Abstract
All life requires the capacity to recover from challenges that are as inevitable as they are unpredictable. Understanding this resilience is essential for managing the health of humans and their livestock. It has long been difficult to quantify resilience directly, forcing practitioners to rely on indirect static indicators of health. However, measurements from wearable electronics and other sources now allow us to analyze the dynamics of physiology and behavior with unsurpassed resolution. The resulting flood of data coincides with the emergence of novel analytical tools for estimating resilience from the pattern of microrecoveries observed in natural time series. Such dynamic indicators of resilience may be used to monitor the risk of systemic failure across systems ranging from organs to entire organisms. These tools invite a fundamental rethinking of our approach to the adaptive management of health and resilience.
Keywords: resilience, health, livestock, tipping points, aging
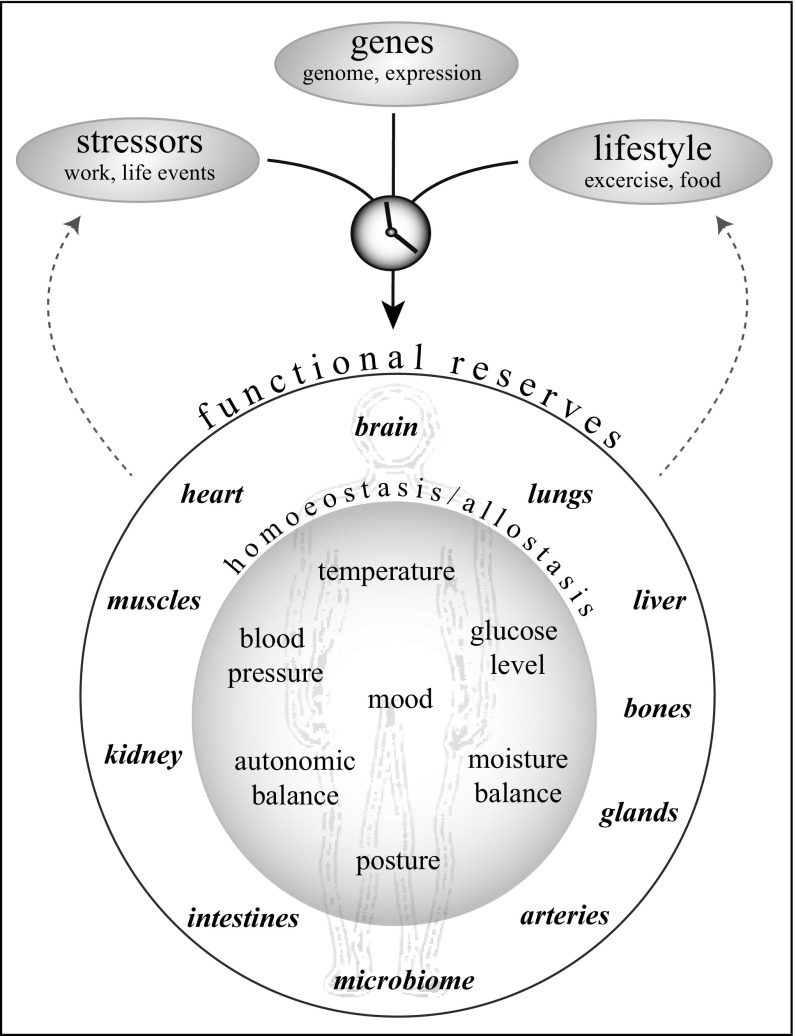
The capacity of animals to regulate critical parameters such as blood pressure, temperature, and glucose levels depends upon the functioning of organs and other subsystems linked through an intricate web of hormonal and neural communication (Fig. 1). The resulting complex dynamical system faces a regime of challenges related to physical strain, food intake, infections, adverse events, and a range of other stressors. If systemic resilience—the capacity to bounce back to normal functioning after a perturbation—decreases, risks of morbidity and mortality increase. Here, we address the question of how such systemic resilience may be understood as a unifying construct and how it can be quantified objectively. To see the relevance of this, consider three societal issues in which understanding the resilience of the system is essential for effective management: the pollinator crisis, industrial livestock production, and frailty in humans. Each of these examples vividly illustrates the need to look beyond single factors and take an integrative approach to manage and measure systemic resilience.
Fig. 1.
The mechanisms that regulate systemic resilience in humans and animals. The resilience of the whole depends on the resilience of subsystems that regulate vital parameters such as temperature, glucose level, and mood. Those in turn depend, among other things, upon the functional reserves (overcapacity) of organs that inevitably wear down with aging, depending on stressors, lifestyle, and genetic make-up.
The “pollinator crisis” is a term used to describe the decline of bees and other insects threatening pollination services on which the majority of our crops depend (1, 2). Causes of bee decline include parasites, exposure to pesticides, and a lack of appropriate flowers, but the roles of these and other factors are heavily debated (3). The stakes are high, as illustrated by the turmoil over the European Union ban on neonicotinoids, a class of crop-protection pesticides that have become widely used in farming and horticulture (4). While pathogens and parasites are often regarded as prime suspects in bee declines, a less visible underlying driver may be impairment of their immune systems induced by exposure to neonicotinoids or by lack of food, further complicated by impacts of neonicotinoids on the navigation and communication systems exacerbating food stress (3, 5). Testing the effects of the separate drivers in isolation is insufficient to explain bee decline (3). Instead, we need to understand how multiple stressors act to shape resilience in these animals and the superorganisms into which they are organized as eusocial insects.
Industrial livestock production is associated with concerns regarding animal welfare (6) as well as worries about risks to human health arising from antibiotic-resistant bacteria (7, 8) and disease outbreaks (9–11). This has led to the global One Health movement focusing on the collaborative effort of multiple disciplines to attain optimal health for people, animals, and our environment (12–15). One problem is that the conditions that facilitate highly efficient production of meat, milk, and eggs tend to come at a cost in the animals’ resilience (16–19). It is becoming clear that not only genetic make-up (17, 20) and feed composition (21) but also early-life conditions supporting the development and expression of important behaviors and the establishment of social relationships (22, 23) have an impact on health and survival (24–26). However, we lack a quantitative understanding of how these multiple factors interact to shape animal resilience.
Human resilience is a focus of increasing interest in geriatric medicine, where indices of frailty are now being developed to mark the risk of cascading declines in function (27–30). For instance, in the frail elderly, a fall may cause hip fracture but also trigger subsequent mental deterioration, social isolation, and eating problems, provoking a cascading transition into a weakened state. While normal aging eventually results in frailty, loss of resilience can happen earlier in life, too. If the risk of systemic failure is high, patients may require intensive care. Not surprisingly, efforts to understand resilience in humans are relatively advanced in geriatric and critical-care medicine (31), but the topic is also of great interest in psychiatry, in which the breakdown of resilience characterizes various disorders (32–34). Despite these efforts, healthcare diagnoses and treatment still remain focused mostly on single issues (35). In the face of a broad range of public health challenges and an aging population, there is a strong demand for better ways of assessing human resilience and unraveling factors that contribute to it.
The idea that systemic resilience may be a useful concept to integrate the complex multifactorial character of health is supported by work on the nematode Caenorhabditis elegans. The lifespan of this tiny worm can vary from days to months, depending on factors such as genes, food, temperatures, and toxic compounds. Experiments exploring the effects of such diverse factors (36) unequivocally point to the existence of a single integrating characteristic of the animals on which all stressors act and that ultimately shapes the risk of death from any proximate cause, an aspect we may call “resilience” (37).
Despite overwhelming evidence for its importance and vast literature on relevant aspects, there is no common framework for understanding systemic resilience and guiding its management. This void may be due in part to the difficulty of quantifying resilience. As we will show, we are now in a position to change this situation radically as a result of two recent developments. First, a novel family of dynamical indicators of resilience has emerged, providing surprisingly generic risk markers for the collapse of complex systems ranging from financial markets and ecosystems to the climate system and physiological systems (31, 38–44). Second, the dynamic time series that such methods require are starting to be ubiquitously available for humans and livestock thanks to the rapid rise of technologies for automated recordings. This holds the promise of an entirely new approach to quantifying and monitoring resilience of animals and humans.
The Concepts of Resilience and Tipping Points
While the concept of resilience may seem intuitively straightforward, it is worth noting that it has been used in different ways across scientific disciplines and also outside academia. Its use in fields as diverse as ecology, engineering, environmental sciences, social sciences, economics, and psychology may be explained in part by the malleability of the concept (45). Resilience takes on different meanings, depending on the context and the field in which it is used (46, 47). Nonetheless, definitions invariably relate to the ability of a system to maintain specific functions in the face of change (45).
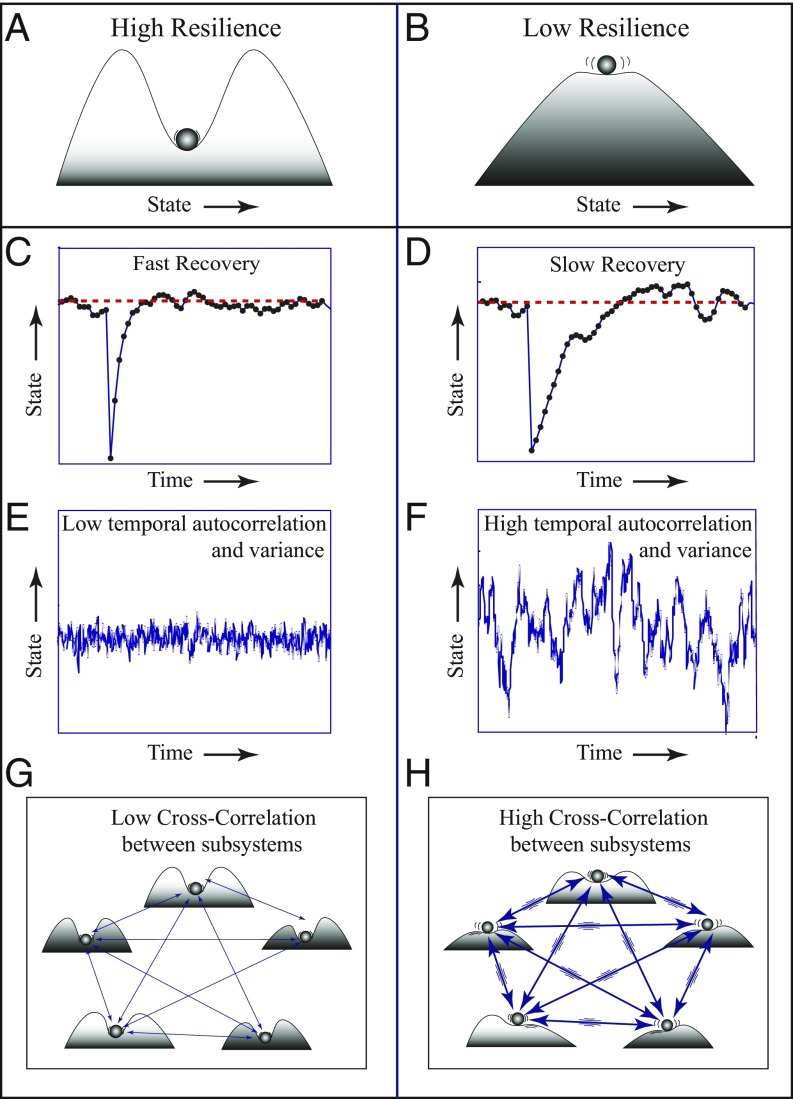
The interpretation of resilience takes a special twist in systems that have a tipping point: a threshold at which a self-reinforcing mechanism propels a critical transition to a contrasting state (48, 49). Efforts to understand such sharp transitions have a long history. In physics, abrupt phase transitions such as freezing may occur even when variables such as temperature change gradually. Also, slow endogenous change may build up a tension that brings a system to a critical point for radical change (50). Nearly 50 years ago the mathematician Thom (51) created a framework called “catastrophe theory” to characterize some of the abrupt transitions that could occur in dynamical systems. Although catastrophe theory fell out of favor because it was initially oversold, the mathematical framework is robust and is now recognized as relevant across a broad range of systems (49). Especially, in ecology, researchers early on recognized the potential for systems to reach a tipping point. To capture the risk of such critical transitions, ecologists defined resilience as the capacity to tolerate disturbance without collapsing (https://www.resalliance.org/). As this “ecological resilience” approaches zero, a critical transition can be invoked even by a tiny nudge (52). It is intuitively straightforward to see how the potential for critical transitions is relevant to organisms (Fig. 2) in which aging and stressors can reduce the resilience of the healthy state. In ecology, resilience (53) was traditionally thought of as the magnitude of the perturbation needed to actually cause the shift (“push it over the ridge” in the representation of Fig. 2). However, this view raises the question of whether such resilience might in some way be quantified without invoking the shift. This would allow detecting situations in which special attention is needed to prevent an unwanted transition [e.g., falling into a depression (44)] or in which the “bad resilience” of an unwanted state is dwindling to the point that a small nudge could push it out of such a trap [e.g., recovering from a depression (44)].
Fig. 2.
DIORs discussed in the main text. (Left) A resilient system. (Right) A frail system with low resilience. (A and B) Resilience is represented as the basin of attraction around a healthy state. Slopes correspond to rates of change. When resilience is low (B vs. A), slopes around the equilibrium are less steep, implying slower return rates to equilibrium. (C and D) Simulated recovery rates upon a small perturbation. (E and F) Simulated dynamics in a system subject to a stochastic regime of perturbations illustrating that fluctuations are larger and slower in a frail system (F vs. E), as reflected in higher variance and higher temporal autocorrelation. (G and H) Interactive dynamics of subsystems (e.g., mood, posture, cognition) are predicted to become more correlated in a network with low systemic resilience (H vs. G).
Dynamic Indicators of Resilience
Borrowing from the literature in physics, it has been found that subtle changes in the dynamics of systems may often be used to quantify the proximity of a tipping point and to allow steps to be taken to avoid the transition (or to encourage it, if the system is in an unfavorable state to begin with). The most important of those early warning indicators are based on the phenomenon of “critical slowing down” (40). Phrased simply, slower recovery from small perturbations (e.g., the recovery of mood after a bad experience) is an indicator that the system is becoming fragile and that a tipping point (e.g., into depression) may be near. In mathematical terms, critical slowing down happens in continuous-time systems when the real part of the dominant eigenvalue of the Jacobian matrix of the linearized model about a steady state tends to zero as a bifurcation point is approached. Although there are transitions in dynamical systems that may not be characterized in this simple way (40, 54, 55), empirical studies across widely different systems suggest that critical slowing down is a surprisingly generic indicator of reduced resilience (38).
The cause of critical slowing down can be seen in an intuitive way from stability landscapes (Fig. 2). As the basin of attraction becomes smaller and shallower, its slopes become less steep (Fig. 2 B vs. A), implying that the return rate to equilibrium upon small perturbations becomes slower (Fig. 2 D vs. C). In principle, measuring this requires an experimental perturbation. However, there is a way around that. All complex systems (including our body) are continuously subject to stochastic variations in external conditions. The effect of this natural regime of perturbations can be used to infer loss of resilience from a change in the nature of fluctuations in the state of a system (38–40, 56, 57). Explaining the mathematical background of this universal principle would go beyond the scope of this review, but the essence can be grasped intuitively. Stochastic fluctuations in the state of a system in part reflect microrecoveries from small, natural perturbations. Therefore, as the intrinsic recovery rate from perturbations becomes slower, fluctuations in the state will become slower overall, which can be seen from an elevated correlation between the state in subsequent moments, the so-called “temporal autocorrelation,” which tends to go hand-in-hand with an increase in variance (Fig. 2 F vs. E) (38–40, 56, 57).
Changes in recovery rates and the associated temporal autocorrelations are not the only class of generic indicators of resilience. Many complex systems can be seen as networks of subsystems, and organisms are no exception (Fig. 1). In such systems, the capacity to bounce back from challenges implies a capacity to avoid a cascading collapse that brings the entire network down. It has been shown that in networks in which the elements depend on each other (facilitative networks), a rising correlation between the fluctuation in the time series of different elements may indicate the risk of such a systemic collapse (38). This makes intuitive sense for organisms. Malfunctioning of one subsystem (e.g., inflammation) will affect the outcome of other subsystems (e.g., cognition and gait) more strongly if those other subsystems already have a low resilience. Thus, as individual elements become less resilient, sensitivity to fluctuations in the functioning of other elements increases, which may lead to a rising cross-correlation between the ups and downs in the functioning of the different elements of the system (Fig. 2H) (44, 58). The network perspective also helps show why resilience is an emergent property and why changes in components will combine to shape systemic resilience of the organism as a whole.
It should be noted that, as indicators of critical slowing down relate to changing dynamics around equilibria, they cannot be linked in simple ways to the resilience of systems characterized by cycles or chaotic dynamics. Critical transitions in such systems have a complex nature and are difficult to foresee (40). The heart and the brain are examples. Possible early warning signals for seizures and particular kinds of heart failure have been linked to phenomena in dynamical systems theory but remain challenging to pick up from data (59–61). Here, we limit ourselves to the relatively intuitive class of resilience indicators related to slowing down. Slowing down will not happen before all transitions, and even if it does, it can be challenging to detect. On the other hand, the generic nature of this phenomenon implies a strikingly wide scope of potential applications. Indeed indicators of slowing down have been shown to signal the loss of resilience before critical transitions in systems ranging from populations of yeast (62, 63), zooplankton (64), and cyanobacteria (65) to complex systems such as the climate (66), tropical forests (67), and Neolithic societies (68).
In the framework of critical slowing down, the capacity of the system to bounce back upon perturbation can be reflected in three characteristics: variance, temporal autocorrelation (correlations between states on subsequent moments), and cross-correlation (between different elements of the system). We coin those indicators “dynamic indicators of resilience” (DIORs) to contrast them with the traditional static correlates of the condition or health of a system. While static indicators have long dominated medicine and animal science, technological advances now allow the assessing such DIORs. An extensive practical guide to the methods used for computing indicators of resilience is published elsewhere (69) linked to a website with freely available open-source software tools.
Resilience of the Subsystems
One way to deal with the complexity of studying animals and humans is to view them as sets of subsystems linked through a web of fast (nervous system) and slower (autocrine, paracrine, endocrine systems) feedbacks. The links allow organs to work together to maintain vital parameters within safe limits (Fig. 1). Depending on the functional reserve (overcapacity) of organs and the effectiveness of their coordination, subsystems regulating critical factors such as temperature and body posture may run into disorder when challenged. Some subsystems may gradually lose their function. However, others are “tipping elements” (70, 71) in the sense that failure has a sharp, all-or-none character often associated with severe health problems (SI Appendix, Table S1) (72).
A well-known example of a tipping element in humans is blood pressure. Pressure drops if a person suddenly stands up, but this is quickly sensed and corrected by contraction of blood vessels and increased pumping by the heart. If such rapid regulation fails, blood pressure in the head drops, resulting in syncope (fainting) (31). There are also tipping elements that are not limited to our internal physiology. The mood system is an example. While depressed feelings are transient in most persons, for others stressful life events may trigger a state of clinical depression that involves disturbances in several other subsystems such as sleep and appetite and from which recovery can be difficult (73, 74). The profoundness and irreversibility of such depressed states are due in part to a set of reinforcing feedback mechanisms. For instance, a depressed person is likely to encounter negativity in relationships, take less physical exercise, make less social contact, and eat less healthily, all of which may deepen the depression (32, 75).
The microbiomes on which humans and animals depend are complex ecosystems in their own right, tightly linked to the host system in intricate ways. Just like wetlands (76), forests (77) and coral reefs (78), such microbiomes may tip to an alternative state. A well-known example is acute rumen acidosis in beef cattle (79). If animals are fed a high level of rapidly digestible carbohydrates, fermentation increases, resulting in lower pH of the rumen (first stomach). This decrease can favor acid-tolerant bacteria that in turn produce more lactic acid that drives pH down even more, thereby potentially tipping the rumen into a highly acidic state, causing a severe crisis and potentially leading to death (79, 80).
Shifts between alternative states are typically triggered by stochastic events and therefore can never be accurately predicted. However, there are good reasons to expect that DIORs may be used to aid risk assessments. In the human health literature, there are already various lines of evidence that slowing down of recovery may signal reduced resilience for a range of subsystems (SI Appendix, Table S2). For instance, subjects with a slow rise in blood pressure following exercise have a five times higher risk of ischemic stroke (81), and persons with a slow rate of recovery of blood pressure upon standing up are more likely to experience syncope (fainting) (82, 83). In psychiatry, slowness of mood change (reflected in elevated temporal correlation and variance of emotions) has been found to be indicative of the risk of falling into a clinical depression later (44, 84). In the elderly, a cohort study found an increase of correlation and variance of self-reported mood and physical well-being with (independently assessed) frailty (85), while in another group of elderly the DIORs assessed from the rapid dynamics of postural balance have been found to correlated with successful aging (86).
Resilience of the Network
Although assessing the risk of critical transitions in subsystems can be useful, the central challenge we wish to address is finding ways to assess systemic resilience of the whole. Could there be reliable generic ways to quantify systemic resilience? Measuring recovery rates upon health crises is an obvious angle. However, there is evidence that declining systemic resilience of the whole may also be reflected in measurable declines in the resilience of a range of subsystems. For instance, overall mortality risk is correlated with longer recovery time in blood pressure upon standing up (87, 88) and also with slowness in the recovery of heart rate during the first minute after exercise (89) as well as hand grip strength, gait speed, and a range of other frailty indicators (27).
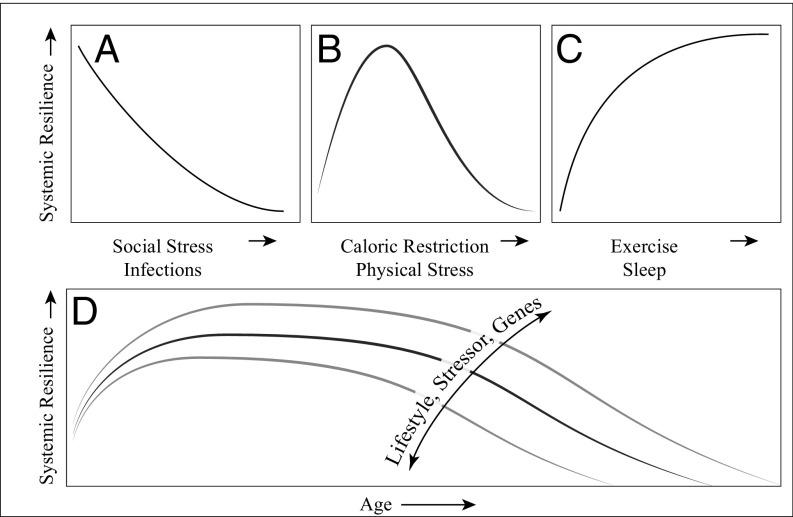
It may seem surprising at first that so many different indicators correlate with the risk of all-cause death. However, this observation can also be interpreted as evidence that the resiliencies of the components and of the whole are linked. Such coupling becomes particularly apparent during the process of aging. In most animals, beyond some age mortality risk rises exponentially with the years (36). For instance, in humans, starting around age 30 years, the likelihood of death doubles roughly every eight years. This rising mortality risk reflects the loss of resilience with age, which is inevitable, but does depend on genetic make-up, stressors, and lifestyle (Fig. 3). The loss of resilience is of course not completely homogeneous across the body. Some subsystems often remain stronger and compensate in part for the weakening of others. Nonetheless, there is a tendency for the whole and its subsystems to decline in concert.
Fig. 3.
Schematic representation of possible effects of different factors on systemic resilience. While effects of some factors are only detrimental (A) or positive (C), the effect of other factors on resilience peaks at intermediate levels (B). Effects of aging on resilience (D) are moderated by the mechanisms summarized in Fig. 1.
Part of an explanation may be that stresses influencing parameters such as reactive oxygen species (ROS), chaperon protein regulation, autophagy, and the accumulation of senescent cells affect tissues throughout the organism (90–92). However, the network of mutual dependencies may also cause correlation between resilience of the parts, as the malfunctioning of one subsystem (e.g., glucose regulation) can raise the stress on other subsystems (e.g., water balance by increased urinary output, cognition by glycation of proteins), causing them to deteriorate also. In addition, organisms must distribute resources over subsystems, implying that increased demand from one system may be met at the expense of others.
This view of organisms as complex adaptive networks has implications for our understanding of systemic resilience and for the possibilities of assessment. It may explain why resilience of the elements can be predictive of the systemic resilience of the whole but also suggests that rising correlation between the ups-and-downs of elements in a network might indicate an elevated risk of systemic failure (Fig. 2 H vs. G) (38). So far, few studies have addressed this possibility, although a recent study of a cohort of Italian elderly indeed revealed that correlation between self-reported mood and physical well-being increases with (independently assessed) frailty (85). In summary, there is emerging evidence that humans and animals may be seen as complex networks in which systemic resilience can be assessed from DIORs, which may be estimated directly from the interactive dynamics of vital parameters such as blood pressure, activity, temperature, postural balance, and mood.
Managing Resilience
Although quantification of systemic resilience has long remained elusive, a long-standing literature documents the ways in which genes, lifestyle, diseases, and other stressors affect healthy dynamic functioning. Indeed, while a systematic approach to managing resilience is missing, the different knobs to turn are basically known. Chronic stress in animals and humans is perhaps the best-known condition that may undermine resilience. The body responds to stress through a suite of reactions that allow energy bursts for fight or flight reactions. When such a state becomes sustained too long, the resulting “allostatic load” causes wear and tear on the body (93). As a result, the reactive scope of the organism to mount an adequate response to challenges gradually erodes (94). Chronic stress is also one of the leading adverse life events that have been shown to increase the risk of mental disorders such as depression (95).
While the effects of prolonged stress on health have been long known, the mechanisms explaining the multifactorial nature of resilience with all its cross-linkages are only starting to be unraveled. One line of work is now revealing how stressors to the mood system may affect the immune system. For instance, experiments on monkeys reveal how up-regulation of proinflammatory genes in response to perceived social isolation comes at the cost of impaired response to viral infectious challenge and increased risk of chronic disease and mortality (96). However, this is only one of many mechanisms that may help explain correlations between life conditions and the risks of morbidity and mortality. The complexity of this issue is well illustrated by work on the relationship between income and disease (93, 97). Numerous mechanisms contribute to the high disease burden of low-income groups, and malnutrition is one of the obvious elements (98). As inflammatory responses and postresponse repair are costly in terms of energy and proteins, malnutrition limits the capacity to fend off disease. This mechanism is vividly illustrated by the sharp rise of active tuberculosis in human populations when food becomes limiting (98, 99). The cost of mounting an immune response in turn also affects further resilience of an organism. For instance, in bumblebees an experimentally induced immune response led to increased mortality if the animals were not allowed to increase their feeding rate to compensate for the increased energy costs (100). Last, the effects of poverty on systemic resilience may have a cognitive component. Worries related to decisions that could affect resources tend to impede overall cognitive functioning (101). This in turn affects the quality of other choices, potentially leading to further negative health effects (102).
Taking a health-management perspective, an obvious way to promote resilience is to minimize stressors that undermine it. However, there is an interesting paradox. While strong challenges can damage an organism, many forms of moderate challenge are known to promote longevity. For instance, moderate caloric restriction tends to promote longevity in animals (103, 104). Similarly, while extreme physical activity can have negative effects (105), the life-extending effect of moderate to vigorous exercise is well documented (106). Indeed, the proverbial observation “if you don’t use it, you lose it” applies to many functional aspects. On the other hand, “what doesn’t kill you makes you stronger” is too much of an extrapolation. For example, heat- and drought-stressed individuals of the Australian white-plumed honeyeaters lost weight and were less likely to be recaptured in the following spring, presumably because they had died (107). On a cellular level, the dual effect of challenge on longevity has been linked to the effects of ROS. While ROS can cause cellular damage, low levels of ROS triggered by challenges such as caloric restriction, hypoxia, temperature stress, and physical activity may actually promote longevity by inducing an adaptive response (108). While such molecular insights are fascinating, moderate challenge may also help maintain function in simpler ways. For instance, exercise in the elderly helps maintain muscle strength (109), which is an essential asset for systemic resilience in many ways.
So far, the effects of single factors on resilience are often poorly understood (Fig. 3), let alone the full picture of how different mechanisms interact to shape systemic resilience (Fig. 1). One limitation of research into this issue is the fact that the dependent variable is typically lifespan or mortality rates. This implies the need for large cohorts and limits the scope for experimental studies. The possibility of quantifying and monitoring systemic resilience of live animals and humans dynamically would greatly enhance the power of studies considering the interactive effects of different drivers. Moreover, lifespan itself is an end point of limited value. In geriatric care, “adding life to years” may often be more valued than “adding years to life.”
Prospect
The dazzling web of mechanisms that shape resilience may seem disappointingly complex. However, even if the details are not resolved, taking a resilience-based approach need not be complicated. It may seem challenging to choose between the many potentially relevant actions, but the multifactorial nature of resilience implies that the precise choice actually may not matter too much. Often, working on any of the plausibly related elements should help. Take the three examples from the introduction. To halt the demise of pollinators, it will help to increase the availability of flowers in a landscape and also to reduce exposure to pesticides (3). To reduce premature morbidity and mortality of piglets, it will help to choose genetically more resilient varieties and also to provide an enriched environment increasing their resistance to infectious challenge (110). To enhance the systemic resilience of the elderly, it will help to ensure a nourishing diet and also to promote physical and mental activity (111). Often some aspects are more difficult or costly to manage than others, allowing strategic choices to be made based on the identification of the nature of the problem.
While for humans caring about general health is broadly embraced, healthcare diagnoses and treatments still remain focused mostly on single issues (35). Singling out well-defined sources of illness remains important. However, many, if not most, health issues are related to interactions involving multiple subsystems of the organism. This becomes vividly clear in cases in which severe loss of resilience causes multiple failures to arise simultaneously. For instance, in dairy cows the onset of lactation may trigger symptoms ranging from infections and metabolic disorders to entire collapse. Similarly, in the frail elderly a broad set of somatic, mood, cognitive, and social problems tend to coincide. Clearly, such multimorbidity is the tip of the iceberg. A tightly knit web of subsystems shapes systemic resilience in all organisms.
The view of organisms as a complex adaptive network logically requires an holistic approach to managing resilience in animals and man. It is easy to forget that fixing a problem with medication or other specific treatment may carry the risk of reducing overall systemic resilience. The accumulation of chronic illnesses despite ever more sophisticated drugs and devices suggests that sustaining and restoring resilience should itself become a major activity. The novel possibilities of measuring resilience may become a game changer in this respect. Routinely providing real-time monitoring of all individuals in herds of thousands of electronically marked dairy cows allows early detection of deviations that hint at individuals that are not doing well (112). Similarly, wearable sensors are starting to allow the remote monitoring of large groups of patients. Moreover, the general public is beginning to share data from individuals’ own wearable electronics for analysis and comparison online. The emergence of DIORs thus comes at a moment where a growing flood of data may help tip human and animal science from a reductionist to a systemic paradigm. Diagnoses could become based on analyses of network resilience including essential elements ranging from mood and social conditions to different somatic subsystems. Meanwhile, management could become more adaptive, monitoring effects throughout the network and retuning medication and other variables over longer trajectories. In short, the recent technological and theoretical advances invite a fundamental rethinking of our approach to managing health and resilience.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810630115/-/DCSupplemental.
References
- 1.Potts SG, et al. Global pollinator declines: Trends, impacts and drivers. Trends Ecol Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Biesmeijer JC, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and The Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- 3.Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347:1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- 4.Stokstad E. Pesticides under fire for risks to pollinators. Science. 2013;340:674–676. doi: 10.1126/science.340.6133.674. [DOI] [PubMed] [Google Scholar]
- 5.Blacquière T, Smagghe G, van Gestel CA, Mommaerts V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology. 2012;21:973–992. doi: 10.1007/s10646-012-0863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garnett T, et al. Agriculture. Sustainable intensification in agriculture: Premises and policies. Science. 2013;341:33–34. doi: 10.1126/science.1234485. [DOI] [PubMed] [Google Scholar]
- 7.Marshall BM, Levy SB. Food animals and antimicrobials: Impacts on human health. Clin Microbiol Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Boeckel TP, et al. Reducing antimicrobial use in food animals. Science. 2017;357:1350–1352. doi: 10.1126/science.aao1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleaveland S, Laurenson MK, Taylor LH. Diseases of humans and their domestic mammals: Pathogen characteristics, host range and the risk of emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:991–999. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liverani M, et al. Understanding and managing zoonotic risk in the new livestock industries. Environ Health Perspect. 2013;121:873–877. doi: 10.1289/ehp.1206001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Bank 2009. Livestock and public health externalities. Minding the Stock: Bringing Public Policy to Bear on Livestock Sector Development Report No 44010-GLB, ed World Bank (World Bank, Washington, DC), pp 47–56.
- 12.Davis MF, et al. Occurrence of Staphylococcus aureus in swine and swine workplace environments on industrial and antibiotic-free hog operations in North Carolina, USA: A One Health pilot study. Environ Res. 2018;163:88–96. doi: 10.1016/j.envres.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson TP, et al. Antibiotic resistance is the quintessential One Health issue. Trans R Soc Trop Med Hyg. 2016;110:377–380. doi: 10.1093/trstmh/trw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyar OJ, et al. Antibiotic use in people and pigs: A One Health survey of rural residents’ knowledge, attitudes and practices in Shandong province, China. J Antimicrob Chemother. 2018;73:2893–2899. doi: 10.1093/jac/dky240. [DOI] [PubMed] [Google Scholar]
- 15.Purohit MR, et al. Antibiotic resistance in an Indian rural community: A ‘One-Health’observational study on commensal coliform from humans, animals, and water. Int J Environ Res Public Health. 2017;14:E386. doi: 10.3390/ijerph14040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broom DA, Fraser AF. Domestic Animal Behaviour and Welfare. 5th Ed. CABI; Wallingford, UK: 2015. pp. 1–462. [Google Scholar]
- 17.Rauw WM, Kanis E, Noordhuizen-Stassen EN, Grommers FJ. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livest Prod Sci. 1998;56:15–33. [Google Scholar]
- 18.Prunier A, Heinonen M, Quesnel H. High physiological demands in intensively raised pigs: Impact on health and welfare. Animal. 2010;4:886–898. doi: 10.1017/S175173111000008X. [DOI] [PubMed] [Google Scholar]
- 19.Jensen P, et al. Genetics and genomics of animal behaviour and welfare—Challenges and possibilities. Appl Anim Behav Sci. 2008;113:383–403. [Google Scholar]
- 20.Ellen ED, et al. The prospects of selection for social genetic effects to improve welfare and productivity in livestock. Front Genet. 2014;5:377. doi: 10.3389/fgene.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Knegsel ATM, et al. Effect of glucogenic vs. lipogenic diets on energy balance, blood metabolites, and reproduction in primiparous and multiparous dairy cows in early lactation. J Dairy Sci. 2007;90:3397–3409. doi: 10.3168/jds.2006-837. [DOI] [PubMed] [Google Scholar]
- 22.Bolhuis JE, Schouten WGP, Schrama JW, Wiegant VM. Effects of rearing and housing environment on behaviour and performance of pigs with different coping characteristics. Appl Anim Behav Sci. 2006;101:68–85. [Google Scholar]
- 23.Oostindjer M, Kemp B, van den Brand H, Bolhuis JE. Facilitating ‘learning from mom how to eat like a pig’ to improve welfare of piglets around weaning. Appl Anim Behav Sci. 2014;160:19–30. [Google Scholar]
- 24.Molenaar R, et al. High eggshell temperatures during incubation decrease growth performance and increase the incidence of ascites in broiler chickens. Poult Sci. 2011;90:624–632. doi: 10.3382/ps.2010-00970. [DOI] [PubMed] [Google Scholar]
- 25.Molenaar R, et al. High environmental temperature increases glucose requirement in the developing chicken embryo. PLoS One. 2013;8:e59637. doi: 10.1371/journal.pone.0059637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon K, de Vries Reilingh G, Bolhuis JE, Kemp B, Lammers A. Early feeding and early life housing conditions influence the response towards a noninfectious lung challenge in broilers. Poult Sci. 2015;94:2041–2048. doi: 10.3382/ps/pev189. [DOI] [PubMed] [Google Scholar]
- 27.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Kempen JAL, Schers HJ, Philp I, Olde Rikkert MGM, Melis RJF. Predictive validity of a two-step tool to map frailty in primary care. BMC Med. 2015;13:287. doi: 10.1186/s12916-015-0519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C, et al. Association of frailty with recovery from disability among community-dwelling older adults: Results from two large US cohorts. J Gerontol A Biol Sci Med Sci. April 10, 2018 doi: 10.1093/gerona/gly080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varadhan R, Walston JD, Bandeen-Roche K. Can a link be found between physical resilience and frailty in older adults by studying dynamical systems? J Am Geriatr Soc. 2018;66:1455–1458. doi: 10.1111/jgs.15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olde Rikkert MGM, et al. Slowing down of recovery as generic risk marker for acute severity transitions in chronic diseases. Crit Care Med. 2016;44:601–606. doi: 10.1097/CCM.0000000000001564. [DOI] [PubMed] [Google Scholar]
- 32.Borsboom D, et al. Kinds versus continua: A review of psychometric approaches to uncover the structure of psychiatric constructs. Psychol Med. 2016;46:1567–1579. doi: 10.1017/S0033291715001944. [DOI] [PubMed] [Google Scholar]
- 33.Nelson B, McGorry PD, Wichers M, Wigman JTW, Hartmann JA. Moving from static to dynamic models of the onset of mental disorder: A review. JAMA Psychiatry. 2017;74:528–534. doi: 10.1001/jamapsychiatry.2017.0001. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann SG, Curtiss J, McNally RJ. A complex network perspective on clinical science. Perspect Psychol Sci. 2016;11:597–605. doi: 10.1177/1745691616639283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greene JA, Loscalzo J. Putting the patient back together–Social medicine, network medicine, and the limits of reductionism. N Engl J Med. 2017;377:2493–2499. doi: 10.1056/NEJMms1706744. [DOI] [PubMed] [Google Scholar]
- 36.Stroustrup N, et al. The temporal scaling of Caenorhabditis elegans ageing. Nature. 2016;530:103–107. doi: 10.1038/nature16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pincus Z. Ageing: A stretch in time. Nature. 2016;530:37–38. doi: 10.1038/nature16873. [DOI] [PubMed] [Google Scholar]
- 38.Scheffer M, et al. Anticipating critical transitions. Science. 2012;338:344–348. doi: 10.1126/science.1225244. [DOI] [PubMed] [Google Scholar]
- 39.Dakos V, Carpenter SR, van Nes EH, Scheffer M. Resilience indicators: Prospects and limitations for early warnings of regime shifts. Philos Trans R Soc Lond B Biol Sci. 2015;370:20130263. [Google Scholar]
- 40.Scheffer M, et al. Early-warning signals for critical transitions. Nature. 2009;461:53–59. doi: 10.1038/nature08227. [DOI] [PubMed] [Google Scholar]
- 41.Lenton TM. Early warning of climate tipping points. Nat Clim Chang. 2011;1:201–209. [Google Scholar]
- 42.Scheffer M, et al. Climate and conservation. Creating a safe operating space for iconic ecosystems. Science. 2015;347:1317–1319. doi: 10.1126/science.aaa3769. [DOI] [PubMed] [Google Scholar]
- 43.Battiston S, et al. Complex systems. Complexity theory and financial regulation. Science. 2016;351:818–819. doi: 10.1126/science.aad0299. [DOI] [PubMed] [Google Scholar]
- 44.van de Leemput IA, et al. Critical slowing down as early warning for the onset and termination of depression. Proc Natl Acad Sci USA. 2014;111:87–92. doi: 10.1073/pnas.1312114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baggio JA, Brown K, Hellebrandt D. Boundary object or bridging concept? A citation network analysis of resilience. Ecol Soc, 2015;20:2. [Google Scholar]
- 46.Bahadur AV, Ibrahim M, Tanner T. 2010. The resilience renaissance? Unpacking of resilience for tackling climate change and disasters (Univ of Sussex, Brighton, United Kingdom), Strengthening Climate Resilience (SCR) Discussion Paper 1.
- 47.Martin-Breen P, Anderies JM. 2011 Resilience: A literature review. Available at https://opendocs.ids.ac.uk/opendocs/handle/123456789/3692. Accessed October 24, 2018.
- 48.van Nes EH, et al. What do you mean, ‘tipping point’? Trends Ecol Evol. 2016;31:902–904. doi: 10.1016/j.tree.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Scheffer M. Critical Transitions in Nature and Society. Princeton Univ Press; Princeton: 2009. p. 400. [Google Scholar]
- 50.Bak P, Sneppen K. Punctuated equilibrium and criticality in a simple model of evolution. Phys Rev Lett. 1993;71:4083–4086. doi: 10.1103/PhysRevLett.71.4083. [DOI] [PubMed] [Google Scholar]
- 51.Thom R. Topological models in biology. Topology. 1969;8:313–335. [Google Scholar]
- 52.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 53.Holling CS. Resilience and stability of ecological systems. Annu Rev Ecol Syst. 1973;4:1–23. [Google Scholar]
- 54.Boettiger C, Hastings A. Quantifying limits to detection of early warning for critical transitions. J R Soc Interface. 2012;9:2527–2539. doi: 10.1098/rsif.2012.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boettiger C, Ross N, Hastings A. Early warning signals: The charted and uncharted territories. Theor Ecol. 2013;6:255–264. [Google Scholar]
- 56.Ives AR, Gross K, Klug JL. Stability and variability in competitive communities. Science. 1999;286:542–544. doi: 10.1126/science.286.5439.542. [DOI] [PubMed] [Google Scholar]
- 57.Carpenter SR, Brock WA. Rising variance: A leading indicator of ecological transition. Ecol Lett. 2006;9:311–318. doi: 10.1111/j.1461-0248.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- 58.Dakos V, van Nes EH, Donangelo R, Fort H, Scheffer M. Spatial correlation as leading indicator of catastrophic shifts. Theor Ecol. 2010;3:163–174. [Google Scholar]
- 59.Skinner JE, Pratt CM, Vybiral T. A reduction in the correlation dimension of heartbeat intervals precedes imminent ventricular fibrillation in human subjects. Am Heart J. 1993;125:731–743. doi: 10.1016/0002-8703(93)90165-6. [DOI] [PubMed] [Google Scholar]
- 60.Mormann F, Andrzejak RG, Elger CE, Lehnertz K. Seizure prediction: The long and winding road. Brain. 2007;130:314–333. doi: 10.1093/brain/awl241. [DOI] [PubMed] [Google Scholar]
- 61.Glass L. Dynamical disease: Challenges for nonlinear dynamics and medicine. Chaos. 2015;25:097603. doi: 10.1063/1.4915529. [DOI] [PubMed] [Google Scholar]
- 62.Dai L, Korolev KS, Gore J. Slower recovery in space before collapse of connected populations. Nature. 2013;496:355–358. doi: 10.1038/nature12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dai L, Vorselen D, Korolev KS, Gore J. Generic indicators for loss of resilience before a tipping point leading to population collapse. Science. 2012;336:1175–1177. doi: 10.1126/science.1219805. [DOI] [PubMed] [Google Scholar]
- 64.Drake JM, Griffen BD. Early warning signals of extinction in deteriorating environments. Nature. 2010;467:456–459. doi: 10.1038/nature09389. [DOI] [PubMed] [Google Scholar]
- 65.Veraart AJ, et al. Recovery rates reflect distance to a tipping point in a living system. Nature. 2011;481:357–359. doi: 10.1038/nature10723. [DOI] [PubMed] [Google Scholar]
- 66.Dakos V, et al. Slowing down as an early warning signal for abrupt climate change. Proc Natl Acad Sci USA. 2008;105:14308–14312. doi: 10.1073/pnas.0802430105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verbesselt J, et al. Remotely sensed resilience of tropical forests. Nat Clim Chang. 2016;6:1028–1031. [Google Scholar]
- 68.Downey SS, Haas WR, Jr, Shennan SJ. European Neolithic societies showed early warning signals of population collapse. Proc Natl Acad Sci USA. 2016;113:9751–9756. doi: 10.1073/pnas.1602504113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dakos V, et al. Methods for detecting early warnings of critical transitions in time series illustrated using simulated ecological data. PLoS One. 2012;7:e41010. doi: 10.1371/journal.pone.0041010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lahti L, Salojärvi J, Salonen A, Scheffer M, de Vos WM. Tipping elements in the human intestinal ecosystem. Nat Commun. 2014;5:4344. doi: 10.1038/ncomms5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lenton TM, et al. Tipping elements in the Earth’s climate system. Proc Natl Acad Sci USA. 2008;105:1786–1793. doi: 10.1073/pnas.0705414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trefois C, Antony PM, Goncalves J, Skupin A, Balling R. Critical transitions in chronic disease: Transferring concepts from ecology to systems medicine. Curr Opin Biotechnol. 2015;34:48–55. doi: 10.1016/j.copbio.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 73.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 74.Cramer AOJ, et al. Major depression as a complex dynamic system. PLoS One. 2016;11:e0167490. doi: 10.1371/journal.pone.0167490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kendler KS. The nature of psychiatric disorders. World Psychiatry. 2016;15:5–12. doi: 10.1002/wps.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Green AJ, et al. Creating a safe operating space for wetlands in a changing climate. Front Ecol Environ. 2017;15:99–107. [Google Scholar]
- 77.Hirota M, Holmgren M, Van Nes EH, Scheffer M. Global resilience of tropical forest and savanna to critical transitions. Science. 2011;334:232–235. doi: 10.1126/science.1210657. [DOI] [PubMed] [Google Scholar]
- 78.Hughes TP, et al. Coral reefs in the Anthropocene. Nature. 2017;546:82–90. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 79.Nagaraja TG, Titgemeyer EC. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J Dairy Sci. 2007;90(Suppl 1):E17–E38. doi: 10.3168/jds.2006-478. [DOI] [PubMed] [Google Scholar]
- 80.Owens FN, Secrist DS, Hill WJ, Gill DR. Acidosis in cattle: A review. J Anim Sci. 1998;76:275–286. doi: 10.2527/1998.761275x. [DOI] [PubMed] [Google Scholar]
- 81.Le V-V, Mitiku T, Sungar G, Myers J, Froelicher V. The blood pressure response to dynamic exercise testing: A systematic review. Prog Cardiovasc Dis. 2008;51:135–160. doi: 10.1016/j.pcad.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Saal DP, Thijs RD, van Dijk JG. Tilt table testing in neurology and clinical neurophysiology. Clin Neurophysiol. 2016;127:1022–1030. doi: 10.1016/j.clinph.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 83.Olde Rikkert MGM, et al. Early warning signs for acute episodes in chronic diseases. Ned Tijdschr Geneeskd. 2015;159:A8150. Dutch. [Google Scholar]
- 84.Wichers M, Groot PC. Psychosystems; ESM Group; EWS Group Critical slowing down as a personalized early warning signal for depression. Psychother Psychosom. 2016;85:114–116. doi: 10.1159/000441458. [DOI] [PubMed] [Google Scholar]
- 85.Gijzel SMW, et al. Dynamical resilience indicators in time series of self-rated health correspond to frailty levels in older adults. J Gerontol A Biol Sci Med Sci. 2017;72:991–996. doi: 10.1093/gerona/glx065. [DOI] [PubMed] [Google Scholar]
- 86.Gijzel SMW, et al. Dynamical indicators of resilience in postural balance time series are related to successful aging in high-functioning older adults. J Gerontol A Biol Sci Med Sci. July 25, 2018 doi: 10.1093/gerona/gly170. [DOI] [PubMed] [Google Scholar]
- 87.Romero-Ortuno R, Cogan L, O’Shea D, Lawlor BA, Kenny RA. Orthostatic haemodynamics may be impaired in frailty. Age Ageing. 2011;40:576–583. doi: 10.1093/ageing/afr076. [DOI] [PubMed] [Google Scholar]
- 88.Lagro J, et al. Impaired systolic blood pressure recovery directly after standing predicts mortality in older falls clinic patients. J Gerontol A Biol Sci Med Sci. 2014;69:471–478. doi: 10.1093/gerona/glt111. [DOI] [PubMed] [Google Scholar]
- 89.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 90.Schumpert CA, Anderson C, Dudycha JL, Patel RC. Involvement of Daphnia pulicaria Sir2 in regulating stress response and lifespan. Aging (Albany NY) 2016;8:402–417. doi: 10.18632/aging.100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gladyshev VN. The origin of aging: Imperfectness-driven non-random damage defines the aging process and control of lifespan. Trends Genet. 2013;29:506–512. doi: 10.1016/j.tig.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 94.Romero LM, Dickens MJ, Cyr NE. The reactive scope model - a new model integrating homeostasis, allostasis, and stress. Horm Behav. 2009;55:375–389. doi: 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 95.Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. 2007;164:1521–1529, quiz 1622. doi: 10.1176/appi.ajp.2007.06091564. [DOI] [PubMed] [Google Scholar]
- 96.Cole SW, et al. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc Natl Acad Sci USA. 2015;112:15142–15147. doi: 10.1073/pnas.1514249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ngonghala CN, et al. Poverty, disease, and the ecology of complex systems. PLoS Biol. 2014;12:e1001827. doi: 10.1371/journal.pbio.1001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schaible UE, Kaufmann SH. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anuradha R, et al. Malnutrition is associated with diminished baseline and mycobacterial antigen–Stimulated chemokine responses in latent tuberculosis infection. J Infect. 2018 doi: 10.1016/j.jinf.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moret Y, Schmid-Hempel P. Survival for immunity: The price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- 101.Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013;341:976–980. doi: 10.1126/science.1238041. [DOI] [PubMed] [Google Scholar]
- 102.Haaksma ML, et al. The clinical course and interrelations of dementia related symptoms. Int Psychogeriatr. 2018;30:859–866. doi: 10.1017/S1041610217000321. [DOI] [PubMed] [Google Scholar]
- 103.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 104.Mattison JA, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rubies C, et al. Extreme physical activity reverses beneficial vascular effects of moderate physical activity. Study in an animal model. Am Heart Assoc. 2015;132(Suppl 3):A18108. [Google Scholar]
- 106.Ekblom-Bak E, Ekblom B, Vikström M, de Faire U, Hellénius M-L. The importance of non-exercise physical activity for cardiovascular health and longevity. Br J Sports Med. 2014;48:233–238. doi: 10.1136/bjsports-2012-092038. [DOI] [PubMed] [Google Scholar]
- 107.Gardner JL, Amano T, Sutherland WJ, Clayton M, Peters A. Individual and demographic consequences of reduced body condition following repeated exposure to high temperatures. Ecology. 2016;97:786–795. [PubMed] [Google Scholar]
- 108.Ristow M, Schmeisser K. Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS) Dose Response. 2014;12:288–341. doi: 10.2203/dose-response.13-035.Ristow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Vries NM, et al. Patient-centred physical therapy is (cost-) effective in increasing physical activity and reducing frailty in older adults with mobility problems: A randomized controlled trial with 6 months follow-up. J Cachexia Sarcopenia Muscle. 2016;7:422–435. doi: 10.1002/jcsm.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Dixhoorn ID, et al. Enriched housing reduces disease susceptibility to co-infection with porcine reproductive and respiratory virus (PRRSV) and Actinobacillus pleuropneumoniae (A. pleuropneumoniae) in young pigs. PLoS One. 2016;11:e0161832. doi: 10.1371/journal.pone.0161832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mirelman A, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): A randomised controlled trial. Lancet. 2016;388:1170–1182. doi: 10.1016/S0140-6736(16)31325-3. [DOI] [PubMed] [Google Scholar]
- 112.Caja G, Castro-Costa A, Knight CH. Engineering to support wellbeing of dairy animals. J Dairy Res. 2016;83:136–147. doi: 10.1017/S0022029916000261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.