Abstract
Four novel bisquaternary aldoxime cholinesterase reactivators differing in their chemical structure were prepared. Afterwards, their biological activity was evaluated for their ability to reactivate acetylcholinesterase (AChE; EC 3.1.1.7) and butyryl-cholinesterase (BuChE; EC 3.1.1.8) inhibited by paraoxon. Their reactivation activity was compared with standard reactivators—pralidoxime, obidoxime and HI-6—which are clinically used at present. As it resulted, none of the prepared compounds surpassed obidoxime, which is considered to be the most potent compound if used for reactivation of AChE inhibited by paraoxon. In case of BuChE reactivation, two compounds (K053 and K068) achieved similar results as obidoxime.
Keywords: acetylcholinesterase, butyrylcholinesterase, reactivator, nerve agent, oxime, pesticide, scavenger
Introduction
Acetylcholinesterase (AChE; EC 3.1.1.7) reactivators are a group of drugs originally developed as antidotes for the treatment of nerve agent poisonings [1]. They are administered by soldiers using autoinjectors in case of need as immediate help if they are intoxicated by nerve agents [2]. With the increasing demands on the agricultural production, several kinds of pesticides are being extensively used. Among them, organophosphorus pesticides play a very important role [3]. Unfortunately, these compounds act biochemically very similarly to nerve agents (e.g., sarin). They inhibit the enzymes AChE and butyrylcholinesterase (BuChE; 3.1.1.8) [2,4]. If AChE is considered, its inhibition is a life threatening process, because AChE terminates nerve impulses on the synaptic clefts of the nerve system [2]. After the inhibition, it cannot degrade the neuromediator acetylcholine (ACh), ACh cumulates on the synaptic clefts, it overstimulates receptors and the intoxicated organism can die because of cholinergic crisis [2].
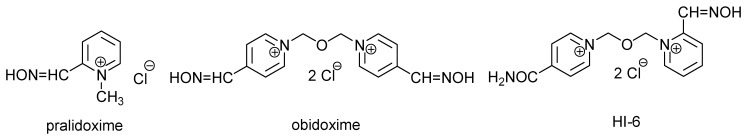
Standard therapy of such intoxications consists of administration of anticholinergic drugs (mostly atropine), AChE reactivators (pralidoxime, obidoxime, HI-6 are clinically used; Figure 1) and anticonvulsives (diazepam or avizafone) [5]. The choice of anticholinergics and anticonvulsives is relatively resistant to changes. On the contrary, many new derivatives among the group of AChE reactivators are described.
Figure 1.
Chemical structures of clinically used acetylcholinesterase reactivators.
At the end of the 20th century, novel approaches for the pre-treatment of nerve agent intoxications, bioscavengers, were investigated. Bioscavengers (cholinesterases, phosphotriesterase, or human paraoxonase) could neutralize nerve agents in the blood stream before they reach their physiological targets. The most investigated, human serum BuChE (EC 3.1.1.8), can be used successfully with relatively high protective potency [6,7].
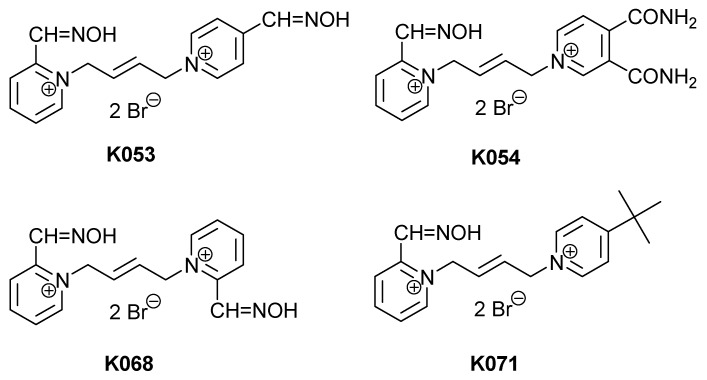
In this study, we have prepared four novel bisquaternary aldoxime cholinesterase reactivators (K053, K054, K068 and K071) with the aim of obtaining new more promising oxime candidates which could in future replace the clinically used reactivators (Figure 2). Paraoxon (POX) was selected as an appropriate organophosphate inhibitor of cholinesterases in our experiments. The newly prepared compounds were also tested for their reactivation of BuChE. BuChE reactivation is at present time well-investigated to get a so-called “pseudocatalytic scavenger” able to act as prophylaxis or treatment of nerve agent poisonings [8,9,10].
Figure 2.
Chemical structures of novel acetylcholinesterase reactivators.
Results and Discussion
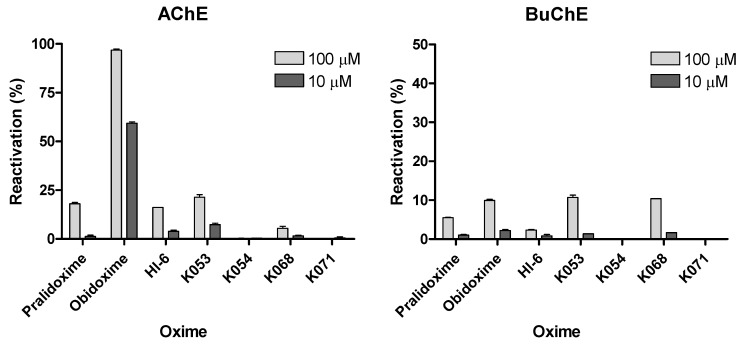
All obtained results are summarized in Table 1 and for better visualization also in Figure 3. As resulted, obidoxime was the most potent reactivator in treatment of paraoxon-inhibited AChE at both concentrations tested (10 µM and 100 µM). Newly prepared oxime K053 together with pralidoxime and HI-6 reached comparable results which are considered to be satisfactory for survival of the intoxicated organism [2]. All other evaluated oximes were not effective in case of AChE reactivation. In case of BuChE reactivation, much more bad results were obtained. This result corresponds with the general finding described already earlier, that reactivation of BuChE is very difficult and different to that for AChE [11,12]. In this case, obidoxime and two novel oximes (K053 and K068) achieved reactivation around 10%. No other oximes (including pralidoxime and HI-6) were able to reactivate sufficiently the POX-inhibited BuChE.
Table 1.
Potency of the tested oximes to reactivate POX- inhibited human erythrocyte AChE and plasma BuChE at concentrations 100 µM and 10 µM. (%, mean value of three independent determinations, time of inhibition by paraoxon 120 min; time of reactivation by AChE reactivators - 10 min; pH 7.4; temperature 25 °C).
| Reactivation (%) | ||||||||
| AChE | BuChE | |||||||
| Concentration | 100 µM | 10 µM | 100 µM | 10 µM | ||||
| Reactivator | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. |
| Pralidoxime6 | 18.0 | 0.7 | 1.3 | 0.7 | 5.5 | 0.1 | 1.0 | 0.2 |
| Obidoxime6 | 96.9 | 0.7 | 59.4 | 0.7 | 9.9 | 0.3 | 2.2 | 0.3 |
| HI-6 6 | 16.1 | 0 | 3.9 | 0.7 | 2.3 | 0.2 | 0.8 | 0.4 |
| K053 | 21.4 | 1.4 | 7.3 | 0.8 | 10.7 | 0.6 | 1.4 | 0 |
| K054 | 0 | 0 | 0.4 | 0 | 0.2 | 0 | 0.2 | 0 |
| K068 | 5.4 | 1.0 | 1.5 | 0.4 | 10.4 | 0 | 1.7 | 0 |
| K071 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Figure 3.
Reactivation of paraoxon-inhibited AChE and BuChE by the novel bisquaternary aldoxime reactivators.
If the obtained results are compared, the new AChE reactivators are not better than the clinically used ones, so that their further investigation cannot be recommended. Due to this, novel structurally different oximes derived from clinically used ones (especially from obidoxime) should be designed and tested for their reactivation potency against POX. On the contrary, if BuChE reactivation is considered, only two oximes (K053 and K068) achieved 10% reactivation. Due to this, if novel BuChE reactivators are to be designed in the future, the results of this study could be used as first approximation to the desired structure with higher BuChE reactivation potency.
Experimental
General
All chemicals used in this study were of reagent grade. They were obtained from commercial sources (Sigma-Aldrich, Czech Republic). Paraoxon (POX; O,O-diethyl-O-4-nitrophenylphosphate, 95% purity) was purchased from Dr. Ehrenstorfer (Augsburg, Germany). 1H- and 13C-NMR spectra were recorded at 300 and 75 MHz, respectively, on a Varian Mercury 300 spectrometer, using D2O as solvent. All experiments were carried out in compliance with the current law of Czech Republic.
Synthesis
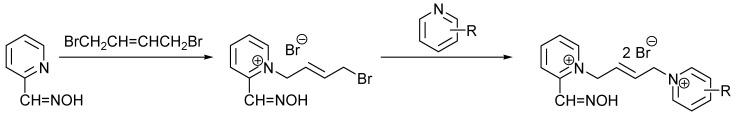
All newly prepared reactivators were prepared using the standard synthetic approach described previously by Musilek et al. [13,14]. As can be clearly seen from the reactivators´ structure, in the case of asymmetric reactivators there is the need to prepare them through the monoquaternary intermediate. To obtain the monoquaternary compound, there is a need of mild conditions to prevent a creation of symmetric bisquaternary compound (Scheme 1). NMR data together with melting points and yields of prepared compounds are listed below:
Scheme 1.
General scheme of the synthesis of novel acetylcholinesterase reactivators.
trans-2,4′-Bis[(hydroxyimino)methyl]-1,1′-(but-2-ene-1,4-diyl)bispyridinium dibromide (K053): yield 68%; m.p. 194-196 °C; 1H-NMR δ: 5.52 (d, 2H, J = 4.5 Hz, CH2), 5.89 (d, 2H, J = 8 Hz, CH2), 6.03 (dt, 1H, J = 16, 4.5 Hz, CH=), 6.34 (dt, 1H, J = 16, 8 Hz, CH=), 8.07 (t, 1H, J = 6 Hz, arom H-5), 8.21 (d, 1H, J = 6 Hz, arom H-3), 8.24 (d, 2H, J = 6 Hz, arom H-3′, H-5′), 8.38 (s, 1H, CH=N), 8.61 (t, 1H, J = 6 Hz, arom H-4), 8.63 (s, 1H, CH=N), 8.85 (d, 1H, J = 6 Hz, arom H-6), 8.87 (d, 2H, J = 6 Hz, arom H-2′, H-6′). The signals of =NOH disappeared in deuterated solvent; 13C-NMR δ: 48.37 (CH2), 62.23 (CH2), 126.94 (CH=), 126.96 (CH=), 127.02 (CH-3′,5′), 129.90 (CH-3), 132.85 (CH-5), 146.95 (CH-2′,6′), 146.99 (CH-6), 148.18 (C-4′), 148.21 (CH-4), 148.74 (CH=N), 151.41 (CH=N), 157.37 (C-2).
trans-3,4-Dicarbamoyl-2′-(hydroxyimino)methyl-1,1′-(but-2-ene-1,4-diyl)bispyridinium dibromide (K054): yield 55%; m.p. 209-211 °C; 1H-NMR δ: 5.40 (d, 2H, J = 7 Hz, CH2), 5.54 (d, 2H, J = 5 Hz, CH2), 6.04 (dt, 1H, J = 16, 7 Hz, CH=), 6.42 (dt, 1H, J = 16, 5 Hz, CH=), 8.09 (t, 1H, J = 6 Hz, arom H-5′), 8.34 (d, 1H, J = 6 Hz, arom H-3′), 8.43 (d, 1H, J = 7 Hz, arom H-5), 8.59 (t, 1H, J = 6 Hz, arom H-4′), 8.64 (s, 1H, CH=N), 8.86 (d, 1H, J = 6 Hz, arom H-6′), 9.11 (d, 1H, J = 7 Hz, arom H-6), 9.25 (s, 1H, arom H-2). The signals of CONH2 and =NOH disappeared in deuterated solvent; 13C-NMR δ: 48.48 (CH2), 61.25 (CH2), 127.51 (CH=), 129.08 (CH=), 129.30 (CH-5), 129.91 (CH-3′), 130.27 (CH-5′), 135.86 (C-3), 144.12 (CH-6), 146.76 (CH-6′), 148.37 (CH-4′), 148.76 (CH-2), 149.88 (CH=N), 152.04 (C-4), 157.32 (C-2′), 167.90 (CONH2), 169.49 (CONH2).
trans-2,2′-Bis[(hydroxyimino)methyl]-1,1′-(but-2-ene-1,4-diyl)bispyridinium dibromide (K068): yield 60%; m.p. 196-199 °C; 1H-NMR δ: 5.28 (d, 4H, J = 5 Hz, 2 x CH2), 6.06 (t, 2H, J = 5 Hz, 2 x CH=), 8.08 (dt, 2H, J = 8, 1.5 Hz, 2 x arom H-5), 8.38 (dd, 2H, J = 8, 1.5 Hz, 2 x arom H-3), 8.60 (dt, 2H, J = 8, 1.5 Hz, 2 x arom H-4), 8.78 (s, 2H, 2 x CH=N), 8.87 (dd, 2H, J = 8, 1.5 Hz, 2 x arom H-6). The signal of =NOH disappeared in deuterated solvent; 13C-NMR δ: 48.53 (CH2), 127.45 (CH=), 129.80 (CH-3), 130.53 (CH-5), 145.01 (CH-6), 148.57 (CH-4), 148.74 (CH=N), 157.31 (C-2).
trans-4′-tert-Butyl-2-(hydroxyimino)methyl-1,1′-(but-2-ene-1,4-diyl)bispyridinium dibromide (K071): yield 47%; m.p. > 300 °C; 1H-NMR δ: 1.25 (s, 9H, 3 x CH3), 5.22 (d, 2H, J = 5 Hz, CH2), 5.84 (d, 2H, J = 8 Hz, CH2), 6.04 (dm, 1H, J = 16 Hz, CH=), 6.32 (dm, 1H, J = 16 Hz, CH=), 8.02-9.25 (m, 8H, arom), 8.64 (s, 1H, CH=N). The signal of =NOH disappeared in deuterated solvent; 13C-NMR δ: 31.55 (CH3), 38.45 (C), 48.38 (CH2), 64.37 (CH2), 126.61 (CH-3′,5′), 127.47 (CH=), 127.54 (CH=), 128.30 (CH-3), 129.84 (CH-5), 142.96 (CH-2′,6′), 146.05 (CH-6), 146.69 (CH-4), 148.70 (CH=N), 157.94 (C-2), 166.88 (C-4′).
Biochemical
Purity of novel compounds was checked once again using TLC technique and HPLC technique immediately prior the experiment [15,16]. Reactivation activity of the synthesized reactivators was tested using our in vitro reactivation test [9]. A short description of this method is summarized here: human erythrocyte AChE or plasma BuChE were inhibited by solution of paraoxon to 5% of their original activity. Time of enzyme inhibition with paraoxon (2 hours, corresponding to 7 × T1/2) was calculated from experimentally determined half life (T1/2) of reaction between enzyme and paraoxon. Then, the inhibited enzyme was incubated for 10 min with a solution of reactivator at concentration 10-4 M and 10-5 M. Activity of AChE (BuChE) was measured spectrophotometrically by modified method according to Ellman with acetylthiocholine (butyrylthiocholine) as substrate [17]. The reactivation potency was calculated from the formula:
| %R = (1 − (a0 − ar)/(a0 − ai)) × 100 |
where %R is percent of reactivation, a0 is activity of intact enzyme, ai is activity of inhibited enzyme and ar is activity of reactivated enzyme minus oximolysis and spontaneous hydrolysis. Each measurement was repeated three times and was conducted under standard laboratory temperature (25 °C). Calculations were performed using software GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego California USA (www.graphpad.com).
Acknowledgements
Authors would like to thank Petr Stodulka for his excellent technical help. This work was supported by the Ministry of Defence—project no. FVZ0000604.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Delfino R.T., Ribeiro T.S., Figueroa-Villar J.D. Organophosphorus Compounds as Chemical Warfare Agents: A Review. J. Braz. Chem. Soc. 2009;20:407–428. doi: 10.1590/S0103-50532009000300003. [DOI] [Google Scholar]
- 2.Bajgar J. Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- 3.Eddleston M., Eyer P., Worek F., Sheriff M.H., Buckley N.A. Predicting outcome using butyrylcholinesterase activity in organophosphorus pesticide self-poisoning. QJM. 2008;101:467–474. doi: 10.1093/qjmed/hcn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorke D.E., Petroianu G.A. New series of monoquaternary pyridinium oximes: Synthesis and reactivation potency for paraoxon-inhibited electric eel and recombinant human acetylcholinesterase. J. Appl. Toxicol. 2009;29:459–469. doi: 10.1002/jat.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petroianu G.A., Lorke D.E. Pyridinium oxime reactivators of cholinesterase inhibited by diisopropyl-fluorophosphate (DFP): Predictive value of in-vitro testing for in-vivo efficacy. Mini Rev. Med. Chem. 2008;8:1328–1342. doi: 10.2174/138955708786369555. [DOI] [PubMed] [Google Scholar]
- 6.Saxena A., Sun W., Luo C., Myers T.M., Koplovitz I., Lenz D.E., Doctor B.P. Bioscavenger for protection from toxicity of organophosphorus compounds. J. Mol. Neurosci. 2006;30:145–148. doi: 10.1385/JMN:30:1:145. [DOI] [PubMed] [Google Scholar]
- 7.Doctor B.P., Saxena A. Bioscavengers for the protection of humus against organophosphate toxicity. Chem. Biol. Interact. 2005;157–158:167–171. doi: 10.1016/j.cbi.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Jun D., Musilova L., Kuca K., Kassa J., Bajgar J. Potency of several oximes to reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by paraoxon and methyl-paraoxon in vitro. Chem. Biol. Interact. 2008;175:421–424. doi: 10.1016/j.cbi.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Musilova L., Kuca K., Jung Y.S., Jun D. In vitro oxime-assisted reactivation of paraoxon-inhibited human acetylcholinesterase and butyrylcholinesterase. Clin. Toxicol. 2009;47:545–550. doi: 10.1080/15563650903058914. [DOI] [PubMed] [Google Scholar]
- 10.Musilova L., Jun D., Kuca K., Pohanka M., Katalinic M., Kovarik Z. Development of new antidotes of organophosphate intoxications: Oxime-assisted reactivation of dimethoxy- and diethoxy-phosphorylated human butyrylcholinesterase for construction of “pseudo catalytic” bioscavengers. Toxicol. Lett. 2009;189:S216. doi: 10.1016/j.toxlet.2009.06.561. [DOI] [Google Scholar]
- 11.Kovarik Z., Vrdoljak A.L., Berend S., Katalinic M., Kuca K., Musilek K., Radic B. Evaluation of oxime K203 as antidote in tabun poisoning. Arh. Hig. Rada Toksikol. 2009;60:19–26. doi: 10.2478/10004-1254-60-2009-1890. [DOI] [PubMed] [Google Scholar]
- 12.Carletti E., Aurbek N., Gillon E., Loiodice M., Nicolet Y., Fontecilla-Camps J.C., Masson P., Thiermann H., Nachon F. Structure-activity analysis of aging and reactivation of human butyrylcholinesterase inhibited by analogues of tabun. Biochem. J. 2009;421:97–106. doi: 10.1042/BJ20090091. [DOI] [PubMed] [Google Scholar]
- 13.Musilek K., Lipka L., Racakova V., Kuca K., Jun D., Dohnal V., Dolezal V. New methods in synthesis of acetylcholinesterase reactivators and evaluation of their potency to reactivate cyclosarin-inhibited AChE. Chem. Papers. 2006;60:48–51. doi: 10.2478/s11696-006-0008-x. [DOI] [Google Scholar]
- 14.Musilek K., Holas O., Jun D., Dohnal V., Gunn-Moore F., Opletalova V., Dolezal M., Kuca K. Monooxime reactivators of acetylcholinesterase with (E)-but-2-ene linker – Preparation and reactivation of tabun and paraoxon-inhibited acetylcholinesterase. Biorg. Med. Chem. 2007;15:6733–6741. doi: 10.1016/j.bmc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Jun D., Stodulka P., Kuca K., Koleckar V., Dolezal B., Simon P., Veverka M. HPLC analysis of HI-6 dichloride and dimethanesulfonate – antidotes against nerve agents and organophosphorus pesticides. Anal. Lett. 2007;40:2783–2787. doi: 10.1080/00032710701588531. [DOI] [Google Scholar]
- 16.Jun D., Stodulka P., Kuca K., Koleckar V., Dolezal B., Simon P., Veverka M. TLC analysis of intermediates arising during the preparation of oxime HI-6 dimethanesulfonate. J. Chrom. Sci. 2008;46:316–319. doi: 10.1093/chromsci/46.4.316. [DOI] [PubMed] [Google Scholar]
- 17.Ellman G.L., Courtney K.D., Andres V., Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]