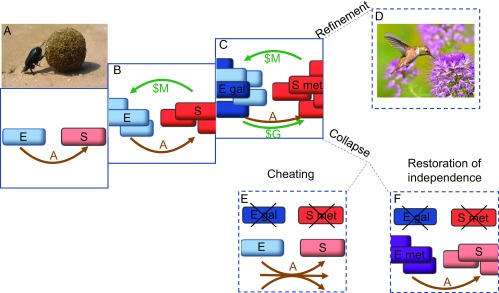
Our natural world is enriched by mutualisms, where organisms engage in a complex and often colorful trade of goods and services for their mutual benefit (Fig. 1D). An enduring question in evolutionary biology is how does this multispecies complexity emerge from simpler interactions? One-way beneficial relationships, such as those between a dung beetle and a mammal, have been proposed as a starting point for the elaboration of mutualisms (1–3) (Fig. 1A) and provide a vivid framing of the puzzle: When and how does it pay a dung beetle to help mammals make more dung? Harcombe et al. (4) took a microbial analog of a mammal–dung beetle relationship and used experimental evolution to probe the potential coevolutionary pathways. In their PNAS paper, Harcombe et al. report a striking result: the evolution of a costly bidirectional mutualism in the laboratory.
Fig. 1.
Step-wise evolution of a costly bidirectional mutualism between Salmonella enterica and an E. coli methionine auxotroph. Costly products are indicated in green and costless products are indicated in brown. (A) The E. coli methionine auxotroph (light blue) produces acetate as a costless byproduct of lactose metabolism. Salmonella (light red) utilizes this acetate waste. This relationship is analogous to the dung beetle–mammal relationship. Image courtesy of Pixabay/Topi_Pigula. (B) Evolution of costly methionine secretion in Salmonella (dark red), enhancing the growth of both species (3). (C) Evolution of costly galactose secretion in E. coli (dark blue), enhancing growth of both species (4). (D–F) Future directions. (D) Interspecific cooperation becomes increasingly elaborate and codependent. Image courtesy of Flickr/Tom Koerner/USFWS. (E) E. coli galactose cheats take over in a well-mixed environment. (F) Restoration of independence via reversion of E. coli methionine auxotrophy to wild-type (purple).
In an earlier study (3), the quest began with the design and experimental evolution of a two-species system with ingredients primed to favor the evolution of mutualisms (Fig. 1A). On one side was a strain of Escherichia coli engineered to be nutritionally deficient (a methionine auxotroph), and on the other a “dung feeder” (Salmonella) that was dependent on a waste product (acetate) produced by E. coli. Following ∼10 generations of evolution in a spatially structured environment, Harcombe (3) reported an innovation in the dung-feeding Salmonella—the emergence of strains that overproduced methionine (Fig. 1B)—a costly investment in E. coli growth that returned more acetate to the new Salmonella strain.
Harcombe et al. (4) now report a striking innovation in their two-species system that marks a transition into a bidirectional costly mutualism. Following another 200+ generations of experimental evolution, the authors report the emergence of a novel E. coli strain that secretes a costly sugar, galactose, that can then be utilized by Salmonella (Fig. 1C). This sugar-secreting super-cooperative E. coli phenotype arose repeatedly across multiple replicate evolutionary lineages, in each case due to a different frameshift mutation in galK that blocked galactose metabolism. These mutations are inevitably very costly when E. coli grows on lactose, as each lactose molecule yields one molecule of glucose and one of galactose, now available to support Salmonella growth. Harcombe et al. demonstrate that this “one for me, one for you” carbon sharing by E. coli leads to substantial costs when grown alone, but triggers net benefits to both partners when cultured together on an agar plate.
Despite enhancing the growth of both species in coculture, the sugar-producing E. coli mutants did not fix in any lineage. On first assessment, this could simply represent an intermediate observation along a transition toward fixation and accelerating mutualism. To test this hypothesis, Harcombe et al. (4) ran competition experiments between the ancestral and galactose-secreting E. coli at different frequencies (always in partnership with the methionine-secreting Salmonella) and instead found a signature of negative-frequency dependence: whichever E. coli strain was rare had a fitness advantage. This result, together with explicit metabolic simulations (4, 5), suggests that the E. coli polymorphism represents an ongoing intraspecific social tension between the individual cost of secreting half of their carbon source and the collective benefit of increased methionine, mediated by their Salmonella partner. From a social evolution perspective, these results map out a fascinating model system where each species plays an intraspecific “public goods” game, and is simultaneously a dynamical component in their partner species’ public good (6, 7).
What is next for these two entwined lineages? By reciprocating gifts of methionine and galactose that support each other’s growth, there is the theoretical possibility of an “orgy of mutual benefaction” (8), an ecological explosion in numbers that could open new evolutionary paths or environmentally mediated collapse. In the absence of immediate ecological destabilization, there are evolutionary threats to the stability of this mutualism. The clear social conflict within the E. coli population highlights the threat of cheats. The spatially structured environment limits the ability of cheats to outcompete the galactose-secreting cooperator (4), but an environmental shift toward greater mixing would risk loss of the cooperator genotypes and therefore collapse of the mutualism (3, 9) (Fig. 1E). Finally, there is the threat of autonomy: if one species loses its dependency on the other, then the incentive to feed their partner is also gone. Autonomy could arise via simple environmental changes: for example, a rich growth medium containing methionine and free carbon. Autonomy could also arise via gain-of-function mutations (10): for example, the restoration of methionine synthesis in E. coli would remove the incentive for trade (Fig. 1F). We encourage Harcombe et al. (4) to keep evolving their fascinating system, and we look forward to finding out what new surprises are in store.
Footnotes
The authors declare no conflict of interest.
See companion article on page 12000.
References
- 1.Connor RC. Pseudo-reciprocity: Investing in mutualism. Anim Behav. 1986;34:1562–1566. [Google Scholar]
- 2.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. The evolution of cooperation. Q Rev Biol. 2004;79:135–160. doi: 10.1086/383541. [DOI] [PubMed] [Google Scholar]
- 3.Harcombe W. Novel cooperation experimentally evolved between species. Evolution. 2010;64:2166–2172. doi: 10.1111/j.1558-5646.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- 4.Harcombe WR, Chacón JM, Adamowicz EM, Chubiz LM, Marx CJ. Evolution of bidirectional costly mutualism from byproduct consumption. Proc Natl Acad Sci USA. 2018;115:12000–12004. doi: 10.1073/pnas.1810949115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harcombe WR, et al. Metabolic resource allocation in individual microbes determines ecosystem interactions and spatial dynamics. Cell Reports. 2014;7:1104–1115. doi: 10.1016/j.celrep.2014.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown SP, Taddei F. The durability of public goods changes the dynamics and nature of social dilemmas. PLoS One. 2007;2:e593. doi: 10.1371/journal.pone.0000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weitz JS, Eksin C, Paarporn K, Brown SP, Ratcliff WC. An oscillating tragedy of the commons in replicator dynamics with game-environment feedback. Proc Natl Acad Sci USA. 2016;113:E7518–E7525. doi: 10.1073/pnas.1604096113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May R, McLean AR. Theoretical Ecology: Principles and Applications. Oxford Univ Press; Oxford: 2007. [Google Scholar]
- 9.Foster KR, Wenseleers T. A general model for the evolution of mutualisms. J Evol Biol. 2006;19:1283–1293. doi: 10.1111/j.1420-9101.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 10.Dragoš A, et al. Collapse of genetic division of labour and evolution of autonomy in pellicle biofilms. Nat Microbiol. 2018 doi: 10.1038/s41564-018-0263-y. [DOI] [PubMed] [Google Scholar]