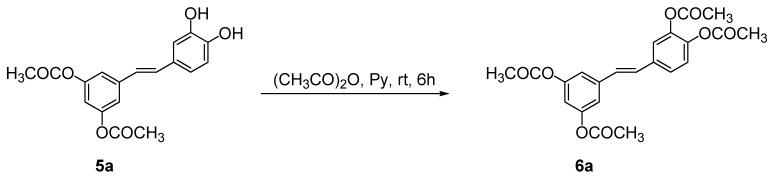Abstract
Piceatannol (E-3,5,3’,4’-tetrahydroxystilbene) is a phytoalexin synthesized in grapes in response to stress conditions. It exhibits strong antioxidant and antileukaemic activities due to the presence of the catechol moiety. To modify some physical properties like solubility, and miscibility in non-aqueous media some new previously unreported piceatannol derivatives having lipophilic chains on the A-ring were prepared in good yields by a simple and efficient procedure. The key step was a chemo- and regioselective aromatic hydroxylation with 2-iodoxybenzoic acid (IBX). The new compounds showed antioxidant activity and seemed promising for possible applications as multifunctional emulsifiers in food, cosmetic and pharmaceutical fields.
Keywords: aromatic hydroxylation, 2-iodoxybenzoic acid (IBX), lipophilic piceatannol derivatives, lipophilic antioxidants
1. Introduction
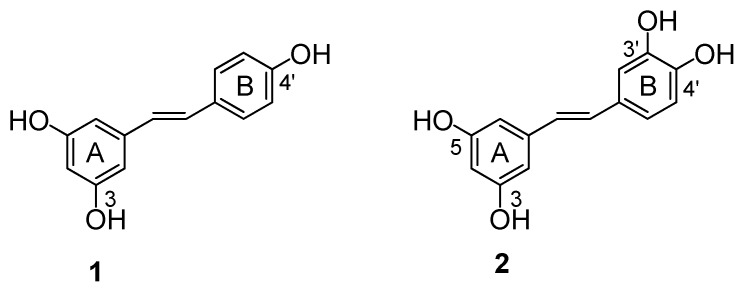
In the last few years, stilbene-based compounds have attracted the attention of many researchers due to their wide range of positive biological effects [1,2,3]. One of the most relevant and extensively studied compounds is resveratrol (E-3,5,4’-trihydroxystilbene 1, Figure 1), a phenolic compound found mainly in grapes, where it is synthesized in response to stress conditions such as fungal infection and trauma [4,5]. In red wines it is present at concentrations of mg/L and it is well known that a moderate alcohol consumption has some protective effects against coronary heart disease [6]. Several studies have demonstrated that resveratrol exhibits a wide range of biological and pharmacological activities [7,8]. For example, it has been shown to have cancer preventive properties [9,10,11], anti-inflammatory activities [12], and a role in the prevention of atherosclerosis and coronary diseases [13,14].
Figure 1.
Chemical structure of natural stilbenes 1 and 2.
Structurally similar to resveratrol is piceatannol (E-3,5,3’,4’-tetrahydroxystilbene 2) which differs from 1 in that it possesses an extra hydroxyl group adjacent to the active 4’-OH of resveratrol. It is present in low quantity in grapes [15], peanuts [16], Euphorbia lagascae [17] and Vaccinium berries [18] and, like resveratrol, it is a phytoalexin. Piceatannol (2) shows many biological activities [19,20,21]. It has known anticancer and antileukaemic properties, inducing apoptosis in several cell lines and animal models; it inhibits a variety of tyrosinase kinases involved in cell proliferation [22,23]. A recent study has demonstrated that the cancer preventative properties of resveratrol (1) are related to its natural conversion into metabolite piceatannol (2) in living cells by the enzyme CYP1B1 (belongs to the cytochrome P450 enzyme family) that is over-expressed in a wide variety of human tumors [24]. Other experimental evidences showed that piceatannol has a higher level of antioxidant activity compared to resveratrol [25]. This result is according to the evidence that a catechol moiety present in a compound increases the cytotoxic and antioxidant activity in vitro [26,27].
As all naturally occurring phenolic antioxidants, stilbene derivatives 1 and 2 are hydrophilic and thus scarcely soluble in aprotic solvents, oils and emulsions. Consequently, their application in the food, pharmaceutical and cosmetic fields is strongly limited.
In recent times, more attention has been turned on the development of possible strategies to modify and to enhance the functional properties of natural antioxidants without altering the functionality responsible for the biological activities. For example, the hydrophobicity of antioxidant phenolic acids has been enhanced by esterifying the carboxylic acid moiety of the phenolic acid with a fatty alcohol by chemical and enzymatic procedures [28]. In a similar way, phenethyl alcohols have been lipophilized by esterifying the alcoholic function with acid chlorides or acid anhydrides [29]. In this way amphiphilic phenolic compounds that are able to distribute between the aqueous phase and the lipophilic phase and interact with an emulsifier at the interface were obtained. Recently, it has been reported that these compounds are potentially interesting for possible applications in nanotechnology [30]. To the best of our knowledge, lipophilic piceatannol derivatives have not been reported in the literature and no chemical or enzymatic synthetic procedures are described.
In the last few years, our research has been devoted to the preparation of biologically active catecholic compounds from naturally occurring phenols by oxidative procedures. For example, we obtained in very good yields 2-(3’,4’-dihydroxyphenyl)ethanol (hydroxytyrosol) and its lipophilic derivatives, useful molecules for cosmetic and pharmaceutical applications [31,32,33,34,35]; novel lignans showing antioxidant activity [36]; endogenous catecholamines utilized for the treatment of many clinical disorders, including hypertension and cardiovascular diseases [37]. The oxidant of choice was 1-hydroxy-1-oxo-1H-1λ5-benzo[d][1,2]iodoxol-3-one (2-iodobenzoic acid, IBX, Figure 2), the most important representative of pentavalent iodine heterocycles first prepared by Hartmann and Mayer [38] which has attracted increasing interest during the last decade because of its selective, mild and environmentally friendly properties as an oxidant in organic synthesis [39,40,41]. Among its synthethic potential, it is particularly interesting the ability to perform a regioselective oxidation of phenols into ortho-quinones [42,43,44]. When the oxidative step was followed by in situ reduction, a catechol moiety was produced with a selectivity similar to that of cytochrome P450 enzymes [45].
Figure 2.
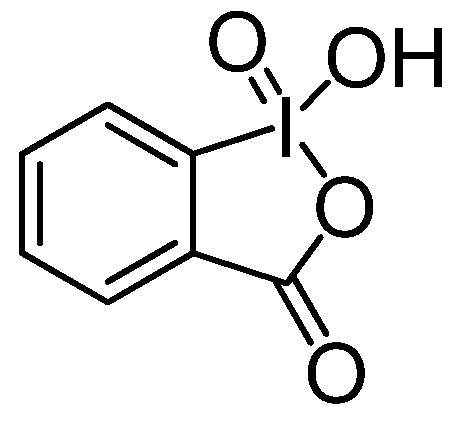
Chemical structure of 2-iodoxybenzoic acid (IBX).
In this paper we describe a simple and efficient route to prepare lipophilic piceatannol derivatives displaying free-catecholic functionality in the B ring and long alkyl chains in the A ring by an oxidative procedure using IBX and subsequent reduction with sodium dithionite (Na2S2O4). As examples of chains with different length, we choose acetyl (C2), butanoyl (C4), octanoyl (C8) and palmitoyl (C16). These new compounds were then evaluated for their radical-scavenging activity using the DPPH method [46].
2. Results and Discussion
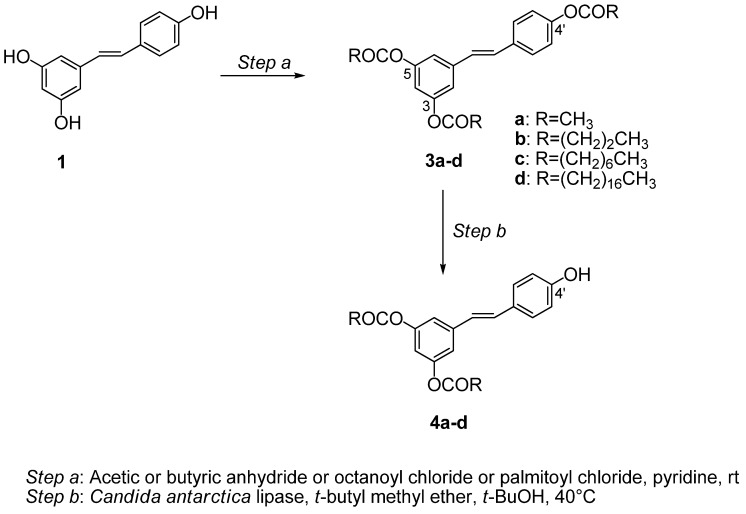
2.1. Preparation of 3,5-di-O-acyl resveratrol derivatives 4a-d
Firstly, we prepared 3,5-di-O-acyl resveratrol derivatives 4a-d by a two-step procedure (Scheme 1). Peracylated derivatives [3,5,4’-tri-O-acetyl resveratrol (3a); 3,5,4’-tri-O-butanoyl resveratrol (3b); 3,5,4’-tri-O-octanoyl resveratrol (3c) and 3,5,4’-tri-O-palmitoyl resveratrol (3d)] were obtained in 90–95% yields from resveratrol (1) using acetic and butyric anhydride in pyridine and octanoyl and palmitoyl chloride in pyridine, respectively (step a); then, compounds 3a-d were dispersed in t-butyl methyl ether and n-butanol and subjected to enzymatic alcoholysis with Candida antarctica lipase to isolate the corresponding 3,5-di-O-acetylated resveratrol derivatives 4a-d in 60–90% yields (step b) [47,48].
Scheme 1.
Synthesis of 3,5-di-O-acetylresveratrol derivatives 4a-d.
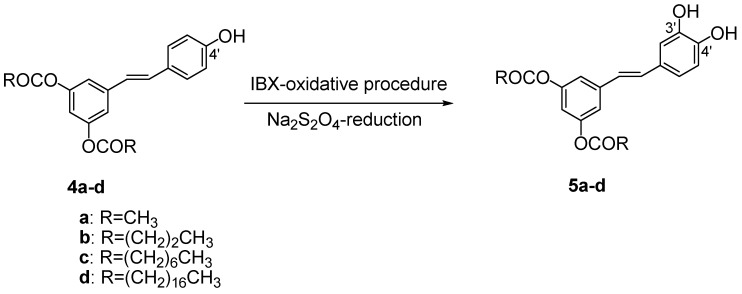
2.2. Aromatic hydroxylation of compounds 4a-d
The key reaction for the preparation of 3,5-di-O-acyl piceatannol derivatives 5a-d was the insertion of an hydroxyl group in C-3’ (B ring) of compounds 4a-d (Scheme 2). The model substrate for the optimization of the reaction conditions was 3,5-di-O-acetyl resveratrol (4a).
Scheme 2.
Aromatic hydroxylation of 3,5-di-O-acyl resveratrol derivatives 4a-d.
We performed some experiments varying the solvent, reaction time, and temperature (Table 1). In all cases an excess of IBX was used (1.2 equiv.); Na2S2O4 in tetrahydrofuran (1.0 M) was the reducing solution. Reactions were monitored by chromatographic analysis (TLC and HPLC). On the basis of the literature data [49], first we used methanol as solvent and a low temperature reaction but under these experimental conditions 3,5-di-O-acetyl piceatannol (5a) was isolated in low yield (Table 1, entry 1). The best result in terms of solubility, conversion and yield was obtained by dissolving compound 4a in dimethyl sulfoxide (DMSO) at room temperature. In these experimental conditions, piceatannol derivative 5a was isolated in quantitative yield after only 1 h (Table 1, entry 4). The oxidative step was both chemoselective and regioselective the double bond present of 5a being unaffected.
Table 1.
Aromatic hydroxylation of 3,5-di-O-acetyl resveratrol (4a) with IBX/Na2S2O4 procedure.
| Entry | Solvent | T (°C) | Time (h) | Conv. (%) | Yield of 5a (%) |
|---|---|---|---|---|---|
| 1 | Methanol | 0 | 1 | 20 | 20 |
| 2 | Methanol/Chloroform=1/1 | 0 | 1 | 70 | 68 |
| 3 | Dimethyl carbonate | 25 | 2 | 50 | 46 |
| 4 | Dimethyl sulfoxide | 25 | 1 | >98 | 95 |
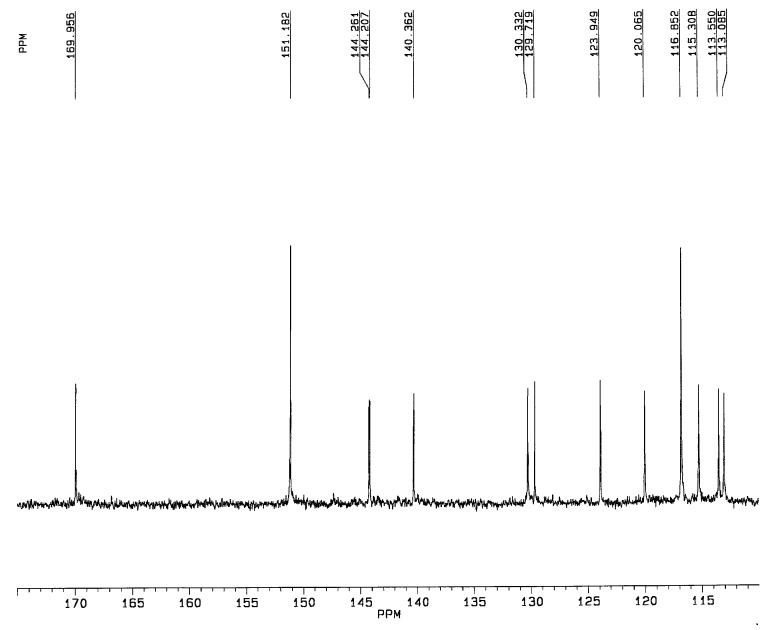
A significative region of the 13C-NMR spectrum of the new synthesized 3,5-di-O-acetyl piceatannol (5a) is shown in Figure 4. The presence of the catecholic moiety in the B-ring of 3,5-di-O-acetyl piceatannol (5a) was confirmed by analyzing this spectroscopic region, which showed the lack of symmetry of the B-ring, two quaternary carbon atoms (C-3’ and C-4’) at 140.4 and 144.2 ppm and three C-H carbons (C-2’, C-5’, C-5’) at 113.5, 115.3 and 116.8 ppm, respectively.
Figure 4.
13C-NMR spectrum (180-110 ppm region) of 3,5-di-O-acetyl piceatannol (5a).
Furthermore, the presence of two free hydroxyl groups in 5a was chemically demonstrated by an esterification reaction of 5a under classical conditions with acetic anhydride in pyridine (Scheme 3). As expected, the proton magnetic resonance spectrum (1H-NMR) of the resulting product 6a evidenced the presence of two singlets at 2.23 and 2.25 ppm attributable to the twelve protons of the four acetyl groups of 6a.
Scheme 3.
Acetylation of 3,5-di-O-acyl piceatannol derivative 4a.
As already reported, a plausible ionic mechanism for the regioselective aromatic hydroxylation of phenol derivatives was reported by Pettus et al. [42]. The phenol added to the iodine-(V) center of IBX to give a λ5-iodanyl intermediate and to extrude H2O. The intramolecular (and regioselective) delivery of an oxygen atom from this specie lead to a λ3-iodanyl intermediate which undergoes tautomerization and oxidatively collapses to produce an ortho-quinone derivative and 2-iodobenzoic acid (IBA), the only by-product of the reaction. The catecholic moiety was finally obtained after the in situ reductive step.
On the basis of the good results, this simple efficient and useful synthetic procedure was applied to 3,5,4’-tri-O-butanoyl resveratrol (4b); 3,5,4’-tri-O-octanoyl resveratrol (4c) and 3,5,4’-tri-O-palmitoyl resveratrol (4d) (Scheme 2). The corresponding lipophilic piceatannol derivatives 5b-d were isolated in satisfactory yields (Table 2, entry 1-3) and with a high degree of purity.
Table 2.
Aromatic hydroxylation of resveratrol derivatives 4a-d with IBX/Na2S2O4 procedure. a
| Entry | Substrate | Time (h) | Conv. (%) | Yield (%) |
|---|---|---|---|---|
| 1 | 4b | 1 | 92 | 5b: 88 |
| 2 | 4c | 2 | 90 | 5c: 84 |
| 3 | 4d | 2 | 90 | 5d: 82 |
a Oxidation reactions were performed in DMSO at room temperature.
2.3. Evaluation of the antioxidant activity of 5a-d
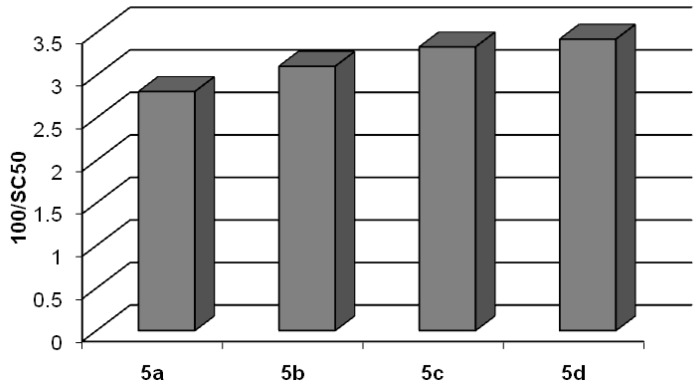
After their preparation, we evaluated the radical scavenging activity of compounds 5a-d using the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH•) radical commonly and widely utilized for testing this activity in natural phenolic compounds. Experimental results are reported in Table 3 and Figure 5. The parameter SC50 is the Scavenging Capacity that is the concentration of compound (expressed in μM) able to quench the 50% of DPPH radical in a 184 μM solution (see Experimental section for details). As shown, SC50 values of lipophilic derivatives 5a-d were in the range 28.9–35.6 μM. All tested compounds exhibited an antioxidant activity superior to that of resveratrol 1 and comparable to that of piceatannol 2 [50]. The length of the alkyl chain does not influence the radical-scavenging activity.
Table 3.
DPPH• scavenging activity of compounds 5a-d expressed as SC50.
| Compound | SC50 (μM)a ± SD |
|---|---|
| 5a | 35.6 ± 2.1 |
| 5b | 32.2 ± 2.5 |
| 5c | 30.0 ± 1.6 |
| 5d | 28.9 ± 1.4 |
a Each reported value is the mean of three separate measurements.
Figure 5.
DPPH• scavenging activity of compounds 5a-d expressed as 100/SC50.
3. Experimental
3.1. Materials and methods
Resveratrol was purchased from Aldrich Company (Milan, Italy) as well all other solvents and reagents. All chemicals used were of analytical grade. IBX was prepared in our laboratory as described in the literature [51]. The Candida antarctica lipase (Chirazyme L-2, c. –f., C2, Lyo) was a gift from Roche. HPLC analyses were performed on a Varian Prostar 325 apparatus equipped with a Varian Pursuit 5μ C18 column (150 × 4.6 mm) and a dual wavelength UV-Vis detector selected on λ = 280 nm. Elutions were carried out at a 1 mL/min flow rate using a H2O/CH3CN mixture (90:10, v/v) for the first minute and a gradient to 40:60 within the following 30 minutes. 1H- and 13C-NMR spectra were recorded in CD3COCD3 or CDCl3/CD3OD using a Bruker 200 MHz spectrometer. All chemical shifts are expressed in parts per million (δ scale) and referenced to either the residual protons or carbon of the solvent. HRMS were recorded with Micromass Q-TOF Spectrometer (Waters). Compounds 4a-d were purified by flash chromatography on silica gel using the Buchi-Biotage FLASH 12™ system equipped with prepacked silica gel cartridges 12S. Analytical Thin-Layer Chromatography (TLC) were performed on silica gel (Merck 60 F254) plates using cerium sulphate as developing reagent.
3.2. Preparation of 3,5-di-O-acyl piceatannol derivatives 5a-d
Step a. Acylation reactions of resveratrol (1). Peracylated derivatives [3,5,4’-tri-O-acetylresveratrol (3a); 3,5,4’-tri-O-butanoylresveratrol (3b); 3,5,4’-tri-O-octanoylresveratrol (3c) and 3,5,4’-tri-O-palmitoylresveratrol (3d)] were prepared using acetic and butyric anhydride in pyridine (1:1) and octanoyl and palmitoyl chloride in pyridine (1:1), respectively. The solutions were stirred at room temperature for 6-12 h; then, they were acidified with HCl 2N and extracted with ethyl acetate; the organic layer was washed with a solution of NaHCO3, and dried with Na2SO4. The solvent was removed under reduced pressure to afford the peracylated derivatives 3a-d in 90-95% yields.
Step b. Enzymatic alcoholysis of 3,5,4’-triO-acylresveratrol derivatives 3a-d. Compounds 3a-d (200 mg) were dispersed in t-butyl methyl ether (20 mL) and n-butanol (0.8 mL); Candida antarctica lipase (200 mg) was added to the solution of 3a-d and the mixture was shaken (400 rpm) at 40 °C. Finally, reactions were quenched by filtering off the enzyme and the filtrate was evaporated in vacuo. The residue was subjected to flash chromatography to obtain the di-O-acyl derivatives 4a-d in 60–90% yields. MS-FAB and 1H-NMR spectra of 3a-d and 4a-d were identical to those reported in the literature [47,48].
3.3. Aromatic hydroxylation of 3,5-di-O-acyl resveratrol derivatives 4a-d
Compounds 4a-d (0.5 mmol) were dissolved in dimethyl solfoxide (5 mL) and then 2-iodoxy-benzoic (IBX) was added (1.2 eq.). The mixture was kept under magnetic stirring at room temperature and monitorated by TLC. When the substrates disappeared, water (5 mL) and sodium dithionite (0.5 mmol) were added. A chromatic change of the solution from red to colorless was observed. After 5 minutes, the mixtures were extracted with ethyl acetate (3 × 10 mL). The organic phases were washed with a solution of NaHCO3and then dried on Na2SO4. The organic solvent was evaporated under reduced pressure obtaining a crude. Final products 5a-d were purified by chromatographic purification on silica gel and characterized by spectroscopic analysis.
3,5-Di-O-acetylpiceatannol (5a). Colorless oil, quantitative yield. 1H-NMR (CD3COCD3) δ 2.27 (s, 6H, 2 × CH3), 2.99 (2H, OH), 6.80-7.20 (m, 8H, CHα=CHβ, H-2,4,6,2’,5’,6’); 13C-NMR (CD3COCD3) δ 21.1, 113.1, 113.6, 115.3, 116.9, 120.1, 123.9, 129.7, 130.3, 140.4, 144.2, 144.3, 151.2, 170.0; HRMS found: 328.22; C18H16O6 requires 328.09.
3,5-Di-O-butanoyl piceatannol (5b). Yellow oil, 88% yield. 1H-NMR (CD3COCD3) δ 1.02 (t, 6H, J = 7.3 Hz, 2 × CH3), 1.65-1.83 (m, 4H, 2 × CH2CH3), 2.56 (t, J = 9.8 Hz, 4H, OCOCH2), 6.79-7.20 (m, 8H, CHα=CHβ, H-2,4,6,2’,5’,6’); 13C-NMR (CD3COCD3) δ 13.7, 18.9, 36.4, 114.0, 114.9, 116.2, 117.3, 120.3, 124.8, 130.1, 131.5, 141.1, 146.1, 146.5, 152.6, 172.1; HRMS found: 384.34; C22H24O6 requires 384.16.
3,5-Di-O-octanoyl piceatannol (5c). Yellow oil, 84% yield. 1H-NMR (CD3COCD3) δ 0.86 (m, 6H, 2 × CH3), 1.28-1.42 (m, 16H, 8 × CH2), 1.62-1.76 (m, 4H, 2 × CH2), 2.56 (t, J = 7.2 Hz, 4H, 2 × CH2), 6.75-7.15 (m, 8H, CHα=CHβ, H-2,4,6,2’,5’,6’); 13C-NMR (CD3COCD3) δ 14.2, 23.2, 25.5, 29.6, 29.7, 32.3, 34.5, 114.0, 114.9, 116.2, 117.3, 120.3, 124.8, 130.1, 131.5, 141.0, 146.1, 146.5, 152.6, 172.1; HRMS found: 496.08; C30H40O6 requires 496.28.
3,5-Di-O-palmitoyl piceatannol (5d). Yellow oil, 82% yield. 1H-NMR (CDCl3+CD3OD) δ 0.83 (m, 6H, 2 × CH3), 1.12-1.38 (m, 48H, 24 × CH2), 1.66-1.73 (m, 4H, 2 × CH2), 2.50 (t, J = 7.3 Hz, 4H, 2 × CH2), 6.68-7.01 (m, 8H, CHα=CHβ, H-2,4,6,2’,5’,6’); 13C-NMR (CDCl3+CD3OD) δ 14.0, 22.6, 24.8, 29.1, 29.2, 29.3, 29.4, 29.6, 29.7, 31.9, 34.4, 112.7, 113.7, 115.1, 116.5, 119.8, 124.5, 129.5, 130.6, 140.1, 144.5, 144.8, 151.3, 172.2; HRMS found: 720.21; C42H72O6 requires 720.53.
3.4. Acetylation of 3,5-di-O-acetyl piceatannol (5a)
Compound 5a (1.0 mmol) was solubilized in pyridine (10 mL) and acetic anhydride was added (10 mL) overnight. At the end of the reaction, a 1 M solution of HCl (10 mL) was added and the final product was extracted with ethyl acetate (3 × 10 mL). The combined organic phases were washed with a solution of NaHCO3 (20 mL) and with a saturated solution of NaCl (20 mL), then dried over Na2SO4. After evaporation of the solvent, and chromatographic purification on silica gel, 3,5,3’,4’-tetra-O-acetyl piceatannol (6a) was isolated in quantitative yield and characterized by spectroscopic data. Colorless oil; 1H-NMR (CDCl3) δ 2.23 (s, 6H, 2 × CH3), 2.25 (s, 6H, 2 × CH3), 6.84 (t, J = 2.0 Hz, 1H, H-4), 6.96 (d, J = 16 Hz, 2H, CH=CHβ), 7.02 (d, J = 16 Hz, 2H, CHα=CH), 7.10 (d, J = 2.0 Hz, H-2,6), 7.18 (d, J = 8.5 Hz, H-5’), 7.30 (d, J = 1.5 Hz, H-2’), 7.34 (dd, J = 8.5 and 1.5 Hz, H-6’). 13C-NMR (CDCl3) δ 20.6, 21.1, 114.6, 117.0, 121.2, 123.6, 125. 0, 128.2, 128.8, 139.2, 141.7, 141.7, 142.3, 151.3, 168.2, 168.9; HRMS found: 412.24; C18H16O6 requires 412.12.
3.5. Measure of antioxidant activity of compounds 5a-d
The radical scavenging activity of compounds 5a-d was evaluated in the presence of stable radical DPPH• according to the method of Brand-Williams et al. [46]. The initial concentration of DPPH• in acetone was controlled for every experiment from a calibration curve made by measuring the absorbance at λ = 517 nm of standard DPPH• solutions at different concentrations. The equation of the curve was ABS515nm = 10,865 × [DPPH•], as determined by linear regression. The compounds 5a-d were tested at three different concentrations and each concentration was analyzed in triplicate. In a typical procedure, 10, 20 and 30 μL of freshly prepared acetone solution of compounds 5a-d were added to 2 mL of freshly prepared 184 μM acetone solution of DPPH•. The solutions were shaken for 30 min in the dark at 25 °C. The results were plotted as the percentage of reacted DPPH• [(ABS0h – ABS30min)/ABS0h × 100] vs the concentration (μM) of the added compounds 5a-d. The results were expressed as SC50 that is the concentration of compound required to quench 50% of the initial DPPH• radicals under the experimental conditions given.
4. Conclusions
In summary, we have described a simple and efficient procedure to prepare new piceatannol derivatives from naturally occurring resveratrol. These compounds possess a free-catecholic moiety in the B ring and long alkyl chains on the A ring. With these chemical features they exhibited an antioxidant activity similar to that of piceatannol, but a greater lipophilicity, therefore, they may be advatageously used as food antioxidants in bulk lipids or emulsions, and could be further evaluated for possible applications in the pharmaceutical, nutritional and cosmetic fields.
Acknowledgments
The authors like to thank Ministero dell’Università e della Ricerca Scientifica e Tecnologica (PRIN 2007) and University of Viterbo and Catania (Progetti di Ricerca di Ateneo, Italy) for financial support.
Footnotes
Sample Availability: Samples of the compounds 5a-d are available from the authors.
References and Notes
- 1.Murias M., Handler N., Erker T., Pleban K., Ecker G., Saiko P., Szekeres T., Jager W. Resveratrol analogues as selective cyclooxygenase-2-inhibitors: Synthesis and structure-activity relationship. Bioorg. Med. Chem. 2004;12:5571–5578. doi: 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Murias M., Jager W., Handler N., Erker T., Horvath Z., Szekeres T., Nohl H., Gille L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure-activity relationship. Biochem. Pharm. 2005;69:903–912. doi: 10.1016/j.bcp.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Riviére C., Richard T., Quentin L., Krisa S., Mérillon J.M., Monti J.P. Inhibitory activity of stilbenes on Alzheimer’s B-amyloid fibrils in vitro. Bioorg. Med. Chem. 2007;15:1160–1167. doi: 10.1016/j.bmc.2006.09.069. [DOI] [PubMed] [Google Scholar]
- 4.Langcake P., Pryce R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976;9:77–86. doi: 10.1016/0048-4059(76)90077-1. [DOI] [Google Scholar]
- 5.Siemann E.H., Creasy L.L. Concentration of the phytoalexin resveratrol in wine. Am. J. Enol. Vitic. 1992;43:49–52. [Google Scholar]
- 6.Renaud S., De Lorgeril M. Wine, alcohol, platelets and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- 7.Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/S0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 8.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 9.Gusman J., Malonne H., Atassi G. A reappraisal of the potential chemopreventive properties of resveratrol. Carcinogenesis. 2001;22:1111–1117. doi: 10.1093/carcin/22.8.1111. [DOI] [PubMed] [Google Scholar]
- 10.Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 11.Dong Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat. Res. 2003;523-524:145–150. doi: 10.1016/S0027-5107(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 12.Ramon M.A., Villegas I., La-Casa C., de La Lastra A.C. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulate apoptosis during early colonic inflammation in rats. Biochem. Pharm. 2004;67:1399–1410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Tadolini B., Juliano C., Piu L., Franconi F., Cabrini L. Resveratrol inhibition of lipid peroxidation. Free Radic. Res. 2000;33:105–114. doi: 10.1080/10715760000300661. [DOI] [PubMed] [Google Scholar]
- 14.Lembège-Varache M., Teguo P.W., Richard T., Monti J.P., Deffiuex G., Vercauteren J., Mérillon J.M., Nuhrich A. Structure-activity relationships of polyhydroxystilbene derivatives extracted from Vitis vinifera cell cultures as inhibitors of human platetelet aggregation. Med. Chem. Res. 2000;10:253–267. [Google Scholar]
- 15.Bavaresco L., Fregoni M., Trevisan M., Mattivi F., Vrhovsek U., Flachetti R. The occurrence of the stilbene piceatannol in grapes. Vitis. 2002;41:133–136. [Google Scholar]
- 16.Ku K.L., Chang P.S., Cheng Y.C., Lien C.Y. Production of stilbenoids from the callus of Arachis hypogaea: A novel source of the anticancer compound piceatannol. J. Agr. Food Chem. 2005;53:3877–3881. doi: 10.1021/jf050242o. [DOI] [PubMed] [Google Scholar]
- 17.Ferrigni N.R., Mclaughlin J.L., Powell R.G., Smith C.R. Isolation of piceatannol as the antileukaemic principle from the seeds of Euphorbia lagascae. J. Nat. Prod. 1984;47:347–352. doi: 10.1021/np50032a019. [DOI] [PubMed] [Google Scholar]
- 18.Rimando A.M., Kalt W., Magee J.B., Dewey J., Ballington J. Resveratrol, pterostilbene and piceatannol in Vaccinium berries. J. Agr. Food Chem. 2004;52:4713–4719. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 19.Larrosa M., Tomas-Barberan F.A., Espin J.C. The grape and wine polyphenol piceatannol is a potent inducer of apoptosis in human SK-Mel-28 melanoma cells. Eur. J. Nutr. 2004;43:275–284. doi: 10.1007/s00394-004-0471-5. [DOI] [PubMed] [Google Scholar]
- 20.Wieder T., Prokop A., Bagci B., Essmann F., Bernicke D., Schulze-Osthoff K., Dorken B., Schmalz H.G., Daniel P.T., Henze G. Piceatannol, a hydroxylated analogue of the chemopreventive agent resveratrol, is a potent inducer of apoptosis in the lymphoma cell line BJAB and in primary, leukemic lymphoblasts. Leukemia. 2001;15:1735–1742. doi: 10.1038/sj.leu.2402284. [DOI] [PubMed] [Google Scholar]
- 21.Wieder F., Prokop A., Bagci B., Essmann F., Bernicke D., Schulze-Osthoff K., Dorken B., Schmalz H.G., Daniel P.T., Henze G. Piceatannol, a hydroxylated analog of the chemopreventive agent resveratrol, is a potent inducer of apoptosis in the lymphoma cell line BJAB and in primary, leukemic lymphoblasts. Leukemia. 2001;15:1735–1742. doi: 10.1038/sj.leu.2402284. [DOI] [PubMed] [Google Scholar]
- 22.Larrosa M., Tomas-Barberan F.A., Espin J.C. The grape and wine polyphenol piceatannol is a potent inducer of apoptosis in human SK-Mel-28 melanoma cells. J. Nutr. 2002;13:369–372. doi: 10.1007/s00394-004-0471-5. [DOI] [PubMed] [Google Scholar]
- 23.Ovesna Z., Kozics K., Bader Y., Saiko P., Handler N., Erker T., Szkeres T. Antioxidant activity of resveratrol, piceatannol and 3,3’,4,4’,5,5’-hexahydroxy-trans-stilbene in three leukemia cell lines. Oncol. Rep. 2006;16:617–624. doi: 10.3892/or.16.3.617. [DOI] [PubMed] [Google Scholar]
- 24.Potter G.A., Patterson L.H., Wanogho E., Perry P.J., Butler P.C., Ijaz T., Ruparelia K.C., Lamb J.H., Farmer P.B., Stanley L.A., Burke M.D. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br. J. Cancer. 2002;86:774–778. doi: 10.1038/sj.bjc.6600197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai Y.I., Wei Q.Y., Fang J.G., Yang L., Liu Z.L., Wyche J.H., Han Z. The 3,4-dihydroxyl groups are important for trans-resveratrol analogs to exhibit enhanced antioxidant and apoptotic activities. Anticancer Res. 2004;24:999–1002. [PubMed] [Google Scholar]
- 26.Visioli F., Galli C. Olive oil phenols and their potential effects on human health. J. Agric. Food Chem. 1998;46:4292–4296. doi: 10.1021/jf980049c. [DOI] [Google Scholar]
- 27.Tuck K.L., Hayball P.J., Stupans I. Structural characterization of the metabolites of hydroxytyrosol, the principal phenolic component in olive oil, in rats. J. Agric. Food Chem. 2002;50:2404–2409. doi: 10.1021/jf011264n. [DOI] [PubMed] [Google Scholar]
- 28.Figueroa-Espinoza M.C., Villeneuve P. Phenolic acids enzymatic lipophilization. J. Agric. Food Chem. 2005;53:2779–2787. doi: 10.1021/jf0484273. [DOI] [PubMed] [Google Scholar]
- 29.Buisman G.J.H., Helteren C.T.W., Kramer G.F.H., Veldsink J.W., Derksen J.T.P., Cuperus F.P. Enzymatic esterification of functionalized phenols for the synthesis of lipophilic antioxidants. Biotecn. Lett. 1998;20:131–136. doi: 10.1023/A:1005368222340. [DOI] [Google Scholar]
- 30.Pignataro B., Sardone L., Marletta G. Self-organizing fiberlike nanostructures and wrapping-up processes in Languimir-Blodgett films. Langmuir. 2003;19:5912–5917. doi: 10.1021/la034557e. [DOI] [Google Scholar]
- 31.Bernini R., Mincione E., Barontini M., Crisante F. Method for preparing hydroxytyrosol and hydroxytyrosol derivatives. WO Pat. 2008110908. 2008
- 32.Bernini R., Mincione E., Barontini M., Crisante F. Convenient synthesis of hydroxytyrosol and its lipophilic derivatives from tyrosol or homovanillyl alcohol. J. Agric. Food Chem. 2008;56:8897–8904. doi: 10.1021/jf801558z. [DOI] [PubMed] [Google Scholar]
- 33.Bernini R., Cacchi S., Fabrizi G., Filisti E. 2-Arylhydroxytyrosol derivatives via Suzuki-Miyaura cross-coupling. Org. Lett. 2008;10:3457–3460. doi: 10.1021/ol8012292. [DOI] [PubMed] [Google Scholar]
- 34.Bernini R., Mincione E., Crisante F., Barontini M., Fabrizi G. A novel use of the recyclable polymer-supported IBX: An efficient chemoselective and regioselective oxidation of phenolic compounds. The case of hydroxytyrosol derivatives. Tetrahedron Lett. 2009;50:1307–1310. doi: 10.1016/j.tetlet.2009.01.037. [DOI] [Google Scholar]
- 35.Bernini R., Mincione E., Crisante F., Barontini M., Fabrizi G. Oxidation of tyrosol derivatives using polymer-supported IBX. Synfacts. 2009;5:571. [Google Scholar]
- 36.Bernini R., Barontini M., Mosesso P., Pepe G., Willför S.M., Sjöholm R.E., Eklund P.C., Saladino R. A selective de-O-methylation of guaiacyl lignans to corresponding catechol derivatives by 2-iodoxybenzoic acid (IBX). The role of the catechol moiety on the toxicity of lignans. Org. Bio. Chem. 2009;7:2367–2377. doi: 10.1039/b822661j. [DOI] [PubMed] [Google Scholar]
- 37.Bernini R., Crisante F., Barontini M., Fabrizi G. A new and efficient route for the synthesis of naturally occurring catecholamines. Synthesis. 2009;22:3838–3842. doi: 10.1055/s-0029-1217027. [DOI] [Google Scholar]
- 38.Hartmann C., Mayer V. Ueber Jodobenzoësäure. Chem. Ber. 1893;26:1727–1732. doi: 10.1002/cber.189302602109. [DOI] [Google Scholar]
- 39.Varvoglis A. Hypervalent Iodine in Organic Synthesis. Academic Press; London, UK: 1997. [Google Scholar]
- 40.Zhdankin V.V., Stang P.J. Recent development in the chemistry of polyvalent iodinee compounds. Chem. Rev. 2002;102:2523–2584. doi: 10.1021/cr010003+. [DOI] [PubMed] [Google Scholar]
- 41.Ladziata U., Zhadankin V.V. Hypervalent iodine (V) reagents in organic synthesis. ARKIVOC. 2006;IX:26–58. doi: 10.1002/chin.200712265. [DOI] [Google Scholar]
- 42.Magdziak D., Rodriguez A.A., Van De Water R.W., Pettus T.R.R. Regioselective oxidation of phenols to o-quinones with o-iodoxybenzoic acid. Org. Lett. 2002;4:285–288. doi: 10.1021/ol017068j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozanne A., Pouységu L., Depernet D., Francois B., Quideau S. A stabilized formulation of IBX (SIBX) for safe oxidation reactions including a new oxidative demethylation of phenolic methyl aryl ethers. Org. Lett. 2003;5:2903–2906. doi: 10.1021/ol0349965. [DOI] [PubMed] [Google Scholar]
- 44.Quideau S., Pouysegu L., Deffieux D., Ozanne A., Gagnepain J., Fabre I., Oxoby M. Iodane-mediated and electrochemical oxidative transformations of 2-methoxy-and 2-methylphenols. Arkivoc. 2003;VI:106–119. [Google Scholar]
- 45.Ulrich R., Hofrichter M. Enzymatic hydroxylation of aromatic compounds. Cell. Mol. Life Sci. 2007;64:271–293. doi: 10.1007/s00018-007-6362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brand-Williams W., Cuvelier M.E., Berzet C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 47.Nicolosi G., Spatafora C., Tringali C. Chemo-enzymatic preparation of resveratrol derivatives. J. Mol. Cat. B Enzym. 2002;16:223–229. doi: 10.1016/S1381-1177(01)00066-2. [DOI] [Google Scholar]
- 48.Cardile V., Lombardo L., Spatafora C., Tringali C. Chemo-enzymatic synthesis and cell-growth inhibition activity of resveratrol analogues. Bioorg. Chem. 2005;33:22–33. doi: 10.1016/j.bioorg.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 49.De Lucia M., Panzella L., Pezzella A., Napolitano A., D’Ischia M. Plant catechols and their S-glutathionyl conjugates as antinitrosating agents: Expedient synthesis and remarkable potency of 5-S-glutathionylpiceatannol. Chem. Res. Toxicol. 2008;21:2407–2413. doi: 10.1021/tx800283d. [DOI] [PubMed] [Google Scholar]
- 50.Teguo P.W., Fauconneau B., Deffieux G., Huguet F., Vercauteren J., Merillon J.M. Isolation, identification, and antioxidant activity of three stilbene glucosides newly extracted from Vitis vinifera cell cultures. J. Nat. Prod. 1998;61:655–657. doi: 10.1021/np9704819. [DOI] [PubMed] [Google Scholar]
- 51.Frigerio M., Santagostino M., Sputore S. A user-friendly entry to 2-iodoxybenzoic acid (IBX) J. Org. Chem. 1999;64:4537–4538. doi: 10.1021/jo9824596. [DOI] [Google Scholar]