Significance
Whereas short-term plasticity is often initiated on one side of the synapse, long-term plasticity involves coordinated changes on both sides, implying extracellular signaling. We have investigated the possible signaling role of an Aplysia neurotrophin (ApNT) and found that it acts as a presynaptic autocrine signal, which forms part of a positive feedback loop that drives consolidation of learning-related synaptic plasticity.
Keywords: Aplysia, neurotrophin, facilitation, presynaptic, autocrine
Abstract
Whereas short-term plasticity is often initiated on one side of the synapse, long-term plasticity involves coordinated changes on both sides, implying extracellular signaling. We have investigated the possible signaling role of an Aplysia neurotrophin (ApNT) in facilitation induced by serotonin (5HT) at sensory-to-motor neuron synapses in culture. ApNT is an ortholog of mammalian BDNF, which has been reported to act as either an anterograde, retrograde, or autocrine signal, so that its pre- and postsynaptic sources and targets remain unclear. We now report that ApNT acts as a presynaptic autocrine signal that forms part of a positive feedback loop with ApTrk and PKA. That loop stimulates spontaneous transmitter release, which recruits postsynaptic mechanisms, and presynaptic protein synthesis during the transition from short- to intermediate-term facilitation and may also initiate gene regulation to trigger the transition to long-term facilitation. These results suggest that a presynaptic ApNT feedback loop plays several key roles during consolidation of learning-related synaptic plasticity.
Memory is stored in the brain as plastic alterations in the strength of neuronal synapses, and both memory and synaptic plasticity can be divided into short-term, intermediate-term, and long-term phases (1–3). In most instances where it has been studied, short-term plasticity is initiated on one side of the synapse, but long-term plasticity involves growth and remodeling of synapses, which require changes on both sides (4). These results imply that there must be extracellular signaling during the transition from short- to intermediate- and long-term plasticity. However, in no case is there a good understanding of the signaling involved in these transitions.
To address this question, we have focused on the cell and molecular mechanisms underlying the transition from short- to intermediate- and long-term facilitation produced by 5-hydroxytryptamine (5HT) at the synapse between a presynaptic sensory neuron (SN) and postsynaptic motor neuron (MN) of the Aplysia gill-withdrawal reflex in isolated cell culture. Because the culture system contains only two neurons that can be selectively manipulated both pharmacologically and genetically (5, 6), it is advantageous for the study of the transitions between the stages of synaptic plasticity, which may involve a complex chain or cascade of anterograde and retrograde interactions between the pre- and postsynaptic neurons (4, 7).
To begin to elucidate this chain, we have also initially focused on the relatively early steps. Facilitation produced by 5-HT at Aplysia sensory to MN synapses, which is a cellular analog of behavioral sensitization, has three phases: short-term facilitation (STF), intermediate-term facilitation (ITF), and long-term facilitation (LTF) (8, 9). The transitions from STF to ITF and then from ITF to LTF are generally thought to occur sequentially (7, 10, 11), although under some circumstances they may also occur in parallel (12). In Aplysia, STF (minutes) appears to be entirely presynaptic and involves covalent modification of preexisting molecules, whereas ITF (1–3 h) requires postsynaptic as well as presynaptic mechanisms and is dependent on translation in both the pre- and postsynaptic neurons (10, 13–16). In addition, LTF (more than 24 h) involves both pre- and postsynaptic growth and remodeling of synapses and requires transcription as well as translation (17–20). Thus, recruitment of postsynaptic mechanisms and activation of protein synthesis in the pre- and postsynaptic neurons are required during the transition from STF to ITF, and synaptic growth and transcription in the pre- and postsynaptic neurons are required during the induction of LTF.
We have previously found that spontaneous release of glutamate from the presynaptic neuron induced by 5HT recruits postsynaptic mechanisms during the induction of ITF and LTF (7, 21). Spontaneous release activates type I metabotropic glutamate receptors (mGluR5) in the postsynaptic neuron, leading to increases in IP3, internal Ca2+, and membrane insertion and recruitment of clusters of AMPA-like receptors. However, although spontaneous release is necessary for the transition to ITF, it is not sufficient, suggesting that other extracellular signaling pathways that may act synergistically with spontaneous release are also required.
To begin to identify those signaling pathways, we have now investigated the role of an Aplysia neurotrophin (ApNT), which is an invertebrate ortholog of the vertebrate brain-derived neurotrophic factor (BDNF) (22). BDNF promotes survival and development of neurons (23, 24) and also regulates functions in the adult brain, including synaptic plasticity (25, 26), in part through regulation of protein synthesis via the PI3K/Akt and Raf/MAPK cascades activated by TrkB receptors (27–29). However, in different studies, BDNF has been reported to act as either an anterograde, retrograde, or autocrine signal during synaptic plasticity (e.g., refs. 30–33), and its pre- and postsynaptic sources and targets remain unclear.
Presynaptic ApNT and ApTrk receptors mediate LTF produced by 5HT in Aplysia (22), suggesting a possible presynaptic autocrine function. Activation of ApTrk receptors also stimulates PLCγ, PI3K, and MAPK signaling, similar to vertebrate TrkB receptors (22). When and how is that signaling first recruited? In vertebrates, short-term exposure (∼5–10 min) of neurotrophin rapidly potentiates spontaneous and evoked synaptic activity at developing neuromuscular synapses in culture (34). This suggested to us that ApNT might also act rapidly and be involved in the transition to ITF as well as LTF.
To address these questions, we have investigated the possible involvement of ApNT/ApTrk signaling during the transition from STF to ITF produced by 5HT. In this first paper, we report that ApNT released from the presynaptic neuron acts as an autocrine signal that forms a positive feedback loop with ApTrk receptors and PKA in the presynaptic neuron. This feedback loop drives an increase in spontaneous release of glutamate that recruits postsynaptic mechanisms, and supplies essential materials for the increase in release through vesicle mobilization and protein synthesis in the presynaptic neuron. In addition, the presynaptic feedback loop increases molecules including PKA and MAPK that initiate transcription necessary for the transition to LTF. In the following paper (35), we report that ApNT also acts as both an anterograde and retrograde signal that forms a transsynaptic feedback loop during the transition to ITF. ApNT activates postsynaptic ApTrk receptors, perhaps cooperating with glutamate acting on postsynaptic mGluR5 receptors (7) to stimulate postsynaptic protein synthesis. These results suggest that ApNT plays several key roles during consolidation of facilitation from STF to ITF and then to LTF.
Results
ITF Induced by 5HT Involves ApNT and ApTrk in the Presynaptic SN.
ApNT is involved during the transition from STF to ITF produced by 5HT at sensorimotor neuron synapses.
To evaluate whether ApNT signaling is involved during the induction of ITF produced by 5HT in sensorimotor neuron cocultures, we applied a Fc-ApTrk receptor body that blocks ApNT signaling (Fig. 1 A and B). There was an increase in the frequency of miniature EPSCs (mEPSCs) in a 10-min recording session beginning 5 min after the start of bath application of 5HT, t(4) = 3.24, P < 0.05, without a significant effect on their amplitude, as reported previously (21). Bath application of Fc-ApTrk receptor body reduced the increase in the frequency of mEPSCs, compared with control, t(9) = 3.35, P < 0.01, without a significant effect on their amplitude (P > 0.05). As a control, Fc-ApTrk did not have a significant effect on the pretest frequency or amplitude (P > 0.05). In addition, bath application of the mature form of ApNT (mApNT) (22) increased the frequency of mEPSCs, compared with control, t(14) = 3.356, P < 0.01, without a clear change in their amplitude (P > 0.05) (Fig. 1C). These results suggest that ApNT signaling is involved during the transition from STF to ITF produced by 5HT at Aplysia sensorimotor neuron synapses. This is similar to what has been reported in vertebrate preparations, where BDNF increases the number of docked vesicles at the active zones of hippocampal excitatory synapses, resulting in an increase in the frequency, but not the amplitude, of AMPA receptor-mediated mEPSCs recorded from CA1 pyramidal neurons in hippocampal slices (36, 37).
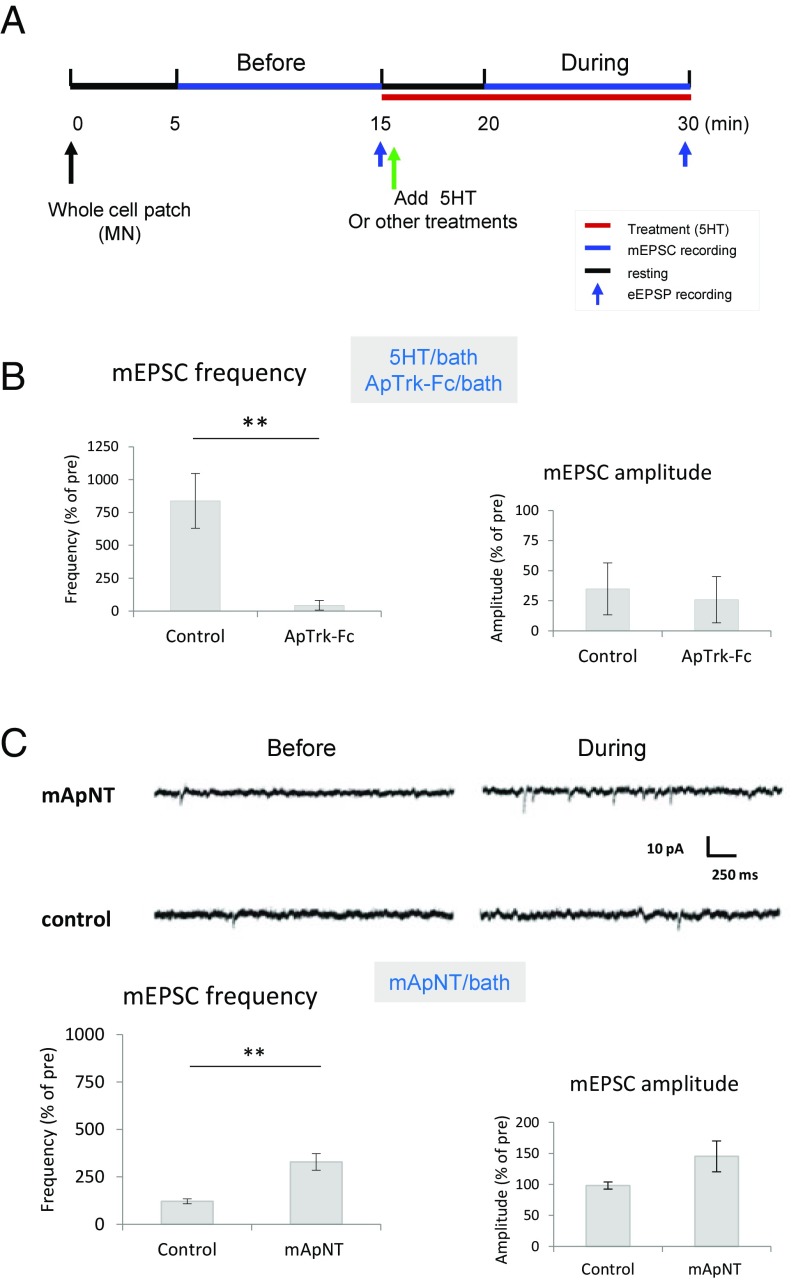
Fig. 1.
ApNT/Aprk signaling is important during the transition from STF to ITF produced by 5HT. (A) The experimental protocol for recording mEPSCs and eEPSPs. Five minutes after obtaining a whole-cell patch of the postsynaptic MN, we recorded mEPSCs for 10 min and checked the eEPSP at the end of the session in current clamp mode. A second 10-min recording session started 5 min after application of either treatment or control solution. The red bar indicates the time when 5HT or other treatments were present, the blue bars indicate the mEPSC recording sessions, and the short blue arrows indicate when the eEPSP was recorded. (B and C) ApNT signaling is involved during the transition from STF to ITF produced by 5HT. (B) Bath application of ApTrk-Fc receptor body (n = 6) reduced the increase in the frequency of mEPSCs produced by 5HT, compared with control (n = 5) (P < 0.01) without a clear effect on mEPSC amplitude (P > 0.05). The average pretest mEPSC values were 38.2 min−1 for frequency and 7.63 pA for amplitude, not significantly different between ApTrk-Fc application and the control group. (C) Bath application of mApNT (n = 9) increased the frequency of mEPSCs, compared with control (n = 7) (P < 0.01), without a clear effect on mEPSC amplitude (P > 0.05). The average pretest mEPSC values were 12 min−1 for frequency and 9 pA for amplitude, not significantly different between mApNT application and control. In this and subsequent figures, the error bars indicate SEMs; **P < 0.01 for the difference between the experimental and control groups.
Presynaptic ApNT is important for the transition from STF to ITF.
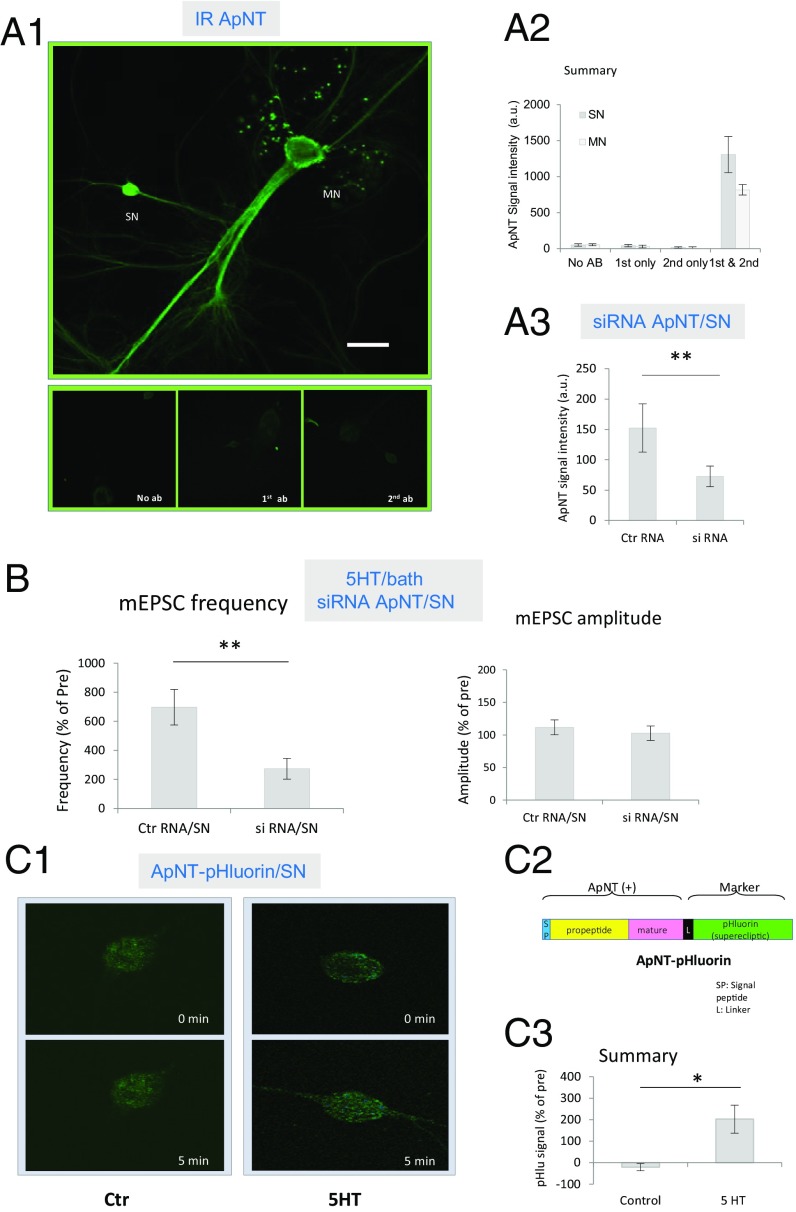
PCR initially revealed ApNT primarily in the SN of cocultures, but there was a weak signal in the MN as well (22). In preliminary studies, we have used qPCR to confirm that ApNT is present in both neurons. As another approach to this question, we performed immunocytochemistry with an antibody raised against mApNT (M50), which similarly showed that ApNT is localized in both sensory and MNs of cocultures (Fig. 2 A1 and A2). However, in the same culture dish, the intensity of ApNT staining is greater in optical sections of the presynaptic SN compared with the postsynaptic MN, F(1, 8) = 10.87, P < 0.05. For each neuron, staining with both the primary and secondary antibodies was significantly stronger than staining with either one alone or no antibody (P < 0.01 in each case). Furthermore, injection into the presynaptic neuron of siRNA against mApNT reduced the signal intensity of ApNT staining in the presynaptic neuron, compared with a control RNA injection, t(8) = 5.03, P < 0.01 (Fig. 2A3). These results suggest that the staining is relatively specific for mApNT.
Fig. 2.
ApNT in the presynaptic neuron is important during the induction of ITF produced by 5HT. (A) ApNT is localized in both presynaptic and postsynaptic neurons of the sensorimotor neuron cocultures. (A1) Representative confocal microscope images of immunoreactivity (IR) using an antibody (M50) raised against mApNT compared with no antibody or the primary or secondary antibody alone. (Scale bar, 100 μm.) In this and subsequent figures, the SN cell body is ∼30 μm, and the MN cell body is ∼100 μm. (A2) There was a significant main effect of antibody on the intensity of ApNT immunostaing in a two-way ANOVA with one repeated measure, F(3, 8) = 11.29, P < 0.01. In addition, staining intensity was greater in optical sections of the presynaptic neuron than the postsynaptic neuron in the same dish (n = 6) (P < 0.05). (A3) Injection of siRNA against mApNT into the presynaptic neuron reduced the intensity of immunostaining for ApNT in the presynaptic SN (n = 5), compared with a control RNA injection (n = 5) (P < 0.01). (B) ApNT in the presynaptic neuron is involved in the increase in the frequency of mEPSCs during the induction of ITF by 5HT. Injection of siRNA against mApNT into the presynaptic neuron (n = 10) reduced the increase in mEPSC frequency produced by 5HT, compared with a control RNA injection (n = 11) (P < 0.001) without a clear effect on mEPSC amplitude (P > 0.05). The average pretest mEPSC values were 15 min−1 for frequency and 9 pA for amplitude, not significantly different between siRNA injection and control RNA injection groups. (C) 5HT stimulates the release of ApNT from SNs. (C1) Representative images before and 5 min after application of either 5HT (20 μM) or control solution to cocultures with SNs expressing ApNTpHluorin. (C2) Schematic diagram of the ApNTpHluorin construct. See the Results. (C3) Five-minute bath application of 5HT (n = 7) significantly increased the release of ApNTpHluorin from the presynaptic SN, compared with control (n = 9) (P < 0.05). *P < 0.05, **P < 0.01 for the difference between the experimental and control groups.
Injection of siRNA against mApNT into the presynaptic neuron also significantly reduced the increase in the frequency of mEPSCs produced by 5HT, compared with a control RNA injection, t(19) = 2.903, P < 0.001, without a clear effect on mEPSC amplitude (P > 0.05) or pretest values (Fig. 2B). To determine whether ApNT is released from the presynaptic neuron, we constructed ApNTpHluorin by adding a pH-sensitive GFP mutant (“superecliptic” pHluorin) to the C-terminal end of the protein. The signal intensity of superecliptic pHluorin is ∼20 times greater when it is released from a cell and binds to receptors than when it is inside the lumen of vesicles (38). A 5-min bath application of 5HT (20 μM) significantly increased the signal intensity of ApNTpHluorin expressed in the presynaptic SN, compared with control, t(14) = 2.921, P < 0.05 (Fig. 2C). These results suggest that ApNT is released from the presynaptic neuron during STF and plays an important role in the induction of ITF by 5HT.
Presynaptic ApTrK is also important for the transition from STF to ITF.
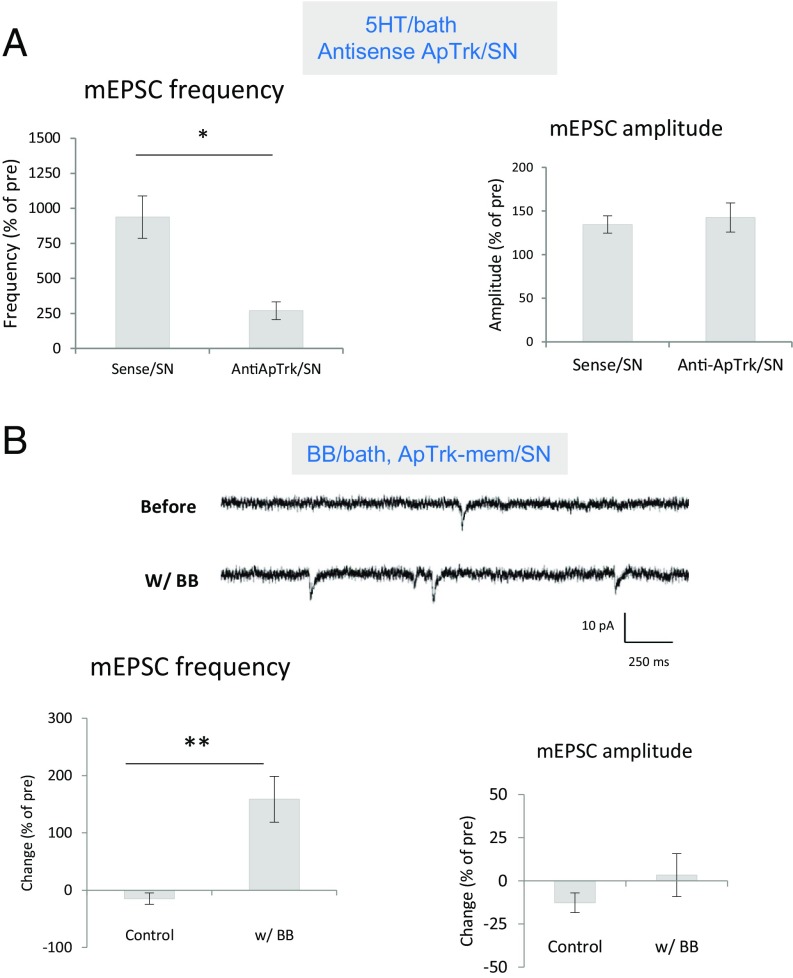
We next checked the role of presynaptic ApTrk receptors during the induction of ITF. Injection of an antisense oligo against ApTrk into the SN significantly reduced the increase in mini frequency produced by 5HT, compared with sense oligo injection, t(9) = 3.170, P < 0.05, without a clear effect on mEPSC amplitude (P > 0.05) or pretest values (Fig. 3A), suggesting that presynaptic ApTrk receptors are involved.
Fig. 3.
ApTrk in the presynaptic neuron is important during the induction of ITF produced by 5HT. (A) Injection of an antisense oligo against ApTrk into the SN (n = 7) reduced the increase in mEPSC frequency produced by 10 min 5HT, compared with sense oligo injection control (n = 4) (P < 0.05) without a clear effect on mEPSC amplitude (P > 0.05). The average pretest eEPSC values were 24 min−1 for frequency and 7 pA for amplitude, not significantly different between antisense oligo injection and sense oligo injection groups. (B) Activation of ApTrk receptor signaling in the presynaptic neuron increased mEPSC frequency. Shown are examples of mEPSCs recorded before and during the activation of presynaptic ApTrk signaling by application of BB dimers to cultures with SNs expressing ApTrkmem (SI Appendix, Figs. S1 and S2). There was a significant increase in mEPSC frequency in the BB ligand group (n = 9), compared with the control ligand group (n = 11) (P < 0.001) without a clear effect on mEPSC amplitude (P > 0.05). The average pretest values for mEPSC frequency were 8.5 min−1 for the BB group and 18.9 min−1 for the control group; the average mEPSC amplitude was 9.5 pA, not significantly different between the two groups. *P < 0.05, **P < 0.01 for the difference between the experimental and control groups.
To activate presynaptic ApTrk receptor signaling selectively, we developed the ApTrk-mem/BB dimer system (SI Appendix, Figs. S1 and S2). The system consists of a hybrid ApTrk receptor (ApTrk-mem) with two artificial binding domains for a specific membrane permeant ligand, BB dimer (iDimerizer system, Clontech), similar to a designer receptor exclusively activated by designer drugs. Activation of presynaptic ApTrk signaling by expression of ApTrk-mem in the SN and bath application of BB dimers significantly increased the frequency of mEPSCs, compared with control ligand application, t(18) = 4.605, P < 0.001, without a clear effect on mEPSC amplitude (P > 0.05) (Fig. 3B).
Collectively, these results suggest that ApNT is released from the presynaptic neuron and activates ApTrk receptors on the presynaptic neuron and thus appears to act as a presynaptic autocrine signal during the transition from STF to ITF. Our previous study in Aplysia had earlier suggested that ApNT and ApTrk receptors in the presynaptic neuron also play an important role in LTF (22). Similarly, in vertebrates, BDNF acts as a presynaptic autocrine signal and contributes to various cellular processes including axon differentiation and growth (39) and some forms of hippocampal LTP (e.g., ref. 30). Because a key component of our assay of facilitation is the frequency of mEPSCs, our results also imply that the ApNT/ApTrk system closely interacts with and in part drives spontaneous release of glutamate from the presynaptic neuron.
ApNT, ApTrk, and PKA Form a Positive Feedback Loop in the Presynaptic SN.
ApNT is released from the presynaptic neuron and acts through ApTrk receptors on the presynaptic neuron during facilitation produced by activation of presynaptic PKA.
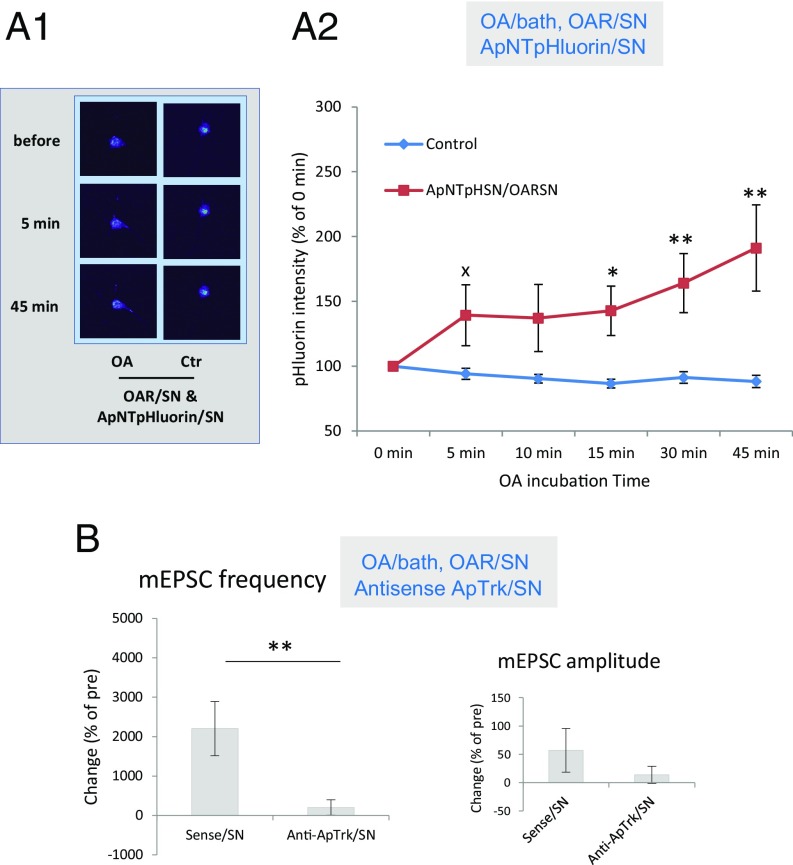
Like ApNT, activation of presynaptic PKA produces an increase in spontaneous release of glutamate during the induction of ITF (21). We therefore examined whether presynaptic PKA might also act in part by increasing release of ApNT from the presynaptic neuron. To activate PKA selectively, we ectopically expressed an octopamine receptor (OAR) that is not normally expressed in sensory or MNs in the presynaptic neuron and activated it by bath application of OA (20 μM). OAR is selectively coupled to cAMP/PKA and not to other signaling systems in the SNs (40). Activation of that pathway significantly increased the signal intensity of ApNTpHluorin released from the presynaptic SN, compared with artificial seawater (ASW) control, F(1, 28) = 6.55, P < 0.05 (Fig. 4A), suggesting that release of ApNT is downstream of PKA. The ApNTpHluorin signal gradually increased over time, F(4, 112) = 7.05, P < 0.001 for the interaction of OA and time.
Fig. 4.
ApNT and ApTrk are downstream of PKA in the SN during the induction of ITF. (A1 and A2) Activation of OAR signaling coupled to PKA in the presynaptic neuron produced release of ApNT from the SN. (A1) Representative images before and 5 and 45 min after application of either OA (20 μM) or control solution to cocultures with SNs expressing OAR. (A2) Bath application of OA (n = 15) significantly increased the release of ApNTpHluorin from the presynaptic SN, compared with application of control solution (n = 15) (P < 0.05). The ApNTpHluorin signal gradually increased over time. (B) The increase in the frequency of mEPSCs produced by activation of OAR signaling coupled to PKA in the presynaptic neuron was reduced by injection of an antisense oligo against ApTrk receptors into the SN (n = 7), compared with sense oligo injection (n = 7) (P < 0.01) without a clear effect on mEPSC amplitude (P > 0.05). The average pretest mEPSC values were 24 min−1 for frequency and 7 pA for amplitude, not significantly different between the antisense oligo injection and sense oligo injection groups. xP < 0.05 one-tail, *P < 0.05, **P < 0.01 for the difference between the experimental and control groups.
We next examined whether ApTrk is also downstream of PKA. The increase in mEPSC frequency produced by activation OAR in the presynaptic neuron was reduced by injection into the presynaptic neuron of antisense oligo against ApTrk receptor, compared with sense oligo injection, t(12) = 3.297, P < 0.01. There was no clear effect on mEPSC amplitude (P > 0.05) or pretest values (Fig. 4B). These results suggest that both ApNT and ApTrk are downstream of PKA.
Activation of ApTrk receptors on the presynaptic neuron activates presynaptic PKA.
We next examined whether the increase in the frequency of mEPSCs produced by ApNT and activation of ApTrk receptors in the presynaptic neuron is mediated through activation of PKA in the presynaptic neuron. Presynaptic injection of a peptide inhibitor of PKA (PKAi 6–22) significantly reduced the increase in mEPSC frequency produced by bath application of mApNT, compared with vehicle injection control, t(21) = 4.697, P < 0.001, without a clear effect on mEPSC amplitude (P > 0.05) or pretest values (Fig. 5A1), suggesting that PKA is downstream of ApNT. Furthermore, 5-min bath application of BB dimers to cocultures in which SNs expressed ApTrkmem increased the signal intensity of immunostaining with an antibody against the catalytic domain of PKA (41), which indicates PKA activation, compared with application of the control ligand, F(1, 69) = 5.98, P < 0.05 (Fig. 5B), suggesting that PKA is also downstream of ApTrk receptor signaling. As positive controls, we activated PKA in the presynaptic neuron either by activation of ectopically expressed OAR receptors in the presynaptic neuron or by bath application of 5HT. Both manipulations also increased staining for the active form of PKA in the presynaptic neuron (P < 0.05 in each case), suggesting that the staining is relatively specific for the catalytic domain of PKA. These results suggest that PKA is downstream of both ApNT and ApTrk signaling in the SN during the induction of ITF.
Fig. 5.
PKA, PKC, and Ca2+ are downstream of ApNT and ApTrk in the SN during the induction of ITF. (A1) Presynaptic PKA is involved in the signal transduction produced by mApNT. The increase in the frequency of mEPSCs produced by mApNT was significantly reduced by injection of a peptide inhibitor of PKA (PKAi 6–22) into the SN (n = 10), compared with vehicle injection (n = 13) (P < 0.001), without a clear effect on mEPSC amplitude (P > 0.05). The average pretest mEPSC values were 27.5 min−1 for frequency and 6 pA for amplitude, not significantly different between PKAi injection and vehicle injection. (A2) PKC in the presynaptic neuron is also involved in mApNT signaling. The increase in the frequency of mEPSCs produced by mApNT was significantly reduced by injection of a peptide inhibitor of PKC (PKCi 19–31) into the SN (n = 3), compared with vehicle injection (n = 13) (P < 0.05), without a clear effect on mEPSC amplitude (P > 0.05). The average pretest mEPSC values were 19.7 min−1 for frequency and 6.43 pA for amplitude, not significantly different between PKCi injection and vehicle injection. (B) Activation of presynaptic ApTrk receptors activates PKA in the presynaptic neuron. Application of BB dimers for 5 min to cocultures with SNs expressing ApTrk-mem (n = 10) increased immunostaining in the SNs with an antibody raised against the catalytic domain (active form) of PKA, compared with control treatment (n = 37) (P < 0.05). As positive controls, we also activated PKA in the presynaptic neuron either by stimulation of ectopically expressed OAR receptors in the presynaptic neuron (n = 7) or by bath application of 5HT (n = 19). Both manipulations also increased staining for the active form of PKA in the presynaptic neuron (P < 0.05 in each case). There was a significant main effect of group in a one-way ANOVA, F(3, 69) = 4.32, P < 0.01. (C1 and C2) Activation of presynaptic ApTrk receptors produced an increase in Ca2+ in the SN. (C1) Representative images of Calcium Orange fluorescence before and 5 and 45 min after application of either BB dimers or control solution to a coculture with a SN expressing ApTrk-mem. (C2) Bath application of BB dimers (n = 5) significantly increased Calcium Orange fluorescence in the presynaptic SN, compared with control (n = 10) (P < 0.05). The Ca2+ signal gradually increased over time. xP < 0.05 one-tail, *P < 0.05, ***P < 0.001 for the difference between the experimental and control groups.
Collectively, our results suggest that during the induction of ITF, activation of PKA increases release of ApNT from the presynaptic neuron, which in turn acts on ApTrk receptors on the presynaptic neuron and activates downstream signaling including PKA. We propose that this positive feedback loop (prePKA → preApNT → preApTrk → prePKA) plays several essential roles during the induction of ITF (see Discussion). In vertebrate preparations, activation of TrkB receptors by BDNF also activates the cAMP/PKA pathway and forms a self-amplifying loop, which is critical for axon development (39).
ApNT and ApTrk receptors on the presynaptic neuron also activate presynaptic PKC and Ca2+.
Since PKC is responsible for vesicle mobilization at the sensorimotor neuron synapses of Aplysia (42–44), we also investigated the role of this protein kinase. The increase in the frequency of mEPSCs produced by mApNT was significantly reduced by injection into the presynaptic SN of a peptide inhibitor of PKC (PKCi 19–31), compared with vehicle injection control, t(14) = 2.451, P < 0.05, without a clear effect on mEPSC amplitude (P > 0.05) or pretest values (Fig. 5A2). These results suggest that PKC in the presynaptic neuron is also downstream of ApNT and ApTrk during the induction of ITF.
In other systems, Trk receptors are coupled to PLCγ, which stimulates production of DAG and activation of PKC (45, 46). PLCγ also stimulates production of IP3 and release of intracellular Ca2+. To begin to test the possible involvement of these pathways, we monitored Ca2+ in the presynaptic neuron by injecting the fluorescent indicator calcium orange. Bath application of BB dimers to cocultures with SNs expressing ApTrk-mem increased the intensity of calcium orange fluorescence compared with control ligand, F(1, 13) = 5.61, P < 0.05 (Fig. 5C). These results suggest that in addition to activating PKC, activation of presynaptic ApTrk receptors produces an increase in presynaptic Ca2+. The Ca2+ may in turn activate an adenyl cyclase, leading to the observed activation of PKA (47). Like the ApNTpHluorin signal (Fig. 4A), the Ca2+ signal gradually increased over time, F(3, 39) = 3.74, P < 0.05 for the interaction of BB dimers and time.
ApTrk Receptor Signaling in the Presynaptic SN Activates MAPK and Protein Synthesis That Build Up over Time.
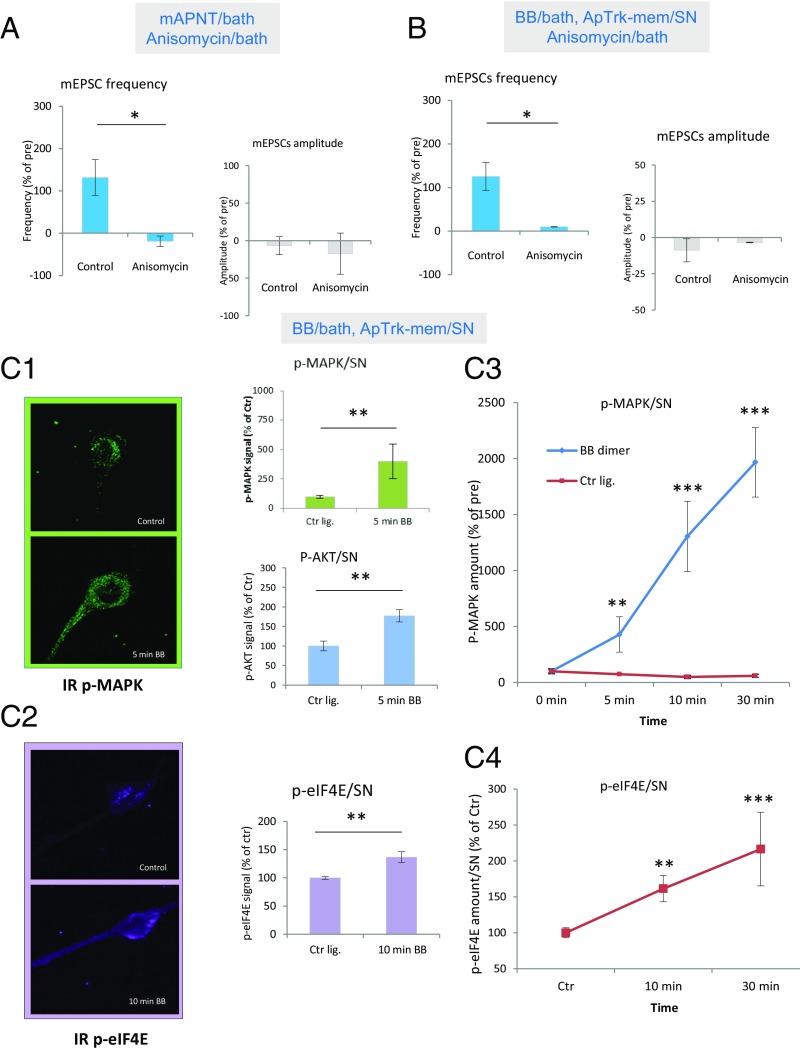
In vertebrate systems, BDNF/TrkB signaling regulates protein synthesis during long-term synaptic plasticity (27, 48). We therefore investigated whether ApNT and ApTrk signaling also act through protein synthesis during the induction of ITF. Bath application of the protein synthesis inhibitor anisomycin reduced the increase in the frequency of mEPSCs produced by mApNT compared with control, t(11) = 3.0672, P < 0.05, without a clear effect on mEPSC amplitude (P > 0.05) or pretest values (Fig. 6A). Anisomycin also significantly reduced the increase in the frequency of mEPSCs produced by bath application of BB dimers to cocultures with SNs expressing ApTrk-mem compared with control, t(10) = 2.433, P < 0.05 (Fig. 6B), without a clear effect on mEPSC amplitude (P > 0.05) or pretest values. These results suggest that ApNT and ApTrk in the presynaptic neuron act in part by regulating protein synthesis during the transition from STF to ITF.
Fig. 6.
Protein synthesis is downstream of ApNT and ApTrk in the SN during the induction of ITF. (A) Protein synthesis is required for the induction of ITF by mApNT. Bath application of anisomycin (n = 4) significantly reduced the increase in the frequency of mEPSCs produced by mApNT, compared with control (n = 9) (P < 0.05), without a clear effect on mEPSC amplitude (P > 0.05). The average pretest mEPSC values were 11.9 min−1 for frequency and 9.6 pA for amplitude, not significantly different between the anisomycin and control groups. (B) Protein synthesis is also required for the induction of ITF by activation of presynaptic ApTrk receptors. Bath application of anisomycin (n = 8) significantly reduced the increase in the frequency of mEPSCs produced by application of BB dimmers to cocultures with SNs expressing ApTrk-mem, compared with control (n = 4) (P < 0.05), without a clear effect on mEPSC amplitude (P > 0.05). The average pretest mEPSC values were 10.6 min−1 for frequency and 7.2 pA for amplitude, not significantly different between the anisomycin and control groups. (C1–C4) Activation of ApTrk receptors on the presynaptic neuron activates MAPK, AKT, and elF4E signaling pathways in the presynaptic neuron. (C1) Bath application of BB ligands to cocultures with SNs expressing ApTrk-mem (n = 4) significantly increased immunostaining for phospho-MAPK (p-MAPK) in the presynaptic SN, compared with control ligand (n = 9) (P < 0.01). BB dimer application (n = 4) also significantly increased immunostaining for phospho-AKT (p-AKT) in the presynaptic neuron, compared with control (n = 7) (P < 0.01). (C2) Activation of ApTrk receptors in the presynaptic neuron increased immunostaining for phospho-eIF4E (p-eIF4E), a major translational factor, in the presynaptic neuron. Bath application of BB dimers to cocultures with SNs expressing ApTrk-mem (n = 13) significantly increased immunostaining for p-eIF4E in the presynaptic SN, compared with control (n = 12) (P < 0.01). (C3 and C4) The immunostaining for both p-MAPK and p-eIF4E gradually increased over time.
In vertebrates, BDNF/TrkB signaling regulates protein synthesis through activation of two major signaling pathways downstream of TrkB: MAPK and PI3K/AKT (28). We investigated whether activation of ApTrk receptors in the presynaptic neuron also activates both MAPK and AKT signaling during the induction of ITF (Fig. 6C1). We applied BB dimers or control ligand for 5 min to cocultures with SNs expressing ApTrk-mem and found a significant increase in immunostaining in the SN with an antibody against phospho-MAPK, t(11) = 3.199, P < 0.01. Activation of presynaptic ApTrk receptor signaling also significantly increased immunostaining in the SN with an antibody against phospho-AKT, t(11) = 3.756, P < 0.01. These results suggest that ApTrk could regulate protein synthesis in the presynaptic neuron through activation of MAPK and AKT.
We next checked whether the eukaryotic translation initiation factor 4E (eIF4E) is also downstream of ApTrk receptors, similar to TrkB receptors (49, 50). eIF4E directs ribosomes to the 5′-cap structure of mRNAs and is the rate-limiting component of the eukaryotic translation apparatus. Almost all cellular mRNA requires eIF4E to be translated into protein. Ten-min activation of ApTrk signaling in the presynaptic neuron significantly increased the signal intensity of immunostaining with an antibody against p-eIF4E in the presynaptic neuron, compared with control, t(23) = 3.0292, P < 0.01 (Fig. 6C2). These results suggest that ApTrk in the presynaptic neuron regulates protein synthesis through activation of p-eIF4E in the presynaptic neuron.
When we repeated these measurements after 0-, 5-, 10-, and 30-min exposure to BB dimers, there was a progressive increase in the signal intensity of immunostaining with an antibody against p-MAPK in the BB group compared with control, with a significant BB Dimer × Time interaction, F(3, 50) = 31.20, P < 0.001, and significant increases at 10 min and 30 min (P < 0.001 in each case) compared with control (Fig. 6C3). There was similarly a progressive increase in the signal intensity of immunostaining with an antibody against p-elF4E, with a significant BB Dimer × Time interaction, F(2, 62) = 3.06, P < 0.05, and significant increases at 10 min and 30 min (P < 0.001) compared with control (Fig. 6C4).
Discussion
Consolidation of memory, the transformation of a labile STM into stable LTM via ITM, is one of the key processes in memory storage. In most systems where it has been studied, STM involves mechanisms on either the pre- or postsynaptic side of the synapse, whereas ITM and LTM involve mechanisms on both sides. These results imply that there must be extracellular signaling during the transitions from STM to ITM and LTM. However, that signaling is poorly understood, in part because of the complexity of most learning-related neuronal systems. To address this problem, we have investigated the signaling in an extremely simple learning-related system, facilitation induced by 5HT at Aplysia sensorimotor synapses of the gill-withdrawal reflex in isolated cell culture. This system also has many technical advantages for a detailed cellular and molecular analysis. Nonetheless, the results are likely to have generality to more complex mammalian systems (4) and ultimately to human disease. As a further simplification, we have initially focused on the transition from STF to ITF, in an attempt to work out the first steps in what we believe to be a complex cascade of back-and-forth signaling ultimately leading to the persistence of LTF (7).
We have previously found that whereas STF appears to be entirely presynaptic, ITF involves both pre- and postsynaptic mechanisms, and spontaneous release of glutamate acts as an anterograde signal to recruit some of the postsynaptic mechanisms (7, 16, 21). We have now investigated the roles of a BDNF-like neurotrophin (ApNT) and its Trk-like receptor (ApTrk) and have found that they also play critical roles in the transition from STF to ITF, adding to the growing list of studies in which neurotrophins have been found to play important roles in learning-related synaptic plasticity (25–27, 51). In those studies, BDNF was found to act as either an anterograde, retrograde, or autocrine messenger, and its exact role has remained controversial. Using a system with only a single presynaptic SN and a single postsynaptic MN, we have been able to address this question rigorously and have found that during the transition from STF to ITF, ApNT can have all three messenger roles: as a presynaptic autocrine messenger, an anterograde messenger, and a retrograde messenger. In this first of two papers, we have focused on the presynaptic autocrine role and have found that ApNT forms part of a positive feedback loop with ApTrk and PKA, which has a number of unexpected properties and consequences. In the following paper (35), we present evidence for anterograde and retrograde signaling by ApNT and show that the positive feedback loop includes transynaptic signaling involving ApNT and ApTrk in the postsynaptic neuron as well.
Presynaptic Autocrine Signaling.
Our results indicate that ApNT acts as a presynaptic autocrine signal during the transition from STF to ITF, and it may also do so during the transition from ITF to LTF (22). Two other growth factor-like molecules are also thought to act as presynaptic autocrine signals during the induction of LTF by 5HT in Aplysia: sensorin (52) and TGF-beta (53). These findings raise the question, Why are these peptides released into the extracellular space only to act back on receptors on the same presynaptic cell, when an intracellular signaling pathway would seem more efficient?
One possible answer concerns the downstream signaling pathways. ApNT, sensorin, and TGF-beta all activate Trk-like receptors that stimulate MAPK and other pathways that are critical for long-term synaptic plasticity and growth (52, 54, 55). It is possible that presynaptic autoreceptors are the best way to activate these signaling pathways, perhaps as a holdover from development (53). Another possibility is that autoreceptors enable positive feedback, which provides signal amplification. A third possibility is that in addition to activating presynaptic autoreceptors, ApNT may spread more widely and activate presynaptic receptors on neighboring SNs (paracrine signaling) or postsynaptic receptors on the MN (anterograde signaling). These possibilities are not mutually exclusive, and indeed, we have obtained evidence supporting all three.
A Presynaptic Positive Feedback Loop.
Our results suggest that a positive feedback loop, which involves PKA, ApNT, and ApTrk receptors in the presynaptic neuron of Aplysia sensorimotor neuron synapses, develops during the transition from STF to ITF produced by 5HT and plays essential roles in that transition (Fig. 7). Such positive feedback is general across species and during various cellular processes including development, survival, and plasticity in the adult brain (39, 56–58). In Aplysia, this feedback drives spontaneous neurotransmitter release from the presynaptic neuron, leading to recruitment of postsynaptic mechanisms and, in addition, activates protein synthesis in the presynaptic neuron, two major requirements that distinguish ITF from STF (16).
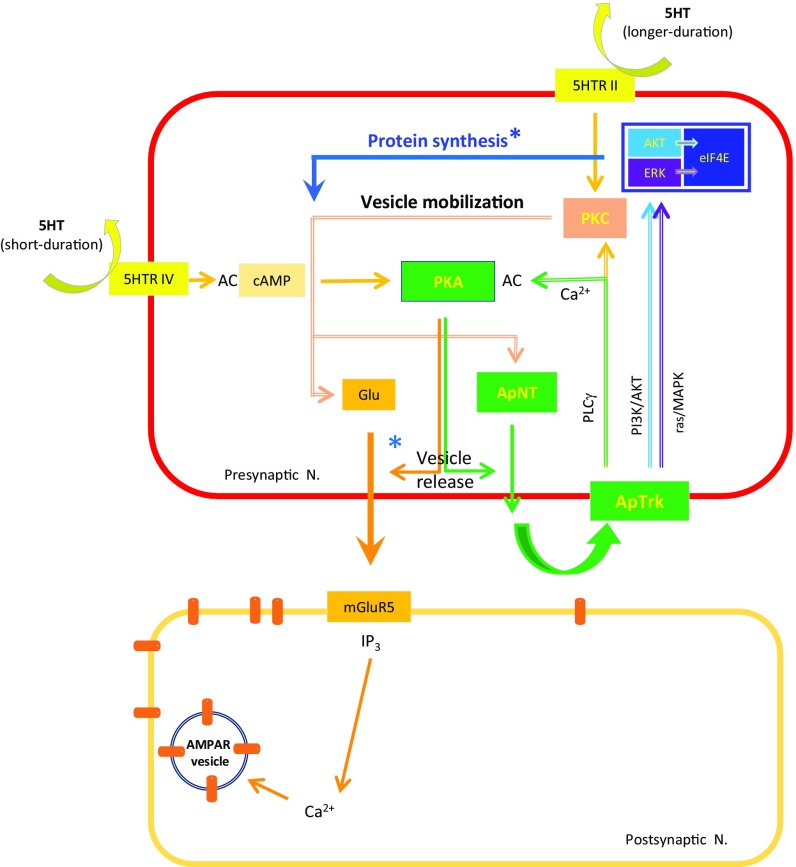
Fig. 7.
Presynaptic signaling pathways involved in the transition from STF to ITF. The induction of ITF involves presynaptic autocrine signaling by ApNT, which forms a presynaptic positive feedback loop with ApTrk receptors and PKA (pre-PKA → pre-ApNT → pre-ApTrk → pre-PKA). This feedback loop enhances spontaneous release of glutamate, which activates mGluR5 receptors and recruits postsynaptic mechanisms, and also stimulates protein synthesis in the presynaptic neuron, both of which are necessary for the transition to ITF. *Protein synthesis is thought to replenish the reserve vesicle pool and other synaptic proteins that support release of neurotransmitters from the presynaptic neuron.
The Feedback Loop Maintains the Release of Glutamate.
The feedback loop maintains the spontaneous release of glutamate during the transition from STF to ITF. The releasable pool of vesicles can be depleted, so refilling the pool through vesicle mobilization is necessary (59). In Aplysia, PKC acting downstream of 5-HT receptors in the SN is thought to play that role, and the contribution of PKC becomes progressively more important as 5HT stimulation continues (42, 44). Our data suggest that PKC is also downstream of ApTrk receptors in the presynaptic neuron (Fig. 5A2). Furthermore, MAPK, which has been suggested to regulate neurotransmitter release through modulation of synapsins (60–62), is also downstream to ApTrk receptors in the presynaptic neuron (Fig. 6C). These results suggest that the positive feedback loop supports recruitment of postsynaptic mechanisms by maintaining the release of glutamate through vesicle mobilization in the presynaptic neuron.
The Feedback Loop Activates Protein Synthesis in the Presynaptic Neuron.
As the induction of the ITF continues, however, even the reserve vesicle pool and other synaptic proteins that support release of neurotransmitter from the presynaptic neuron need to be replenished (26, 60, 63, 64). In addition to transport of proteins from the cell body to the synapse (65), activation of local mRNAs supplies new proteins at the terminal (66, 67). In vertebrates, BDNF stimulates protein synthesis via activation of PI3K/Akt and Raf/MAPK, two major signaling pathways of TrkB receptors (28, 68). PI3K activates translation through mTOR, and MAPK enhances protein synthesis-dependent plasticity (69). Acting in a similar fashion, ApNT released from the presynaptic neuron stimulates protein synthesis back in the presynaptic neuron through activation of PI3K/Akt as well as raf/MAPK signaling downstream of presynaptic ApTrk receptors (Fig. 6). As a result, the feedback loop can continue to enhance transmitter release during the transition from STF to ITF.
The Feedback Loop May also Contribute to the Transition from ITF to LTF Produced by 5HT.
One additional step required for the transition to LTF is the activation of transcription, which requires translocation into the nucleus of both MAPK and PKA. Some transcription factors such as CREB2 and C/EBP require MAPK activity, whereas other transcription factors such as CREB1 require PKA-dependent phosphorylation (70). As we have already discussed, both PKA and MAPK are downstream to ApTrk receptor activation in the presynaptic neuron (Figs. 5 and 6). Furthermore, the feedback loop could also contribute to the persistence of synaptic plasticity and memory by sustaining translation and transcription (25).
The Feedback Loop Can Be Self-Amplifying.
We have found that two molecules in the presynaptic positive feedback loop (ApNT and Ca2+) and two molecules downstream of it (p-MAPK and p-eIF4E) build up over time during ITF (Figs. 4A, 5C, and 6C). These results are consistent with self-amplification by the positive feedback loop, which may be necessary during the transitions from STF to ITF, then to LTF produced by 5HT at the sensorimotor neuron synapses. These transitions involve several thresholds. First, 5HT stimulation of its receptor (5HTR4) needs to be sufficient to activate PKA in the presynaptic neuron during the induction of STF (42). Second, spontaneous release of glutamate from the presynaptic neuron needs to be sufficient to recruit postsynaptic mechanisms of the facilitation during the induction of ITF. In addition, three major signaling pathways downstream of ApTrk receptors—PLCγ, PI3K, and MAPK—need to be activated sufficiently to recruit PKA, PKC, and protein synthesis in the presynaptic neuron. The self-amplifying property of the positive feedback loop may make it possible to overcome these thresholds and to sustain the molecular changes necessary for ITF. This self-amplifying property may also contribute to producing sufficient PKA and MAPK for translocation into the nucleus during the induction of LTF (70) and could, in addition, contribute to the long-term maintenance of plasticity, perhaps by acting cooperatively with memory molecules such as PKMζ (71–75) or CPEB (48, 76, 77) in the pre- or postsynaptic neuron.
Materials and Methods
The methods for cell culture preparation, miniature EPSC (mEPSC) and evoked excitatory postsynaptic potential (eEPSP) recording, expression of constructs for fluorescent fusion proteins, the hybrid system for activating pre- or postsynaptic ApTrk receptors, immunocytochemistry, and statistical analysis were generally as described previously (6, 70, 78, 79). See SI Appendix, Supplementary Methods for details.
Supplementary Material
Acknowledgments
We thank Dr. Honggang Wang for help with patch clamping and Dr. Stylianos Kosmidis for help in immunostaining. This work is supported by grants from the Howard Hughes Medical Institute (to E.R.K.) and National Institute of Health Grant NS083690 (to R.D.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810649115/-/DCSupplemental.
References
- 1.Rosenzweig MR, Bennett EL, Colombo PJ, Lee DW, Serrano PA. Short-term, intermediate-term, and long-term memories. Behav Brain Res. 1993;57:193–198. doi: 10.1016/0166-4328(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 2.Raymond CR. LTP forms 1, 2 and 3: Different mechanisms for the “long” in long-term potentiation. Trends Neurosci. 2007;30:167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins RD, Mayford MR, Kandel ER. A comparative analysis of the molecular mechanisms contributing to implicit and explicit memory storage in Aplysia and in the hippocampus. In: Byrne JH, editor. Learning and Memory: A Comprehensive Reference. 2nd Ed. Elsevier; Oxford: 2016. pp. 5–32. [Google Scholar]
- 5.Rayport SG, Schacher S. Synaptic plasticity in vitro: Cell culture of identified Aplysia neurons mediating short-term habituation and sensitization. J Neurosci. 1986;6:759–763. doi: 10.1523/JNEUROSCI.06-03-00759.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaang BK. Parameters influencing ectopic gene expression in Aplysia neurons. Neurosci Lett. 1996;221:29–32. doi: 10.1016/s0304-3940(96)13279-1. [DOI] [PubMed] [Google Scholar]
- 7.Jin I, et al. Spontaneous transmitter release recruits postsynaptic mechanisms of long-term and intermediate-term facilitation in Aplysia. Proc Natl Acad Sci USA. 2012;109:9137–9142. doi: 10.1073/pnas.1206846109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller U, Carew TJ. Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron. 1998;21:1423–1434. doi: 10.1016/s0896-6273(00)80660-1. [DOI] [PubMed] [Google Scholar]
- 9.Byrne JH, Hawkins RD. Nonassociative learning in invertebrates. Cold Spring Harb Perspect Biol. 2015;7:a021675. doi: 10.1101/cshperspect.a021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of Aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, et al. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron. 2003;40:151–165. doi: 10.1016/s0896-6273(03)00595-6. [DOI] [PubMed] [Google Scholar]
- 12.Emptage NJ, Carew TJ. Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science. 1993;262:253–256. doi: 10.1126/science.8211146. [DOI] [PubMed] [Google Scholar]
- 13.Jin I, Kandel ER, Hawkins RD. Pre- and postsynaptic mechanisms of facilitation at Aplysia sensory-motor synapses: Time and state dependence revisited. Soc Neurosci Abstr. 2004:515.6. [Google Scholar]
- 14.Li Q, Roberts AC, Glanzman DL. Synaptic facilitation and behavioral dishabituation in Aplysia: Dependence on release of Ca2+ from postsynaptic intracellular stores, postsynaptic exocytosis, and modulation of postsynaptic AMPA receptor efficacy. J Neurosci. 2005;25:5623–5637. doi: 10.1523/JNEUROSCI.5305-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonov I, Kandel ER, Hawkins RD. Presynaptic and postsynaptic mechanisms of synaptic plasticity and metaplasticity during intermediate-term memory formation in Aplysia. J Neurosci. 2010;30:5781–5791. doi: 10.1523/JNEUROSCI.4947-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin I, Kandel ER, Hawkins RD. Whereas short-term facilitation is presynaptic, intermediate-term facilitation involves both presynaptic and postsynaptic protein kinases and protein synthesis. Learn Mem. 2011;18:96–102. doi: 10.1101/lm.1949711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montarolo PG, et al. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- 18.Glanzman DL, Kandel ER, Schacher S. Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science. 1990;249:799–802. doi: 10.1126/science.2389145. [DOI] [PubMed] [Google Scholar]
- 19.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 20.Hu JY, Levine A, Sung YJ, Schacher S. cJun and CREB2 in the postsynaptic neuron contribute to persistent long-term facilitation at a behaviorally relevant synapse. J Neurosci. 2015;35:386–395. doi: 10.1523/JNEUROSCI.3284-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin I, et al. Spontaneous transmitter release is critical for the induction of long-term and intermediate-term facilitation in Aplysia. Proc Natl Acad Sci USA. 2012;109:9131–9136. doi: 10.1073/pnas.1206914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassabov SR, et al. A single Aplysia neurotrophin mediates synaptic facilitation via differentially processed isoforms. Cell Rep. 2013;3:1213–1227. doi: 10.1016/j.celrep.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- 24.Klein R. Role of neurotrophins in mouse neuronal development. FASEB J. 1994;8:738–744. doi: 10.1096/fasebj.8.10.8050673. [DOI] [PubMed] [Google Scholar]
- 25.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Leal G, Afonso PM, Salazar IL, Duarte CB. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015;1621:82–101. doi: 10.1016/j.brainres.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Christian K, Lu B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinet C, Pellerin L. Brain-derived neurotrophic factor enhances the expression of the monocarboxylate transporter 2 through translational activation in mouse cultured cortical neurons. J Cereb Blood Flow Metab. 2010;30:286–298. doi: 10.1038/jcbfm.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos AR, Comprido D, Duarte CB. Regulation of local translation at the synapse by BDNF. Prog Neurobiol. 2010;92:505–516. doi: 10.1016/j.pneurobio.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Zakharenko SS, et al. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- 31.Jia Y, Gall CM, Lynch G. Presynaptic BDNF promotes postsynaptic long-term potentiation in the dorsal striatum. J Neurosci. 2010;30:14440–14445. doi: 10.1523/JNEUROSCI.3310-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng YR, et al. Postsynaptic spiking homeostatically induces cell-autonomous regulation of inhibitory inputs via retrograde signaling. J Neurosci. 2010;30:16220–16231. doi: 10.1523/JNEUROSCI.3085-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harward SC, et al. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016;538:99–103. doi: 10.1038/nature19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 35.Jin I, et al. Anterograde and retrograde signaling by an Aplysia neurotrophin forms a trans-synaptic functional unit. Proc Natl Acad Sci USA. 2018;115:E10951–E10960. doi: 10.1073/pnas.1810650115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shinoda Y, et al. BDNF enhances spontaneous and activity-dependent neurotransmitter release at excitatory terminals but not at inhibitory terminals in hippocampal neurons. Front Synaptic Neurosci. 2014;6:27. doi: 10.3389/fnsyn.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashby MC, Ibaraki K, Henley JM. It’s green outside: Tracking cell surface proteins with pH-sensitive GFP. Trends Neurosci. 2004;27:257–261. doi: 10.1016/j.tins.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Cheng PL, et al. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci USA. 2011;108:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang DJ, et al. Activation of a heterologously expressed octopamine receptor coupled only to adenylyl cyclase produces all the features of presynaptic facilitation in aplysia sensory neurons. Proc Natl Acad Sci USA. 2000;97:1829–1834. doi: 10.1073/pnas.97.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrne JH, Kandel ER. Presynaptic facilitation revisited: State and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagakura I, et al. Regulation of protein kinase C Apl II by serotonin receptors in Aplysia. J Neurochem. 2010;115:994–1006. doi: 10.1111/j.1471-4159.2010.06986.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhou L, Baxter DA, Byrne JH. Contribution of PKC to the maintenance of 5-HT-induced short-term facilitation at sensorimotor synapses of Aplysia. J Neurophysiol. 2014;112:1936–1949. doi: 10.1152/jn.00577.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshii A, et al. TrkB and protein kinase Mζ regulate synaptic localization of PSD-95 in developing cortex. J Neurosci. 2011;31:11894–11904. doi: 10.1523/JNEUROSCI.2190-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melemedjian OK, et al. BDNF regulates atypical PKC at spinal synapses to initiate and maintain a centralized chronic pain state. Mol Pain. 2013;9:12. doi: 10.1186/1744-8069-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yovell Y, Kandel ER, Dudai Y, Abrams TW. A quantitative study of the Ca2+/calmodulin sensitivity of adenylyl cyclase in Aplysia, Drosophila, and rat. J Neurochem. 1992;59:1736–1744. doi: 10.1111/j.1471-4159.1992.tb11005.x. [DOI] [PubMed] [Google Scholar]
- 48.Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76:639–656. doi: 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Robinet C, Pellerin L. Brain-derived neurotrophic factor enhances the hippocampal expression of key postsynaptic proteins in vivo including the monocarboxylate transporter MCT2. Neuroscience. 2011;192:155–163. doi: 10.1016/j.neuroscience.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 50.Genheden M, et al. BDNF stimulation of protein synthesis in cortical neurons requires the MAP kinase-interacting kinase MNK1. J Neurosci. 2015;35:972–984. doi: 10.1523/JNEUROSCI.2641-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: On the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu JY, Glickman L, Wu F, Schacher S. Serotonin regulates the secretion and autocrine action of a neuropeptide to activate MAPK required for long-term facilitation in Aplysia. Neuron. 2004;43:373–385. doi: 10.1016/j.neuron.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Zhang F, Endo S, Cleary LJ, Eskin A, Byrne JH. Role of transforming growth factor-beta in long-term synaptic facilitation in Aplysia. Science. 1997;275:1318–1320. doi: 10.1126/science.275.5304.1318. [DOI] [PubMed] [Google Scholar]
- 54.Chin J, Liu RY, Cleary LJ, Eskin A, Byrne JH. TGF-beta1-induced long-term changes in neuronal excitability in aplysia sensory neurons depend on MAPK. J Neurophysiol. 2006;95:3286–3290. doi: 10.1152/jn.00770.2005. [DOI] [PubMed] [Google Scholar]
- 55.Shobe J, Philips GT, Carew TJ. Transforming growth factor β recruits persistent MAPK signaling to regulate long-term memory consolidation in Aplysia californica. Learn Mem. 2016;23:182–188. doi: 10.1101/lm.040915.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acheson A, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 57.Vicario-Abejón C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci. 2002;3:965–974. doi: 10.1038/nrn988. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, et al. Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell Physiol Biochem. 2014;34:715–723. doi: 10.1159/000363036. [DOI] [PubMed] [Google Scholar]
- 59.Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- 60.Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- 61.Chi P, Greengard P, Ryan TA. Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci. 2001;4:1187–1193. doi: 10.1038/nn756. [DOI] [PubMed] [Google Scholar]
- 62.Angers A, et al. Serotonin stimulates phosphorylation of Aplysia synapsin and alters its subcellular distribution in sensory neurons. J Neurosci. 2002;22:5412–5422. doi: 10.1523/JNEUROSCI.22-13-05412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreira A, Kosik KS, Greengard P, Han HQ. Aberrant neurites and synaptic vesicle protein deficiency in synapsin II-depleted neurons. Science. 1994;264:977–979. doi: 10.1126/science.8178158. [DOI] [PubMed] [Google Scholar]
- 64.Fioravante D, Liu RY, Netek AK, Cleary LJ, Byrne JH. Synapsin regulates basal synaptic strength, synaptic depression, and serotonin-induced facilitation of sensorimotor synapses in Aplysia. J Neurophysiol. 2007;98:3568–3580. doi: 10.1152/jn.00604.2007. [DOI] [PubMed] [Google Scholar]
- 65.Puthanveettil SV, et al. A new component in synaptic plasticity: Upregulation of kinesin in the neurons of the gill-withdrawal reflex. Cell. 2008;135:960–973. doi: 10.1016/j.cell.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin KC, Barad M, Kandel ER. Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol. 2000;10:587–592. doi: 10.1016/s0959-4388(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 67.Kim E, Jung H. Local protein synthesis in neuronal axons: Why and how we study. BMB Rep. 2015;48:139–146. doi: 10.5483/BMBRep.2015.48.3.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pérez-Pérez A, et al. MAPK and PI3K activities are required for leptin stimulation of protein synthesis in human trophoblastic cells. Biochem Biophys Res Commun. 2010;396:956–960. doi: 10.1016/j.bbrc.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 69.Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin KC, et al. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 71.Sacktor TC, et al. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ling DS, Benardo LS, Sacktor TC. Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- 73.Mei F, Nagappan G, Ke Y, Sacktor TC, Lu B. BDNF facilitates L-LTP maintenance in the absence of protein synthesis through PKMζ. PLoS One. 2011;6:e21568. doi: 10.1371/journal.pone.0021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai D, Pearce K, Chen S, Glanzman DL. Protein kinase M maintains long-term sensitization and long-term facilitation in aplysia. J Neurosci. 2011;31:6421–6431. doi: 10.1523/JNEUROSCI.4744-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu J, et al. Cell-specific PKM isoforms contribute to the maintenance of different forms of persistent long-term synaptic plasticity. J Neurosci. 2017;37:2746–2763. doi: 10.1523/JNEUROSCI.2805-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Si K, et al. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell. 2003;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- 77.Oe S, Yoneda Y. Cytoplasmic polyadenylation element-like sequences are involved in dendritic targeting of BDNF mRNA in hippocampal neurons. FEBS Lett. 2010;584:3424–3430. doi: 10.1016/j.febslet.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 78.Schacher S. Differential synapse formation and neurite outgrowth at two branches of the metacerebral cell of Aplysia in dissociated cell culture. J Neurosci. 1985;5:2028–2034. doi: 10.1523/JNEUROSCI.05-08-02028.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bao JX, Kandel ER, Hawkins RD. Involvement of pre- and postsynaptic mechanisms in posttetanic potentiation at Aplysia synapses. Science. 1997;275:969–973. doi: 10.1126/science.275.5302.969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.