Abstract
A series of amino acid ester derivatives containing 5-fluorouracil were synthesized using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) and N-hydroxybenzotriazole (HOBt) as a coupling agent. The structures of the products were assigned by NMR, MS, IR etc. The in vitro antitumor activity tests against leukaemia HL-60 and liver cancer BEL-7402 indicated that (R)-ethyl 2-(2-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-(4-hydroxyphenyl) propanoate showed more inhibitory effect against BEL-7402 than 5-FU.
Keywords: 5-fluorouracil, amino acid ester, antitumor activity
1. Introduction
5-Fluorouracil (5-FU) is an antimetabolite of the pyrimidine analogue type, which is frequently used for treating solid tumors, such as colorectal, gastric tract, and liver carcinomas [1,2,3]. However, the clinical applications of 5-FU are greatly limited by its short plasma half-life, poor tumor affinity, myelosuppression, and strong intestinal toxicity. Consequently, numerous research efforts have focused on the discovery of suitable carrier-linked prodrugs, in which 5-FU is conjugated with a wide spectrum of low- or high- molecular-weight carriers including glucose, peptides, and biodegradable polymers such as polysaccharides, liposomes, etc [4,5,6,7,8,9,10]. In general prodrug systems the drug is bound to the carrier through a spacer that incorporates a predetermined breaking point that allows the bound drug to be released at the cellular target site. Therefore, the optimization physicochemical properties of a carrier, the modification of the carrier with 5-FU to preserve the targeting properties of the carrier and ensure a controlled release of 5-FU inside or outside the tumor cells are the critical aspects of 5-FU prodrug design [3,11].
Peptides play an important role in human metabolism. Some peptide derivatives of 5-FU have been reported as an approach to develop chemotherapeutic agents with improved physicochemical and biological characteristics [4,12,13], and we also have previously reported some peptide derivatives of 5-FU [14,15,16]. In continuation of the research, we now describe our studies on the synthesis and assessment of some amino acid ester derivatives containing 5-FU with the aim of finding appropriate biodegradable linkages.
2. Results and Discussion
2.1. Chemistry
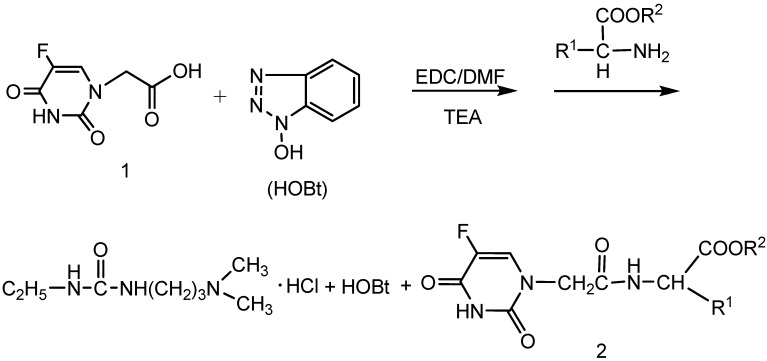
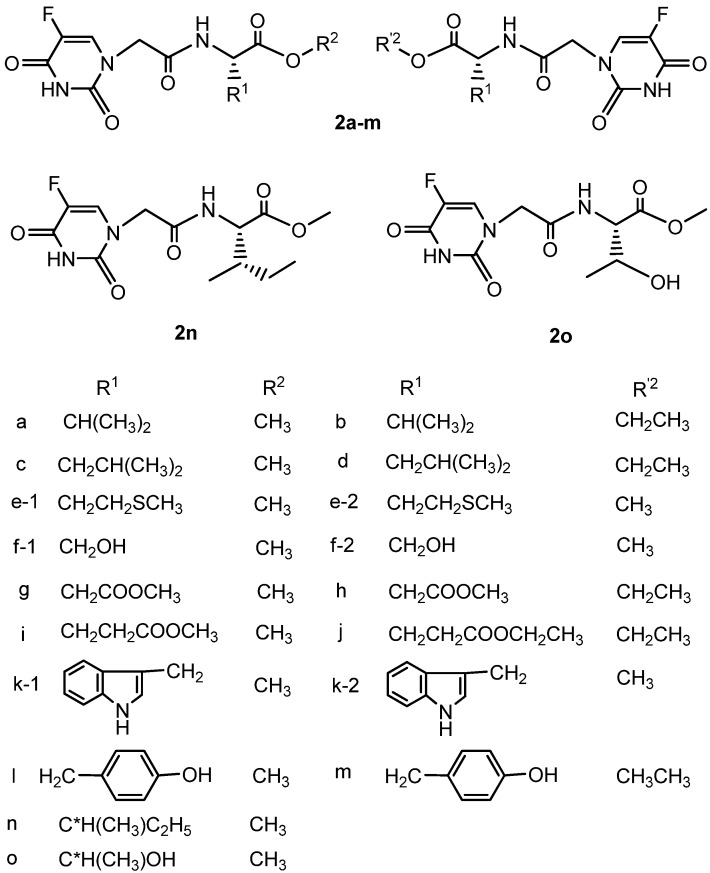
The synthetic route to the target compounds 2a-o is shown in Scheme 1 and Figure 1. The starting material 2-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) acetic acid (or 5-fluorouracil-1-yl acetic acid) (1) could be easily prepared by carboxymethylation of 5-fluorouracil according to the literature [17]. Treatment of compound 1 with a series of amino acid esters using 1-ethyl-3-(3-dimethylamino-propyl)carbodiimide hydrochloride (EDC·HCl) and N-hydroxybenzotriazole (HOBt) as a coupling agent yielded a series of 2-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-aceto amino acid ester derivatives 2. HOBt was reported as a racemisation suppressant in peptide coupling reactions with carbodiimide coupling reagents [18,19,20].
Scheme 1.
Synthesis of 2-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-aceto amino acid ester derivatives 2.
Figure 1.
Structural formulae of compounds 2.
The purity and structures of compounds 2a-o were established on the basis of their melting points, specific rotations and spectral data, which were in full agreement with the proposed molecular structures. The 1H-NMR spectra of all compounds showed doublets at 7.93-8.02 ppm, which corresponded to the coupling of fluorine and hydrogen signals in the FC=CH moieties. Compounds 2e-1 and 2e-2, for example, almost have the same melting point (139-140 °C), the same spectral data, but opposite specfic rotations of −10.4 and +10.4, respectively. In the 1H-NMR their CH2SCH3 fragment methylene protons were observed as multiplets at δ 2.50-2.41 ppm, which overlapped with the signal of the solvent DMSO-d6. The 13C-NMR of 2c and 2d displayed signals at δ 39.7 ppm from the methylene carbon from the CH2CH(CH3)2 moiety which overlapped as well with that of the solvent DMSO-d6,. The assignment of the above four compounds were further proven by 13C-1H COSY spectra.
2.2. In vitro antitumor activity
All target compounds 2a-o were evaluated for their in vitro antitumor activity against the HL-60 leukaemia and BEL-7402 liver cancer cell lines by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazoliumbromide (MTT) [21] and Sulforhodamine B (SRB) assay methods [22], respectively, with 5-FU and the prodrug FT-207 being used for comparisons (Table 1 and Table 2).
Table 1.
Inhibitory rates (%) against HL-60.
| Compounds | Concentration (mol/L) | ||||
|---|---|---|---|---|---|
| 10−4 | 10−5 | 10−6 | 10−7 | 10−8 | |
| 2a | 2.0 | 8.0 | 9,6 | 6.9 | 8.3 |
| 2b | 3.7 | 5.2 | 6.2 | 9.7 | 2.8 |
| 2c | 33.1 | 0.1 | 9.9 | 9.8 | 0 |
| 2d | 27.3 | 0 | 1.7 | 0 | 10.0 |
| 2e-1 | 22.4 | 11.2 | 7.1 | 4.2 | 1.2 |
| 2e-2 | 18.5 | 15.3 | 3.4 | 10.5 | 0 |
| 2f-1 | 3.3 | 10.8 | 6.6 | 12.3 | 0 |
| 2f-2 | 31.8 | 9.2 | 3.7 | 6.2 | 0 |
| 2g | 36.1 | 10.0 | 5.7 | 5.0 | 2.5 |
| 2h | 55.7 | 19.6 | 23.1 | 2.3 | 8.6 |
| 2i | 2.8 | 6.7 | 1.5 | 8.3 | 0.6 |
| 2j | 55.8 | 12.8 | 2.7 | 5.4 | 5.9 |
| 2k-1 | 29.2 | 0 | 3.8 | 8.2 | 2.4 |
| 2k-2 | 51.2 | 15.5 | 9.8 | 12.7 | 8.9 |
| 2l | 42.4 | 7.9 | 5.2 | 9.9 | 5.7 |
| 2m | 65.1 | 0 | 12.6 | 13.0 | 0.3 |
| 2n | 11.4 | 0 | 0 | 0 | 0 |
| 2o | 22.4 | 11.2 | 7.1 | 4.2 | 1.2 |
| 5-FU | 57.4 | 33.5 | 0 | 7.0 | 10.4 |
| FT-207 | 0 | 0 | 0 | 0 | 0 |
Table 2.
Inhibitory rates (%) against BEL-7402.
| Compounds | Concentration (mol/L) | ||||
|---|---|---|---|---|---|
| 10−4 | 10−5 | 10−6 | 10−7 | 10−8 | |
| 2a | 0 | 0 | 0 | 0 | 0 |
| 2b | 9.0 | 8.1 | 0 | 0 | 1.8 |
| 2c | 50.0 | 13.2 | 5.7 | 5.2 | 0 |
| 2d | 13.2 | 0 | 0 | 0 | 0 |
| 2e-1 | 41.2 | 9.7 | 8.8 | 8.2 | 9.1 |
| 2e-2 | 38.4 | 9.1 | 8.8 | 0 | 5.3 |
| 2f-1 | 17.4 | 10.5 | 16.6 | 14.0 | 4.6 |
| 2f-2 | 41.2 | 9.8 | 0 | 0 | 0 |
| 2g | 36.1 | 11.1 | 7.0 | 7.4 | 2.4 |
| 2h | 52.6 | 15.9 | 2.4 | 0.7 | 0 |
| 2i | 14.5 | 8.7 | 8.0 | 4.6 | 11.7 |
| 2j | 34.0 | 9.2 | 4.1 | 3.5 | 5.9 |
| 2k-1 | 35.9 | 0 | 6.9 | 3.0 | 0.7 |
| 2k-2 | 22.4 | 11.9 | 7.1 | 4.2 | 1.2 |
| 2l | 36.2 | 10.2 | 4.9 | 0.5 | 0 |
| 2m | 71.7 | 68.3 | 60.4 | 43.1 | 24.3 |
| 2n | 8.2 | 4.5 | 5.2 | 0 | 0 |
| 2o | 9.7 | 0 | 8.8 | 8.2 | 9.1 |
| 5-FU | 72.6 | 53.8 | 35.0 | 23.8 | 16.6 |
| FT-207 | 58.0 | 8.1 | 0 | 0 | 0 |
As shown in Table 1, all compounds’ in vitro inhibition rates against HL-60 were significantly lower than that of 5-FU, except for the R-type compounds 2h, 2j, 2k-2 and 2m, which exhibited equivalent inhibitory effect as 5-FU at 10-4 mol/L concentration, but the activity decreased rapidly when the concentration declined. The results indicate that these compounds were less sensitive to HL-60 at lower concentrations when the N-1 position of 5-FU was occupied.
In Table 2, almost all the compounds showed less sensitivity to BEL-7402, except 2m, which showed more potent inhibitory effect than 5-FU. The reason maybe was the R-conformation of 2m with a moderately rigid stereo structure, being composed of the pyrimidine ring and the phenyl ring, so it could release 5-FU sufficiently, while other compounds showed either more flexible configurations (such as 2a-e), or a more rigid structure such as the case of 2k [23]. The different inhibition against HL-60 and BEL-7402 between R-type and S-type compounds suggested the complexity of the antitumor mechanism.
3. Experimental
3.1. General
Melting points of synthesized compounds were determined on a Digital Melting Point Appatatus X-4 and were uncorrected. Mass spectra were obtained on a DECAX-30000 LCQ DecaXP Plus instrument. IR spectra were recorded (in KBr) on a Bruker EQUINOX 55. 1H-NMR and 13C-NMR were recorded on Bruker AVANCE-300 at 300 and 75 MHz, respectively in DMSO-d6 solutions with TMS as internal standard.
3.2. General procedure for the synthesis of compounds 2a-o
Synthesis of compounds 2a-o was accomplished as shown in Scheme 1. 2-(5-Fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) acetic acid (10 mmol), HOBt (10 mmol) and DMF (50 mL) were added to a round-bottom flask, then EDC·HCl (13 mmol), L- or D-amino acid ester hydrochloride (10 mmol), and triethylamine (20 mmol) were added to the above mixture. After 10 h reaction at room temperature with thin layer chromatography (TLC) monitoring, the white solid 5-fluorouracil-1-yl-aceto amino acid esters 2a-o were obtained after filtration, reduced pressure distillation of DMF, and silica gel column chromatography separation.
(S)-Methyl 2-(2-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-methylbutanoate (2a). Yield: 68%; m.p. 109-110°C; 1H-NMR δ: 11.80 (s, 1H, NH of 5-FU), 8.54 (d, 1H, NH, J = 8.1 Hz), 8.01 (d, 1H, FC=CH, 3JFH = 6.9 Hz), 4.39 (d, 2H, NCH2, J = 16.8 Hz), 4.22 (t, 1H, NCH, J = 7.2 Hz), 3.65 (s, 3H, OCH3), 2.09-1.98 (m, 1H, CCH, J = 6.6 Hz), 0.89 (d, 3H, CH3, J = 6.6 Hz), 0.87 (d, 3H, CH3, J = 6.6 Hz); 13C-NMR δ: 171.9, 167.1, 157.7(d, 2JFC = 25.6 Hz), 149.8, 139.3 (d, 1JFC = 226.7 Hz), 131.3 (d, 2JFC = 33.6 Hz), 57.7, 51.9, 49.5, 30.4, 19.0, 18.3; IR (cm-1) ν: 3456, 3280, 2969, 1722, 1666, 1560, 1467, 1379, 1227, 1146, 783; MS (ESI) m/z: 300 (M-); -22.0 (c 1.0, DMF).
(R)-Ethyl 2-(2-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-methylbutanoate (2b). Yield: 65%; m.p. 136-137 °C; 1H-NMR δ: 11.83(s, 1H, NH of 5-FU), 8.53 (d, 1H, NH, J = 8.1 Hz), 8.02 (d, 1H, FC=CH, 3JFH = 6.6 Hz), 4.38 (d, 2H, NCH2, J = 16.8 Hz), 4.19 (dd, 1H, NCH, J = 8.1, 6.3 Hz), 4.16-4.05 (m, 2H, COOCH2, J = 7.2 Hz), 2.11-1.98 (m, 1H, CCH), 1.19 (t, 3H, OCH2CH3, J = 7.2 Hz), 0.89 (d, 3H, CH3, J = 6.9 Hz), 0.88 (d, 3H, CH3, J = 6.6 Hz); 13C-NMR δ: 174.0, 169.6, 160.5 (d, 2JFC = 25.3 Hz), 151.6, 141.2 (d, 1JFC = 228.1 Hz), 132.8 (d, 2JFC = 33.5 Hz), 63.3, 59.9, 51.6; 31.6, 20.0, 19.2, 15.2; IR (cm-1) ν: 3295, 3253, 2977, 1707, 1552, 1467, 1377, 1225, 1148; MS (ESI) m/z: 314(M-); +14.0 (c 0.1, DMF).
(S)-Methyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-4-methylpentanoate (2c). Yield: 75%; m.p. 144-145°C; 1H-NMR δ: 11.83 (d, 1H, NH of 5-FU, 4JFH = 5.4 Hz), 8.60 (d, 1H, NH, J = 7.8 Hz), 8.02 (d, 1H, FC=CH, 3JFH = 6.9 Hz), 4.32 (s, 2H, NCH2), 4.35-4.27 (m, 1H, NCH), 3.62 (s, 3H, OCH3), 1.66-1.59 (m, 1H, CCH), 1.54-1.46 (m, 2H, CCH2), 0.88 (d, 3H, CH3, J = 6.3 Hz), 0.83 (d, 3H, CH3, J = 6.3 Hz); 13C-NMR δ 172.9, 167.0, 157.8 (d, 2JFC = 25.5 Hz), 149.8, 139.4 (d, 1JFC = 226.6 Hz), 131.3 (d, 2JFC = 33.8 Hz), 52.2, 50.5, 49.6, 39.7, 24.3, 22.9, 21.6; IR (cm-1) ν: 3323, 3046, 2959, 1666, 1543, 1472, 1384, 1244, 1155; MS (ESI) m/z: 314(M-); -19.2 (c 1.0, DMF).
(R)-Ethyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-4-methylpentanoate (2d). Yield: 72%; m.p. 146-147°C; 1H-NMR δ: 11.81(d, 1H, NH of 5-FU, 4JFH = 5.1 Hz), 8.57(d, 1H, NH, J =7.8 Hz), 8.01(d, 1H, FC=CH, 3JFH = 6.9 Hz), 4.32(s, 2H, NCH2), 4.30-4.24(m, 1H, NCH), 4.08(q, 2H, OCH2, J = 7.2 Hz), 1.67-1.58(m, 1H, CCH), 1.56-1.48(m, 2H, CCH2), 1.17(t, 3H, OCH2CH3, J = 7.2 Hz), 0.89(d, 3H, CH3, J = 6.3 Hz), 0.84(d, 3H, CH3, J = 6.3 Hz); 13C-NMR(75 MHz) δ: 172.3, 166.8, 157.7(d, 2J FC = 25.2 Hz), 149.7, 139.3(d, 1JFC = 226.4 Hz), 131.2 (d, 2JFC = 33.9 Hz), 60.7, 50.6, 49.5, 39.7, 24.3, 22.8, 21.5, 14.1; IR(KBr, cm-1) ν: 3328, 2963, 2818, 1690, 1637, 1555, 1473, 1377, 1238, 1196, 1150; MS(ESI) m/z: 328(M-); +11.6(c 1.0, DMF).
(S)-methyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-4-(methylthio)butanoate (2e-1). Yield: 62%; m.p. 139-140°C; 1H-NMR(300 MHz) δ: 11.84(s, 1H, NH of 5-FU), 8.64(d, 1H, NH, J = 7.5 Hz), 8.02(d, 1H, FC=CH, 3J = 6.9 Hz), 4.46-4.39(m, 1H, NCH), 4.33(s, 2H, NCH2), 3.63(s, 3H, OCH3), 2.50-2.48(m, 2H, CH2S), 2.02(s,3H, SCH3), 1.97-1.79(m, 2H, CCH2); 13C-NMR(75 MHz) δ: 172.2, 167.1, 157.8(d, 2JFC = 25.7 Hz), 149.9, 139.5(d, 1JFC = 226.5 Hz), 131.2(d, 2JFC = 33.8 Hz), 52.3, 51.1, 49.7, 30.9, 29.5, 14.8; IR(KBr, cm-1) ν: 3345, 3042, 2983, 1687, 1662, 1542, 1474, 1428, 1386, 1233; MS(ESI) m/z: 332(M-); -10.4(c 1.0, DMF).
(R)-methyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-4-(methylthio)butanoate (2e-2). Yield: 59%; m.p. 138-140 °C; 1H-NMR(300 MHz) δ: 11.83(s, 1H, NH of 5-FU), 8.63(d, NH, J = 7.6 Hz), 8.02(d, 1H, FC=CH, 3JFH = 6.0 Hz), 4.43-4.38(m, 1H, NCH), 4.33(s, 2H, NCH2), 3.64(s, 3H, OCH3), 2.47-2.43(m, 2H, CH2S), 2.03(s, 3H, SCH3), 1.95-1.87(m, 2H, CCH2); 13C-NMR(75 MHz) δ: 172.1, 167.1, 157.8(d, 2JFC = 25.7 Hz), 149.8, 139.5(d, 1JFC = 226.7 Hz), 131.2 (d, 2JFC = 33.7 Hz), 52.3, 51.1, 49.6, 30.9, 29.5, 14.7; IR(KBr, cm-1) ν: 3348, 3237, 2962, 1725, 1660, 1569, 1478, 1425, 1381, 1345, 1305, 1246, 1162, 794; MS(ESI) m/z: 332(M-); +10.4(c 1.0, DMF).
(S)-methyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-hydroxypropanoate (2f-1). Yield: 54%; m.p. 190-191°C; 1H-NMR(300 MHz) δ: 11.83(d, 1H, NH of 5-FU, 4JHH = 4.5 Hz), 8.63(d, 1H, NH, J = 8.1 Hz), 8.01(d, 1H, FC=CH, 3JFH = 6.9 Hz), 5.11(t, 1H, OH, J = 5.4 Hz), 4.38(s, 2H, NCH2), 4.42-4.36(m, 1H, NCH), 3.65(s, 3H, OCH3), 3.75-3.58(m, 2H, CH2OH, J = 5.4, 10.8 Hz); 13C-NMR(75 MHz) δ: 170.9, 167.0, 157.6(d, 2JFC = 25.7 Hz), 149.8, 139.3(d, 1JFC = 226.7 Hz), 131.2(d, 2JFC = 33.7 Hz), 61.4, 54.8, 52.0, 49.4; IR(KBr, cm-1) ν: 3459, 3295, 2850, 1713, 1692, 1563, 1467, 1416, 1382, 1277, 1251, 1185, 1071; MS(ESI) m/z: 288(M-). -10.0 (c 1.0, DMF).
(R)-methyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-hydroxypropanoate (2f-2). Yield: 55%; m.p. 190-192°C; 1H-NMR(300 MHz) δ: 11.81(s, 1H, NH of 5-FU), 8.62(d, 1H, NH, J = 7.5 Hz), 8.00(d, 1H, FC=CH, 3JFH = 6.9 Hz), 5.12(t, 1H, OH, J = 5.4 Hz), 4.37(s, 2H, NCH2), 4.41-4.35(m, 1H, NCH), 3.63(s, 3H, OCH3), 3.74-3.56(m, 2H, CH2OH, J = 5.4, 10.8 Hz); 13C-NMR(75 MHz) δ: 171.0, 167.0, 157.7(d, 2JFC = 25.6 Hz), 149.8, 139.3(d, 1JFC = 226.6 Hz), 131.4(d, 2JFC = 33.5 Hz), 61.5, 54.9, 52.1, 49.5; IR(KBr, cm-1) ν: 3459, 3295, 2850, 1713, 1692, 1563, 1467, 1416, 1382, 1277, 1251, 1185, 1071; MS(ESI) m/z: 288(M-). +10.0 (c 1.0, DMF).
(S)-dimethyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)succinate (2g). Yield: 65%; m.p. 145-147 °C; 1H-NMR(300 MHz) δ: 11.85(d, 1H, NH of 5-FU, 4JFH = 5.1 Hz), 8.76(d, 1H, NH, J =7.8 Hz), 8.01(d, 1H, FC=CH, 3JFH = 6.6 Hz), 4.68(dd, 1H, NCH, J =7.5, 6.6 Hz), 4.33(s, 2H, NCH2), 3.64(s, 3H, OCH3), 3.62(s, 3H, OCH3), 2.85-2.70(m, 2H, CCH2, J = 6.6, 7.5, 16.8 Hz); 13C-NMR(75 MHz) δ: 170.9, 170.5, 166.9, 157.7(d, 2JFC = 25.7 Hz), 149.8, 139.4(d, 1JFC = 228.0 Hz), 131.1 (d, 2JFC = 33.9 Hz), 52.4, 51.9, 49.5, 48.7, 35.9; IR(KBr, cm-1) ν: 3348, 3191, 2850, 1755, 1697, 1526, 1430, 1380, 1337, 1216, 1047, 980; MS(ESI) m/z: 330(M-); -14.4(c 1.0, DMF).
(R)-diethyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)succinate (2h). Yield: 68%; m.p. 116-118 °C; 1H-NMR(300 MHz) δ: 11.82(d, 1H, NH of 5-FU, 4JFH = 4.8 Hz), 8.71(d, 1H, NH, J = 7.8 Hz), 7.99(d, 1H, FC=CH, 3JFH = 6.8 Hz), 4.63(dd, 1H, NCH, J = 6.6, 7.5 Hz), 4.33(s, 2H, NCH2), 4.12-4.03(m, 4H, OCH2, J = 6.9 Hz), 2.81-2.65(m, 2H, CCH2, J = 6.6, 16.5 Hz), 1.17(t, 3H, CH3, J = 6.9 Hz), 1.16(t, 3H, CH3, J = 6.9 Hz); 13C-NMR(75 MHz) δ: 170.4, 170.0, 166.9, 157.7(d, 2JFC = 25.7 Hz), 149.8, 139.4(d, 1JFC = 228.3 Hz), 131.2 (d, 2JFC = 33.8 Hz), 61.2, 60.6, 49.5, 48.9, 36.1, 14.2, 14.1; IR(KBr, cm-1) ν: 3314, 3217, 2990, 1721, 1691, 1549, 1467, 1378, 1339, 1240, 1167, 1021, 792; MS(ESI) m/z: 358(M-); +16.0 (c 1.0, DMF).
(S)-dimethyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)pentanedioate (2i). Yield: 42%; m.p. 147-148 °C; 1H-NMR(300 MHz) δ: 11.85(s, 1H, NH of 5-FU), 8.63(d, 1H, NH, J =7.8 Hz), 8.02(d, 1H, FC=CH, 3JFH = 6.9 Hz), 4.32(s, 2H, NCH2), 4.37-4.34(m, 1H, NCH), 3.63(s, 3H, OCH3), 3.58(s, 3H, OCH3), 2.37(t, 2H, CH2CH2CO, J = 7.5 Hz), 2.06-1.76(m, 2H, CH2CH2CO); 13C-NMR(75 MHz) δ: 172.7, 171.9, 168.0, 157.7(d, 2JFC = 25.7 Hz), 149.8, 139.4(d, 1JFC = 226.7 Hz), 131.1(d, 2JFC = 33.7 Hz), 52.2, 51.5, 51.3, 49.6, 29.6, 26.4; IR(KBr, cm-1) ν: 3328, 2964, 1716, 1660, 1541, 1449, 1348, 1261, 800; MS(ESI) m/z: 344(M-); -8.39 (c 0.5, DMF).
(R)-diethyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)pentanedioate (2j). Yield: 45%; m.p. 108-109 °C; 1H-NMR(300 MHz) δ: 11.84(s, 1H, NH of 5-FU), 8.61(d, 1H, NH, J = 7.8 Hz), 8.01(d, 1H, FC=CH, 3JFH = 6.9 Hz), 4.33(s, 2H, NCH2), 4.30-4.26(m, 1H, NCH), 4.08(q, 2H, OCH2CH3, J = 6.9 Hz), 4.04(q, 2H, OCH2CH3, J = 7.2 Hz), 2.36(t, 2H, CH2CH2CO, J = 7.5 Hz), 2.03-1.78(m, 2H, CH2CH2CO), 1.17(t, 3H, OCH2CH3, J = 6.9 Hz), 1.16(t, 3H, OCH2CH3, J = 7.2 Hz); 13C-NMR(75 MHz) δ: 172.2, 171.4, 167.0, 157.7(d, 2JFC = 25.7 Hz), 149.8, 139.4(d, 1JFC = 226.9 Hz), 131.2(d, 2JFC = 33.7 Hz), 60.9, 60.1, 51.5, 49.6, 29.9, 26.5, 14.2, 14.1; IR(KBr, cm-1) ν: 3304, 3213, 2924, 1725, 1675, 1546, 1468, 1416, 1379, 1250, 1176, 1023; MS(ESI) m/z: 372 (M-); +12.40 (c 1.0, DMF).
(S)-methyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-(1H-indol-3-yl)pro-panoate (2k-1). Yield: 50%; m.p. 205-206 °C; 1H-NMR(300 MHz) δ: 11.84(s, 1H, NH of 5-FU), 10.90(s, 1H, NH of indole), 8.72(d, 1H, NH, J = 7.2 Hz), 7.93(d, 1H, FC=CH, 3JFH = 6.8 Hz), 7.48(d, 1H, Ar-H, J = 7.5 Hz), 7.33(d, 1H, Ar-H, J = 8.1 Hz), 7.16(d, 1H, =CHN, J = 2.2 Hz), 7.07(t, 1H, Ar-H, J = 8.1, 6.9 Hz), 6.99(t, 1H, Ar-H, J = 7.5, 6.9 Hz), 4.54(dd, 1H, NCH, J = 7.2, 6.6 Hz), 4.33(d, 2H, NCH2, J = 16.5 Hz), 3.56(s, 3H, OCH3), 3.19-3.03(m, 2H, CCH2, J = 7.5, 6.0, 14.4 Hz); 13C-NMR(75 MHz) δ: 172.2, 166.9, 157.8(d, 2JFC = 25.7 Hz), 149.9, 139.4(d, 1JFC = 228.1 Hz), 136.3, 131.3(d, 2JFC = 33.8 Hz), 127.3, 124.1, 121.2, 118.7, 118.2, 111.7, 109.2, 53.6, 52.1, 49.5, 27.4; IR(KBr, cm-1) ν: 3386, 3312, 3069, 2977, 1702, 1545, 1381, 1233, 1062; MS(ESI) m/z: 387(M-); +36.4 (c 1.0, DMF).
(R)-methyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-(1H-indol-3-yl)pro-panoate (2k-2). Yield: 53%; m.p. 205-206 °C; 1H-NMR(300 MHz) δ: 11.84(d, 1H, NH of 5-FU, 4JFH = 5.1 Hz), 10.90(s, 1H, NH of indole), 8.72(d, 1H, NH, J = 7.5 Hz), 7.93(d, 1H, FC=CH, 3JFH = 6.9 Hz), 7.48(d, 1H, Ar-H, J = 7.8 Hz), 7.34(d,1H, Ar-H, J = 8.1 Hz), 7.16(s, 1H, =CHN), 7.07(t, 1H, Ar-H, J = 7.2, 7.8 Hz), 6.99(t, 1H, Ar-H, J = 7.2 Hz), 4.55(dd, 1H, NCH, J = 6.9, 6.6 Hz), 4.33(d, 2H, NCH2, J = 16.5 Hz), 3.57(s, 3H, OCH3), 3.20-3.03(m, 2H, CCH2, J = 7.5, 6.0, 14.8 Hz); 13C-NMR(75 MHz) δ: 172.2, 166.9, 157.8 (d, 2JFC = 25.7 Hz), 149.9, 139.4(d, 1JFC = 228.2 Hz), 136.3, 131.3(d, 2JFC = 33.8 Hz), 127.3, 124.1, 121.2, 118.7, 118.2, 111.7, 109.2, 53.5, 52.1, 49.5, 27.4; IR(KBr, cm-1) ν: 3392, 3320, 3075, 1732, 1648, 1542, 1446, 1385, 1355, 1248, 1219,1099, 744; MS(ESI) m/z: 387(M-); -36.4 (c 1.0, DMF).
(S)-methyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-(4-hydroxyphenyl) propanoate (2l). Yield: 72%; m.p. 192-193 °C; 1H-NMR(300 MHz) δ: 11.85(s, 1H, NH of 5-FU), 9.28(s, 1H, OH), 8.68(d, 1H, NH, J = 7.5 Hz), 7.94(d, 1H, FC=CH, 3JFH = 6.9 Hz), 6.99 (d, 2H, Ar-H, J = 8.4 Hz), 6.66 (d, 2H, Ar-H, J = 8.4 Hz), 4.39(dd, 1H, NCH, J = 6.3, 7.5 Hz), 4.31(d, 2H, NCH2, J = 16.8 Hz), 3.59(s, 3H, OCH3), 2.93-2.77(m, 2H, CH2Ar, J = 6.3, 8.1, 13.8 Hz); 13C-NMR(75 MHz) δ: 171.9, 166.9, 157.7(d, 2JFC = 25.7 Hz), 156.3, 149.8, 139.4(d, 1JFC = 226.9 Hz), 131.2(d, 2JFC = 33.8 Hz), 130.3, 127.0, 115.3, 54.4, 52.0, 49.5, 36.3; IR(KBr, cm-1) ν: 3271, 1739, 1712, 1661, 1552, 1516, 1451, 1386, 1231, 1164, 778; MS(ESI) m/z: 364(M+); +16.6 (c 1.0, DMF).
(R)-ethyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-(4-hydroxyphenyl) propanoate (2m). Yield: 70%; m.p. 156-158 °C; 1H-NMR(300 MHz) δ: 11.82(s, 1H, NH of 5-FU), 9.23(s, 1H, OH), 8.63(d, 1H, NH, J = 7.5 Hz), 7.94(d, 1H, FC=CH, 3JFH = 6.6 Hz), 6.99(d, 2H, Ar-H, J = 8.4 Hz), 6.65(d, 2H, Ar-H, J = 8.4 Hz), 4.36(dd, 1H, NCH, J = 7.5, 6.9 Hz), 4.31(d, 2H, NCH2, J = 16.8 Hz), 4.02(q, 2H, OCH2, J = 7.2 Hz), 2.89-2.77(m, 2H, CH2Ar, J = 7.5, 6.0, 13.8 Hz), 1.10(t, 3H, CH3, J = 7.2 Hz); 13C-NMR(75 MHz) δ: 171.4, 166.9, 157.7(d, 2JFC = 25.7 Hz), 156.3, 149.8, 139.4(d, 1JFC = 228.1 Hz), 131.2(d, 2JFC = 33.9 Hz), 130.3, 127.0, 115.3, 60.7, 54.4, 49.5, 36.4, 14.1; IR(KBr, cm-1) ν: 3336, 3065, 2851, 1691, 1532, 1380, 1340, 1220, 801; MS(ESI) m/z: 378(M-); -24.4 (c 1.0, DMF).
(2S,3S)-methyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-methylpentanoate (2n). Yield: 70%; m.p. 152-154 °C; 1H-NMR(300 MHz) δ: 11.82(d, 1H, NH of 5-FU, 4JFH = 5.1 Hz), 8.57(d, 1H, NH, J = 8.1 Hz), 8.01(d, 1H, FC=CH, 3JFH = 6.9 Hz), 4.36(d, 2H, NCH2, J = 16.8 Hz), 4.25(dd, 1H, NCH, J = 8.1, 6.6 Hz), 3.63(s, 3H, OCH3), 1.77-1.72(m, 1H, CCH), 1.43-1.09(m, 2H, CCH2), 0.84(t, 3H, CH2CH3, J = 7.2 Hz), 0.83(d, 3H, CHCH3, J = 6.9 Hz); 13C-NMR(75 MHz) δ: 172.0, 167.1, 157.7(d, 2JFC = 25.7 Hz), 149.8, 139.3(d, 1JFC = 227.9 Hz), 131.4(d, 2JFC = 33.8 Hz), 56.6, 52.0, 49.5, 36.9, 24.9, 15.6, 11.3; IR(KBr, cm-1) ν: 3275, 3083, 2968, 1712, 1666, 1571, 1460, 1378, 1340, 1243, 1149, 976, 700; MS(ESI) m/z: 314(M-); -3.0 (c 0.5, DMF).
(2S,3S)-methyl 2-(2-5-(fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido)-3-hydroxybutanoate (2o). Yield: 47%; m.p. 207-208 °C; 1H-NMR(300 MHz) δ: 11.79(d, 1H, NH of 5-FU, 4JFH = 5.1 Hz), 8.44(d, 1H, NH, J = 8.4 Hz), 8.01(d, 1H, FC=CH, 3JFH = 6.6 Hz), 5.03(d, 1H, OH, J = 5.1 Hz), 4.42(dd, 2H, NCH2, J = 6.6, 16.5 Hz), 4.32(dd, 1H, NCH, J = 8.4, 3.3 Hz), 4.15-4.08(m, 1H, CHOH, J = 3.3, 5.1, 6.3 Hz), 1.05(d, 3H, CH3, J = 6.3 Hz); 13C NMR(75 MHz) δ 171.0, 167.4, 157.7(d, 2JFC = 25.6 Hz), 149.8, 139.3(d, 1JFC = 226.6 Hz), 131.4(d, 2JFC = 33.5 Hz), 66.5, 58.1, 52.0, 49.6, 20.2; IR(KBr, cm-1) ν: 3484, 3312, 2977, 1718, 1663, 1558, 1376, 1283, 1238, 1140; MS(ESI) m/z: 302(M-); -2.0 (c 0.1, DMF).
4. Conclusions
A serials of amino acid ester derivatives containing 5-fluorouracil were synthesized by EDC/HOBt method and characterized. The in vitro antitumor activity tests indicated that the synthesized compounds had less inhibition rates against HL-60 and BEL-7402 than 5-FU except compound 2m, which showed more potent inhibitory effect against BEL-7402 than 5-FU. This might be explained by the R configuration of compound 2m with the moderate rigid framework composed of pyrimidine ring and hydroxyphenyl ring, which may be easily to give 5-fluorouracil.
Acknowledgements
We thank the National Centre for Drug Screening, Shanghai, China, for evaluation of the in vitro antitumor activities. This research was supported by Zhejiang Provincial Technology Project Foundation (No. 2008C21034) and Wenzhou Technology Project Foundation (No. S20060029).
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Smith N.F., Figg W.D., Sparreboom A. Recent advances in pharmacogenetic approaches to anticancer drug development. Drug Develop. Res. 2004;62:233–253. doi: 10.1002/ddr.10361. [DOI] [Google Scholar]
- 2.Ragnhammar P., Blomgren H.A. How to optimize the effect of 5-fluorouracil modulated therapy in advanced colorectal cancer. Med. Oncol. 1995;12:187–201. doi: 10.1007/BF01571196. [DOI] [PubMed] [Google Scholar]
- 3.Arias J.L. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules. 2008;13:2340–2369. doi: 10.3390/molecules13102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichifor M., Schacht E.H. Synthesis of peptide derivatives of 5-fluorouracil. Tetrahedron. 1994;50:3747–3760. doi: 10.1016/S0040-4020(01)90395-3. [DOI] [Google Scholar]
- 5.Sloan K.B., Wasdo S. Designing for topical delivery: Prodrugs can make the difference. Med. Res. Rev. 2003;23:763–793. doi: 10.1002/med.10048. [DOI] [PubMed] [Google Scholar]
- 6.Krishnaiah Y.S.R., Satyanarayana V., Kumar B.D., Karthikeyan R.S. In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5-fluorouracil. Eur. J. Pharm. Sci. 2002;16:185–192. doi: 10.1016/S0928-0987(02)00081-7. [DOI] [PubMed] [Google Scholar]
- 7.Lee S.M, Lee N.J., Ha C.K., Cho W.J. Syntheses and biological activities of polymers containing methacryloyl-2-oxy-1,2,3-propanetricarboxylic acid of 5-fluorouracil. J. Appl. Polym. Sci. 2004;94:57–64. doi: 10.1002/app.20713. [DOI] [Google Scholar]
- 8.Nichifor M, Schacht E.H., Seymour L.W. Polymeric prodrug of 5-fluorouracil. J. Control. Release. 1997;48:165–178. doi: 10.1016/S0168-3659(97)00048-5. [DOI] [Google Scholar]
- 9.Yang Y.W., Lee J.S., Kim I., Jung Y.J., Kim Y.M. Synthesis and properties of N-nicotinoyl-2-(5-fluorouracil-1-yl)-D,L-glycine ester as a prodrug of 5-fluorouracil for rectal administration. Er. J. Pharm. Biopharm. 2007;66:260–267. doi: 10.1016/j.ejpb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Elorza B., Elorza M.A., Frutos G., Chantres J.R. Characterization of 5-fluorouracil loaded liposomes prepared by reverse-phase evaporation or freezing-thawing extrusion methods: Study of drug release. Biochim. Biophys. Acta. 1993;1153:135–142. doi: 10.1016/0005-2736(93)90398-J. [DOI] [PubMed] [Google Scholar]
- 11.Kratz F., Müller L.A., Ryppa C., Warnecke A. Prodrug strategies in anticancer chemotherapy. ChemMedChem. 2008;3:20–53. doi: 10.1002/cmdc.200700159. [DOI] [PubMed] [Google Scholar]
- 12.Liu X.J., Chen R.Y., Yang Y.Y. Synthesis and anticancer activities of novel 5-fluorouracil-1-yl phosphonotripeptides. Chem. J. Chin. Univ. 2002;23:1299–1303. [Google Scholar]
- 13.Yin P., Hu M.L., Hu L.C. Synthesis, structural characterization and anticarcinogenic activity of a new Gly-Gly dipeptide derivative: Methyl 2-(2-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamido) acetate. J. Mol. Struct. 2008;882:75–79. doi: 10.1016/j.molstruc.2007.09.017. [DOI] [Google Scholar]
- 14.Xiong J., Lei X.X., Hu M.L., Yuan J.X., Cai X.Q. Crystal structure of 2-(5-fluoro-2,4-dioxo-1,2,3,4-tetrahydropyrimidine) glycin, C8H8FN3O5. Z. Kristallogr. NCS. 2006;221:37–38. doi: 10.1524/ncrs.2006.221.1.37. [DOI] [Google Scholar]
- 15.Yin P., Hu M.L., Xiong J., Yuan J.X. Crystal structure of 2(R)-(5-fluorouracil-1-yl)acetyl-2-phenylglycin dimethylformamide solvate, C14H12FN3O5·C3H7NO. Z. Kristallogr. NCS. 2006;221:39–40. [Google Scholar]
- 16.Yin P., Hu M.L., Xiong J., Cai X.Q. Methyl (2S)-2-[2-(5-fluoro-2,4-dioxo-3,4-dihydropy-rimidin-1-yl)acetamido] propionate monohydrate. Acta Crystallogr. E. 2006;E62:o1745–o1746. doi: 10.1107/S1600536806011184. [DOI] [Google Scholar]
- 17.Kosynkina L., Wang W., Liang T.C. A Convenient Synthesis of Chrial Peptide Nucleic Acid (PNA) Monomers. Tetrahedron Lett. 1994;35:5173–5176. doi: 10.1016/S0040-4039(00)77056-0. [DOI] [Google Scholar]
- 18.König W., Geiger R. A new method for synthesis of peptides: activation of the carboxyl group with dicyclohexyl carbodiimide using 1-hydroxybenzotriazoles as additives. Chem. Ber. 1970;103:788–798. doi: 10.1002/cber.19701030319. [DOI] [PubMed] [Google Scholar]
- 19.Suresh Babu V.V., Gopi H.N. Rapid and efficient synthesis of peptide fragments containing α- aminoisobutyric acid using fmoc-amino acid chlorides/potassium salt of 1-hydroxybenzotriazole. Tetrahedron Lett. 1998;39:1049–1050. doi: 10.1016/S0040-4039(97)10724-9. [DOI] [Google Scholar]
- 20.Han S.Y., Kim Y.A. Recent development of peptide coupling reagents in organic synthesis. Tetrahedron. 2004;60:2427–2467. doi: 10.1016/j.tet.2004.01.020. [DOI] [Google Scholar]
- 21.Kuroda M., Mimaki Y., Sashida Y., Hirano T., Oka K., Dobashi A. Novel cholestane glycosides form the bulbs of ornithogalum saundersiae and their cytostatic activity on leukemia HL-60 and Molt-4 Cells. Tetrahedron. 1997;53:11549–11562. doi: 10.1016/S0040-4020(97)00750-3. [DOI] [Google Scholar]
- 22.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Nat. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 23.Mori M., Hatta H., Nishimoto S.I. Stereoelectronic effect on one-electron reductive release of 5-fluorouracil from 5-fluoro-1-(2’-oxocycloalkyl)uracil as a new class of radiation-actvivated antitumor prodrugs. J.Org. Chem. 2000;65:4641–4647. doi: 10.1021/jo000245u. [DOI] [PubMed] [Google Scholar]