Significance
The plant hormone abscisic acid (ABA) is essential for drought-stress responses in plants, and its functions have been well studied; however, the detailed molecular mechanisms of ABA biosynthesis during early drought stress need to be further explored. The present study identified a transcription factor, NGTHA1 (NGA1), which positively regulates ABA accumulation during dehydration stress by activating the NCED3 gene encoding a key ABA biosynthetic enzyme. We also identified a cis-acting element bound by NGA1 in the 5′ untranslated region (5′ UTR) of the NCED3 promoter. The NGA1 protein was degraded under nonstressed conditions, but it was stabilized during dehydration stress in an ABA-independent pathway.
Keywords: drought stress, ABA biosynthesis, transcriptional regulation, NCED3, NGA
Abstract
The plant hormone abscisic acid (ABA) is accumulated after drought stress and plays critical roles in the responses to drought stress in plants, such as gene regulation, stomatal closure, seed maturation, and dormancy. Although previous reports revealed detailed molecular roles of ABA in stress responses, the factors that contribute to the drought-stress responses—in particular, regulation of ABA accumulation—remain unclear. The enzyme NINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3 (NCED3) is essential for ABA biosynthesis during drought stress, and the NCED3 gene is highly induced by drought stress. In the present study, we isolated NGATHAs (NGAs) as candidate transcriptional regulators of NCED3 through a screen of a plant library harboring the transcription factors fused to a chimeric repressor domain, SRDX. The NGA proteins were directly bound to a cis-element NGA-binding element (NBE) in the 5′ untranslated region (5′ UTR) of the NCED3 promoter and were suggested to be transcriptional activators of NCED3. Among the single-knockout mutants of four NGA family genes, we found that the NGATHA1 (NGA1) knockout mutant was drought-stress-sensitive with a decreased expression level of NCED3 during dehydration stress. These results suggested that NGA1 essentially functions as a transcriptional activator of NCED3 among the NGA family proteins. Moreover, the NGA1 protein was degraded under nonstressed conditions, and dehydration stress enhanced the accumulation of NGA1 proteins, even in ABA-deficient mutant plants, indicating that there should be ABA-independent posttranslational regulations. These findings emphasize the regulatory mechanisms of ABA biosynthesis during early drought stress.
Plants have developed various systems to survive adverse and fluctuating environmental conditions, such as drought, high salt, and extreme temperature, as sessile organisms. Drought stress has negative effects on the plant growth and crop yield (1) and often causes severe damage to the agricultural crops (2). The plant hormone abscisic acid (ABA) is known as an essential factor that positively regulates the plant drought-stress responses, such as stomatal closure, induction of drought-inducible genes, and repression of plant growth (3). Previous studies revealed the detailed molecular mechanisms by which ABA is transported (4), received (5) to activate the cellular signal cascades (6, 7), and then induces drought-stress-responsive gene expression (8); however, the factors that regulate early drought-stress responses before ABA accumulation need to be further explored.
Among the various enzymatic proteins involved in several ABA biosynthetic pathways in plants (9), NINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3 (NCED3) is known as an important enzyme for ABA accumulation during drought stress because expression of the NCED3 gene is highly induced in the vascular tissues by drought stress, and its knockout mutants revealed decreased ABA accumulation under drought-stress conditions and drought-stress-sensitive phenotypes (10, 11). The NCED3 promoter has a G-box sequence as a long-range enhancer that is essential for gene induction in the vascular tissues during dehydration stress (12, 13). This G-box is located ∼2.3 kb upstream from its translational start site; however, the detailed molecular mechanisms by which the unidentified transcription factors activate the NCED3 gene before ABA accumulation have not been elucidated. In the present study, we identified a transcription factor, NGATHA1 (NGA1), that activates the NCED3 gene during dehydration stress and characterized a cis-acting sequence, NGA-binding element (NBE), in the 5′ untranslated region (5′ UTR) of the NCED3 promoter that is necessary for gene induction (in this work, we use “promoter” to refer to the region upstream from the translational start site of a protein coding gene and include the 5′ UTR region). Moreover, it also was suggested that ABA-independent posttranslational regulation should be involved in the stabilization of the NGA1 protein. NGA proteins are B3-type transcription factors, and Arabidopsis thaliana has four NGA family proteins. Previous work revealed that the NGA proteins regulate the developmental process in the reproductive organs or leaves (14–16). In the present work, we revealed functions of NGA1, which acts as a positive regulator of ABA biosynthesis during the drought-stress responses by directly activating the expression of NCED3.
Results
NGA2–SRDX-Overexpressing Plants Reveal Repressed Expression of NCED3.
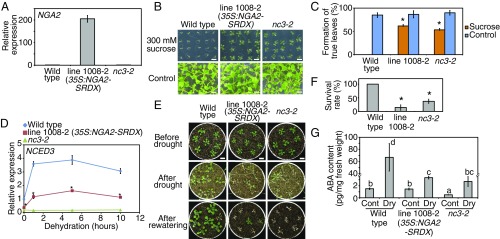
To identify the transcription factors that regulate ABA biosynthesis during early drought responses, we screened a library of transgenic plants overexpressing the transcription factors fused to the repressive domain SRDX (CRES-T; chimeric repressor gene silencing technology) (17). ABA-deficient mutants have been reported to show sucrose-insensitive phenotypes during germination (18). Previous studies reported that sucrose or other sugar-insensitive mutants often carried mutations in the genes involved in ABA biosynthesis (19, 20). Therefore, we screened 1,795 CRES-T plant lines of the library on the basis of the germination phenotypes on high sucrose-containing (300 mM) medium and found four sucrose-insensitive lines. Among these, the line 1008-2 overexpressing NGA2–SRDX indicated strong insensitivity to a high concentration of sucrose during germination (Fig. 1 A–C). Line 1008-2 also exhibited suppressed expression of NCED3 during dehydration stress (Fig. 1D), as well as drought-sensitive phenotypes with decreased leaf temperature, which also were observed in NCED3 knockout mutants (nc3-2) (Fig. 1 E and F and SI Appendix, Fig. S1 A–C). (In this work, “dehydration” and “drought” stresses are defined as water-deficit stress treatments of plants on parafilms from agar plates and plants on soil without a water supply, respectively.) ABA accumulation during dehydration stress was significantly suppressed in line 1008-2 (Fig. 1G) in accord with the repression of NCED3 (Fig. 1D). The low level of ABA accumulation in nc3-2 likely results from low induction of another NCED family gene in nc3-2 that was not induced in the wild-type plant (21). Thus, we hypothesized that the NGA family proteins are involved in the transcription of NCED3 during drought stress.
Fig. 1.
The 35S:NGA2-SRDX plants (line 1008-2) revealed decreased expression of NCED3 and were sensitive to drought stress. (A) Expression levels of NGA2 in the identified line through a screen. The error bars indicate the SD from triplicate technical repeats. (B and C) Sucrose insensitivity of the 35S:NGA2-SRDX plants in germination. (B) Images of plants grown on MS medium with or without 300 mM sucrose for 2 wk. (Scale bars: 1 cm.) (C) Rates of plants forming true leaves grown on medium with or without 300 mM sucrose. The error bars indicate the SD from four replicates (n = 20 each). Asterisks indicate significant differences from the wild-type plants. *P < 0.01 (Bonferroni-corrected Student’s t test). (D) Expression levels of NCED3 in the identified line through a screen. Plants grown on MS medium for 2 wk were treated with dehydration stress. The error bars indicate the SD from triplicate technical repeats. Asterisks indicate significant differences between the 35S:NGA2-SRDX and wild-type plants. *P < 0.01 (Bonferroni-corrected Student’s t test). (E and F) Drought-stress tolerance test of the 35S:NGA2-SRDX and nc3-2 mutant plants. (E) Images of plants before and after drought and after rewatering. Plants grown on MS medium for 2 wk were transferred to soil and grown for 2 d. Water was withheld for 11 d. (Scale bars: 1 cm.) (F) Survival rates of plants after rewatering. The error bars indicate the SD from five replicates (n = 35 total). Asterisks indicate significant differences from the wild-type plants. *P < 0.01 (Bonferroni-corrected Student’s t test). (G) ABA contents of the 35S:NGA2-SRDX and nc3-2 mutant plants during nonstress (Cont) and dehydration stress (Dry). Plants grown on MS medium for 2 wk were treated with dehydration stress for 4 h. The error bars indicate the SD from six replicate samples. The letters above the bars indicate significant differences among plant lines under nonstress and dehydration-stress conditions (P < 0.05, according to Tukey’s multiple range test).
NGA proteins are B3-type transcription factors, and Arabidopsis thaliana has four NGA family proteins and three NGA-like (NGAL) family proteins (SI Appendix, Fig. S1D). RAV subfamily proteins have the conserved B3 domain with high homology to NGA; however, the protein structures are quite different because they also have conserved AP2 domains. An amino acid sequence, K/RLFGV, was found in all of the NGA, NGAL, and RAV subfamily proteins, which was reported as a putative repressor motif (22). More comprehensive and detailed phylogenetic analysis of the NGA family proteins in land plants was reported in a previous study (23).
NGA Family Proteins Directly Bind to and Activate the NCED3 Promoter.
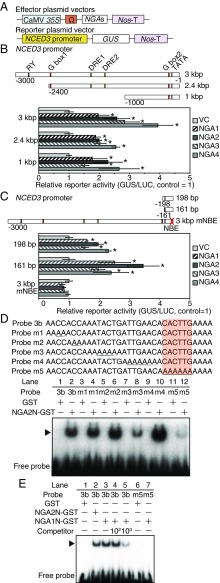
The NGA family proteins contain the putative repression domain RLFGV, but previous studies reported that another K/RLFGV domain-containing transcription factor family, HEAT SHOCK FACTOR B (HsfB), acts as both repressor and activator during heat stress (24, 25). To elucidate whether the NGA proteins function as activators or repressors, we performed transactivation assays using the NCED3 promoter as a reporter in Arabidopsis mesophyll protoplasts (Fig. 2A). Transfections of the NGA family proteins resulted in activation of the NCED3 3-kb promoter (Fig. 2B). The deletion series of the NCED3 promoter revealed that the nearby region of a translational start site (−1 to −161) was sufficient for the activation of NCED3 by NGAs (Fig. 2 B and C). These data indicate that the NGA family proteins function as transcriptional activators of NCED3. We also performed similar transactivation assays using mutated NGA2 and NGA4 within their putative repressor motifs RLFGV to five alanines (AAAAA) as effectors. Interestingly, the mutated proteins did not activate the NCED3 3-kb promoter (SI Appendix, Fig. S2A), which suggested that this motif is necessary for NGA to activate the NCED3 promoter. We confirmed that NGA has transcriptional activating functions by detecting increased mRNA levels of the GUS reporter gene in protoplasts transfected with NGAs (SI Appendix, Fig. S2B). The endogenous NCED3 gene in protoplasts also was activated by transfecting the NGA family proteins (SI Appendix, Fig. S2C). These data indicate that the NGA family proteins function as a transcriptional activator of NCED3.
Fig. 2.
Transactivation assays and EMSAs revealed that the NGA family proteins activated and directly bound to the NCED3 promoter in vitro. (A) Schematic diagram of the reporter and effector constructs. (B and C) Transactivation assays of the NGA family proteins using the various NCED3 promoters as reporters. The error bars indicate the SD from three replicate samples. Asterisks indicate statistically significant differences of reporter activities from the vector control. *P < 0.05 (Bonferroni-corrected Student’s t test). The 35S:ELUC plasmid was also cotransfected in each experiment as an internal control. (B) Reporters of 3, 2.4, and 1 kbp. (C) Reporters of 198-, 161-bp, and 3-kbp mNBE. (D and E) Schematic diagrams of the probes of the NCED3 promoters and EMSA using the recombinant NGA2 (D) and NGA1 (E) protein. The migration positions of the protein–DNA complexes are represented by arrows. Mutated nucleotides in each probe are underlined, and an orange box indicates the position of the NBE. Incubation with 100- or 1,000-fold competitors was performed in the presence of the recombinant NGA1 proteins to confirm specific binding to the probes.
We investigated whether the NGA proteins directly bound to the NCED3 promoter in vitro by electrophoretic mobility shift assay (EMSA) using the −1- to −161-bp promoter region of NCED3. This region was divided into three shorter parts (probes E1–E3). Due to the unsuccessful expression of the full-length NGA proteins in Escherichia coli, the N-terminal region of NGA2 that includes the DNA-binding domain (SI Appendix, Fig. S2D) fused to GST (NGA2N-GST), was expressed. Two band shifts were observed when the NGA2N-GST protein was incubated with the probe E3 (SI Appendix, Fig. S2E), which suggested the possibility that NGA2 directly bound to the NCED3 promoter by forming a dimer. Weak band shifts were observed by using the E1 and E2 probes, but there was a large difference in the intensity of band shifts from probe E3, which suggests that E3 likely contains the NGA binding site. The probe E3 was divided into the two shorter parts (probes 3a and 3b), and subsequent EMSAs suggested that the NGA2N-GST protein directly bound to the probe 3b (SI Appendix, Fig. S2F). This region contains the 6-bp motif CACTTG that was reported as a direct binding motif of NGAL2 (SOD7) on the KLU promoter (26). The mutated probe (probe m1-5) indicated that replacement of the CACTTG motif led to a complete loss of binding (Fig. 2D). This CACTTG motif therefore appears to be a common binding sequence of NGA and NGAL family proteins. The NGA1 protein also directly bound to the CACTTG motif (Fig. 2E). This sequence CACTTG motif was named as the NBE. Additional transactivation assays in protoplasts confirmed that the NGA proteins could not activate the 3-kb NCED3 promoter with NBE mutations, because there was no detection of increased enzymatic activity or mRNA accumulation of the GUS reporter (Fig. 2C and SI Appendix, Fig. S2B). These results suggest that the NGA family proteins directly bind to NBE on the NCED3 promoter and induced gene expression. Two band shifts were not observed in other EMSAs, presumably due to the concentrations of polyacrylamide gel; 6% gel was used for the experiment presented in SI Appendix, Figs. S2E, and 8% was used for the other figures.
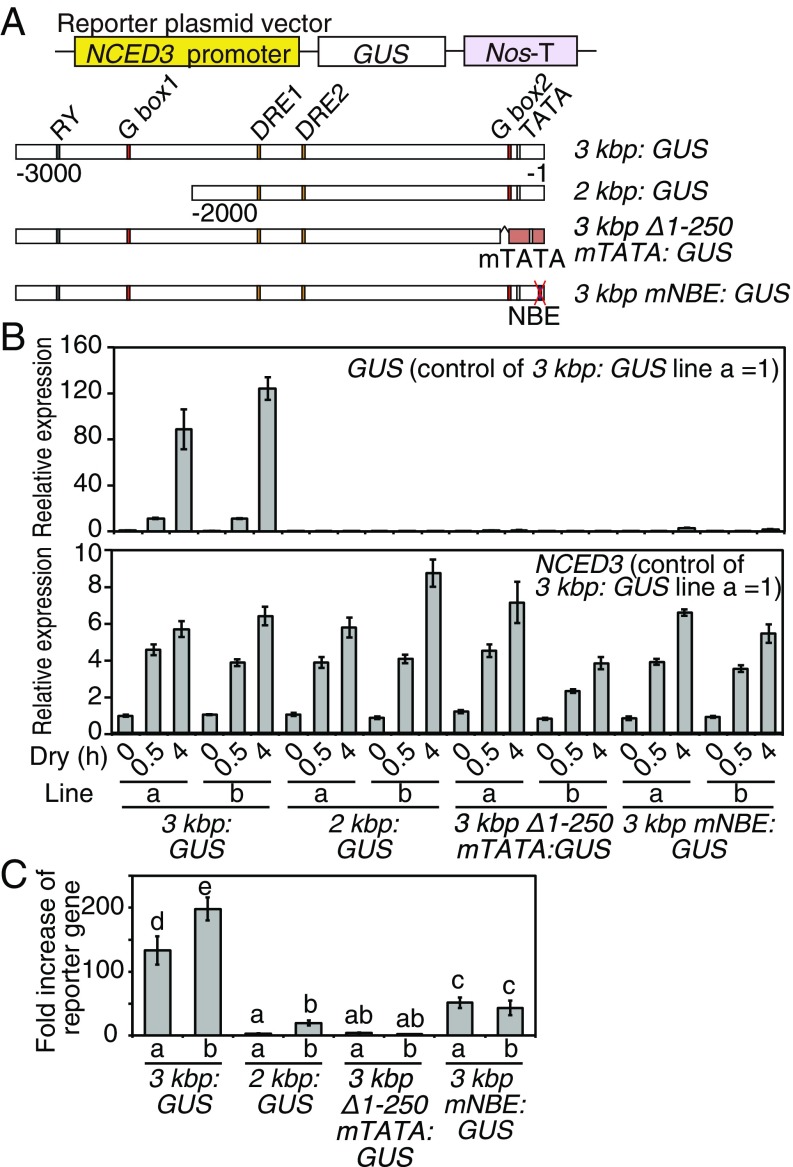
The NBE Sequence Is Important for the NCED3 Promoter Activity in Transgenic Plants During Dehydration Stress.
NBE was found to be a cis-acting element present in the NCED3 promoter. We generated transgenic plants harboring the GUS reporter gene driven by modified NCED3 promoters (Fig. 3A). As previous papers reported (12, 13), the NCED3 3-kb promoter could induce the expression of the reporter gene, whereas the 2-kb promoter could not (Fig. 3B). The promoter of the −250- to −3,000-bp region fused to the RD29A minimal TATA box (3-kbp Δ1–250 mTATA) and the 3-kbp region with mutation in NBE to six adenines (3-kbp mNBE) revealed almost no expression of the reporter genes relative to the expression level of the reporter gene in the 3 kbp:GUS plants (Fig. 3 B, Upper), when all transgenic lines were treated with similar dehydration stress (Fig. 3 B, Lower). In comparison with the expression levels of the reporter genes under control conditions in each line, the 3-kb mNBE promoter still activated the reporter gene, but the reporter gene was significantly suppressed relative to the 3-kbp promoter (Fig. 3C). In addition, reporter activation by the 2- and 3-kbp Δ1–250 mTATA promoter was suppressed more than the 3-kbp mNBE promoter (Fig. 3C). These results suggest that the NBE sequence was necessary for both full activation of the NCED3 promoter during dehydration stress and basal expression under control conditions. The GUS staining was also observed in only the 3 kbp:GUS plants during dehydration stress among the transgenic lines (SI Appendix, Fig. S3 A and B).
Fig. 3.
The NBE sequence in the 5′ UTR of the NCED3 promoter was essential in inducing expression of the reporter gene during dehydration stress in planta. (A) Schematic diagrams of various NCED3 promoters that drive a GUS reporter gene. (B) Expression levels of NCED3 and GUS reporter in the transgenic plants under dehydration-stress conditions (Dry). B, Upper indicates the relative expression levels of GUS during dehydration stress compared with that of the 3 kbp:GUS plants under nonstress conditions. B, Lower indicates the expression levels of the endogenous NCED3 genes as a control. The error bars indicate the SD from triplicate technical repeats. (C) Fold increases of relative expression of the reporter gene during dehydration stress. The fold increases of the reporter expression levels at 4 h of dehydration stress relative to those under control conditions (0 h) in each plant line are shown. The error bars indicate the SD from triplicate technical repeats. The letters above the bars indicate significant differences between the plant lines at each time point (P < 0.05, according to Tukey’s multiple range test).
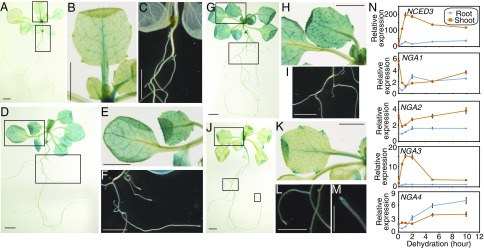
NGA Genes Are Expressed in Various Tissues Including Vascular Ones and Reveal Several Stress-Mediated Expression Patterns.
The 35S:NGA2-SRDX plants ectopically expressed the NGA2 protein fused to the repressor motif, and the transactivation assays in protoplasts suggested the presence of redundant NGA functions in activating NCED3 (Fig. 2 A and B). However, overexpression in plants and protoplasts does not reflect the actual tissue expression patterns or intensity in wild-type plants. To elucidate how each NGA protein contributed to the induction of NCED3 during drought stress, we analyzed tissue-specific expression and stress-inducible expression of NGAs. We generated transgenic plants harboring the GUS reporter gene driven by an ∼3-kb-long promoter of each NGA gene. In the NGA1p:GUS plants, GUS activity was observed in the vascular tissues of aerial organs; in particular, strong activity was observed in the petioles and major veins of true leaves (Fig. 4 A and B). Weak GUS staining was also observed in the lateral roots (Fig. 4C). In the NGA2p:GUS and NGA3p:GUS plants, GUS activity was observed in the whole leaves, including vascular tissues, shoot meristematic region, root–shoot junction, and lateral roots (Fig. 4 D–I). In the NGA4p:GUS plants, GUS activity was observed in the lateral roots, shoot meristematic region, and leaf tips (Fig. 4 J–M). Weak GUS staining was detected in the petioles of true leaves (Fig. 4K). The NCED3 gene was expressed in the vascular tissues (SI Appendix, Fig. S3B) (12), which suggested that the NGA family genes were coexpressed with NCED3. In particular, the GUS staining patterns of the NGA1p:GUS plants exhibited a similar pattern to the NCED3 3 kbp:GUS plants. We analyzed the expression levels of NGAs under dehydration-stress conditions. NGA1, NGA2, and NGA3 were highly expressed in the shoots under the nonstress condition (dehydration: 0 h) (Fig. 4N). During dehydration stress, NGA1, NG2, and NGA4 genes were induced by stress in the root tissues, and NGA3 and NGA4 were transiently and continuously induced in the shoots, respectively (Fig. 4N). Meanwhile, the expression levels of NGA1 and NGA2 were transiently suppressed by dehydration stress in the early phase and were elevated again in the later phase of dehydration (Fig. 4N). Dehydration stress increased the intensity of GUS staining in the NGA3p:GUS and NGA4p:GUS plants in shoot and root tissues, respectively (SI Appendix, Fig. S4A). The NGA3p:GUS plants showed similar tissue-specific expression patterns during dehydration stress, and the NGA4p:GUS plants showed GUS staining over a wider range in lateral roots.
Fig. 4.
Each NGA family gene revealed tissue-specific expression and alteration of expression levels during dehydration stress. (A–M) GUS staining of the NGA1p:GUS (A–C), NGA2p:GUS (D–F), NGA3p:GUS (G–I), and NGA4p:GUS (J–M) plants at 14 d after sowing. Images of the whole plants (A, D, G, and J) and their higher-magnification images are presented as follows: B and C from A, E and F from D, H and I from G, and K–M from J. [Scale bars: 0.5 mm (M) and 1 mm (A–L).] (N) Expression levels of the NCED3 and NGA family genes during dehydration stress in roots and shoots of the wild-type plants. The error bars indicate the SD from triplicate technical repeats. The relative expression levels were determined compared with the expression levels under nonstress conditions in roots.
We also examined expression levels of NCED3 and NGAs during germination on medium with or without high sucrose. The results indicated that during germination, high concentration of sucrose induced higher expression of NCED3 and NGAs relative to control medium (SI Appendix, Fig. S4B). In analyses of spatial expression patterns of the NCED3 and NGAs during germination, the NCED3 3 kbp:GUS and NGAp:GUS plants demonstrated GUS staining in whole embryos on control medium (SI Appendix, Fig. S4C). On a high-sucrose medium, the NCED3 3 kbp:GUS and NGA1p:GUS plants showed stronger GUS staining patterns in the tip regions of cotyledons and radicles of embryos, as well as in the seed coats near the micropylar endosperm. Weak GUS staining was detected in seed coats in the NGA2p:GUS plants (SI Appendix, Fig. S4C). These expression patterns during germination suggest that NGAs might activate the NCED3 gene to inhibit germination on high-sucrose-containing medium.
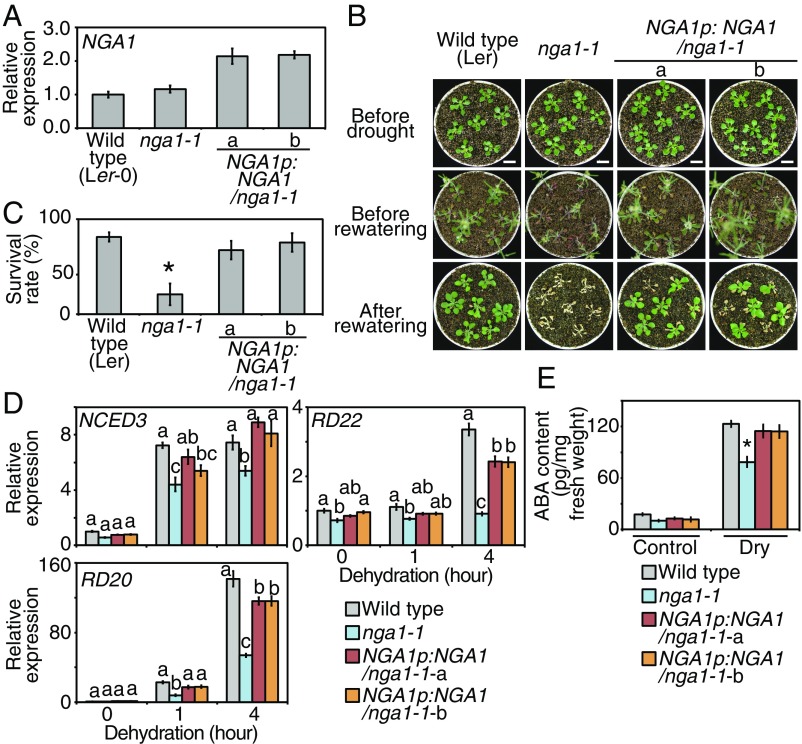
Knockout of NGA1 Results in Drought-Stress Susceptibility and Suppresses the Expression of the NCED3 Gene.
To elucidate the contribution of each NGA gene to drought-stress responses in seedlings, single-knockout mutants of each NGA gene were isolated (nga1-, nga2-, nga3-, and nga4-1) (SI Appendix, Fig. S5 A–C). A point mutation in the nga1-1 resulted in a truncated NGA1 lacking the C-terminal region (SI Appendix, Fig. S5D). Among the NGA single-knockout mutants, only nga1-1 mutants exhibited drought-stress sensitivity, and complementation of the NGA1 expression with the NGA1 genomic region (NGA1pro:NGA1) led to the recovery of drought-stress tolerance (Fig. 5 A–C and SI Appendix, Fig. S5E). The nga2-, nga3-, and nga4-1 did not show any difference in drought-stress tolerance (SI Appendix, Fig. S5 F–K). The nga1-1 mutants exhibited decreased expression levels of NCED3 and other ABA-responsive genes, RD22 and RD20 (Fig. 5D). Greater effects on RD22 and RD20 than NCED3 might reflect amplification of ABA signaling during dehydration stress, and the knockout of NGA1 has greater effects on RD22 than RD20 because the induction of RD20 was suppressed in nga1-1, while RD22 showed almost no induction during dehydration stress, as well as suppressed gene expression under nonstress conditions. The accumulation of ABA during dehydration stress was also decreased in nga1-1 (Fig. 5E). Complemented expression of NGA1 recovered the expression levels of dehydration-inducible genes and ABA accumulation (Fig. 5 D and E). The expression levels of NCED3 during dehydration stress were not significantly different from wild type in nga2-, nga3-, and nga4-1 (SI Appendix, Fig. S5 L and M). These results suggest that among the NGA family genes, NGA1 mainly contributes to induction of NCED3 during drought stress. This is also supported by the vascular-specific expression patterns of the NGA1 gene (Fig. 4 A–C), which are similar to those of NCED3 (11). We also checked whether the truncated form of NGA1 in nga1-1 (NGA1 mut) results in a dominant-negative effect with repressive effects on NCED3 expression. In Arabidopsis mesophyll protoplasts, transfection of the NGA1 mut did not affect the endogenous NCED3 gene expression, and cotransfection of the NGA1 mut with the wild-type NGA1 (NGA1 wt) did not affect induction of NCED3 by NGA1 wt (SI Appendix, Fig. S5N). These data suggest that the NGA1 mut in nga1-1 has no transcriptional activity or repressive effects on NCED3.
Fig. 5.
The nga1-1 single mutants revealed decreased expression of NCED and were sensitive to drought stress. (A) Expression levels of NGA1 in nga1-1 and complemented lines. The error bars indicate the SD from triplicate technical repeats. (B and C) Drought-stress tolerance test of nga1-1 and complemented lines. (B) Images of plants before and after drought and after rewatering. Plants grown on MS medium for 2 wk were transferred to soil and grown for 2 d. Water was withheld for 15 d. (Scale bars: 1 cm.) (C) Survival rates of plants after rewatering. The error bars indicate the SD from five replicates (n = 35 in total). Asterisk indicates significant differences from the wild-type plants. *P < 0.01 (Bonferroni-corrected Student’s t test). (D) Expression levels of NCED3 and other dehydration-inducible genes during dehydration stress in nga1-1 and complemented lines. Plants grown on MS medium for 2 wk were treated with dehydration stress. The error bars indicate the SD from triplicate technical repeats. The letters above the bars indicate significant differences between the plant lines at each time point (P < 0.05, according to Tukey’s multiple range test). (E) ABA contents of nga1-1 and complemented lines during nonstress (Control) and dehydration stress (Dry). Plants grown on MS medium for 2 wk were treated with dehydration stress for 3 h. The error bars indicate the SD from at least four replicate samples. Asterisk indicates significant differences from the wild-type plants. *P < 0.01 (Bonferroni-corrected Student’s t test).
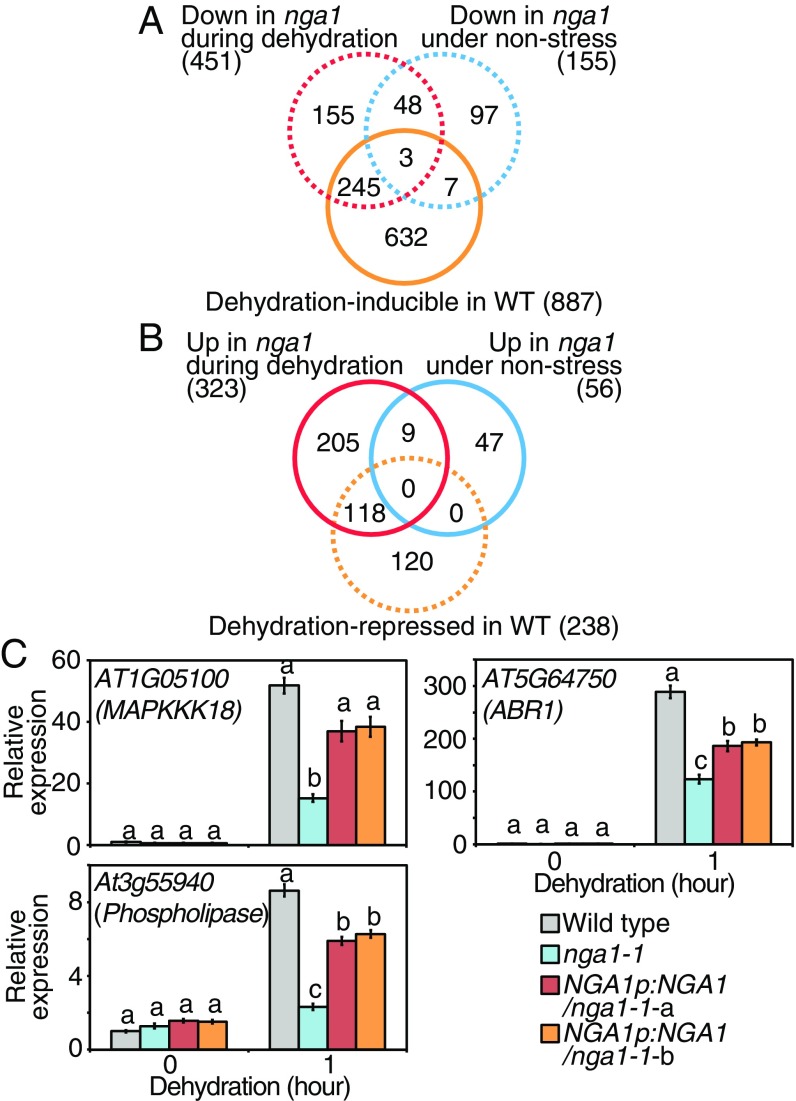
Transcriptomic Analysis Reveals That Dehydration-Inducible Genes Are Down-Regulated in the nga1-1 Mutants.
We analyzed the transcriptomic dynamics in nga1-1 under 1-h dehydration-stress conditions and nonstress conditions by RNA sequencing (Datasets S1–S3). In the wild-type plants, 887 and 238 genes were induced and repressed, respectively, more than fourfold in response to dehydration stress [wild type (control) vs. wild type (dehydration)] (P < 0.05; Fig. 6 A and B). In the nga1-1 mutant, 155 genes and 451 genes were down-regulated under nonstress [wild type (control) vs. nga1-1 (control)] or dehydration-stress conditions [wild type (dehydration) vs. nga1-1 (dehydration)], respectively, more than twofold of the level in the wild-type plants (P < 0.05; Fig. 6A). A total of 248 (245 + 3) genes among the 451 down-regulated genes in nga1-1 during dehydration stress were dehydration-inducible genes, whereas 10 (7 + 3) genes were dehydration-inducible genes under nonstress conditions (Fig. 6A). Among the 451 down-regulated genes, a significant proportion of them were dehydration-inducible genes (55%; 248/451) relative to all Arabidopsis genes (3%; 887/27,417; P < 0.0001, Fisher’s exact test). In comparison with a previous paper on the transcriptomic dynamics in nc3-2 subjected to drought stress (21), 79 genes among the 451 down-regulated genes in nga1-1 during dehydration stress were also down-regulated in nc3-2 under drought stress. Among the 451 down-regulated genes, a significant proportion of them were NCED3-dependent genes (18%; 79/451) relative to all Arabidopsis genes (3%; 759/27,417; P < 0.001, Fisher’s exact test). These data suggested that NGA1 is necessary for the dehydration-inducible transcriptomic changes. Many dehydration-stress-repressed genes (118 genes) were up-regulated in nga1-1 during dehydration stress among all of the up-regulated genes during dehydration stress (323 genes; Fig. 6B). This also suggested that all of the dehydration-stress responses were attenuated in nga1-1, including stress-specific gene suppression. The expression levels of three down-regulated genes in nga1-1 during dehydration stress were confirmed by quantitative RT-PCR (qRT-PCR; MAPKKK18, ABR1, and At3g55940) (Fig. 6C). These three genes were dehydration-stress-inducible, were reported to be involved in drought-stress responses (27–29), and have both NBE and G box motifs in their promoters (Dataset S3).
Fig. 6.
Transcriptome analysis revealed the essential roles of NGA1 during dehydration stress. (A) Venn diagram comparing the dehydration-inducible genes in the wild-type plants and down-regulated genes in nga1-1 under nonstress and dehydration-stress conditions. The total numbers of each set of genes are presented in parentheses. (B) Venn diagram comparing the dehydration-stress-repressed genes in the wild-type plants and up-regulated genes in nga1-1 under nonstress and dehydration-stress conditions. The total numbers of each set of genes are given in parentheses. (C) Expression levels of the dehydration-inducible genes identified by RNA-seq in nga1-1 and complemented lines during dehydration stress. The error bars indicate the SD from triplicate technical repeats. The letters above the bars indicate significant differences between the plant lines at each time point (P < 0.05, according to Tukey’s multiple range test).
A metaprofile analysis was performed by using a public transcriptome database (Genevestigator) with the top 300 down- or up-regulated genes in nga1-1 during dehydration stress. Many genes down-regulated in nga1-1 were suggested to be induced by ABA and dehydration stress treatment (SI Appendix, Fig. S6A). The genes that were up-regulated in nga1-1 during dehydration stress exhibited almost the opposite trend, and especially many of the up-regulated genes in nga1-1 were salt-stress-repressed genes (SI Appendix, Fig. S6B). To characterize the genes whose expression levels were affected by the knockout of NGA1, we compared the frequency of the hexamer sequences in the promoters of the top 300 genes that were up- or down-regulated in nga1-1 during dehydration stress with their normalized frequencies in the promoters of the entire Arabidopsis genome. The Z-scores of all hexamer sequences are presented in the scatter plots. The results revealed that the hexamers related to the ABRE sequences that contain the G-box core sequence (ACGT) were highly enriched on the promoters of the down-regulated genes in nga1-1 (SI Appendix, Fig. S6C). This is consistent with the suppressed expression of NCED3 and other ABA-responsive genes in nga1-1. Meanwhile, these hexamer sequences were not abundant on the promoters of the up-regulated genes in nga1-1 (SI Appendix, Fig. S6D). The NBE sequence was not enriched on the promoters of the down-regulated genes (CACTTG; Z score = 1.44, P = 0.075), presumably because the large effect of the suppressed dehydration-stress responses in ABA signaling might mask the enrichment of fewer down-regulated genes that contain the NBE on their promoters. These suggested that NGA1 should affect the whole transcriptome by regulating the limited numbers of its target genes including NCED3. We performed Gene Ontology (GO) analyses using the public GO database agriGO (Version 2.0) (30). The top 300 genes that were down- or up-regulated in nga1-1 during dehydration stress were analyzed compared with the entire whole genome. Genes encoding proteins involved in ABA (P < 0.005) and reproductive structure development (P < 0.00005) were overrepresented among the down-regulated genes in nga1-1 (SI Appendix, Fig. S6 E and G). This result is consistent with the function of NGA1 as an activator of NCED3 during dehydration stress and in the regulation of reproductive growth as reported (15). Among the up-regulated genes in nga1-1, genes encoding proteins involved in growth related-hormone; auxin, cytokinin, and gibberellin were overrepresented (SI Appendix, Fig. S6F). These data suggest that NGA1 inhibited plant growth during dehydration stress by activating ABA biosynthesis.
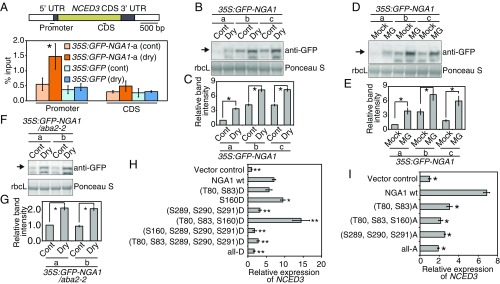
NGA1 Is Posttranslationally Regulated Through ABA-Independent Pathways During Dehydration Stress.
To analyze the binding activity of the NGA protein in planta by chromatin immunoprecipitation (ChIP) assays, the transgenic plants overexpressing NGA1 fused to GFP were generated (35S:GFP-NGA1-a, -b, and -c). GFP-NGA1 was localized in the nuclei (SI Appendix, Fig. S7A). We found that the binding activity of NGA1 to the NCED3 promoter region was enhanced by the dehydration stress (Fig. 7A). In these transgenic plants, protein accumulation of GFP-NGA1 increased due to dehydration stress (Fig. 7 B and C), but mRNA levels were not affected (SI Appendix, Fig. S7B). Treatment of MG132, a 26S proteasome inhibitor, also led to accumulation of GFP-NGA1 (Fig. 7 D and E). Importantly, the accumulation of GFP-NGA1 was observed in the ABA-deficient mutant aba2-2 during dehydration stress (Fig. 7 F and G), and ABA treatment did not affect protein accumulation (SI Appendix, Fig. S7C). These results suggest that NGA1 was degraded by a 26S proteasome under control conditions and that NGA1 was posttranslationally stabilized in an ABA-independent manner. The NGA proteins have been shown to be modified by both phosphorylation and O-linked N-acetylglucosaminylation (O-GlcNAcylation) (31), and NGA1 is believed to have six phosphorylation sites (T80, T83, S160, S289, S290, and S291) and three possible O-GlcNAcylation sites (T3, S6, or T8) (31). We found that modification of these sites is conserved among all or part of the other NGA family proteins (SI Appendix, Fig. S7D). To determine whether these modifications affect the transcriptional activity or protein stability of NGA1, transactivation assays using Arabidopsis mesophyll protoplasts were performed. Among the phosphorylated amino acids, a S160D phospho-mimic mutation significantly increased endogenous NCED3 expression (Fig. 7H), and additional T80D and S83D phospho-mimic mutations further enhanced expression (Fig. 7H). However, alanine mutations at T80, S83, and S160 suppressed the NCED3 expression (Fig. 7I). These data suggest that the phosphorylation of these three amino acids can enhance the transcriptional activity of NGA1 for NCED3 during dehydration stress. However, no differences in protein accumulation were found for the mutated NGA1 proteins (SI Appendix, Fig. S7E), implying that there are different activating mechanisms of NGA1 involved in regulation of protein stability and transcriptional activity. Any combination, including phospho-mimic or alanine mutations of the C-terminal regions (S289, S290, and S291), repressed the transcriptional activity of NGA1 (Fig. 7 H and I). Mutations at the possible O-GlcNAcylated amino acids also caused negative effects on the transcriptional activity of NGA1 (SI Appendix, Fig. S7F). These results suggest that three serine amino acids at positions 289, 290, and 291 and O-GlcNAcylation are involved in regulating transcriptional activity of NGA1 during dehydration stress and that complex regulatory systems of NGA1 protein stability and transcriptional activity likely exist.
Fig. 7.
NGA1 is posttranslationally regulated during dehydration stress. (A) ChIP assays of the NGA1 proteins. A schematic diagram displays the amplified region of the NCED3 genome by qRT-PCR. Plants grown on MS medium for 2 wk were treated with dehydration stress for 1 h. Yellow and closed boxes indicate the coding sequence (CDS) regions and UTRs of NCED3, respectively. Error bars indicate the SD from six technical replicates. Asterisks indicate significant differences from control plants. *P < 0.01 (Bonferroni-corrected Student’s t test). (B–G) Accumulation levels of the GFP-NGA1 protein during dehydration stress (B, C, F, and G) and with treatment of MG132, a 26S proteasome inhibitor (D and E) in the 35S:GFP-NGA1 (lines a, b, and c) (B–E) and 35S:GFP-NGA1/aba2-2 (lines a and b) (F and G). Immunoblotting (B, D, and F) and relative band intensity of GFP-NGA1 (C, E, and G) are shown. Plants grown on MS medium for 2 wk were treated with dehydration stress for 1 h (Dry) or 100 μM MG132 for 4 h (MG). (B, D, and F) Total extracted proteins were analyzed by immunoblotting with an anti-GFP antibody (Upper), and the rubisco large subunits (rbcL) were detected by Ponceau S staining (Lower). Relative band intensity of the GFP-NGA1 proteins from the transgenic line a under control condition was set to 1. The error bars indicate the SD from three replicate samples. Asterisks indicate significant differences between conditions. *P < 0.01 (Bonferroni-corrected Student’s t test). (H and I) Relative expression levels of the endogenous NCED3 in Arabidopsis mesophyll protoplasts transfected with wild-type and mutated NGA1. The effects of mutations to aspartic acid (H) and alanine (I) are shown. The serine and threonine in the brackets show the mutated positions, and “all-D” and “all-A” indicate mutations in the six amino acids (T80, S83, S160, S289, S290, and S291). Relative expression levels of NCED3 among protoplasts transfected with the wild-type and mutated NGA1 were normalized to expression levels in the wild-type and mutated NGA1. The error bars indicate the SD from three replicate samples. Asterisks indicate significant differences in relative expression from protoplasts transfected with the wild-type NGA1. *P < 0.05; **P < 0.01 (Bonferroni-corrected Student’s t test).
Discussion
Recent studies of ABA signaling have revealed the detailed molecular mechanisms in which the varieties of proteins (transporter, receptor, signaling regulator, transcription factor, etc.) function to activate ABA responses in plants during drought stress (4–8, 32); however, the factors that are involved in the early response to dehydration stress to activate NCED3 for ABA accumulation need to be further explored. The present study identified a transcription factor, NGA1, which activated NCED3 during dehydration stress, through a unique screening method (Fig. 1). Among the NGA transcription factors, single-knockout mutants of NGA1 exhibited not only suppressed expression of NCED3 during dehydration stress (Fig. 5F), but also reduced accumulation of ABA (Fig. 5G). This NGA1 transcription factor exhibited a positive effect on the reporter activity driven by the NCED3 promoter in Arabidopsis mesophyll protoplasts (Fig. 2 A and B) and directly bound to the NCED3 promoter in vitro (Fig. 2E) and in vivo (Fig. 7A). These results clearly suggest that NGA1 is a transcriptional activator for the induction of NCED3 under dehydration-stress conditions. Moreover, the transcriptomic changes during dehydration stress (Fig. 6A) and decreased drought-stress tolerance (Fig. 5B) in nga1-1 revealed that NGA1 plays certain critical roles in the dehydration-stress responses and survival during drought stress in plants.
The present study identifies posttranslational activating mechanisms of NGA1 during dehydration stress. The NGA1 proteins appear to be degraded through the 26S proteasome pathways under nonstress conditions (Fig. 7D), but they are stabilized by dehydration stress (Fig. 7B). Importantly, protein stabilization of NGA1 during dehydration stress was detected even in the ABA-deficient mutant (Fig. 7F). These results suggest that the NGA1 protein is posttranslationally regulated through protein stabilization in an ABA-independent pathway in the early responses to drought stress (33) (SI Appendix, Fig. S8). Moreover, the transactivation assays using the mutated NGA1 suggest the presence of complex mechanisms of regulating transcriptional activity of NGA1. Increased transcriptional activity of the phospho-mimic mutations of NGA1 and the adverse effects of the alanine mutations at positions T80, S83, and 160S suggest that phosphorylation of these amino acids is involved in activating mechanisms of NGA1 during drought stress. However, there was no effect of these amino acid mutations on protein accumulation in protoplasts (SI Appendix, Fig. S7E), which implies that the increase in transcriptional activity by phosphorylation at position T80, S83, and 160S might be due to different activating mechanisms from those required for protein stabilization of NGA1. Decreased transcriptional activation due to mutations at possible O-GlcNAcylated amino acids suggests that O-GlcNAcylation also might be involved in the activation of NGA1 (SI Appendix, Fig. S7F).
In the present study, an important cis-acting element NBE (CACTTG) in the 5′ UTR region of the NCED3 promoter was isolated. The NGA proteins bound to the NBE in vitro (Fig. 2 D and E), and activated the NCED3 promoter through NBE in protoplasts (Fig. 2 B and C). Mutation of NBE in the 3-kb promoter of NCED3 decreased the reporter activity in the transgenic plants (Fig. 3). These findings strongly suggest that the NBE sequence is necessary for activation of NCED3 during dehydration stress by NGA. Interestingly, the NBE is located in the 5′ UTR region of NCED3. Recently, several reports have demonstrated that genomic regions other than promoters (intron or 3′-nontranscribed intergenic region) play important roles in the transcriptional activation of genes (34, 35); however, few papers have indicated the importance of cis-acting elements in the 5′ UTR region for transcriptional activation (36). It was also reported that a G-box sequence located ∼2.3 kb upstream from the translational start site was necessary for the induction of NCED3 (Fig. 3) (12, 13), implying that any transcription factors binding to the G-box coordinately activate NCED3 collectively with NGA1 (SI Appendix, Fig. S8). Analysis of the transcriptional mechanisms of NCED3 is important not only for elucidating the early drought responses in plants, but also for studying and proposing an intriguing model of unique transcriptional mechanisms. Moreover, future studies will be necessary to identify the novel transcription factors that bind to the long-range enhancer G-box.
Previous reports revealed that NGAs also are involved in plant growth. Marginal growth of leaves is regulated by NGAs and the TCP transcription factors by restricting meristem activity in leaf distal regions (16). This study revealed that NGA and TCP have negative effects on the expression of KANADI, which encodes a transcription factor involved in the regulation of adaxial–abaxial polarity (16). Other studies reported that KANADI has negative effects on expression of genes involved in ABA signaling (37) and that one of the KANADI-regulating genes encoded a transcription factor ABIG1 (ABA INSENSITIVE GROWTH 1) that was necessary for ABA-mediated growth inhibition (38). GO analysis in the present study suggested that NGA1 is involved in growth inhibition during dehydration stress (SI Appendix, Fig. S6F). Our findings thus present a possibility that the NGA proteins control plant growth or organ polarity during drought stress through ABA biosynthesis.
In conclusion, the present study revealed that: (i) NGA1 is a transcriptional activator of NCED3; (ii) NGA1 regulates NCED3 through a cis-element NBE in the 5′ UTR in the promoter; and (iii) NGA1 is posttranslationally stabilized in an ABA-independent manner (SI Appendix, Fig. S8). These findings provide mechanisms for the early plant response during drought stress before ABA accumulation. However, additional experiments are necessary to elucidate the detailed molecular mechanisms, especially with respect to the posttranslational activation mechanisms of NGA1. We revealed that mutations in putative modified amino acids (31) had positive and negative effects on transcriptional activity of NGA1 (Fig. 7 H and I). Future investigations should determine how these modifications affect the molecular function of NGA1 and what factors regulate the modification and protein stabilization of NGA1 under control and dehydration-stress conditions. A recent study reported that a small peptide CLAVATA3/EMBRYO-SURROUNDING REGIONRELATED 25 (CLE25) induced the NCED3 expression during dehydration stress (39). It is also an intriguing question whether and how the CLE25-dependent signaling cascade is involved in the activation mechanisms of NGA1.
Materials and Methods
Plant Materials and Growth Conditions.
A. thaliana ecotype Columbia (Col-0), Landsberg erecta (Ler-0), or Nossen (Nos-0) plants were grown on germination medium (GM) agar plates and on soil at 22 °C under a 16-h photoperiod at a photon flux density of 40 μmol m−2⋅s−1 as described (40). Knockout mutants of the NGA family genes were provided by John Paul Alvarez, Monash University, Clayton, VIC, Australia (14) (nga2-1, CS110052; nga3-1, CS872330; and nga4-1, N16752). Knockout mutants of NCED3 (nc3-2) and ABA2 (aba2-2) were described in previous studies (21, 41). Transgenic plants were generated through the floral dip method by using Agrobacterium tumefaciens GV3101 (pMP90) cells as described (42). More than two independent transgenic lines were analyzed for each experiment.
Screen for Sucrose-Insensitive Lines in a Plant Library.
Transgenic plants harboring the transcription factors fused to a chimeric repressor SRDX were reported (17). Sterilized seeds of the transgenic plants were sown on Murashige and Skoog (MS) medium (0.8% agar) supplemented with 300 mM sucrose. The plants were grown under control conditions for 2 wk, and phenotypic differences were analyzed.
Stress and Chemical Treatment.
For the dehydration-stress treatment, 14-d-old plants grown on solid GM agar plates were transferred on parafilm. For the drought-stress treatment, 14-d-old plants grown on solid GM agar plates were transferred to soil and grown for 2 d under control conditions. Water supply was withheld until plants withered. During periods of drought-stress treatment, pot weights were measured, and relative water content (RWC) was calculated from the dried pot weight and adjusted among plant lines to ensure equal intensity of drought stress. RWC was calculated as {(pot weight during stress) − (dried pot weight)}/{(initial pot weight) − (dried pot weight)} × 100. Survival rates of plants were calculated 7 d after rewatering under control conditions. For RNA extraction and GUS staining using germinating seeds, sterilized seeds were sown in one layer of filter paper containing 2 mL of liquid GM with or without 300 mM sucrose in 60-mm plastic Petri dishes. The seeds were cold stratified at 4 °C for 3 d in the dark, and then seeds were transferred to the light at 22 °C. For ABA or MG132 treatment, 14-d-old plants were transferred to a Petri dish filled with distilled water containing 100 μM ABA (Sigma-Aldrich) or 100 μM MG132 (Calbiochem).
RNA Preparation and qRT-PCR.
The total RNA from seedlings was isolated with RNAiso plus (TaKaRa) according to the supplier’s instructions. Total RNA from seeds was isolated as described (43). The cDNA was synthesized by using SuperScript IV VILO (Thermo Fisher Scientific), and qRT-PCR was performed by using an Applied Biosystems 7500 Fast Real-Time PCR system and Fast SYBR Green (Thermo Fisher Scientific), as described in the supplier’s instructions. The obtained values were normalized according to the amounts of ACT2.
Measurement of Leaf Temperature and ABA Content.
Leaf temperature was measured by using a FLIR T640 infrared camera and FLIR tool software. The temperature of three spots in each plant was calculated by using seven plants of each line. Extraction of ABA from plants was performed as described (44). Parameters for liquid chromatography tandem mass spectrometry analysis to measure the endogenous ABA and internal control D6-ABA (Icon Isotepes) by Triple TOF 5600+ (SCIEX) are described in SI Appendix, Tables S4 and S5.
Sequence Alignment and Phylogenetic Analysis.
The alignment of the peptide sequences and the construction of phylogenetic trees were performed as described (45). The peptide sequences of B3-type transcription factors were obtained through Phytozome 12 (https://phytozome.jgi.doe.gov/pz/portal.html) using PFAM ID PF02362.
Transient Expression with Arabidopsis Mesophyll Protoplasts and Transactivation Assays.
Isolation and transient transformation of Arabidopsis mesophyll protoplasts was performed as described (46), and transactivation assays were performed as described (45) by using Perkin-Elmer Multimode Plate Reader EnSpire for measurement of the luciferase luminescence and methylumbelliferone fluorescence. For transfection, 3, 2, and 5 μg of the reporter, internal control, and effector plasmids were used.
GFP Fluorescence Observation and Histochemical GUS Staining.
GFP fluorescence was observed by using a confocal laser scanning microscope (Olympus FLUOVIEW FV1000). Histochemical GUS staining was performed as described (45).
EMSA and ChIP Assay.
EMSA was performed as described (47) with minor modifications. A mixture of 10,000 dpm of 32P-labeled probe and 1 μg was incubated in binding buffer [25 mM Hepes–KOH (pH 7.9), 40 mM KCl, 1 mM DTT, 1 mM ETDA, 10% glycerol, 1% BSA, and 1 mg of poly(dI-dC)] for 30 min at room temperature. The reaction mixtures were resolved by electrophoresis through a 6% or 8% polyacrylamide gel in 0.5× Tris–borate–EDTA buffer at 100 V for 50 min. ChIP assays were performed by using an EpiQuik Plant ChIP kit (Epigenetek) according to the user guide. One gram of plants with or without stress treatment was collected. To immunoprecipitate the DNA–protein complex, the polyclonal anti-GFP antibody (48) was used.
RNA Sequencing and Data Analysis.
CDNA libraries were constructed by using the TruSeq RNA Sample Preparation Kit (Version 2; Illumina), and the libraries were sequenced by NextSeq 500 (Illumina). The produced bcl files were converted to fastq files by bcl2fastq (Illumina). The reads were analyzed as described (49). The data were deposited into the DNA Data Bank of Japan (accession no. DRA006360). Metaprofile analysis was performed by using the public transcriptome database Genevestigator (https://genevestigator.com/gv/) (50), and overrepresentation analysis of hexamers on the promoters of sets of genes by RNA-sequencing (RNA-seq) data were performed as described (51) using 1-kb upstream sequences from the translational start sties obtained through Phytozome (Version 12; https://phytozome.jgi.doe.gov/pz/portal.html). GO analysis was performed by using the public GO database agriGO (Version 2.0; systemsbiology.cau.edu.cn/agriGOv2/) (30) and comparing to the TAIR10 whole-genome sequence as a reference.
Protein Immunoblot Analysis.
Total protein was extracted from seedlings and protoplasts by using protein extraction buffer (8 M urea, 5% 2-mercaptoethanol, 2 mM EDTA, 1% SDS, and50 mM Tris⋅HCl, pH 6.8) and centrifuged at 20,000 × g for 10 min at room temperature. The supernatant was boiled at 95 °C for 3 min. The resultant extracts, which corresponded to a fresh weight of 3 mg of seedling or 1 × 104 protoplasts, were separated by SDS-polyacrylamide gel electrophoresis. Immunoblotting was performed by using a polyclonal anti-GFP antibody (48) as the primary antibody and goat anti-Rabbit IgG peroxidase-conjugate (Thermo Fisher Scientific) as a secondary antibody. The signals were developed by SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific) according to the manufacturer’s protocol and detected with an image analyzer (ChemiDoc MP; Bio-Rad). Ponceau S (APRO SCIENCE) staining was performed according to the manufacturer’s instructions. Band intensity was measured by using Image Lab Software (Bio-Rad) and normalized to the intensity of the rubisco large subunit (rbcL).
Supplementary Material
Acknowledgments
We thank Dr. John Paul Alvarez and Dr. Yuval Eshed for providing materials for the nga knockout mutants; and Ms. Fuyuko Shimoda, Ms. Saho Mizukado, Ms. Saya Kikuchi, Ms. Hiroko Kobayashi, Ms. Kumiko Matsuo, Ms. Michie Etoh, Ms. Ayami Furuta, and Ms. Tomomi Shinagawa for their excellent technical assistance. This work was supported by Grant-in-Aid for Scientific Research for Young Scientists (B) 16K21626 (to H.S.) and Grants-in-Aid for Scientific Research on Innovative Areas JP16H01475 and JP18H04792 (to F.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Transcriptome datasets have been deposited in the DNA Data Bank of Japan (accession no. DRA006360).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811491115/-/DCSupplemental.
References
- 1.Lipiec J, Doussan C, Nosalewicz A, Kondracka K. Effect of drought and heat stresses on plant growth and yield: A review. Int Agrophys. 2013;27:463–477. [Google Scholar]
- 2.Lesk C, Rowhani P, Ramankutty N. Influence of extreme weather disasters on global crop production. Nature. 2016;529:84–87. doi: 10.1038/nature16467. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci. 2014;5:170. doi: 10.3389/fpls.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuromori T, et al. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc Natl Acad Sci USA. 2010;107:2361–2366. doi: 10.1073/pnas.0912516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santiago J, et al. The abscisic acid receptor PYR1 in complex with abscisic acid. Nature. 2009;462:665–668. doi: 10.1038/nature08591. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 7.Fujita Y, et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 2009;50:2123–2132. doi: 10.1093/pcp/pcp147. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida T, et al. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2015;38:35–49. doi: 10.1111/pce.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo M, Koshiba T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002;7:41–48. doi: 10.1016/s1360-1385(01)02187-2. [DOI] [PubMed] [Google Scholar]
- 10.Iuchi S, et al. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001;27:325–333. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 11.Endo A, et al. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 2008;147:1984–1993. doi: 10.1104/pp.108.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behnam B, et al. Characterization of the promoter region of an Arabidopsis gene for 9-cis-epoxycarotenoid dioxygenase involved in dehydration-inducible transcription. DNA Res. 2013;20:315–324. doi: 10.1093/dnares/dst012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YZ, Tan BC. A distal ABA responsive element in AtNCED3 promoter is required for positive feedback regulation of ABA biosynthesis in Arabidopsis. PLoS One. 2014;9:e87283. doi: 10.1371/journal.pone.0087283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez JP, et al. Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell. 2006;18:1134–1151. doi: 10.1105/tpc.105.040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez JP, Goldshmidt A, Efroni I, Bowman JL, Eshed Y. The NGATHA distal organ development genes are essential for style specification in Arabidopsis. Plant Cell. 2009;21:1373–1393. doi: 10.1105/tpc.109.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez JP, Furumizu C, Efroni I, Eshed Y, Bowman JL. Active suppression of a leaf meristem orchestrates determinate leaf growth. eLife. 2016;5:e15023. doi: 10.7554/eLife.15023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsuda N, et al. 2011. CRES-T, an effective gene silencing system utilizing chimeric repressors. Plant Transcription Factors: Methods and Protocols, Methods in Molecular Biology, eds Yuan L, Perry S (Humana, New York) Vol 754, pp 87–105.
- 18.Finkelstein RR, Gibson SI. ABA and sugar interactions regulating development: Cross-talk or voices in a crowd? Curr Opin Plant Biol. 2002;5:26–32. doi: 10.1016/s1369-5266(01)00225-4. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Li CY, Biddle KD, Gibson SI. Identification, cloning and characterization of sis7 and sis10 sugar-insensitive mutants of Arabidopsis. BMC Plant Biol. 2008;8:104. doi: 10.1186/1471-2229-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laby RJ, Kincaid MS, Kim D, Gibson SI. The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J. 2000;23:587–596. doi: 10.1046/j.1365-313x.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 21.Urano K, et al. Analysis of plant hormone profiles in response to moderate dehydration stress. Plant J. 2017;90:17–36. doi: 10.1111/tpj.13460. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda M, Ohme-Takagi M. A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol. 2009;50:970–975. doi: 10.1093/pcp/pcp048. [DOI] [PubMed] [Google Scholar]
- 23.Pfannebecker KC, Lange M, Rupp O, Becker A. Seed plant-specific gene lineages involved in carpel development. Mol Biol Evol. 2017;34:925–942. doi: 10.1093/molbev/msw297. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol. 2011;157:1243–1254. doi: 10.1104/pp.111.179036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharti K, et al. Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB binding protein ortholog HAC1. Plant Cell. 2004;16:1521–1535. doi: 10.1105/tpc.019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, et al. Transcription factors SOD7/NGAL2 and DPA4/NGAL3 act redundantly to regulate seed size by directly repressing KLU expression in Arabidopsis thaliana. Plant Cell. 2015;27:620–632. doi: 10.1105/tpc.114.135368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, et al. Arabidopsis MAPKKK18 positively regulates drought stress resistance via downstream MAPKK3. Biochem Biophys Res Commun. 2017;484:292–297. doi: 10.1016/j.bbrc.2017.01.104. [DOI] [PubMed] [Google Scholar]
- 28.Pandey GK, et al. ABR1, an APETALA2-domain transcription factor that functions as a repressor of ABA response in Arabidopsis. Plant Physiol. 2005;139:1185–1193. doi: 10.1104/pp.105.066324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perera IY, Hung CY, Moore CD, Stevenson-Paulik J, Boss WF. Transgenic Arabidopsis plants expressing the type 1 inositol 5-phosphatase exhibit increased drought tolerance and altered abscisic acid signaling. Plant Cell. 2008;20:2876–2893. doi: 10.1105/tpc.108.061374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian T, et al. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017;45:W122–W129. doi: 10.1093/nar/gkx382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu SL, et al. Proteomic analysis reveals O-GlcNAc modification on proteins with key regulatory functions in Arabidopsis. Proc Natl Acad Sci USA. 2017;114:E1536–E1543. doi: 10.1073/pnas.1610452114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umezawa T, et al. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:17588–17593. doi: 10.1073/pnas.0907095106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrero JM, et al. Both abscisic acid (ABA)-dependent and ABA-independent pathways govern the induction of NCED3, AAO3 and ABA1 in response to salt stress. Plant Cell Environ. 2006;29:2000–2008. doi: 10.1111/j.1365-3040.2006.01576.x. [DOI] [PubMed] [Google Scholar]
- 34.Maruyama-Nakashita A, et al. Sulfur-responsive elements in the 3′-nontranscribed intergenic region are essential for the induction of SULFATE TRANSPORTER 2;1 gene expression in Arabidopsis roots under sulfur deficiency. Plant Cell. 2015;27:1279–1296. doi: 10.1105/tpc.114.134908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallegos JE, Rose AB. Intron DNA sequences can be more important than the proximal promoter in determining the site of transcript initiation. Plant Cell. 2017;29:843–853. doi: 10.1105/tpc.17.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mironova VV, Omelyanchuk NA, Wiebe DS, Levitsky VG. Computational analysis of auxin responsive elements in the Arabidopsis thaliana L. genome. BMC Genomics. 2014;15(Suppl 12):S4. doi: 10.1186/1471-2164-15-S12-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinhart BJ, et al. Establishing a framework for the Ad/abaxial regulatory network of Arabidopsis: Ascertaining targets of class III homeodomain leucine zipper and KANADI regulation. Plant Cell. 2013;25:3228–3249. doi: 10.1105/tpc.113.111518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu T, Longhurst AD, Talavera-Rauh F, Hokin SA, Barton MK. The Arabidopsis transcription factor ABIG1 relays ABA signaled growth inhibition and drought induced senescence. eLife. 2016;5:e13768. doi: 10.7554/eLife.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi F, et al. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature. 2018;556:235–238. doi: 10.1038/s41586-018-0009-2. [DOI] [PubMed] [Google Scholar]
- 40.Sakuma Y, et al. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell. 2006;18:1292–1309. doi: 10.1105/tpc.105.035881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nambara E, Kawaide H, Kamiya Y, Naito S. Characterization of an Arabidopsis thaliana mutant that has a defect in ABA accumulation: ABA-dependent and ABA-independent accumulation of free amino acids during dehydration. Plant Cell Physiol. 1998;39:853–858. doi: 10.1093/oxfordjournals.pcp.a029444. [DOI] [PubMed] [Google Scholar]
- 42.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 43.Martin RC, Liu P-P, Nonogaki H. Simple purification of small RNAs from seeds and efficient detection of multiple microRNAs expressed in Arabidopsis thaliana and tomato (Lycopersicon esculentum) seeds. Seed Sci Res. 2005;15:319–328. [Google Scholar]
- 44.Yoshimoto K, et al. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell. 2009;21:2914–2927. doi: 10.1105/tpc.109.068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato H, et al. Arabidopsis DPB3-1, a DREB2A interactor, specifically enhances heat stress-induced gene expression by forming a heat stress-specific transcriptional complex with NF-Y subunits. Plant Cell. 2014;26:4954–4973. doi: 10.1105/tpc.114.132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- 47.Kidokoro S, et al. The phytochrome-interacting factor PIF7 negatively regulates DREB1 expression under circadian control in Arabidopsis. Plant Physiol. 2009;151:2046–2057. doi: 10.1104/pp.109.147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka H, et al. Abiotic stress-inducible receptor-like kinases negatively control ABA signaling in Arabidopsis. Plant J. 2012;70:599–613. doi: 10.1111/j.1365-313X.2012.04901.x. [DOI] [PubMed] [Google Scholar]
- 49.Notaguchi M, Higashiyama T, Suzuki T. Identification of mRNAs that move over long distances using an RNA-seq analysis of Arabidopsis/Nicotiana benthamiana heterografts. Plant Cell Physiol. 2015;56:311–321. doi: 10.1093/pcp/pcu210. [DOI] [PubMed] [Google Scholar]
- 50.Hruz T, et al. Genevestigator v3: A reference expression database for the meta-analysis of transcriptomes. Adv Bioinforma. 2008;2008:420747. doi: 10.1155/2008/420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maruyama K, et al. Identification of cis-acting promoter elements in cold- and dehydration-induced transcriptional pathways in Arabidopsis, rice, and soybean. DNA Res. 2012;19:37–49. doi: 10.1093/dnares/dsr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.