCl (m.w. 541.5): C, 50.99/51.45; H, 3.35/4.02; N, 15.51/16.19.
(5Z)-3́'-(2-Chloroethyl)-5-(4-bromophenyl)-3-({3'-nitrophenyl}amino)-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (8e): Yield: 75%; m.p. 300 °C (dec.); IR: (ν, cm-1) 3,050 (C-Har.), 2,990-2,895 (C-HalIph.), 1,685 (C=O), 1,640 (C=N), 1,612 (C=Calkene); 1H-NMR: δ (ppm) 2.28-2.47 (t, N-CH2), 3.18 (s, C=CH-), 3.42-3.69 (t, CH2-Cl), 6.37-6.82 (d, 4H, ArH), 7.45-7.81 (d, 4H, ArH), 8.33 (s, N=CH-), 8.81 (s, 1H, imidazole); Anal. % calc./found for C22H15N7O5BrCl (m.w. 572.5): C, 46.13/46.86; H, 2.64/3.75; N, 17.12/18.58.
(5Z)-3́'-(2-Chloroethyl)-5-(4-bromophenyl)-3-({2'-nitrophenyl}amino)-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (8f): Yield: 71%; m.p. 275-278°C; IR: (ν, cm-1) 3,060 (C-Har.), 2,986-2,795 (C-Haliph.), 1,660 (C=O), 1,627 (C=N), 1,607 (C=Calkene); 1H-NMR: δ (ppm) 2.33-252 (t, N-CH2), 3.17 (s, C=CH-), 3.42-3.71 (t, CH2-Cl), 6.62-6.92 (d, 4H, ArH), 7.32-7.61 (d, 4H, ArH), 8.25 (s, N=CH-), 8.69 (s, 1H, imidazole); Anal. % calc./found for C22H15N7O5BrCl (m.w. 572.5): C, 46.13/44.68; H, 2.64/2.07; N, 17.12/16.65.
(5Z)-3́'-(2-Chloroethyl)-5-(4-chlorophenyl)-3-({4'-methylphenyl}amino)-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (8g): Yield: 68%; m.p. 291-293°C; IR: (ν, cm-1) 3,055 (C-Har.), 2,978-2,865 (C-Haliph.), 1,655 (C=O), 1,631 (C=N), 1,610 (C=Calkene); 1H-NMR: δ (ppm) 1.34 (s, CH3), 2.31-2.49 (N=CH2), 3.21 (s, C=CH-), 3.45-3.83 (t, CH2-Cl), 6.58-6.83 (d, 4H, ArH), 7.35-7.85 (d, 4H, ArH), 8.29 (s, N=CH-), 8.67 (s, 1H, imidazole); Anal. % calc./found for C23H18N6O3Cl2 (m.w. 497): C, 55.55/55.79; H, 3.65/3.90; N, 16.90/16.84.
(5Z)-3́'-(2-Chloroethyl)-5-(4-chlorophenyl)-3-({3'-nitrophenyl}amino)-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (8h): Yield: 70%; m.p. 250-253°C; IR: (ν, cm-1) 3,063 (C-Har.), 2,990-2,895 (C-Haliph.), 1,643 (C=O), 1,633 (C=N), 1,617 (C=Calkene); 1H-NMR: δ (ppm) 2.36-256 (t, N-CH2), 3.37 (s, C=CH-), 3.47-3.88 (t, CH2-Cl), 6.32-678 (d, 4H ArH),7.33-780 (d, 4H, ArH), 8.23 (s, N=CH-), 8.58 (s, 1H, imidazole); Anal. % calc./found for C22H15N7O3Cl2 (m.w. 528): C, 50.02/52.07; H, 2.86/3.83; N, 18.56/19.78.
(5Z)-3́'-(2-Chloroethyl)-5-(4-chlorophenyl)-3-({2'-nitrophenyl}amino)-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (8i): Yield: 86%; m.p. 303 °C (dec.); IR: (ν, cm-1) 3,057 (C-Har.), 2,977-2,863 (C-Haliph.), 1,650 (C=O), 1,629 (C=N), 1,620 (C=Calkene); 1H-NMR: δ (ppm) 2.39-265 (t, N-CH2), 3.40 (s, C=CH-), 3.59-3.96 (t, CH2-Cl), 6.39-6.83 (d, 4H, ArH), 7.32-7.85 (d, 4H ArH), 8.27 (s, N=CH-), 8.89 (s, 1H, imidazole); Anal. % calc./found for C22H15N7O3Cl2 (m.w. 528): C, 50.02/51.98; H, 2.86/3.08; N, 18.56/19.21.
3.9. Synthesis of ethyl {[3'-(2-chloroethyl)-4-arylidene-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]amino}acetates 9a-c
The corresponding compound 7 (0.01 mole) was refluxed with an equivalent amount of sodium in absolute ethanol for 2 hrs. Then, ethyl bromoacetate (1.81 g, 0.01 mole) was added and refluxed for an additional 5 hrs. After evaporating the solvent under reduced pressure, a solid appeared that was recrystallized from ethanol to afford the desired compound.
Ethyl {[3'-(2-chloroethyl)-4-(4-nitrophenyl)-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]-amino}acetate (9a): Yield: 56%; m.p. 245-248°C; IR: (ν, cm-1) 3,250 (NH), 3,070 (C-Har.), 2,970-2,860 (C-Haliph.), 1,727 (C=Oester), 1,680 (C=Oimidazole), 1,610 (C=Calkene) 1,270 (C-O); 1H-NMR: δ (ppm) 1.35-152 (t, CH3-CH2-), 2.31-2.52 (t, N-CH2), 3.10 (s, C=CH), 3.35-3.49 (q, CH2-CH3), 3.65-3.92 (t, CH2-Cl), 5.22 (s, N-CH2-CO), 6.63-6.88 (d, 2H, ArH), 7.41-7.62 (d, 2H, ArH), 8.53 (s, 1H, imidazole), 10.72 (s, NH); Anal. % calc./found for C19H18N7O7Cl (m.w. 491.5): C, 46.40/45.97; H, 3.96/3.65; N,19.93/18.05.
Ethyl {[3'-(2-chloroethyl)-4-(4-bromophenyl)-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]-amino}acetate (9b): Yield: 66%; m.p. 210-212°C; IR: (ν, cm-1) 3,210 (NH), 3,080 (C-Har.), 2,966-2,890 (C-Haliph.), 1,715 (C=Oester), 1,656 (C=Oimidazole), 1,612 (C=Calkene), 1,300 (C-O); 1H-NMR: δ (ppm) 1.41-166 (t, CH3-CH2-), 2.36-2.48 (t, N-CH2), 3.02 (s, C=CH), 3.59-3.78 (q, CH2-CH3), 3.81-4.05 (t, CH2-Cl), 5.53 (s, N-CH2-CO), 6.71-6.93 (d, 2H, ArH), 7.37-7.52 (d, 2H, ArH), 8.69 (s, 1H, imidazole), 10.59 (s, NH); Anal. % calc./found for C19H18N6O5BrCl (m.w. 525): C, 43.41/43.67; H, 3.45/4.32; N, 15.99/15.11.
Ethyl {[3'-(2-chloroethyl)-4-(4-chlorophenyl)-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]-amino}acetate (9c): Yield: 65%; m.p. 189-192°C; IR: (ν, cm-1) 3,205 (NH), 3,080 (C-Har.), 2,979-2,885 (C-Haliph.), 1,730 (C=Oester), 1,670 (C=Oimidazole), 1,620 (C=Calkene) 1,250 (C-O); 1H-NMR: δ (ppm) 1.52-178 (t, CH3-CH2-), 2.39-2.50 (t, N-CH2), 3.15 (s, C=CH), 3.31-3.60 (q, CH2-CH3), 3.92-4.22 (t, CH2-Cl), 5.01 (s, N-CH2-CO), 6.57-6.88 (d, 2H, ArH), 7.41-7.59 (d, 2H, ArH), 8.32 (s, 1H, imidazole), 10.53 (s, NH); Anal. % calc./found for C19H18N6O5Cl2 (m.w. 481): C,47.42/47.97; H, 3.77/4.29;N, 17.46/17.86.
3.10. Synthesis of 2-{[3'-(2-chloroethyl)-4-arylidene-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]amino}acetohydrazides 10a-c
A mixture of compound 9 (0.01, mole) and hydrazine hydrate (99%, 0.32 g, 0.01 mole) in ethanol (25 mL) was refluxed for 8 hrs. Upon cooling the solution a solid appeared. This was recrystallized from ethanol to afford the desired compound.
2-{[3'-(2-Chloroethyl)-4-(4-nitrophenyl)-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]-amino}acetohydrazide (10a): Yield: 75%; m.p. 241-244°C; IR: (ν, cm-1) 3,390-3,344 (NH2), 3,180 (NH), 3,060 (C-Har.), 2,950-2,880 (C-Haliph.), 1,690 (C=Oimidazole), 1,650 (C=Oamide), 1,210 (C-N); 1H-NMR: δ (ppm) 2.45-2.70 (t, N-CH2), 3.21 (s, C=CH), 3.51-3.72 (t, CH2-Cl), 4.65 (s, N-CH2-CO), 6.32 (s, NH2), 6.81-7.02 (d, 2H, ArH), 7.62-7.83 (d, 2H, ArH), 8.81 (s, 1H, imidazole), 10.83 (s, NH), 11.32 (s, CO-NH-N); Anal. % calc./found for C17H16N9O6Cl (m.w. 477): C, 42.73/42.99; H, 3.38/4.06; N, 26.38/27.69.
2-{[3'-(2-Chloroethyl)-4-(4-bromophenyl)-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]-amino}acetohydrazide (10b): Yield: 64%; m.p. 167-169°C; IR: (ν, cm-1) 3,351-3,311 (NH2), 3,160 (NH), 3,080 (C-Har.), 2,987-2,880 (C-Haliph.), 1685 (C=Oimidazole), 1,639 (C=Oamide), 1,230 (C-N); 1H-NMR: δ (ppm) 2.63-2.90 (t, N-CH2), 3.30 (s, C=CH), 3.62-3.83 (t, CH2-Cl), 4.73 (s, N-CH2-CO), 6.41 (s, NH2), 6.73-6.92 (d, 2H, ArH), 7.51-7.72 (d, 2H, ArH), 8.67 (s, 1H, imidazole), 10.92 (s, NH), 11.82 (s, CO-NH-N); Anal. % calc./found for C17H16N8O4BrCl (m.w. 511.5): C, 39.90/40.65; H, 3.15/3.95; N, 21.90/22.73.
2-{[3'-(2-Chloroethyl)-4-(4-chlorophenyl)-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]-amino}acetohydrazide (10c): Yield: 68%; m.p. 179-181°C; IR: (ν, cm-1) 3,363-3,300 (NH2), 3,180 (NH), 3,075 (C-Har.), 2,989-2,890 (C-Haliph.), 1,695 (C=Oimidazole), 1,640 (C=Oamide), 1,220 (C-N); 1H-NMR: δ (ppm) 2.73-2.95 (t, N-CH2), 3.42 (s, C=CH), 3.67-3.80 (t, CH2-Cl), 4.72 (s, N-CH2-CO), 6.52 (s, NH2), 6.82-7.99 (d, 2H, ArH), 7.32-7.52 (d, 2H, ArH), 8.82 (s, 1H, imidazole), 10.85 (s, NH), 11.75 (s, CO-NH-N); Anal. % calc./found for C17H16N8O4Cl2 (m.w. 467): C, 43.70/43.12; H, 3.95/3.32; N, 23.98/25.00.
3.11. Synthesis of N-[(amino-λ4-sulfanylidyne)methyl-2-{[3'-(2-chloroethyl)-4-arylidene-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]amino} acetothiosemicarbazides 11a-c
These compounds were synthesized by the same procedure used for compounds 10a-c.
N-[(Amino-λ4-sulf{4-nitrophenyl})methyl-2-{[3'-(2-chloroethyl)-4-arylidene-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]amino}acetothiosemicarbazide (11a): Yield: 38%; m.p. 286-289°C; IR: (ν, cm-1) 3,390-3,355 (NH2), 3,280 (NHthiosemicarbazide), 3,170 (NH), 3,050 (C-Har.), 2,983-2,890 (C-Haliph.), 1,693 (C=Oimidazole), 1,645 (C=Oamide), 1,270 (C=S); 1H-NMR: δ (ppm) 2.56-2.78 (t, N-CH2), 3.27 (s, C=CH), 3.53-3.72 (t, CH2-Cl), 4.83 (s, N-CH2-CO), 6.32 (s, NH2), 6.58-6.71 (d, 2H, ArH), 7.61-7.85 (d, 2H, ArH), 8.86 (s, 1H, imidazole), 10.45 (s, NH), 10.91 (s, N-NH-CS), 11.72 (s, CO-NH-N); Anal. % calc./found for C18H17N10O6SCl (mw. 536.5): C, 40.27/41.87; H, 3.19/3.76; N, 26.09/25.55.
N-[(Amino-λ4-sulf{4-bromophenyl})methyl-2-{[3'-(2-chloroethyl)-4-arylidene-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]amino}acetothiosemicarbazide (11b): Yield: 42%; m.p. 282-284°C; IR: (ν, cm-1) 3,356-3,327 (NH2), 3,259 (NHthiosemicarbazide), 3,189 (NH), 3,080 (C-Har.), 2,987-2,890 (C-Haliph.), 1,700 (C=Oimidazole), 1,650 (C=Oamide), 1,268 (C=S); 1H-NMR: δ (ppm) 2.42-2.67 (t, N-CH2), 3.31 (s, C=CH), 3.59-3.71 (t, CH2-Cl), 4.78 (s, N-CH2-CO), 6.13 (s, NH2), 6.42-6.68 (d, 2H, ArH), 7.53-7.72 (d, 2H, ArH), 8.65 (s, 1H, imidazole), 10.33 (s, NH), 10.75 (s, N-NH-CS), 11.23 (s, CO-NH-N); Anal. % calc./found for C18H17N9O4SBrCl (m.w. 570.5): C, 37.87/36.31; H, 3.00/3.75; N, 22.08/23.54.
N-[(Amino-λ4-sulf{4-chlorophenyl})methyl-2-{[3'-(2-chloroethyl)-4-arylidene-2'-nitro-5-oxo-4,5-dihydro-1H,3'H-2,4'-biimidazol-1-yl]amino}acetothiosemicarbazide (11c): Yield: 35%; m.p. 293-294°C; IR: (ν, cm-1) 3,402-3,381 (NH2), 3,275 (NHthiosemicarbazide), 3,140 (NH), 3,090 (C-Har.), 2,981-2,883 (C-Haliph.), 1,683 (C=Oimidazole), 1,639 (C=Oamide), 1,259 (C=S); 1H-NMR: δ (ppm) 2.45-2.70 (t, N-CH2), 3.23 (s, C=CH), 3.62-3.80 (t, CH2-Cl), 4.86 (s, N-CH2-CO), 6.33 (s, NH2), 6.50-6.69 (d, 2H, ArH), 7.43-7.58 (d, 2H, ArH), 8.75 (s, 1H, imidazole), 10.46 (s, NH), 10.82 (s, N-NH-CS), 11.84 (s, CO-NH-N); Anal. % calc./found for C18H17N9O4SCl (m.w. 526): C, 41.07/41.58; H, 3.26/4.08; N, 23.95/23.21.
3.12. Synthesis of 3'-(2-chloroethyl)-5- arylidene-3-{[5-mercapto-1,3,4-oxadiazol-2-yl-methyl] amino}-2'-nitro--3,5dihydro-3'H,4H,2,4'-biimidazol-4-ones 12a-c
The corresponding compound 10 (0.01 mole) and CS2 (0.6 mL, 0.01 mole) were added to a solution of KOH (0.56 g, 0.01 mole) in ethanol (30 mL). The reaction mixture was refluxed for 3 hrs. After evaporation under reduced pressure to dryness, a solid was obtained. This was dissolved in H2O (200 mL) and acidified with conc. HCl. The precipitate was filtered off, washed with water and recrystallized from ethanol to afford the desired compound.
3'-(2-Chloroethyl)-5-(4-nitrophenyl)-3-{[5-mercapto-1,3,4-oxadiazol-2-yl-methyl]amino}-2'-nitro-3,5-dihydro-3'H,4H,2,4'-biimidazol-4-one (12a): Yield: 63%; m.p. 302-303°C; IR: (ν, cm-1) 3,220 (NH), 3,080 (C-Har.), 2,987-2,890 (C-Haliph.), 2,490 (SH), 1,670 (C=Oimidazole), 1,265 (C=S); 1H-NMR: δ (ppm) 2.52-2.72 (t, N-CH2), 3.27 (s, C=CH), 3.51-3.78 (t, CH2-Cl), 4.63 (s, N-CH2-oxadiazole), 6.45-6.62 (d, 2H, ArH), 7.39-7.60 (d, 2H, ArH), 8.89 (s, 1H, imidazole), 10.52 (s, NH), 12.55 (s, NH + SH of oxadiazole); Anal. % calc./found for C18H14N9O6Cl (m.w. 519.5): C, 41.59/41.83; H, 2.71/3.57; N, 24.25/23.61.
3’-(2-Chloroethyl)-5-(4-bromophenyl)-3-{[5-mercapto-1,3,4-oxadiazol-2-yl-methyl]amino}-2'-nitro-3,5-dihydro-3'H,4H,2,4'-biimidazol-4-one (12b): Yield: 65%; m.p. 295-297°C; IR: (ν, cm-1) 3,235 (NH), 3,085 (C-Har.), 2,976-2,855 (C-Haliph.), 2,470 (SH), 1,685 (C=Oimidazole), 1,280 (C=S); 1H-NMR: δ (ppm) 2.91-3.22 (t, N-CH2), 3.52 (s, C=CH), 3.69-3.80 (t, CH2-Cl), 4.51 (s, N-CH2-oxadiazole), 6.34-6.50 (d, 2H, ArH), 7.50-7.69 (d, 2H, ArH), 8.49 (s, 1H, imidazole), 10.66 (s, NH), 12.95 (s, NH + SH of oxadiazole); Anal. % calc./found for C18H14N8O4BrCl (m.w. 553.5): C, 39.04/38.53; H, 2.55/2.97; N, 20.23/21.78.
3’-(2-Chloroethyl)-5- (4-chlorophenyl)-3-{[5-mercapto-1,3,4-oxadiazol-2-yl-methyl]amino}-2'-nitro-3,5-dihydro-3'H,4H,2,4'-biimidazol-4-one (12c): Yield: 69%; m.p. 287-289°C; IR: (ν, cm-1) 3,215 (NH), 3,065 (C-Har.), 2,978-2,860 (C-Haliph.), 2,580 (SH), 1,690 (C=Oimidazole), 1,210 (C=S); 1H-NMR: δ (ppm) 2.49-2.70 (t, N-CH2), 3.71 (s, C=CH), 3.92-4.00 (t, CH2-Cl), 4.42 (s, N-CH2-oxadiazole), 6.46-6.70 (d, 2H, ArH), 7.45-7.65 (d, 2H, ArH), 8.12 (s, 1H, imidazole), 10.59 (s, NH), 13.91 (s, NH + SH of oxadiazole); Anal. % calc./found for C18H14N8O4SCl2 (m.w. 509): C, 42.45/42.65; H, 2.77/3.23; N, 22.00/23.65.
3.13. Synthesis of 3-{[(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)methyl]amino}-3'-(2-chloroethyl)-5-arylidene-2'-nitro-3',5-dihydro-3'H,4H-2,4'-biimidazol-4-ones 13a-c
The corresponding compound 10 (0.01 mole) and CS2 (0.6 mL, 0.01 mole) in ethanol (20 mL) were stirred for 12 hrs. Then, diethyl ether (18 mL) was added. The precipitated solid thus obtained was filtered, washed with cold diethyl ether, and without isolation and purification dissolved in water (10 mL) and hydrazine hydrate (99%, 0.34 g, 0.01 mole) was added. The reaction mixture was refluxed for 1 hr. cooled, diluted with water and acidified with acetic acid. The precipitate was filtered off, washed with water and recrystallized from ethanol to afford the desired compound.
3-{[(4-Amino-5-mercapto-4H-1,2,4-triazol-3-yl)methyl]amino}-3'-(2-chloroethyl)-5-(4-nitrophenyl)-2'-nitro-3',5-dihydro-3'H,4H-2,4'-biimidazol-4-one (13a): Yield: 53%; m.p. 243-246°C; IR: (ν, cm-1) 3,400-3,370 (NH2), 3,200 (NH), 3,060 (C-Har.), 2,966-2,872 (C-Haliph.), 2,460 (SH), 1,695 (C=Oimidazole), 1,220 (C=S); 1H-NMR: δ (ppm) 2.51-2.70 (t, N-CH2), 3.38 (s, C=CH), 3.55-3.69 (t, CH2-Cl), 4.81 (s, N-CH2-triazole), 6.42 (s, NH2), 6.57-6.70 (d, 2H, ArH), 7.35-7.60 (d, 2H, ArH), 8.71 (s, 1H, imidazole), 10.50 (s, NH), 13.20 (s, NH + SH of triazole); Anal. % calc./found for C18H16N11O5SCl (m.w. 533.5): C, 37.90/36.54; H, 3.00/3.76; N, 27.01/27.68.
3-{[(4-Amino-5-mercapto-4H-1,2,4-triazol-3-yl)methyl]amino}-3'-(2-chloroethyl)-5-(4-bromophenyl)-2'-nitro-3',5-dihydro-3'H,4H-2,4'-biimidazol-4-one (13b): Yield: 55%; m.p. 232-235°C; IR: (ν, cm-1) 3,480-3,300 (NH2), 3,205 (NH), 3,075 (C-Har.), 2,980-2,880 (C-Haliph.), 2,550 (SH), 1,700 (C=Oimidazole), 1,250 (C=S); 1H-NMR: δ (ppm) 2.62-280 (t, N-CH2), 3.45 (s, C=CH), 3.61-3.80 (t, CH2-Cl), 4.52 (s, N-CH2-triazole ), 6.53 (s, NH2), 6.70-6.92 (d, 2H, ArH), 7.42-7.60 (d, 2H, ArH), 8.80 (s, 1H, imidazole), 10.94 (s, NH), 13.22 (s, NH + SH of triazole); Anal. % calc./found for C18H16N10O3BrSCl (m.w. 567.7): C, 38.07/37.53; H, 2.84/3.72; N, 24.67/45.51.
3-{[(4-Amino-5-mercapto-4H-1,2,4-triazol-3-yl)methyl]amino}-3'-(2-chloroethyl)-5-(4-chlorophenyl)-2'-nitro-3',5-dihydro-3'H,4H-2,4'-biimidazol-4-one (13c): Yield: 48%; m.p. 274-276°C; IR: (ν, cm-1) 3,410-3,360 (NH2), 3,190 (NH), 3,070 (C-Har.), 2,971-2,890 (C-Haliph.), 2,560 (SH), 1,700 (C=Oimidazole), 1,270 (C=S); 1H-NMR: δ (ppm) 2.36-2.52 (t, N-CH2), 3.67 (s, C=CH), 3.81-3.96 (t, CH2-Cl), 4.50 (s, N-CH2-triazole), 6.46 (s, NH2), 6.62-6.89 (d, 2H, ArH), 7.35-7.58 (d, 2H, ArH), 8.62 (s, 1H, imidazole), 10.62 (s, NH), 13.21 (s, NH + SH of triazole); Anal. % calc./found for C18H16N10O3SCl (m.w. 523): C, 38.62/36.99; H, 3.06/3.25; N, 25.02/25.93.
3.14. Synthesis of 3'-(2-chloroethyl)-5-arylidene-2'-nitro-3-[(4H-1,2,4-triazol-3-yl-methyl )amino]-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-ones 14a-c
A mixture of compound 11 (0.01 mole) and sodium hydroxide (0.01 mole, as 4% solution) was stirred for 4 hrs. After cooling, the solution was acidified with conc. HCl and the precipitate was filtered and recrystallized from ethanol to afford the desired compound.
3'-(2-Chloroethyl)-5-(4-nitrophenyl)-2'-nitro-3-[(4H-1,2,4-triazol-3-yl-methyl)amino]-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (14a): Yield: 43%; m.p. 202-205°C; IR: (ν, cm-1) 3,300 (triazole, NH), 3,210 (NH), 3,066 (C-Har.), 2,982-2,877 (C-Haliph.), 2,625 (SH), 1,696 (C=Oimidazole), 1230 (C=S); 1H-NMR: δ (ppm) 2.32-2.50 (t, N-CH2), 3.31 (s, C=CH), 3.62-3.81 (t, CH2-Cl), 4.68 (s, N-CH2-triazole), 6.61-6.82 (d, 2H, ArH), 7.42-7.65 (d, 2H, ArH), 8.72 (s, 1H, imidazole), 10.39 (s, NH), 11.32 (s, triazole NH) 13.37 (s, NH + SH of triazole); Anal. % calc./found for C18H15N10O5SCl (m.w. 518.5): C, 41.66/42.61; H, 2.91/3.61; N, 26.99/26.13.
3'-(2-Chloroethyl)-5-(4-bromophenyl)-2'-nitro-3-[(4H-1,2,4-triazol-3-yl-methyl)amino]-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (14b): Yield: 61%; m.p. 213-215°C; IR: (ν, cm-1) 3,353 (triazole, NH), 3,190 (NH), 3,082 (C-Har.), 2,991-2,879 (C-Haliph.), 2,630 (SH), 1,700 (C=Oimidazole), 1,300 (C=S); 1H-NMR: δ (ppm) 2.41-2.66 (t, N-CH2), 3.42 (s, C=CH), 3.67-3.82 (t, CH2-Cl), 4.65 (s, N-CH2-triazole), 6.41-6.60 (d, 2H, ArH), 7.32-7.60 (d, 2H, ArH), 8.80 (s, 1H, imidazole), 10.42 (s, NH), 11.49 (s, triazole NH) 13.41 (s, NH + SH of triazole); Anal. % calc./found for C18H15N9O5SBrCl (m.w. 552.5): C, 39.11/40.71; H, 2.74/3.43; N, 22.80/23.41.
3'-(2-Chloroethyl)-5-(4-chlorophenyl)-2'-nitro-3-[(4H-1,2,4-triazol-3-yl-methyl)amino]-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (14c): Yield: 45%; m.p. 241-245°C; IR: (ν, cm-1) 3,300 (triazole, NH), 3254 (NH), 3,030 (C-Har.), 2,980-2,855 (C-Haliph.), 2,570 (SH), 1,689 (C=Oimidazole), 1,256 (C=S); 1H-NMR: δ (ppm) 2.51-2.79 (t, N-CH2), 3.63 (s, C=CH), 3.60-3.85 (t, CH2-Cl), 4.71 (s, N-CH2-triazole), 6.32-6.56 (d, 2H, ArH), 7.52-7.81 (d, 2H, ArH), 8.69 (s, 1H, imidazole), 10.52 (s, NH), 11.63 (s, triazole NH) 13.39 (s, NH + SH of triazole); Anal. % calc./found for C18H15N9O3SCl2 (m.w. 508): C, 42.53/42.00; H, 2.97/2.13; N, 24.80/45.39.
3.15. Synthesis of 3-{[(5-amino-1,3,4-thiadiazol-2-yl)methyl]amino}-3'-(2-chloroethyl)-5-arylidene-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-ones 15a-c
The corresponding compound 11 (0.01 mole) was dissolved in cold conc. sulfuric acid (10 mL) and stirred at room temperature for 24 hrs. Then, the reaction mixture was poured into crushed ice and diluted with water; the precipitate was filtered, washed with water and recrystallized from ethanol to afford the desired compound.
3-{[(5-Amino-1,3,4-thiadiazol-2-yl)methyl]amino}-3'-(2-chloroethyl)-5-(4-nitrophenyl-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (15a): Yield: 69%; m.p. 263-265°C; IR: (ν, cm-1) 3,402-3,366 (NH2), 3,205 (NH), 3,065 (C-Har.), 2,990-2,879 (C-Haliph.), 1688 (C=Oimidazole); 1H-NMR: δ (ppm) 2.34-2.62 (t, N-CH2), 3.59 (s, C=CH), 3.65-3.78 (t, CH2-Cl), 4.82 (s, N-CH2-thiadiazole), 6.41 (s, NH2), 6.47-6.62 (d, 2H, ArH), 7.35-7.47 (d, 2H, ArH), 8.87(s, 1H, imidazole), 10.43 (s, NH); Anal. % calc./found for C18H15N10O5SCl (m.w. 518.5): C, 41.66/41.08; H, 2.91/3.86; N, 26.99/26.12.
3-{[(5-Amino-1,3,4-thiadiazol-2-yl)methyl]amino}-3'-(2-chloroethyl)-5-(4-bromophenyl-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (15b): Yield: 78%; m.p. 271-273°C; IR: (ν, cm-1) 3,387-3,308 (NH2), 3,195 (NH), 3,076 (C-Har.), 2,983-2,890 (C-Haliph.), 1,703 (C=Oimidazole); 1H-NMR: δ (ppm) 2.53-2.67 (t, N-CH2), 3.31 (s, C=CH), 3.72-3.95 (t, CH2-Cl), 4.63 (s, N-CH2-thiadiazole), 6.45 (s, NH2), 6.68-6.79 (d, 2H, ArH), 7.36-7.50 (d, 2H, ArH), 8.73(s, 1H, imidazole), 10.53 (s, NH); Anal. % calc./found for C15H15N9O3SBrCl (m.w. 552.5):C, 39.11/40.87; H, 2.74/2.06; N, 22.80/22.01.
3-{[(5-Amino-1,3,4-thiadiazol-2-yl)methyl]amino}-3'-(2-chloroethyl)-5-(4-chlorphenyl-2'-nitro-3,5-dihydro-3'H,4H-2,4'-biimidazol-4-one (15c): Yield: 62%; m.p. 300-302°C; IR: (ν, cm-1) 3,410-3,345 (NH2), 3,215 (NH), 3086 (C-Har.), 2957-2861 (C-Haliph.), 1,702 (C=Oimidazole); 1H NMR: δ (ppm) 2.56-2.70 (t, N-CH2), 3.43 (s, C=CH), 3.65-3.81 (t, CH2-Cl), 4.83 (s, N-CH2-thiadiazole), 6.48 (s, NH2), 6.63-6.82 (d, 2H, ArH), 7.66-7.73 (d, 2H, ArH), 8.75(s, 1H, imidazole), 10.73 (s, NH); Anal. % calc./found for C18H15N9O3SCl2 (m.w. 508): C, 42.53/42.99; H, 2.97/2.22; N, 24.80/23.10.
Footnotes
Sample Availability: Samples of compounds 1 - 15a-c are available from the authors.
References
- 1.Khabnadideh S., Rezaei Z., Khalafi A.N., Eskandari E. Synthesis of metronidazole derivatives as antigiardiasis agents. DARU. 2007;15:17–20. [Google Scholar]
- 2.Foster E.J., Lavigueur C., KE Y.C., Williams V.E. Synthesis of alkyl 2-(benzoylamino)-3-(4,5-dicyano-1H-imidazol-1-yl) propenoates. J. Mater. Chem. 2005;37:4062–4068. [Google Scholar]
- 3.Brunsveld L., Zong H., Glasbeek M. E.W., Meijer E.W. RNA Cleavage by a DNA Enzyme with Extended Chemical Functionality. J. Am. Chem. Soc. 2000;122:6175–6182. doi: 10.1021/ja0005237. [DOI] [PubMed] [Google Scholar]
- 4.Sang H.S., Gregory N.T., Chang J.Y. Lyotropic columnar liquid crystals based on polycatenar 1H-imidazole amphiphles and their assembly into bundles at the surface of silicon. J. Soft Mater. 2006;2:889–891. doi: 10.1039/b606870g. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen H.T., Destrade C., Malthete J. Synthesis, spectroscopy, thermal studies and supramolecular structures of two new alkali-earth 4-nitrobenzoate complexes containing coordinated imidazole. Adv. Mater. 1997;9:375–388. doi: 10.1002/adma.19970090503. [DOI] [Google Scholar]
- 6.Borisch K., Diele S, Goring P., Kresse H., Tschierske C. Synthesis of Novel Organometallic Polymers Based Upon N-Heterocyclic Carbenes. J. Mater. Chem. 1998;8:529–543. doi: 10.1039/a705359b. [DOI] [Google Scholar]
- 7.Sharba A.H., Al-Bayati R.I., Aouad M., Rezki N. Synthesis of Thiadiazoles and 1, 2, 4-Triazoles Derived from Cyclopropane Dicarboxylic acid. Molecules. 2005;10:1153–1160. doi: 10.3390/10091153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank P.V., Girish K.S., Kalluraya B. Solvent-free microwave-assisted synthesis of oxadiazoles containing imidazole moiety. J. Chem. Sci. 2007;119:41–46. doi: 10.1007/s12039-007-0007-7. [DOI] [Google Scholar]
- 9.Norris J.F. Experimental Organic Chemistry. 2nd ed. Mc Graw-Hill Book Company, Inc.; New York, NY, USA: 1924. Preparation of Terephthalic Acid from p-Toluic Acid; p. 173. [Google Scholar]
- 10.Vogel A.I. A Text Book of Practical Organic Chemistry. 4th ed. Longmans Green and Co. Inc.; London, UK: 1954. Hippuric acid; p. 561. [Google Scholar]
- 11.Shuka S. B., Horan A. A. Synthesis of Amino [ 4-{dis(2- Hydroxy Ethyl)Amino} Thenyl] Acetic Acid. Chim. Acta. 1997;40:80–85. [Google Scholar]
- 12.Prakanyi C., Schmidt D.S. Synthesis of 5-Chloro-2-methyl-3-(5-methylthiazol-2-yl)-4(3H)-quinazolinone and Related Compounds with potential Biological Activity. J. Heterocycl. Chem. 2000;37:725–729. doi: 10.1002/jhet.5570370409. [DOI] [Google Scholar]
- 13.Sharma R.S., Bahel S.C. Synthesis of Aryloxy /Acetyl Thiosemicarbazides, Substituted 1,3,4-Oxadiazolles,1,3,4-thiadiazoles, 1,2,4-Triazoles and Related Compounds as Potential Fungicides. J. Ind. Chem. Soc. 1982;LIX:877–880. [Google Scholar]
- 14.Domirbas N., Domirbas A., Karaoylu S., Celik E. Synthesis and antimicrobial activities of some new[1,2,4]-triazolo[3,4-b]thiadiazoles and [1,2,4]triazolo[3,4-b][1,3,4]thiadiazines. ARKIVOC. 2005:75–91. [Google Scholar]
- 15.Kraft M.Y., Kochergin T.P.M., Tsyganova A.M., Shlikhunove V.S. Synthesis of metronidazole from ethylenediamine. Pharm. Chem. J. 1989;23:861–863. doi: 10.1007/BF00764821. [DOI] [Google Scholar]