In a recent issue of PNAS, Lenc et al. (1) report that steady-state responses (SSRs) induced by rhythmic auditory stimuli are enhanced when listeners attend to low-frequency sounds, particularly when these sounds reflect salient periodicities like the beat or the meter. Lenc et al. (1) interpret this finding as an entrainment of sensory-motor networks important for planning and controlling movements, underlying humans’ spontaneous tendency to move to music and particularly to low-frequency sounds produced by instruments such as bass or drums (2).
We propose a more parsimonious interpretation of these findings: The EEG periodicities contributing to the enhanced SSRs may not reflect an actual neural entrainment but, instead, might be driven by a sequence of auditory event-related potentials (ERPs). In an earlier study, Nozaradan et al. (3) dismissed this alternative interpretation by simply reporting that only the first stimulus of the sequence elicited an ERP, whereas the following stimuli did not elicit “measurable” ERPs. The latter point, however, was merely qualitative and not supported by statistical evidence. Relevant to this discourse is a recent study disclosing that, although habituated over repetition, small-amplitude ERPs are reliably elicited even following tens of consecutive stimuli presented at 1 Hz (4). These preserved ERPs included the supramodal, biphasic negative–positive (N–P) vertex wave and the contingent negative variation (CNV), a slow-rising negativity anticipating predictable events. Notably, these ERPs are commonly evoked by auditory stimuli (5, 6).
A modulation of either of these ERPs could explain the results reported by Lenc et al. (1), because both the N–P vertex wave and the CNV are modulated by both temporal attention and anticipation (5–7). If a participant allocates more attentional resources to the periodic events marking the beat or the meter (or to any predictable or salient event within a pattern), then the ERP amplitude associated with these events becomes larger. It follows that such time-locked modulations would enhance the power at beat- and meter-relevant frequencies (Fig. 1). Supporting this alternative interpretation, we highlight that (i) the reported SSRs had a scalp topography similar to that of both the CNV and the negative vertex wave (auditory N1); (ii) the SSRs’ enhancement was contingent on attentional allocation (5, 6); (iii) remarkably, the N–P vertex wave has larger amplitude when elicited by low-frequency sounds (8); and (iv) the N–P vertex wave can also be evoked by imagery of transient auditory events that are typical of syncopated rhythms (9).
Fig. 1.
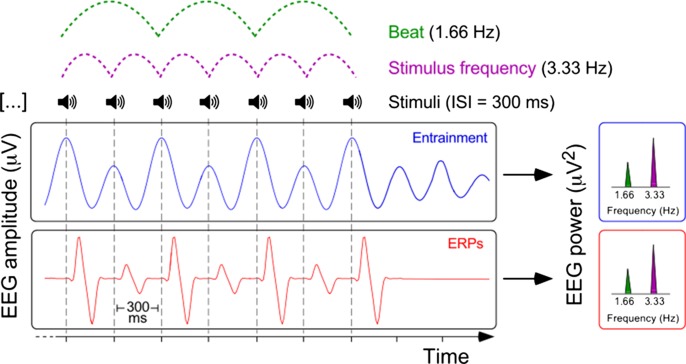
Two possible physiological interpretations of the EEG SSRs reported by Lenc et al. (1). The entrainment account postulates sustained oscillatory activity outlasting the stimulus offset (blue waveform) at the frequency of stimulus presentation and the perceived beat. The ERP account postulates that ERPs (red waveform; in this example, the N–P vertex wave) are evoked by each stimulus, with greater amplitude recorded when stimuli reflect salient periodicities like the beat. Both accounts predict a similar enhancement of EEG power (Right) at the frequencies corresponding to both stimulus presentation (Top, purple dashed lines) and the perceived beat (Top, green dashed lines). ISI, interstimulus interval.
This alternative interpretation could be easily tested by analyzing the EEG data in the time domain and statistically assessing the presence of ERPs in the waveforms time-locked to each stimulus onset (4). Should this analysis reveal the presence of ERPs, two fundamental implications of the results reported by Lenc et al. (1) follow. First, the notion that these SSRs tag neural entrainment should be reconsidered: They might not reflect stimulus-induced sustained oscillatory activity, but rather repeated stimulus-evoked transient ERPs (10). Second, the cortical network underlying the results of Lenc et al. (1) and Nozaradan et al. (3) would subserve attention to, and anticipation of, salient events (5–7).
Footnotes
The authors declare no conflict of interest.
References
- 1.Lenc T, Keller PE, Varlet M, Nozaradan S. Neural tracking of the musical beat is enhanced by low-frequency sounds. Proc Natl Acad Sci USA. 2018;115:8221–8226. doi: 10.1073/pnas.1801421115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hove MJ, Marie C, Bruce IC, Trainor LJ. Superior time perception for lower musical pitch explains why bass-ranged instruments lay down musical rhythms. Proc Natl Acad Sci USA. 2014;111:10383–10388. doi: 10.1073/pnas.1402039111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nozaradan S, Peretz I, Missal M, Mouraux A. Tagging the neuronal entrainment to beat and meter. J Neurosci. 2011;31:10234–10240. doi: 10.1523/JNEUROSCI.0411-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancini F, et al. Characterizing the short-term habituation of event-related evoked potentials. eNeuro. 2018;5:ENEURO.0014-18.2018. doi: 10.1523/ENEURO.0014-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: A salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Nobre AC, van Ede F. Anticipated moments: Temporal structure in attention. Nat Rev Neurosci. 2018;19:34–48. doi: 10.1038/nrn.2017.141. [DOI] [PubMed] [Google Scholar]
- 7.Breska A, Deouell LY. Neural mechanisms of rhythm-based temporal prediction: Delta phase-locking reflects temporal predictability but not rhythmic entrainment. PLoS Biol. 2017;15:e2001665. doi: 10.1371/journal.pbio.2001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wunderlich JL, Cone-Wesson BK. Effects of stimulus frequency and complexity on the mismatch negativity and other components of the cortical auditory-evoked potential. J Acoust Soc Am. 2001;109:1526–1537. doi: 10.1121/1.1349184. [DOI] [PubMed] [Google Scholar]
- 9.Hubbard TL. Auditory imagery: Empirical findings. Psychol Bull. 2010;136:302–329. doi: 10.1037/a0018436. [DOI] [PubMed] [Google Scholar]
- 10.Barczak A, et al. Top-down, contextual entrainment of neuronal oscillations in the auditory thalamocortical circuit. Proc Natl Acad Sci USA. 2018;115:E7605–E7614. doi: 10.1073/pnas.1714684115. [DOI] [PMC free article] [PubMed] [Google Scholar]