Abstract
Novel polyfunctional small amphiphilic peptide dendrimers characterized by incorporation of a new core compounds – tris-amino acids or tetrakis-amino alcohols that originated from a series of basic amino acids – were efficiently synthesized. These new core elements yielded molecules with multiple branching and (+5)/(+6) charge at the 1-st dendrimer generation. Dendrimers exhibited significant antimicrobial potency against Gram(+) and Gram(-) strains involving also multiresistant reference strains (S. aureus ATCC 43300 and E. coli ATCC BAA-198). In addition, high activity against fungi from the Candida genus was detected. More charged and more hydrophobic peptide dendrimers expressed hemolytic properties.
Keywords: dendrimeric peptides, antimicrobial, antibiotic resistance
1. Introduction
The rapid emergence of multi-drug resistance against conventional antibiotics is one of the greatest challenges of modern medical science. In places like health care units, the food industry, dental or implant materials that are confronted every day with microbial invasion new microbicides and disinfectants are an urgent need. Public awareness of the slow discovery of novel antibiotics practiced by pharmaceutical companies during last decades has recently prompted a vigorous search for complementary and alternative antimicrobial medicines characterized by new structure(s) and acting on new cellular targets [1]. Nanomedicine is one of the very promising research directions in designing novel multifunctional materials that offer better therapeutic properties in many areas of medicine, including the treatment of microbial infections [2].
Dendrimers - synthetic macromolecules of nanoscopic dimensions built from several layers of branches located around a central core [3,4,5] are among several groups of macromolecules that are considered as promising nanopharmaceuticals with high potential. The multivalent nature of these compounds, their unambiguous composition, reliability and versatility of their synthesis, make this type of carriers well-suited in various applications for diagnostic purposes, [6] protein mimetics, [7] antiviral agents, [8,9] vaccines, [10,11] versatile prospective drugs [12,13] and gene delivery vehicles [14]. The problem of designing antimicrobial compounds emerged in the early days of dendrimer chemistry. Cationic dendrimers with terminal ammonium groups originated from PAMAM [15], PPI [16], or carbosilane [17] dendrimers have been shown to affect the integrity of negatively charged bacterial membranes resulting in high bacteriostatic and bactericidal potency. PAMAM dendrimers have been used as carriers of quinolone [18] and penicillin V [19] antibiotics with improved bioavailability or as containers for biocidal silver nanoparticles [20].
Natural antimicrobial peptides (AMPs) constitute another groups of macromolecules that are considered a good source of new strategies and new nanopharmaceuticals [21]. Their structural diversity and mechanism of action (modification of the membrane permeability), which differs from that of the conventional antibiotics, makes them an interesting alternative to classic antibiotics. The majority of natural AMPs contain a 10-50 amino acids sequence that includes several basic amino acids (Lys, Arg) and up to 60% of these with lipophilic side chains (Phe, Tyr, Ala, etc.). Such a composition provides a positive charge and enables formation of amphipatic structure(s) essential for interactions with microbial membranes.
Intensive research on economically feasible smaller analogs has allowed the introduction of several compounds into clinical trials [1,22]. Based on these natural compounds Tam and co-workers designed lysine (Lys) dendrimers that have been used as carriers of two to eight copies of tetra- or octapeptide fragments of the antimicrobial peptide tachyplesin. The obtained dendrimers expressed high potency against broad range of microorganisms while being at the same concentration less toxic than natural precursors [23].
An interesting novel application of dendrimers designed to act as new microbial targets has been reviewed recently by Rojo and Deldgado [24 and references cited therein]. These authors summarized the work of several groups focused on the synthesis of dendrimers carrying multiple copies of polysaccharides or lipopolysaccharides [25] and their intended use as infection preventing anti-adhesive agents.
According to our concept of the “non-sequential” pharmacophore [26,27] dendrimer trees can be used not only as carriers of antimicrobial fragments, but also for de novo design of positively charged amphiphilic molecules. This approach allowed for the successful synthesis of a novel class of low molecular mass amphiphilic dendrimeric peptides (tetra- to octapeptides) having considerable antimicrobial activity against Gram(+) and less activity against Gram(-) bacteria with structure-dependent cytotoxicity [28,29]. According to DSC studies, these cationic amphiphilic molecules based on the Lys(Lys)2 scaffold interact stronger with anionic dimirystoyl phosphatidyl glycerol (DMPG)-prepared model multilamelar vesicles that emulate the properties of microbial membranes than with vesicles prepared from neutral phospholipids [30].
These studies confirmed that their selectivity is shifted towards negatively charged microbial ones rather than towards neutral human cell membranes. Although it is generally acknowledged that the structural complexity of biological membranes is a source of the selective response of microorganisms towards antibiotics the last thirty years of intensive research has provided no specific rules relating structure and biological activity of natural peptides. In the case of macromolecular dendrimeric compounds the efficiency of their interactions with heterogenic negatively charged microbial membranes depends on the number and distribution of the charged and hydrophobic groups (polyvalency) and on their structural flexibility, i.e. adaptability of the dendrimer to the dynamic structure of the membrane.
Here we present the synthesis and spectroscopic data for two groups of cationic, amphiphilic peptide dendrimers which utilize novel multibranched scaffolds of amino acid origin. Use of these new building blocks as a core element yielded first generation peptide dendrimers characterized by high charge (+5 or +6) and high polyvalency.
The antimicrobial activity of these multicharged small cationic dendrimers against both Gram(+), Gram(-) bacteria and fungi from the Candida genus was studied and results were compared with the activity of the previously studied less charged and less branched analogs [28]. The obtained results demonstrate that the new small but highly charged, more branched amphiphilic peptide dendrimers express better properties: along with high activity against Gram(+) bacteria, they show significant activity against Gram(-) bacteria and fungi, including antibiotic resistant strains. Hemotoxicity towards human red blood cells for more hydrophobic compounds was detected, however.
2. Results and Discussion
2.1. Synthesis
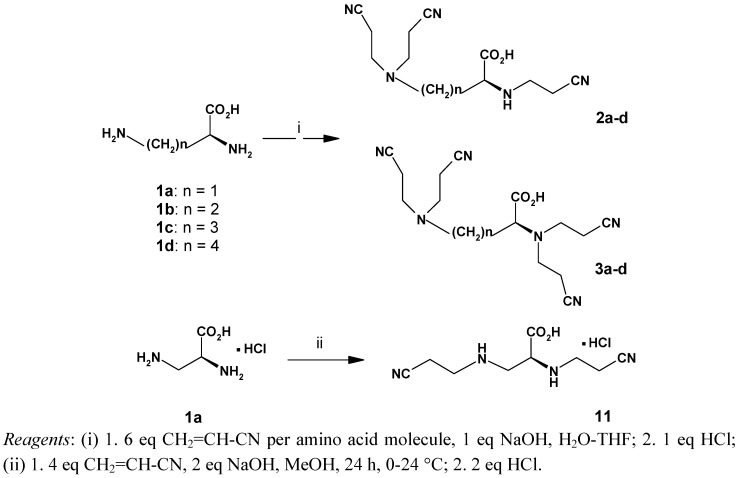
Preparation of the new cores involved cyanoethylation of the respective basic amino acids (Scheme 1), followed by reduction of the nitriles to polyamines. Michael addition of acrylonitrile to polyamines (ethylene- or propylenediamine) and subsequent reduction of nitriles to yield amines is one of the classic routes in the dendrimer chemistry [31]. Condensation of acrylonitrile with several α-amino acids in aqueous solution in the presence of alkali salts, yielding mono-, di-, and tricyanoethyl derivatives was studied by McKinney [32,33].
Scheme 1.
Synthesis of N-cyanoethylated derivatives of 1.
Synthesis of a tris-N-cyanoethyl derivative of lysine in low yield (22.3%) was reported by Riehm and Scheraga without isolation and identification, however, of the second product – the corresponding tetrakis-N-cyanoethyl derivative [34]. In the case of basic amino acids, reaction with acrylonitrile gave a mixture of tris- and tetrakis-N-cyanoethyl derivatives with the ratio depending on the reaction conditions (Scheme 1). For example, the bis-N-cyanoethyl derivative 11 was initially observed as an impurity. However, when the reaction was performed at 0 °C in methanol, using four equivalents of acrylonitrile per amino group, the N,N-bis(cyanoethyl)-α,β-diaminopropionic acid 11 was obtained exclusively. Under the same conditions, the other three amino acids yielded tris-cyanoethyl derivatives as the major products. Most of the reduction procedures of the multiple nitrile groups involve catalytic hydrogenation with the use of the Raney-Ni or Co [35,36]. Unexpectedly, there was a pronounced difference in reactivity between tris- and tetrakis-N-cyanoethyl derivatives.
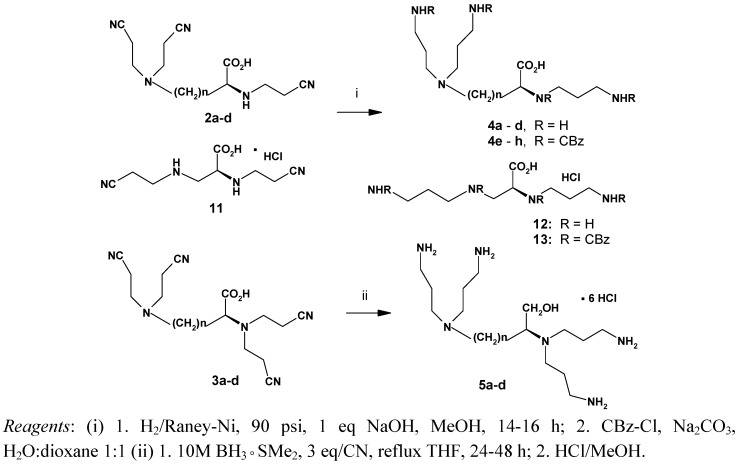
While catalytic reduction of the tris-N-cyanoethyl amino acids 2 in MeOH using Raney-Ni in a Parr hydrogenation apparatus gave the respective N-propylamino derivatives 4 in almost quantitative yields (Scheme 2), reduction of tetrakis-N-cyanoethyl derivatives 3 under the above conditions left 90% of the original substrate unconverted. Finally, a family of compounds 5 was obtained using three equivalents of 10 M borane dimethylsulfide complex per nitrile group with extention of the reaction time to 24-48 h (Scheme 2) [37].
Scheme 2.
Reduction of N-cyanoethylated derivatives 2 and 3.
The above procedures provide a rapid access to new templates for preparation of branched peptides or other dendrimers.
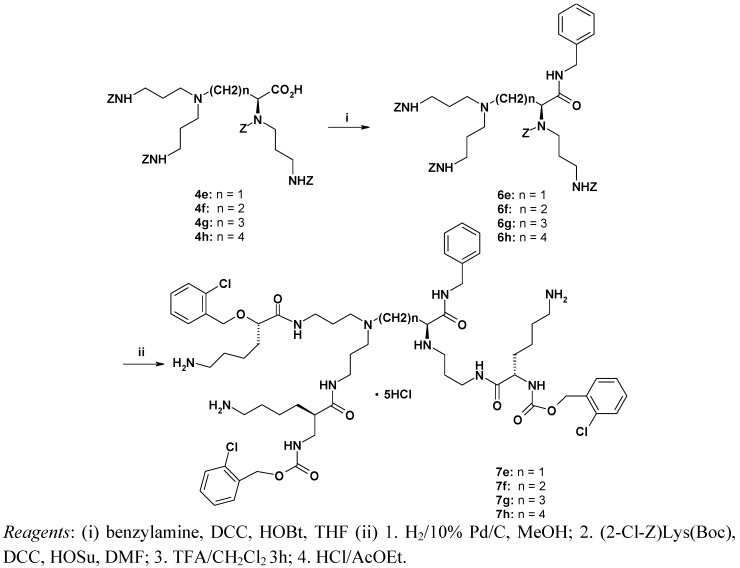
The synthesis of the lysine-functionalized first generation dendrimers 7e-h from the N-protected trimeric scaffolds 4e-h started with coupling with benzylamine, yielding the amides 6e-h. Then the amino group deprotection was followed by coupling with (2-Cl-Z)Lys(Boc) to afford 7e-h in 67-71% overall yield (Scheme 3).
Scheme 3.
Synthesis of the dendrimers 7e-h.
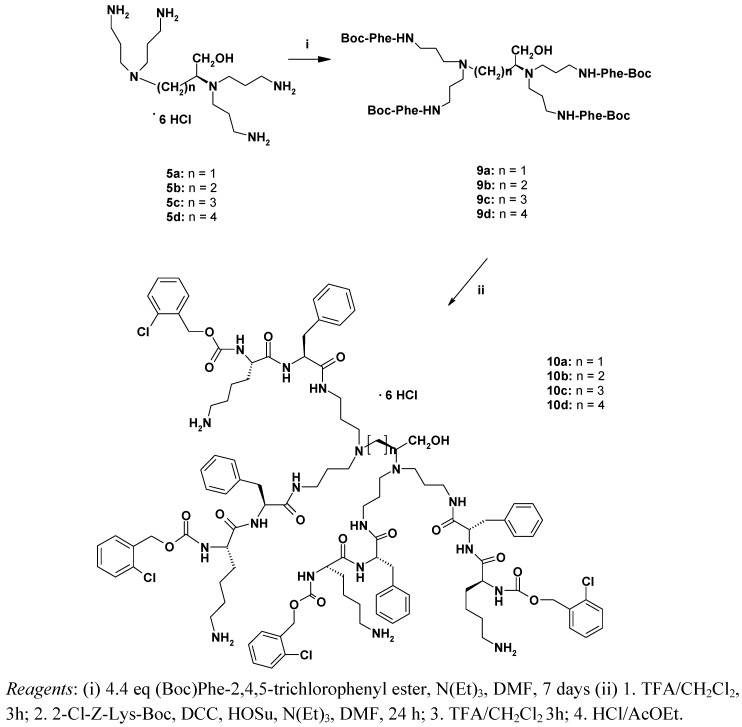
Synthesis of the tetra-functional branched compound using the hexahydrochloride of 5c as a starting material was complicated by the fact that 5a-d are practically insoluble in the commonly used organic solvents. Eventually, they were dissolved in hot DMF (50-60 °C) with addition of a large excess of triethylamine. In this case, coupling with 2,4,5-trichlorophenyl ester of (Boc)Phe was carried out for 7 days affording the compound 9a-d in 54% yield (Scheme 4).
Scheme 4.
Synthesis of the dendrimers 10a-d.
2.1. Antimicrobial and hemolytic activities
The results of in vitro antimicrobial activity of the amphiphilic dendrimers 7e-h, 10a-d and two reference compounds 5c-d assayed against two Gram(+) strains, six Gram(-) strains, and three strains from the Candida genus are shown in Table 1. Due to application in the synthesis 3- or 4-arm cores the families of compounds 7e-h vs. 10a-d are structurally different regarding the relative distribution of cationic and lipophilic groups and net charge. In the compounds 7e-h, 3-arm cores are N-terminated with lysine hydrochloride residues substituted at Nα with 2-chlorobenzyloxycarbonyl- residue (2-Cl-Z) and with a benzyl group located at the C-end (Scheme 3). In the compounds 10a-d, all four arms of the core are elongated with phenylalanine residues (Phe) that are followed by substituted lysine residues similar to those above (Scheme 4). Within each family, compounds have the same net charge - (+5) or (+6) and differ in the number of methylene groups in the core.
Table 1.
Minimal inhibitory concentrations (MICs) of studied dendrimers against Gram(+), Gram(-) and fungi and two reference compounds 5c and 5d (μM). a
| Compound | 7e | 7f | 7g | 7h | 10a | 10b | 10c | 10d | 5c | 5d |
|---|---|---|---|---|---|---|---|---|---|---|
| Scaffold/charge b | DAPc | DAB d | Orn | Lys | DAP | DAB | Orn | Lys | Orn | Lys |
| (+)5 | (+)5 | (+)5 | (+)5 | (+)6 | (+)6 | (+)6 | (+)6 | (+)6 | (+)6 | |
| S. aureus | 2.8 | 11.7 | 10.9 | 21.6 | 1.7 | 1.3 | 27.2 | 3.4 | 113 | 221 |
| ATCC 25923 | ||||||||||
| S. aureus | 11.8 | 10.3 | 10.9 | 21.6 | 3.4 | 7.3 | 13.6 | 1.7 | - | - |
| ATCC 43300 | ||||||||||
| P. aeruginosa | 24.3 | 48 | 21.8 | 86 | 30.2 | 47 | 54 | 14 | - | - |
| ATCC 27853 | ||||||||||
| B. bronchiseptica | 11 | 11 | 2.7 | 5.4 | 3.4 | 3.4 | 6.8 | 1.7 | - | - |
| ATCC 4617 | ||||||||||
| A. baumani | >89 | >88 | 5.4 | >86 | 13.8 | 13.8 | 27.3 | 3.4 | - | - |
| ATCC 19606 | ||||||||||
| K. pneumoniae | >89 | >88 | 87 | >86 | >55 | >55 | 54 | >54 | - | - |
| ATCC 13882 | ||||||||||
| E.coli | 11.8 | 48 | 21.8 | 86 | 7.3 | 7.3 | 27.3 | 6.8 | >226 | >221 |
| ATCC 25922 | ||||||||||
| E. coli | >88 | >89 | 10.9 | 43 | 6.9 | 6.9 | 13.6 | 3.4 | - | - |
| ATCC BAA-198 | ||||||||||
| C. albicans | >88 | >89 | 10.9 | >86 | 6.8 | 6.9 | 54 | 6.8 | - | - |
| ATCC 90028 | ||||||||||
| C. krusei | 44 | 44 | 5.4 | 43 | 6.8 | 6.9 | 27.3 | 3.4 | - | - |
| ATCC 6258 | ||||||||||
| C. parapsilosis | 88 | 89 | 5.4 | 86 | 6.8 | 6.9 | 54 | 3.4 | - | - |
| ATCC 22019 |
a MICs of the reference compounds: Penicillin G against S. aureus ATCC 25923 – 6.6 (μM); polymyxin B against E. coli ATCC 25922 and P. auruginosa ATCC 27853 – 0.55 (μM); amphotericin B against C. krusei ATCC 6258 – 1.1 μM; indolicidin against S. aureus ATCC 25923 – 2.1 (μM) and E. coli ATCC 25922 – 4.2 (μM); b charge assigned by elementary analysis from Cl content; c diaminopropionic acid core; d diaminobutyric acid core.
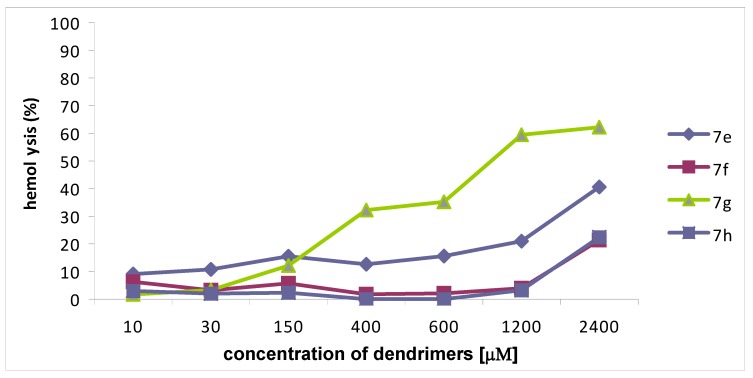
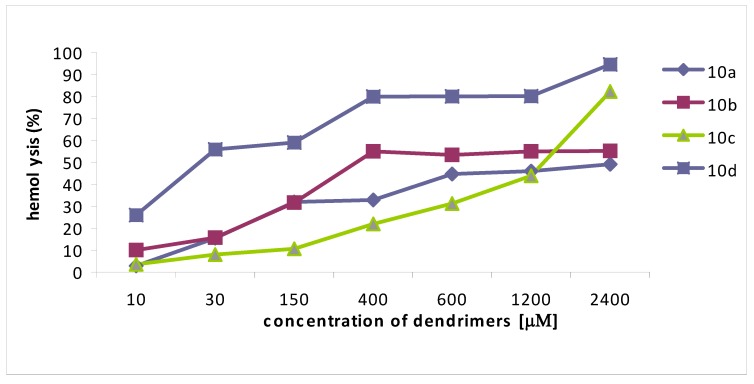
Dendrimers 7e,f,h characterized by the MIC values in the range 2.8-21.6 μM displayed less than 30% hemotoxicity up to a concentration 1,200 μM (extrapolated), whereas dendrimer 7g, exhibited 32% toxicity at a concentration of 400 μM (Figure 1). Compounds of the second series 10a,b,c characterized by MICs in the 1.3-54 μM range displayed 30% hemolysis at 400, 150 and 600 μM concentrations, respectively (Figure 2). Although hemotoxicity of the most potent compound 10d characterized by the MIC values in the range 1.7-14 μM reaches 25% in the region of antimicrobial activity. From this group of compounds the best antimicrobial activity against Candida genus and low hemolytic effect correspond to dendrimer 7g built around the smallest diaminopropionic acid core (DAP), and dendrimer 10a, that presents a similar antimicrobial profile and significantly lower hemotoxicity than compound 10d.
Figure 1.
Hemolytic activity of the three-arm compounds 7e-h.
Figure 2.
Hemolytic activity of the four-arm compounds 10a-d.
Selectivity of molecule-membrane interactions has been the main concern of numerous studies on biomedical applications of both multi-charged antimicrobial peptides [39,40] and dendrimers (PAMAM, PPI, etc.) [41,42]. Multiple positive charge and amphiphatic structure are proposed as a source of higher affinity of natural peptides to microbial versus human membranes [22]. In case of the title peptide dendrimers, the charge comes from the protonated amino groups of lysines (3 or 4) and two protonated ternary N-atoms from the core. Accumulation of positively charged groups in relatively small branched molecules should promote extended “carpet-like” conformations, facilitating multiple electrostatic interactions with microbial membranes composed mainly from acidic phospholipids (phosphatidylglycerol and cardiolipin). In contrast, outer leaflets of mammalian membranes are mainly composed from zwitterionic phospholipids (phosphatidylcholine and sphingomyelin) with only minor contribution of negatively charged species. Low impact of cationic compounds on red blood cells is regarded as an indication of a compound’s selectivity. In the present case, differences in hemolytic properties are observed within compounds belonging to the same series and even more pronounced between structurally different 7e-h and 10a-d series. Four-arm dendrimers 10a-d, [(+6) charge] containing four additional hydrophobic side chains from phenylalanine residues expressed higher antimicrobial potency associated however, with enhancement of hemolytic properties. Similarly, recent data on cationic, amphiphilic polymethacrylates revealed that lower mole fraction of hydrophobic groups is one of the important factors in design of potent, non-hemolytic antimicrobial polymers [43].
The presented data show that designing more charged amphiphilic peptide dendrimers resulted in better antimicrobial properties. It appears however, that an increase of charge from (+5) to (+6) is not sufficient for significant enhancement of antimicrobial potency between 7e-h vs. 10a-d series and for shift in antimicrobial profile. Both, antimicrobial activity and hemolytic properties of dendrimeric peptides depend also on distribution of charged and lipophylic groups, i.e. on structural factors. Unlike many other dendrimer types, dendrimeric peptides constructed from, or terminated with basic amino acids allow to distribute on the surface independently two different types of residues – e.g. charged and lipophilic. Their number, relative sizes and orientations may be varied providing an opportunity to obtain dendrimers with good antimicrobial activity and low toxicity.
3. Experimental
3.1. General
All solvents and reactants were of analytical grade and were used without further purification. Mass spectra were recorded with a Mariner ESI time-of-flight mass spectrometer (PerSeptive Biosystems) for the samples prepared in MeOH. Proton and carbon NMR spectra were recorded using a Bruker Avance spectrometer at 500 or 400 MHz, respectively, using deuterated solvents and TMS as an internal standard. Chemical shifts are reported as δ values in parts per million and coupling constants are given in hertz. The optical rotations were measured with JASCO J-1020 digital polarimeter. Melting points were recorded on a Köfler hot-stage apparatus and are uncorrected. Thin layer chromatography (TLC) was performed on aluminum sheets with silica gel 60 F254 from Merck. Column chromatography (CC) was carried out using silica gel (230-400 mesh) from Merck or Sephadex LH20. The TLC spots were visualized by treatment with 1% alcoholic solutions of ninhydrin and heating.
3.2. Antifungal susceptibility testing
Yeasts: Candida albicans ATCC 90028, Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were cultivated onto Sabouraud dextrose agar (Difco) during 24 hrs at 35 °C.
Medium: RPMI – 1640 (Sigma) supplemented with 0.2% (wt/vol) glucose, 0.3 g/L L-glutamine, 0.0053 g/L phenol red, buffered with morpholinopropanesulfonic acid (MOPS; Sigma) at a final concentration of 0.165 M and adjusted to pH 7.0 was prepared.
Reference Method: The broth microdilution susceptibility test was performed as described in Clinical and Laboratory Standards Institute (CLSI) reference method M27 – A2 (1) [44]. A series of the twofold dendrimers and amphotericin B dilutions in DMSO were diluted 1:49 with RPMI. Aliquots (100 µL0 were dispensed into microdilution sterile plates (Mar-Four). Then, yeast inoculum (100 µL) containing 1 × 103 to 5 × 103 CFU/mL was added to each well. The final concentration of dendrimers ranged from 128 to 2 µg/mL [or 105 to 1.6 μM for an average molecular mass for 7 series (1,213) and 61 to 0.9 μM for an average molecular mass for 10 series (2,103)], and amphotericin B from 1 to 8 µg/mL (1.1 to 8.1 μM, respectively), all in twofold dilution steps. The plates were incubated at 35 °C and read after 24 and 48 hrs. MIC’s (Minimal Inhibitory Concentration) was defined as the lowest drug concentration that reduced growth by 100%.
3.3. Antibacterial susceptibility testing
Bacteria. Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 43300 were cultivated on tryptone - soy agar (TSA; Oxoid). Klebsiella pneumoniaeATCC 13882, Bordetella bronchiseptica ATCC 4617, Acinetobacter baumannii ATCC 19606 were cultivated onto nutrient agar (Difco). Escherichia coli ATCC BAA-198 was cultivated on TSA with ceftazidime (10 µg/mL or 15.7 μM; Sigma). All strains were incubated during 24 hrs at 37 °C.
Medium. Mueller – Hinton Broth (Oxoid) was supplemented with cations: 12.5 mg Mg ++/L and25 mg Ca++/L. The pH of the medium after sterilization is between 7.2 and 7.4 [cation–adjusted Mueller – Hinton Broth” (CAMHB)].
Reference method. Broth microdilution susceptibility test was performed as described in Committee Laboratory Standards (CLSI) reference method M7-A7 (2) [45]. A series of the twofold dendrimers dilutions in DMSO and twofold polymyxin B and penicillin G dilutions in CAMHB were diluted 1: 94 with CAMHB. Aliquots (95 µL) were dispensed into microdilution sterile plates (Mar-Four). Then, bacteria inoculum (5 µL) containing 5 × 104 CFU/mL were added. The final concentration of dendrimers ranged from 128 to 2 µg/mL [or 105 to 1.6 μM for an average molecular mass for 7 series (1213) and 61 to 0.9 μM for an average molecular mass for 10 series(2,103)], polymyxin B and penicillin G from 8 – 0.15 µg /mL (or 5.8 to 0.1 μM and 21.5 to 0.4 μM, respectively for polymyxin B and penicillin G), all in twofold dilution steps. The plates were incubated at 35 °C and read after 18 or 24 hours depending on bacterial strain. MICs (Minimal Inhibitory Concentration) was defined as the lowest drug concentration that reduced growth by 100%.
3.4. Hemolysis assay
Dendrimer induced hemolysis was observed as previously reported [18]. Briefly, serum free human red blood cells obtained from the Institute of Hematology and Transfusion Medicine in Warsaw, were suspended in phosphate buffered saline (PBS, pH 7.4). Prepared suspension of 1% hematocrit was incubated with serial concentration of dendrimers for 30 min at 23 °C. After centrifugation (1,000 rpm, 5 min) the absorbance of supernatant was measured at 540 nm (Jasco 630, Japan). A value of 100% hemolysis was determined by incubation of erythrocytes with double-distilled water (30 min at 23o). In a control experiment, cells were incubated in buffer without peptide and absorbance at 540 nm value was used as a blank.
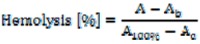 |
A - absorbance of the samples incubated with dendrimers; Ab – absorbance of the blank samples; A100% - absorbance of the reference; Ac - absorbance of red blood cells in PBS, hematocrit 1%.
3.5. Synthesis
3.5.1. Substrate preparation
The monohydrochlorides of L-α,β-diaminopropionic acid (1a) and L-α,γ-diaminobutyric acid (1b) were obtained according to the Rao protocol as white crystals, with the correct melting points, elemental analysis and optical rotation values: α[D]28,5°C = +25 ± 1º and +24 ± 1º (C=2 in 1M HCl) for 1a and 1b, respectively, corresponding to the literature data [46].
To a stirred aqueous solution containing 0.1 mol of a basic amino acid and 0.1 mol of NaOH, an excess of acrylonitrile (0.6 mol) and THF (30-40 mL) was added. The mixture was stirred for 48 h at room temperature and then refluxed for 3 h. To a cold reaction mixture, 0.1 mol of conc. HCl was added and left in refrigerator overnight. The crystalline product was separated and washed with several portions of cold MeOH-H2O (1:1). The washings were collected and evaporated to dryness. Then acetone (150 mL) was added to the oily residue and filtered. Two crops of precipitate were combined and recrystallized from hot water or MeOH-H2O (7:3) giving the tris-N-cyanoethylated amino acids 2a-d as white crystals in 49.5-69.2% yield.
The acetone filtrate was evaporated to dryness and purified by flash chromatography, eluting with an 8:2 mixture of ethyl acetate and hexane plus 3% of methanol, yielding the tetrakis-N-cyanoethylated derivatives 3a-d as amber-colored gums (except Nα,Nβ-tetrakis(cyanoethyl)-L-α,β-diaminopropionic acid, which slowly crystallized to give a white solid) in 21.1-28.6% yield. Both the yields and the 2:3 ratio depend on the reaction temperature. The yield of 3 can reach 50% when the reaction is performed in a boiling mixture of water-methanol (1:1) overnight.
N,N,N’-tris(cyanoethyl)-L-α,β-diaminopropionic acid (2a): Yield: 13.0 g (49.5%); mp 178-180 °C (dec.); ESI MS 264 (M+1), 286 (M+23); 1H-NMR (D2O) δ 2.74 (m, 4H, βCH2CN), 2.97 (m, 2H, αCH2CN), 2.99 (dd, J = 3.96 Hz, 1H, βCHa), 3.05 [m, 4H, Nβ-(CH2)2], 3.15 (dd, J = 3.96 Hz, 1H, βCHb), 3.6 (t, J = 6.96 Hz, 2H, NHα-CH2), 3.9 (dd, J = 3.96 Hz, 1H, αCH); 13C-NMR δ 15.2 (αCH2-CN), 15.4 (βCH2-CN), 42.7 (NHα-CH2), 48.8 [Nβ-(CH2)2], 53.2 (βC), 61.1 (αC), 117.6 (αCN), 121.0 (βCN), 170.9 (COOH); Anal. Calcd for C12H17O2N5: C, 54.7; H, 6.5; N, 26.6. Found: C, 54.53; H, 6.42; N, 26.44; α[D]26.2 °C = +24.3 ± 1° (c = 2 in 1M HCl).
N,N,N’-tris(cyanoethyl)-L-α,γ-diaminobutyric acid (2b): Yield: 17.3 g (62.6%); mp 170-172 °C; ESI MS 278 (M+1), 300 (M+23); 1H-NMR (D2O) δ 2.09 (q, J = 6.55 Hz, 2H, βCH2), 2.73 (m, 6H, CH2CN), 2.93 [m, 4H, Nγ-(CH2)2], 3.03 (t, J = 6.85 Hz, 2H, NHα-CH2), 3.46 (t, J = 6.85 Hz, 2H, γCH2), 3.84 (t, J = 6.08 Hz, 1H, αCH); 13C-NMR δ 15.5 (αCH2-CN), 15.5 (γCH2-CN), 26.9 (βC), 42.7 (NHα-CH2), 48.2 [Nγ-(CH2)2], 50.0 (γC), 62.4 (αC), 117.9 (αCN), 121.2 (γCN), 172.9 (COOH); Anal. Calcd for C13H19O2N5: C, 56.30; H, 6.90; N, 25.25. Found: C, 56.05; H, 6.80; N, 25.20; α[D]27.8°C = +24.2 ° 1° (c = 2 in 1M HCl).
N,N,N’-tris(cyanoethyl)-L-ornithine (2c): Yield: 18.4 g (63.3%); mp 192-194 °C; ESI MS 292 (M+1), 314 (M+23); 1H-NMR (D2O) δ 1.51, 1.92 (2m, 4H, γCH2, βCH2), 2.62 (t, J = 7.28 Hz, 2H, αCH2CN), 2.69 (t, J = 6.65 Hz, 4H, δCH2CN), 2.91 [t, J = 6.73 Hz, 4H, Nδ-(CH2)2], 3.02 (t, 2H, NHα-CH2), 3.42 (m, 2H, δCH2), 3.72 (dd, J = 4.95 Hz, 1H, αCH); 13C-NMR δ 15.3 (αCH2-CN), 15.7 (δCH2-CN), 22.0 (γC), 27.6 (βC), 42.3 (NHα-CH2), 48.5 [Nδ-(CH2)2], 52.3 (δC), 62.9 (αC), 117.9 (αCN), 121.1 (δCN), 173.4 (COOH); Anal. Calcd for C14H21O2N5: C, 57.71; H, 7.26; N, 24.03. Found: C, 57.52; H, 7.28; N, 23.85; α[D]27.5°C = +24.5 ± 1° (c = 2 in 1M HCl).
N,N,N’-tris(cyanoethyl)-L-lysine (2d): Yield: 21.1 g (69.2%); mp 214-216 °C; ESI MS 306 (M+1), 328 (M+23); 1H-NMR (DMSO) δ 1.30-1.38 (bm, 4H, γCH2, δCH2), 1.50-1.55 (2m, 2H, βCH2), 2.45 (t, J = 7.05 Hz, 2H, εCH2), 2.55 (m, 6H, CH2CN), 2.73 [t, J = 6.76 Hz, 4H, Nε-(CH2)2], 2.65, 2.81 (2m, 2H, NHα-CH2), 3.15 (t, J = 6.42 Hz, 1H, αCH); 13C-NMR δ 15.5 (εCH2-CN), 17.7 (αCH2-CN), 22.8 (γC), 26.5 (δC), 32.0 (βC), 42.9 (NHα-CH2), 48.4 [Nε-(CH2)2], 52.3 (εC), 60.2 (αC), 119.7 (αCN), 119.9 (εCN), 175.2 (COOH); Anal. Calcd for C15H23O2N5: C, 58.99; H, 7.59; N, 22.9. Found: C, 58.9; H, 7.47; N, 22.89; α[D]22°C = +23 ± 1° (c = 2 in 1M HCl).
N,N’,N,N’-tetrakis(cyanoethyl)-L-α,β-diaminopropionic acid (3a): Yield: 9.05 g (28.6%); mp 126-128 °C; ESI MS 317 (M+1), 339 (M+23); 1H-NMR (DMSO) δ 2.57-2.67 (bm, 9H, CH2CN, βCHa), 2.81-2.98 [bm, 9H, N-(CH2)2, βCHb], 3.48 (dd, J = 6.24 Hz, 1H, αCH), 12.64 (s, 1H, COOH); 13C-NMR δ 15.3, 17.4 (αCH2-CN, βCH2-CN), 47.1, 48.6 [Nα-(CH2)2, Nβ-(CH2)2], 53.3 (βC), 61.7 (αC), 119.68, 119.72 (αCN, βCN), 172.9 (COOH); Anal. Calcd for C15H20O2N6: C, 56.95; H, 6.37; N, 26.56. Found: C, 56.99; H, 6.37; N, 26.67; α[D]29.1°C = -71° (±1°, c=2 in acetone). Monocrystals of rac-3a prepared in a separate procedure were subjected to X-ray analysis [31].
N,N’,N,N’-tetrakis(cyanoethyl)-L-α,γ-diaminobutyric acid (3b): Yield: 7.1 g (21.1%); semisolid; ESI MS 353 (M+23); 1H-NMR (DMSO) δ 1.61 (m, 2H, βCH2), 2.47 (t, J=6.95 Hz, 2H, γCH2), 2.58 [m, 8H, CH2CN), 2.75 [m, 8H, N-(CH2)2], 3.67 (t, J = 6.00 Hz, 1H, αCH), 11.55 (s, 1H, COOH); 13C-NMR δ 15.6, 18.0 (αCH2-CN, γCH2-CN), 31.1 (βC), 47.8, 48.4 [Nα-(CH2)2, Nγ-(CH2)2], 51.6 (γC), 65.1 (αC), 119.1, 120.0 (αCN, γCN), 174.6 (COOH); Anal. Calcd for C16H22O2N6: C, 58.16; H, 6.71; N, 25.43. Found: C, 57.95; H, 6.62; N, 25.23; α[D]29.1°C = -64° (±1°, c = 2 in acetone).
N,N’,N,N’-tetrakis(cyanoethyl)-L-ornithine (3c): Yield: 8.2 g (23.6%); semisolid; ESI MS 345 (M+1), 367 (M+23); 1H-NMR (CDCl3) δ 1.40, 1.54, 1.68 (3m, 4H, γCH2, βCH2), 2.50 (m, 2H, δCH2), 2.59 [m, 8H, CH2CN), 2.76, 2.88 [2m, 8H, N-(CH2)2], 3.34 (dd, J = 4.45 Hz, 1H, αCH); 13C-NMR δ 15.5, 17.4 (αCH2-CN, δCH2-CN), 23.5 (γC), 27.1 (βC), 46.9, 48.4 [Nα-(CH2)2, Nδ-(CH2)2], 51.9 (δC), 62.5 (αC), 119.86, 119.91 (αCN, δCN), 174.2 (COOH); Anal. Calcd for C17H24O2N6: C, 59.28; H, 7.02; N, 24.40. Found: C, 59.04; H, 6.96; N, 24.19; α[D]29.1°C = -65.2° (±1°, c = 2 in acetone).
N,N’,N,N’-tetrakis(cyanoethyl)-L-lysine (3d): Yield: 8.5 g (23.8%); semisolid; ESI MS 359 (M+1), 381 (M+23); 1H-NMR (CDCl3) δ 1.52, 1.68, 1.85 (3m, 6H, γCH2, δCH2, βCH2), 2.52 (m, 10H, CH2CN, εCH2), 2.85, 3.04 [2m, 8H, N-(CH2)2], 3.38 (dd, J = 5.34 Hz, 1H, αCH); 13C-NMR δ 16.9, 18.4 (αCH2-CN, εCH2-CN), 24.1 (γC), 26.9 (δC), 29.6 (βC), 47.5, 49.5 [Nα-(CH2)2, Nε-(CH2)2], 52.9 (εC), 63.4 (αC), 118.77, 118.84 (αCN, εCN), 177.2 (COOH); Anal. Calcd for C18H26O2N6: C, 60.3; H, 7.3; N, 23.4. Found: C, 60.08; H, 7.26; N, 23.15; α[D]28.6°C = -63° (±1°, c = 2 in acetone).
3.5.2. Synthesis of N,N-bis(cyanoethyl)-L-α,β-diamino propionic acid (11)
L-α,β-Diaminopropionic acid hydrochloride (14.05 g, 0.1 mol) was suspended in MeOH (100 mL), followed by slow addition of NaOH (8 g) in MeOH (100 mL). The reaction mixture was cooled to 0 °C in an ice-water bath. Then CH2=CH-CN (26.33 mL, 0.4 mol) was added in small portions and vigorous stirring was continued overnight, followed by addition of concentrated HCl (16.9 mL, 0.2 mol). The resulting white precipitate was collected on a filter and recrystallized from a MeOH-H2O mixture (1:1, v/v) to give 16.9 g (66.92%) of 11. Yield: 16.9g (66.9%); mp 180-182 °C (dec.); ESI MS 211 (M+1), 233 (M+23), 265 (M+23+32); 1H-NMR (D2O) δ 3.03, 3.07 (2t, J = 6.74 Hz, 4H, α, βCH2CN), 3.45-3.65 (bm, 6H, N-CH2), 4.06 (dd, J = 5.50 Hz, 1H, αCH); 13C-NMR δ 15.3, 15.8 (α, βCH2-CN), 42.74, 43.78 (α, βNH-CH2-), 45.8 (βC), 56.4 (αC), 117.7, 118.0 (α, βCN), 170.2 (COOH); Anal. Calcd for C9H15O2N4Cl: C, 43.82; H, 6.12; N, 22.71; Cl, 14.37. Found: C, 43.66; H, 6.20; N, 22.55; Cl, 14.41; α[D]24.5°C = +24.2 ± 1° (c = 2 in 1 M HCl).
3.5.3. General procedure for the catalytic reduction of the nitriles 2a-d to the tris-N-propylamino derivatives 4a-d and the synthesis of the Z-protected derivatives 4e-h
To a methanolic solution (150 mL) containing a tris-N-cyanoethylated derivative of basic amino acid 2a-d (5 g, ca. 15 mmol) and NaOH (0.64 g, 16 mmol), Raney-Ni suspension (5 g) was added. The resulting mixture was agitated at room temperature for 14-16 h under 90 psi (6 bar) of H2 pressure. The progress of the reaction was monitored by TLC using 1% ninhydrin in ethanol. After completion of the reaction, the catalyst was separated by filtration on Celite and the remaining solution was evaporated to dryness giving the compounds 4a-d as white hygroscopic oils in almost quantitative yields. The crude products were used for the next step without further purification.
N,N,N’-tris(3-aminopropyl)-L-α,β-diaminopropionic acid (4a): Yield: ~100%; ESI MS 298 (M+23), 330 (M+23+32); 1H-NMR (D2O) δ 1.50-1.70 (m, 6H, C-CH2-C), 2.50-2.70 (bm, 14H, N-CH2), 3.15 (dd, J = 5.65 Hz, 1H, αCH), 3.34 (s, 6H, NH2); 13C-NMR δ 28.3 (βCH2-CH2NH2), 28.9 (αCH2-CH2NH2), 38.9 (αCH2NH2), 39.3 (βCH2NH2), 44.9 (NHα-CH2), 49.1 [Nβ-(CH2)2], 62.0 (αC), 181.8 (COO-).
N,N,N’-tris(3-aminopropyl)-L-α,γ-diaminobutyric acid (4b): Yield: ~100%; ESI MS 344 (M+23+32); 1H-NMR (D2O) δ 1.55-1.75 (bm, 8H, C-CH2-C), 2.40-2.80 (bm, 14H, N-CH2), 2.98 (dd, J = 5.33 Hz, 1H, αCH), 3.35 (s, 6H, NH2); 13C-NMR δ 28.8 (γCH2-CH2NH2), 29.0 (αCH2-CH2NH2), 32.0 (βC), 38.9 (αCH2NH2), 39.4 (γCH2NH2), 45.1 (NHα-CH2), 49.1 [Nγ-(CH2)2], 50.9 (γC), 62.67 (αC), 182.3 (COO-).
N,N,N’-tris(3-aminopropyl)-L-ornithine (4c): Yield: ~100%; ESI MS 326 (M+23); 1H-NMR (D2O) δ 1.39 (m, 4H, β,γCH2), 1.53 (m, 6H, C-CH2-C), 2.30-2.60 (bm, 14H, N-CH2), 2.95 (dd, J = 6.02 Hz, 1H, αCH), 3.28 (s, 6H, NH2); 13C-NMR δ 21.5 (αCH2-CH2NH2), 28.1 (δCH2-CH2NH2), 30.7 (γC), 31.6 (βC), 38.4 (αCH2NH2), 38.9 (δCH2NH2), 44.6 (NHα-CH2), 48.6 [Nδ-(CH2)2], 50.4 (δC), 63.4 (αC), 182.3 (COO-).
N,N,N’-tris(3-aminopropyl)-L-lysine (4d): Yield: ~100%; ESI MS 318 (M+1); 1H-NMR (D2O) δ 1.50 (m, 2H, γCH2), 1.81 (m, 2H, δCH2), 2.06 (m, 2H, βCH2), 2.15 (m, 6H, C-CH2-C), 3.10 (t, J = 7.7 Hz, 6H, CH2NH2), 3.20-3.30 (bm, 8H, Nε-CH2, NHα-CH2), 3.34 (s, 6H, NH2), 3.97 (dd, J = 5.09 Hz, 1H, αCH); 13C-NMR δ 21.5 (αCH2-CH2NH2), 21.7 (εCH2-CH2NH2), 22.9(γC), 23.9 (δC), 28.6 (βC), 36.6 (εCH2NH2), 36.7 (αCH2NH2), 43.9 (NHα-CH2), 50.1 [Nε-(CH2)2], 52.7 (εC), 60.7 (αC), 181.5 (COO-).
N,N-bis(3-aminopropyl)-L-α,β-diaminopropionic acid (12): Yield: ~100%; ESI MS 272 (M+NH4++ 2H2O), 273 (M+Na++MeOH), 275 (M+K++H2O); 1H-NMR (D2O) δ 1.60 (m, 4H, C-CH2-C), 2.50-2.75 (bm, 10H, βCH2, N-CH2), 3.19 (t, J = 6.46 Hz, 1H, αCH), 3.35 (s, 6H, NH); 13C=NMR δ 31.9, 32.1 (α, βCH2-CH2NH2), 38.9 (α, βCH2NH2), 45.4, 46.4 (NH-CH2), 51.4 (βCH2), 63.2 (αC), 181.44 (COO-).
3.5.4. General synthesis of the Z-protected derivatives 4e-h
To the crude products from the previous reaction (8.5-9.5 g, ca. 15 mmol, 100%), a dioxane-water mixture (1:1, v/v, 100 mL) was added, followed by addition of benzyloxycarbonyl chloride (12 mL, 85.7 mmol). The pH value of the mixture was maintained between 9 and 10 by adding 1M NaOH (88 mL). The reaction mixture was cooled in an ice-water bath; after addition of substrates, it was allowed to warm to room temperature and was stirred overnight. Then the aqueous phase was washed three times with diethyl ether (50 mL; the ether extracts were discarded), acidified using 10% citric acid, and extracted three times with chloroform (100 mL). The CHCl3 layers were combined, dried over Na2SO4 overnight and concentrated in vacuo. The residue was purified by flash chromatography (CHCl3:MeOH 8:1) to give 4e-h (58.7-69%) as pale colored gums.
Nα-benzyloxycarbonyl-N,N,N’-tris(benzyloxycarbonyl-3-aminopropyl)-L-α,β-diaminopropionic acid (4e): Yield: 9.05g (58.7%); yellow gum; ESI MS 826 (esterification in MeOH in the spectrometer!); 1H-NMR (DMSO, 350K) δ 1.60-1.80 (m, 6H, C-CH2-C), 2.80-3.30 (bm, 12H, N-CH2), 3.60 (m, 2H, βCH2), 4.30 (m, 1H, αCH), 5.04 (s, 8H, CH2Ar), 7.29 (m, 20H, Ar-H); 13C-NMR δ 28.5, 29.5 (C-CH2-C), 39.79, 39.81 (CH2NH), 43.7 (Nα-CH2), 49.6 [Nβ-(CH2)2], 51.8 (βC), 59.1 (αC), 65.4, 66.8 (CH2-Ar), 127.7, 127.8, 128.3, 137.0 (CAr), 155.1, 156.4 (O-CO-N-), 172.4 (COOH); α[D]26.6°C = -10.30 ± 0.5° (c=2 in acetone).
Nα-benzyloxycarbonyl-N,N,N’-tris(benzyloxycarbonyl-3-aminopropyl)-L-α,γ-diaminobutyric acid (4f): Yield: 10.25 g (69%); colorless gum; ESI MS 839 (M’ + 1, esterification in MeOH in the spectrometer!), 916 (M + C7H7+); 1H-NMR (DMSO, 350K) δ 1.49-1.66 (bm, 8H, C-CH2-C), 3.0-3.6 (bm, 15H, N-CH2) 4.15 (m, 1H, αCH), 5.02 (s, 8H, CH2-Ar), 7.33 (bs, 20H, Ar-H); 13C-NMR δ 25.6, 26.1 (C-CH2-C), 28.5 (βC), 38.5 (CH2NH), 45.0 (NHα-CH2), 50.7 [Nγ-(CH2)2], 51.0 (γC), 60.1 (αC) 64.8, 66.0 (CH2-Ar), 126.8, 127.0, 127.8, 136.9 (CAr), 155.6 (O-CO-N-), 172.3 (COOH); α[D]26.3°C = -10.1 ± 0.5° (c = 2 in acetone).
Nα-benzyloxycarbonyl-N,N,N’-tris(benzyloxycarbonyl-3-aminopropyl)-L-ornithine (4g):Yield: 8.16 g (56.6%); pale yellow gum; ESI MS 840 (M+1), 854 (M’ + 1, esterification in MeOH in the spectrometer!); 1H-NMR (DMSO, 350K) δ 1.48, 2.31 (2m, 10H, C-CH2-C, β, γCH2), 2.90-3.80 (bm, 14H, N-CH2), 4.24 (m, 1H, αCH), 5.02, 5.18 (s, 8H, CH2Ar), 7.31 (m, 20H, Ar-H); 13C-NMR δ 23.2 (γC), 26.8 (C-CH2-C), 29.4 (βC), 38.7 (CH2NH), 45.1 (Nα-CH2), 50.8 [Nδ-(CH2)2], 52.8 (δC), 59.8 (αC), 65.1, 66.3 (CH2-Ar), 127.8, 128.3, 137.3 (CAr), 155.4, 156.0 (O-CO-N-), 171.5 (COOH); α[D]26.5°C = -10.12 ± 0.5° (c = 2 in acetone).
Nα-benzyloxycarbonyl-N,N,N’-tris(benzyloxycarbonyl-3-aminopropyl)-L-lysine (4h): Yield: 8.50 g (62.27%); yellow gum; ESI MS 854 (M+1), 944 (M + C7H7+), 1034 (M-1+2C7H7+); 1H-NMR (DMSO, 350K) δ 1.15-2.0 (bm, 12H, β, γ, δCH2, C-CH2-C), 2.50, 3.10 (2 bm, 14H, NCH2), 4.10 (m, 1H, αCH), 4.97, 5.03 (bs, 8H, CH2Ar), 7.29 (bm, 20H, Ar-H); 13C-NMR δ 22.6 (γC), 28.6 (δC), 28.8 (C-CH2-C), 29.8 (βC), 37.6, 38.5 (CH2NH), 44.5 (Nα-CH2), 50.6 [Nε-(CH2)2], 52.2 (εC), 58.4 (αC), 66.3, 66.9 (CH2-Ar), 127.9, 128.3, 136.7, 136.8 (CAr), 156.6, 157.1 (O-CO-N-), 172.7 (COOH); α[D]23.6°C = -10.33 ± 0.5° (c = 2 in acetone).
N,Nβ-bis(benzyloxycarbonyl)-Nα,Nβ-bis(benzyloxycarbonyl-3-aminopropyl)-L-α,β-diaminopropionic acid (13): Yield: 11.1g (61.83%); amber-colored gum; ESI MS 777 (M+23, CHCl3); 1H-NMR (DMSO, 350K) δ 1.60-1.70 (m, 4H, C-CH2-C), 2.96 (m, 4H, CH2NH), 3.20, 3.30 (2 m, 4H, N-CH2), 3.70 (m, 2H, βCH2), 4.25 (m, 1H, αCH), 5.04 (s, 8H, CH2Ar), 7.29 (m, 20H, Ar-H); 13C-NMR δ 28.7 (C-CH2-C), 38.8 (CH2NH), 45.8 (NH-CH2), 47.1 (βC), 59.2 (αC), 64.9, 66.0 (CH2-Ar), 128.1, 128.7, 137.79, 137.82 (CAr), 155.7, 156.5 (O-CO-N-), 171.39 (COOH); α[D]24.5°C = -52.50 ± 1° (c = 2 in acetone).
3.5.5. General procedure for the reduction of the nitriles to the corresponding tetrakis-N-propylamino derivatives 5a-d
To a derivative 3a-d (5 g, ca. 15 mmol) dissolved in dry THF (250 mL), 10 M solution of BH3 × SMe2 (Aldrich, 15.8 mL) were added. The reaction mixture was heated to reflux and stirred vigorously overnight. After cooling to room temperature, 1M HCl in MeOH (94.8 mL) and additional MeOH (100 mL) were added. The mixture was evaporated in vacuo, the oily residue was treated three times with 50 mL of MeOH and evaporated to dryness, to give 8.5-9 g (ca. 100%) of a white, very hygroscopic foam. The elemental analysis shows a mixture of penta- and hexahydrochlorides of 5a-d.
N,N’,N,N’-tetrakis(3-aminopropyl)-L-α,β-diaminopropanol (5a): Yield: 8.9g (~100%); ESI MS 319 (M+1); 1H-NMR (D2O) δ 1.90-2.10 (m, 8H, C-CH2-C), 3.00-3.70 (3m, 20H, N-CH2, CH2OH), 3.90 (2m, 1H, αCH); 13C-NMR δ 26.7, 29.3 (αC-CH2-C, βC-CH2-C), 37.6, 38.0 (CH2NH2), 48.7, 57.8 [Nα-(CH2)2, Nβ-(CH2)2], 58.34 (βC), 59.2 (αC), 61.7 (CH2OH); α[D]26.5 °C = +15.6° (±1°, c = 2 in 1M HCl).
N,N’,N,N’-tetrakis(3-aminopropyl)-L-α,γ-diaminobutanol (5b): Yield: 8.8g (~100%); ESI MS 353 (M+23); 1H-NMR (D2O) δ 1.60-2.2 (bm, 10H, βCH2, C-CH2C), 2.80-3.60 (bm, 20H, N-CH2, CH2OH), 3.87 (m, 1H, αCH); 13C-NMR δ 21.7, 22.9 (C-CH2-C), 28.8 (βC), 36.5, 36.6 (CH2NH2), 49.0, 50.1 [Nα-(CH2)2, Nγ-(CH2)2], 52.1 (γC), 57.35 (αC), 61.9 (CH2OH); α[D]29.1°C = +12.7° (±1°, c=2 in 1M HCl).
N,N’,N,N’-tetrakis(3-aminopropyl)-L-α,δ-diaminopentanol (5c): Yield: 8.7g (~100%); ESI MS 347 (M+1); 1H-NMR (CDCl3) δ 1.55, 1.80, 2.10 (3m, 12H, β, γCH2, C-CH2-C), 2.90-3.40 (bm, 20H, N-CH2, CH2OH), 3.95 (2m, 1H, αCH); 13C-NMR δ 21.8 (C-CH2-C), 22.9 (γC), 27.9 (βC), 36.6, 36.7 (CH2NH2), 50.0 [α, δN-(CH2)2], 52.9 (δC), 57.8 (αC), 61.6 (CH2OH); α[D]29.1°C = +12.3° (±1°, c=2 in 1M HCl).
N,N’,N,N’-tetrakis(3-aminopropyl)-L-α,ε-diaminohexanol (5d): Yield: 8.9 g (~100%); ESI MS 361 (M+1), 383 (M+23); 1H-NMR (CDCl3) δ 1.50-2.30 (3m, 14H, β, γ, δCH2, C-CH2-C), 2.90-3.40 (bm, 20H, N-CH2, CH2OH), 3.95 (m, 1H, αCH); 13C-NMR δ 22.0 (C-CH2-C), 23.3 (γC), 23.9 (δC), 26.3 (βC), 36.9, 37.3 (CH2NH2), 49.2, 50.21 [α, ε N-(CH2)2], 53.2 (εC), 58.9 (αC), 61.8 (CH2OH); α[D]28.6°C = +13.2 ± 1°, (c = 2 in 1M HCl).
3.5.6. Preparation of dendrimeric compounds 7e-7h
To benzylamine (3.21 g, 3.3 mL = 30 mmol) dissolved in dry THF (15 mL), 4g (2.55 g, 3 mmol), HOBt (0.46 g, 3 mmol) and DCC (0.63 g, 3 mmol) were added, the mixture was stirred for 24 h and the solvent was evaporated in vacuo. The residue was dissolved in CHCl3 (50 mL) and washed consecutively with 10% Na2CO3, H2O, 1% citric acid and saturated NaCl solution, then dried over Na2SO4 and evaporated to dryness: yield 2.07 g (74.3%) of the amide 6g as a light yellow gum.
N,N,N’-tris(benzyloxycarbonyl-3-aminopropyl)-L-diaminoalanine benzylamide (6e): C51H60O9N6, Yield: 1,92g (65.3%); yellow gum; ESI MS 901 (M+H+), 923 (M+Na+); 1H-NMR (DMSO, 350K) δ 1.66-1.85 (m, 6H, C-CH2-C), 2.79-3.38 (bm, 12H, N-CH2), 3.63 (m, 2H, βCH2), 4.23 (s, 2H, Ar-CH2-NH), 4.3 (m, 1H, αCH), 5.05, 5.09 (2s, 8H, CH2-Ar), 7.2-7.3 (bm, 25H, Ar-H); 13C-NMR δ 28.6, 29.1 (C-CH2-C), 40.1 (CH2NH), 42.3 (Ar-CH2-NH), 43.7 (Nα-CH2), 49.5 [Nβ-(CH2)2], 51.1 (βC), 59.6 (αC), 65.1, 66.3 (CH2-Ar), 127.7, 127.8, 128.2 (CHAr), 136.6, 136.9 (CAr), 155.0, 156.2 (O-CO-N-), 173.1 (CONH).
N,N,N’-tris(benzyloxycarbonyl-3-aminopropyl)-L-diaminobutyric benzylamide (6f): Yield 2.15 g (70.1%); pale yellow gum; ESI MS 915 (M+H+), 937 (M+Na+); 1H-NMR (DMSO, 350K) δ 1.45-1.7 (3m, 8H, βCH2, C-CH2-C), 2.9-3.5 (bm, 14H, N-CH2), 4.2 (s, 2H, Ar-CH2-NH), 4.28 (m, 1H, αCH), 5.07, 5.11 (2s, 8H, CH2-Ar), 7.2-7.3 (bm, 25H, Ar-H). 13C-NMR δ 25.5, 26.4 (C-CH2-C), 29.1 (βC), 38.5 (CH2NH), 42.4 (Ar-CH2-NH), 44.5 (NHα-CH2), 50.3 [Nγ-(CH2)2], 51.1 (γC), 59.8 (αC), 65.0, 66.1 (CH2-Ar), 126.9, 127.0, 127.8, 128.0 (CHAr), 136.8, 137.0 (CAr), 155.3, 156.2 (O-CO-N-), 173.0 (CONH).
N,N,N’-tris(benzyloxycarbonyl-3-aminopropyl)-L-ornithine benzylamide (6g): Yield: 74.3%; C53H64O9N6, pale yellow gum; ESI MS 929 (M+1), 951 (M+23), 967 (M+39), 1,019 (M + 91 = C7H7+); 1H-NMR (DMSO, 350K) δ 1.40 - 2.30 (2m, 10H, C-CH2-C, β, γCH2,), 2.85-3.70 (bm, 14H, N-CH2), 4.22 (s, 2H, Ar-CH2-NH), 4.31 (m, 1H, αCH), 5.05, 5.20 (s, 8H, CH2Ar), 7.29 (m, 25H, Ar-H); 13C- NMR δ 23.2 (γC), 26.3 (C-CH2-C), 29.6 (βC), 38.6 (CH2NH), 42.2 (Ar-CH2-NH), 44.2 (Nα-CH2), 50.6 [Nδ-(CH2)2], 52.6 (δC), 59.7 (αC), 65.0, 66.3 (OCH2-Ar), 127.6, 127.7, 128.3, 137.3 (CAr), 155.2, 156.1 (O-CO-N-), 171.3 (CONH).
N,N,N’-tris(benzyloxycarbonyl-3-aminopropyl)-L-lysine benzylamide (6h): Yield: 78.6%; C54H66O9N6, yellow gum; ESI MS 943 (M+1), 965 (M+23), 981 (M+39) 1,033 (M+91 = C7H7+); 1H-NMR (DMSO, 350K) δ 1.2-2.1 (bm, 12H, β, γ, δCH2, C-CH2-C), 2.52, 3.12 (2 bm, 14H, NCH2), 4.19 (m, 1H, αCH), 4.26 (s, 2H, Ar-CH2-NH), 5.03, 5.1 (bs, 8H, CH2Ar), 7.15-7.3 (bm, 25H, Ar-H); 13C-NMR δ 22.7(γC), 28.4 (δC), 28.5 (C-CH2-C), 29.4 (βC), 37.4, 38.1 (CH2NH), 42.1 (Ar-CH2-NH), 43.9 (Nα-CH2), 49.9 [Nε-(CH2)2], 52.1 (εC), 58.5 (αC), 66.4, 66.8 (OCH2-Ar), 127.9, 128.4, 136.8, 136.9 (CAr), 156.6, 156.9 (O-CO-N-), 171.9 (CONH).
A) 6g (1.5 g, 1.6 mmol) dissolved in MeOH (25 mL) was stirred for 24 h with 10% Pd/C (150 mg) under an atmospheric pressure of H2. Then the catalyst was separated on Celite® and washed with MeOH. The collected methanol fractions were evaporated to dryness yielding 0.59 g (93.1%) of deprotected 6g as a white waxy solid. To the solution of deprotected 6g in DMF (25 mL), (2-Cl-Z)Lys(Boc) (2.04 g, 4.95 mmol), HOSu (0.57 g, 4.95 mmol) and DCC (1.02 g, 4.95 mmol) were added. The mixture was stirred for 24 h (until disappearance of free amino groups in the ninhydrin test), filtered and evaporated in vacuo. The residue was dissolved in CHCl3 (50 mL) and washed with 10% Na2CO3, H2O, 1% citric acid, dried over Na2SO4 overnight and evaporated to dryness. The residue was purified by flash chromatography (CHCl3-MeOH 8:1) to give 1.7 g (71.3%) of Boc-protected 7g as a dark yellow resin.
B) Boc-protected 7g (0.5 g, 0.32mmol) was dissolved in CH2Cl2 (5 mL) and trifluoroacetic acid (TFA, 5 mL) was added. The reaction mixture was stirred at room temperature for 3 h. Then the reaction mixture was evaporated in vacuo, the residue was dissolved in ethyl acetate (5 mL) and evaporated (3 times) and then in diethyl ether (5 mL) and evaporated (twice) – to remove all remaining trifluoroacetic acid. Trifluoroacetate ions were replaced by chlorides by dissolving the oily residue in HCl-saturated ethyl acetate and evaporation in vacuo (four times) to give 470 mg (99%) of 7g hexahydrochloride as a yellow glassy gum.
N,N,N’-tris[(Nα-2-chlorobenzyloxycarbonyl)-L-lysil-3-aminopropyl]-L-diaminoalanine benzylamide pentahydrochloride (7e): C61H87O10N12Cl3·5HCl; Yield 0.99g (97.5%); ESI MS 627 (M+2H+)2+ - base peak, 647 (M+H++Na++H2O)2+, 1,253 (M+H+); 1H-NMR (DMSO, 350K) δ1.1 – 2.1 (4bm, 24H, C-CH2-C, β, γ,δCH2 G-1 Lys), 2.6-3.5 (3 bm, 20H, αNH-CH2, βN-(CH2)2, CH2-NH core, εCH2 G-1 Lys), 3.53 (m, 1H, αCH core), 4.18 (m, 3H, αCH G-1 Lys), 4.30 (Ar-CH2-NH), 5.11, 5.18 (2bs, 6H, Ar-CH2O- from 2-Cl-Z), 7.2-7.4 (bm; 17H, Ar-H); 13C-NMR δ 22.2 (γC G-1 Lys), 25.1 (C-CH2-C core), 29.7 (δC G-1 Lys), 31.0 (βC G-1 Lys), 38.5 (εC G-1 Lys), 40.1 (CH2NH core), 43.4 (ArCH2-NH), 44.6 (Nα-CH2 core), 50.9 [Nβ-(CH2)2 core], 52.7 (βC core), 55.4 (αC G-1 Lys), 59.5 (αC core Lys), 62.1, 62.3 (Ar-CH2-O), 126.5, 126.6, 127.4, 127.8, 128.7, 129.0, 129.1, 129.2 (CHAr), 132.0 (CAr-Cl), 133.7 (CAr-CH2-NH), 134.1 (CAr-CH2-O), 155.6, 156.7 (O-CO-NH-), 171.3 (CONH G-1 Lys), 172.2 (CONH core); Anal. Calcd for C61H87O10N12Cl3·5HCl, (12 days/P2O5): C, 51.0; H, 6.45; N, 11.70; Cl, 19.73. Found: C, 50.67; H, 6.51; N, 11.43; Cl, 19.5.
N,N,N’-tris[(Nα-2-chlorobenzyloxycarbonyl)-L-lysil-3-aminopropyl]-L-diaminobutyro benzylamide pentahydrochloride (7f): C62H89O10N12Cl3·5HCl; Yield 1.0g (98.3%); hygroscopic yellow gum; ESI MS 634 (M+2H+)2+- base peak, 654 (M+H++Na++H2O)2+, 1,267 (M+H+); 1H-NMR (DMSO, 350K) δ 1.1 – 2.1 (bm, 26H, C-CH2-C, βCH2 core and β, γ,δCH2 G-1 Lys), 2.5-3.55 (2 bm, 20H, αNH-CH2, γ N-(CH2)2, CH2-NH core, εCH2 G-1 Lys), 3.68 (m, 1H, αCH core), 4.15 (m, 3H, αCH G-1 Lys), 4.28 (Ar-CH2-NH), 5.09, 5.15 (2bs, 6H, Ar-CH2O- grup 2-Cl-Z), 7.2-7.4 (bm; 17H, Ar-H); 13C-NMR δ 22.1 (γC G-1 Lys), 25.3 (C-CH2-C core), 29.1 (βC core), 29.6 (δC G-1 Lys), 31.1 (βC core Lys), 38.2 (εC G-1 Lys), 39.9 (CH2NH core), 43.2 (ArCH2-NH), 44.9 (Nα-CH2 core), 50.8 [Nγ-(CH2)2 core], 53.1 (γC core), 55.3 (αC G-1 Lys), 59.7 (αC core), 62.2, 62.4 (Ar-CH2-O), 126.5, 126.7, 127.3, 127.8, 128.7, 129.0, 129.1, 129.2 (CHAr), 131.9 (CAr-Cl), 133.8 (CAr-CH2-NH), 134.0 (CAr-CH2-O), 155.7, 156.8 (O-CO-NH-), 171.4 (CONH G-1 Lys), 172.2 (CONH core); Anal. Calcd for C62H89O10N12Cl3·5HCl, (12 days/P2O5): C, 51.3; H, 6.52; N, 11.58; Cl, 19.54. Found: C, 50.96; H, 6.61; N, 11.31; Cl, 19.42.
N,N,N’-tris[(Nα-2-chlorobenzyloxycarbonyl)-L-lysil-3-aminopropyl]-L-ornithine benzylamide penta- hydrochloride (7g): Yield: 99%; C63H91O10N12Cl3·5HCl, hygroscopic, glass-like gum; ESI MS 641 (M+2)2+, 1,281 (M+1), 1,299 (M+18+1); 1H-NMR (DMSO, 350K) δ 1.3 – 2.2 (bm, 28H, core β, γCH2, C-CH2-C, G1 β, γ,δCH2), 2.4-3.3 (bm, 20H, core αNH-CH2, δN-(CH2)2, CH2-NH, G1 εCH2), 3.60 (m, 1H, core αCH), 4.19 (m, 3H, G1 αCH), 4.38 (Ar-CH2-NH), 5.17 (m, 6H, G1 Ar-CH2O-), 7.2-7.4 (bm; 17H, Ar-H); 13C-NMR δ 22.4 (G1 γC), 23.1 (core γC), 27.6 (core C-CH2-C), 29.3 (G1 δC), 29.5 (core βC), 32.3(G1 βC), 36.3 (core CH2NH), 39.8, 40.0 (G1 εC), 43.2 (ArCH2-NH), 44.1 (core Nα-CH2), 49.7 [core Nδ-(CH2)2], 51.6 (core δC), 55.0, 55.2 (G1 αC), 58.8 (core αC), 66.5, 66.8 (Ar-CH2-O), 126.67, 126.7, 126.8, 127.6, 128.5, 128.9, 129.0, 129.3 (CHAr), 132.8 (G1 CAr-Cl), 133.9 (CAr-CH2-NH), 134.4 (G1 CAr-CH2-O), 155.8, 156.0 (G1 O-CO-N-), 174.0 (core CONH), 175.2 (G1 CONH); Anal. Calcd for C63H91O10N12Cl3·5HCl: C, 51.65; H, 6.26; N, 11.47; Cl, 19.35. Found: C, 51.43; H, 6.45; N, 10.31; Cl, 19.05.
N,N,N’-tris[(Nα-2-chlorobenzyloxycarbonyl)-L-lysil-3-aminopropyl]-L-lysine benzylamide penta- hydrochloride (7h): Yield: 99%; C64H93O10N12Cl3·5HCl, hygroscopic, glassy solid; ESI MS 648 (M+2)2+, 659 (M+1+23)2+ 1,295 (M+1), 1,313 (M+18+1); 1H-NMR (DMSO, 350K) δ 1.1–2.1 (bm, 30H, C-CH2-C, core and G1 β, γ,δCH2), 2.6-3.5 (2 bm, 20H, core αNH-CH2, εN-(CH2)2, CH2-NH, G1 εCH2), 3.65 (m, 1H, core αCH), 4.05 (m, 3H, G1 αCH), 4.33 (Ar-CH2-NH), 5.12 (m, 6H, G1 Ar-CH2O-), 7.2-7.4 (bm; 17H, Ar-H); 13C-NMR δ 21.9 (G1 γC), 22.1 (core γC), 24.5 (core δC) 25.6 (core C-CH2-C), 28.8 (core βC), 29.8 (G1 δC), 31.0 (G1 βC), 38.2 (G1 εC), 39.7 (core CH2NH), 43.1 (ArCH2-NH), 45.3 (core Nα-CH2), 51.4 [core Nε-(CH2)2], 53.6 (core εC), 55.6 (G1 αC), 59.9 (core αC), 62.4, 62.5 (Ar-CH2-O), 126.5, 126.7, 127.1, 127.8, 128.7, 129.0, 129.1, 129.2 (CHAr), 131.8 (G1 CAr-Cl), 133.9 (CAr-CH2-NH), 134.0 (G1 CAr-CH2-O), 156.7, 157.0 (G1 O-CO-N-), 171.5 (G1 CONH), 172.0 (core CONH); Anal. Calcd for C64H93O10N12Cl3*5HCl: C, 51.9; H, 6.67; N, 11.36; Cl, 19.17. Found: C, 51.63; H, 6.8; N, 11.19; Cl, 18.93.
3.5.7. Preparation of the dendrimeric compounds 9a-d
5c (450 mg, 0.72 mmol) was suspended in DMF (15 mL) with addition of N(Et)3 (2.40 mL, 17.28 mmol) and stirred at 40 °C until all of 5c was dissolved. Then (Boc)-L-Phe-2,4,5-trichlorophenyl ester 1.76 g (3.96 mmol, 5.5 eq) was added and the reaction mixture was stirred at room temperature for 7 days until the complete disappearance of free amino groups in the ninhydrin test. The solution was evaporated in vacuo, the residue was dissolved in CHCl3 (50 mL) and washed with 20% K2CO3 and saturated NaCl solution, dried over Na2SO4 overnight, filtered and evaporated to dryness. The residue was purified by flash chromatography (CHCl3-MeOH, 8:1) to give 520 mg of 9c (54%) as a white foam.
N,N',N,N’-tetrakis(Boc-L-phenylalanyl-3-aminopropyl)-L-α,δ-diaminopropanol (9a): Yield 2.76g (66%); C71H106O13N10; white foam; ESI MS 654 (M+2H+)2+, 1,307 (M+H+); 1H-NMR (DMSO, 350K) δ1.2-1.3 (m, 36H, Boc C-CH3), 1.47 (m, 8H, C-CH2-C), 2.33 (m, 4H, Nβ-CH2), 2.39 – 2.43 (m, 6H, βCH2, Nα-CH2), 2.83, 2.93 (dd, J 5.3, 8.5 Hz, 8H, Ar-CH2), 3.18 (m, 8H, CH2-NH), 3.42 (m, 2H, CH2OH), 3.86 (m, 1H, αCH core), 4.23 (m, 4H, αCH Phe), 7.2 (m; 20H, Ar-H). 13C-NMR δ 21.2 (C-CH2-C), 28.2 (Boc C-CH3), 36.8, 36.9 (α, βCH2NH core), 50.0 [α, βN-(CH2)2], 52.8 (βC), 58.4 (αC), 62.0 (CH2OH), 77.7 (Boc C-CH3), 125.2, 125.6, 127.6, 127.8, 128.7, 128.8 (CHAr), 137.6, 138.5 (CAr), 154.4, 154.8 (O-CO-NH), 172.2 (CONH Phe).
N,N',N,N’-tetrakis(Boc-L-phenylalanyl-3-aminopropyl)-L-α,δ-diaminobutanol (9b): Yield 3.9 g (71%); C72H108O13N10; ESI MS 662 (M+2H+)2+, 1,321 (M+H+); 1H-NMR (DMSO, 350K) δ1.2-1.3 (m, 36H, Boc C-CH3), 1.46 (m, 10H, βCH2, C-CH2-C), 2.35 (m, 6H, Nγ-CH2), 2.38 (m, 4H, Nα-CH2), 2.82, 2.94 (dd, J 5.3, 8.5 Hz, 8H, Ar-CH2), 3.15 (m, 8H, CH2-NH), 3.39 (m, 2H, CH2OH), 3.96 (m, 1H, αCH core), 4.22 (m, 4H, αCH Phe), 7.2 (m; 20H, Ar-H); 13C-NMR δ 21.6 (C-CH2-C), 27.8 – 28.4 (βC, Boc C-CH3), 36.6, 36.7 (α, γCH2NH core), 50.1 [α, γN-(CH2)2], 52.6 (γC), 57.7 (αC), 61.7 (CH2OH), 77.8 (Boc C-CH3), 125.2, 125.7, 127.6, 127.7, 128.6, 128.8 (CHAr), 137.7, 138.6 (CAr), 154.3, 154.5 (O-CO-NH), 171.3 (CONH Phe).
N,N',N,N’-tetrakis(Boc-L-phenylalanyl-3-aminopropyl)-L-α,δ-diaminopentanol (9c): Yield: 54%; C73H110O13N10, white foam; ESI MS 1,335 (M+1); 1H-NMR (350K, DMSO) δ 1.25-1.35 (m, 36H, C-CH3, Boc), 1.48 (m, 12H, core β, γCH2, C-CH2-C), 2.34 (m, 6H, core Nδ-CH2), 2.40 (m, 4H, core Nα-CH2), 2.80, 2.97 (dd, J = 5.26, 8.55 Hz, 8H, G1 Ar-CH2), 3.10 (m, 8H, core CH2-NH), 3.41 (m, 2H, core CH2OH), 3.98 (m, 1H, core αCH), 4.20 (m, 4H, G1 αCH), 7.19 (m; 20H, G1 Ar-H). 13C-NMR δ 26.5 (γC, δ core C-CH2-C), 28.5 (βC, αC-CH2-C), 36.7, 36.8 (α, δ CH2NH), 37.7 (G1 Ar-CH2), 47.5, 50.9 [core α, δN-(CH2)2], 53.6 (core δC), 55.5, 55.7 (core and G1 αC), 61.1 (core CH2OH), 77.7 [O-C-(CH3)3], 125.2, 125.6, 127.5, 127.2, 128.7, 128.9 (G1 CHAr), 137.6, 138.6 (G1 CAr), 154.3, 154.5 (G1 O-CO-NH), 170.7 (G1 CO-NH).
N,N',N,N’-tetrakis(Boc-L-phenylalanyl-3-aminopropyl)-L-α,ε-diaminohexanol (9d): Yield: 73.5%; C74H112O13N10, white foam; ESI MS 675 (M+2)2+, 1,349 (M+1)+; 1H-NMR (350K, DMSO) δ 1.24-1.36 (m, 36H, C-CH3, Boc), 1.43 (m, 14H, core β, γ, δCH2, C-CH2-C), 2.31 (m, 6H, core Nε-CH2), 2.42 (m, 4H, core Nα-CH2), 2.79, 2.95 (dd, J = 5.27, 8.54 Hz, 8H, G1 Ar-CH2), 3.15 (m, 8H, core CH2-NH), 3.43 (m, 2H, core CH2OH), 3.99 (m, 1H, core αCH), 4.22 (m, 4H, G1 αCH), 7.20 (m; 20H, G1 Ar-H).13C-NMR δ 24.1 (core γC), 26.6 (core δC, ε C-CH2-C), 28.5 (core βC, αC-CH2-C), 36.7, 36.8 (core α, ε CH2NH), 37.7 (G1 Ar-CH2-CH), 47.8, 51.0 [core α, εN-(CH2)2], 53.8 (core εC), 54.9, 55.1 (core and G1 αC), 61.1 (core CH2OH), 77.8 [O-C-(CH3)3], 125.2, 125.5, 127.2, 128.7, 129.0 (G1 CHAr), 137.7, 138.7 (G1 CAr), 154.7, 154.7 (G1 O-CO-NH), 170.6 (G1 CO-NH).
3.5.8. Preparation of the dendrimeric compound 10c
A) 9c (0.5 g, 0.375 mmol) was dissolved in CH2Cl2 (5 mL) and TFA (5 mL) was added. The reaction mixture was stirred at room temperature for 3 h. Then the reaction mixture was evaporated in vacuo, the residue was dissolved in ethyl acetate (5 mL) and evaporated (three times) and then in diethyl ether (5 mL) and evaporated (twice) – to remove all remaining trifluoroacetic acid. Dark-orange oil (0,4g) was used for the next step without purification.
B) (2-Cl-Z)L-Lys(Boc) (0.68 g, 1.65 mmol) and N-hydroxysuccinimide (HOSu, 0.19 g 1.66 mmol) was dissolved in THF (10 mL) followed by addition of DCC (0.34 g, 1.66 mmol). The solution was stirred for 0.5 h and added to the solution of Boc-deprotected 9c and N(Et)3 (1 mL, 7.13 mmol) in DMF (5 mL). The final solution was stirred for 48 h at room temperature, then DCU was filtered off and the solvents was evaporated in vacuo. The residue was dissolved in CHCl3 (50 mL) and washed with 10% Na2CO3 and saturated NaCl solution (twice), dried over Na2SO4 overnight, filtered and evaporated to dryness. The residue was purified by flash chromatography (CHCl3:MeOH, 15:1) to give 0.729 g of Boc protected 10c (77.1%) as a white foam.
C) Boc-10c (360 mg, 0.14 mmol) was deprotected according to procedure A). Trifluoroacetate ions were replaced by chlorides by dissolving the oily residue in HCl-saturated ethyl acetate and evaporation in vacuo (four times) to give 330 mg of 10c hexahydrochloride (99%) as a white, hygroscopic foam.
N,N',N,N’-tetrakis[(Nα-2-chlorobenzyloxycarbonyl)-L-lysil-L-phenylalanyl-3-aminopropyl]-L-α,δ-diaminopropanol hexahydrochloride (10a): Yield 1.0 g (98%); C107H142O17N18Cl4·6HCl; pale yellow foam; ESI MS 1,046 (M+2H+)2+, 1,057 (M+H++Na+)2+, 2,105 (M+H+); 1H-NMR (DMSO, 298K) δ 1–2 (bm, 32H, γ,β,δCH2 G-1 Lys, C-CH2-C core arms), 2.6-3.7 (4 bm, 37H, CH2-NH core, εCH2 G-1 Lys i βCH2 core, α, βN-(CH2)2 core, Ar-CH2-CH Phe, CH2OH & αCH core), 4.1, 4.6 (2 bm, 8H, αCH Lys & Phe, respectively), 5.16 (4s, 8H, Ar-CH2 from 2-Cl-Z), 7.2 – 7.45 (m; 36H, Ar-H); 13C-NMR δ 22.1, 22.3 (γC G-1 Lys), 23.4 (α, γC-CH2-C core), 26.6 (δC G-1 Lys), 28.7 (βC core), 30.3, 31.1 (βC G-1 Lys), 36.3, 36.5 (α, βCH2NH core), 37.7 (Ar-CH2-CH), 38.3, 38.6 (εCH2NH2 G-1 Lys), 48.8, 49.7 [α, βN-(CH2)2 core], 52.3 (βC core), 54.2, 55.3 (αC Phe and Lys, respectively), 58.8 (αC core), 60.3 (CH2OH core) 62.7, 62.8 (Ar-CH2-O), 126.3, 127.2, 128.2, 129.1, 129.3, 129.6, 129.7, 129.8 (CHAr), 132.1, 133.2 (Car-Cl), 134.3, 134.4 (Car-CH2Phe), 136.9 (Car-CH2O), 155.4, 155.5 (O-CO-NH), 171.3, 172.4 (CONH Phe and L-Lys, respectively); Anal. Calcd for C107H142O17N18Cl4·6HCl: C, 55.6; H, 6.45; N, 10.9; Cl, 15.33. Found: C, 55.38; H, 6.63; N, 10.58; Cl, 15.02.
N,N',N,N’-tetrakis[(Nα-2-chlorobenzyloxycarbonyl)-L-lysil-L-phenylalanyl-3-aminopropyl]-L-α,δ-diaminobutanol hexahydrochloride (10b): Yield 1.01 g (99%); C108H144O17N18Cl4·6HCl, white hygroscopic foam; ESI MS 1,053 (M+2H+)2+, 1,064 (M+H++Na+)2+, 2,105 (M+H+); 1H-NMR (DMSO, 298K) δ 1 – 2 (bm, 34H, βCH2 core i γ,β,δCH2 G-1 Lys, C-CH2-C core arms), 2.65-3.75 (3 bm, 37H, CH2-NH core, εCH2 G-1 Lys & γCH2 core, α, γN-(CH2)2 core, Ar-CH2-CH Phe, CH2OH and αCH core), 4.15, 4.57 (2 bm, 8H, αCH Lys and Phe, respectively), 5.11 (4s, 8H, Ar-CH2 from 2-Cl-Z), 7.2–7.45 (m; 36H, Ar-H). 13C-NMR δ 22.1, 22.2 (γC G-L-Lys), 23.5 (α, δC-CH2-C core), 26.5 (δC G-1 Lys), 28.8 (βC core), 30.2, 31.1 (βC G-1 Lys), 36.2, 36.3 (α, γCH2NH core), 37.6 (Ar-CH2-CH), 38.4, 38.5 (εCH2NH2 G-1 Lys), 48.9, 49.7 [α, γN-(CH2)2 core], 52.0 (γC core), 54.1, 55.1 (αC Phe & Lys, respectively), 58.4 (αC core), 60.1 (CH2OH core) 62.6, 62.8 (Ar-CH2-O), 126.2, 127.3, 128.1, 129.1, 129.3, 129.5, 129.7, 129.8 (CHAr), 132.2, 133.3 (Car-Cl), 134.2, 134.3 (Car-CH2Phe), 137.0 (Car-CH2O), 155.3, 155.6 (O-CO-NH), 171.2, 172.6 (CONH Phe and Lys, respectively); Anal. Calcd for C108H144O17N18Cl4*6HCl: C, 55.74; H, 6.5; N, 10.83; Cl, 15.23. Found: C, 55.5; H, 6.7; N, 10.51; Cl, 14.94.
N,N',N,N’-tetrakis[(Nα-2-chlorobenzyloxycarbonyl)-L-lysil-L-phenylalanyl-3-aminopropyl]-L-α,δ-diaminopentanol hexahydrochloride (10c): Yield: 99%; C109H146O17N18Cl4*6HCl, white hygroscopic foam; ESI MS 1,051 (M-18+2)2+, 1,060 (M+2)2+, 1,071 (M+1+23)2+, 2,119 (M+1)+; 1H-NMR (298K, DMSO); NMR: δ 1 – 2 (bm, 36H, core γ, βCH2, G2 γ,β,δCH2, core C-CH2-C), 2.7-3.8 (3 bm, 37H, core CH2-NH, G2 εCH2, core δCH2, core α, δN-(CH2)2, G1 Ar-CH2-CH, core CH2OH and αCH), 4.1, 4.55 (2 bm, 8H, G1 and G2 αCH), 5.15 (m, 8H, G2 Ar-CH2), 7.25 (m; 36H, Ar-H), 8.15 (bm, 21H, N-H). 13C=NMR δ 22.2, 22.3 (G2 γC), 23.2 (core α, δC-CH2-C), 23.9 (core γC), 26.4 (G2 δC), 28.4 (core βC), 30.3, 31.0 (G2 βC), 35.9, 36.2 (core α, δ CH2NH), 37.7 (G1 Ar-CH2-CH), 38.3, 38.6 (G2 εCH2NH2), 48.9, 49.6 [core α, δN-(CH2)2], 52.1 (core δC), 54.1, 55.0 (G1 and G2 αC), 57.9 (core αC), 60.3 (core CH2OH) 62.7, 62.9 (G2 CH2Ar), 126.2, 127.3, 128.0, 129.1, 129.4, 129.6, 129.7, 129.7 (CHAr), 132.1, 133.3 (G2 Car-Cl), 134.3, 134.4 (G1 Car-CH2), 136.9 (G2 Car-CH2), 155.2, 155.7 (G2 O-CO-NH), 171.3, 172.8 (G1 and G2 CO-NH); Anal. Calcd for C109H146O17N18Cl4·6HCl: C, 55.92; H, 6.54; N, 10.77; Cl, 15.14. Found: C, 55.68; H, 6.65; N, 10.43; Cl, 14.96.
N,N',N,N’-tetrakis[(Nα-2-chlorobenzyloxycarbonyl)-L-lysil-L-phenylalanyl-3-aminopropyl]-L-α,ε-diaminohexanol hexahydrochloride (10d): Yield: 99%; C110H148O17N18Cl4*6HCl, white hygroscopic foam; ESI MS 1,058 (M-18+2)2+, 1,067 (M+2)2+, 1,078 (M+1+23)2+, 2,133 (M+1)+; 1H-NMR (298K, DMSO) δ 1–2 (bm, 38H, core and G2 γ,β,δCH2, core C-CH2-C), 2.6-3.8 (3 bm, 37H, core CH2-NH, core and G2 εCH2, core α, εN-(CH2)2, G1 Ar-CH2-CH, core CH2OH, core αCH), 4.0, 4.49 (2 bm, 8H, G1 and G2 αCH), 5.10 (m, 8H, G2 Ar-CH2), 7.20 (m; 36H, G1 and G2 Ar-H), 8.11–8.7 (3 bm, 21H, N-H); 13C-NMR δ 22.2, 22.3 (G2 γC), 23.0 (core α, εC-CH2-C), 24.4 (core γC), 25.2 (core δC), 26.3, 26.4 (G2 δC), 28.6 (core βC), 30.0, 31.2 (G2 βC), 36.0, 36.2 (core α, ε CH2NH), 37.7 (G1 Ar-CH2-CH), 38.3, 38.4 (G2 εCH2NH2), 48.5, 49.8 [core α, εN-(CH2)2], 51.3 (core εC), 53.7, 54.0, 54.7 (G1 and G2 αC), 57.4 (core αC), 60.4 (core CH2OH) 62.8, 62.9 (G2 CH2Ar), 126.2, 127.2, 127.9, 129.2, 129.5, 129.6, 129.7 (G1 and G2 CHAr), 132.1, 133.2 (G2 Car-Cl), 134.2, 134.3 (G1 Car-CH2), 137.6 (G2 Car-CH2), 155.6, 155.8 (G2 O-CO-NH), 171.0, 171.4, 172.7 (G1 and G2 CO-NH); Anal. Calcd for C110H148O17N18Cl4*6HCl: C, 56.1; H, 6.6; N, 10.7; Cl, 15.05. Found: C, 55.65; H, 6.76; N, 10.35 Cl, 14.91.
4. Conclusions
Two series of new low molecular weight amphiphilic peptide dendrimers were efficiently synthesized and characterized. They contain novel core elements - basic tris-amino acids 3 and tetrakis-amino alcohols 4. Their application in the synthesis of amphiphilic peptide dendrimers yielded molecules with (+5)/(+6) charge and multiple distribution of cationic and lipophylic groups in the 1st dendrimer generation. These cationic dendrimeric peptides were significantly more potent against Gram(+) and Gram(-) bacteria and fungi from C. albicans genus, with single micromolar MICs, than previously designed derivatives built around a Lys(Lys)2 core. Interestingly, for the first time high activity of the dendrimeric species against antibiotics resistant MRSA ATCC 43300 and ESBL ATCC BAA-198 pathogens was detected. Both, higher antimicrobial potency and higher hemolytic properties are associated with higher charge and hydrophobicity of the present dendrimers. The high potency and broad range of activity of the new and previously described compounds evidences rationality of searching for simple, economically attractive antimicrobial compounds in the class of low molecular weight peptide dendrimers.
Acknowledgements
Financial support from the Ministry of Science and Higher Education of Poland, grant No 3T09B 115 28 and EC STREP program NORMOLIFE (CT-2006-037733) are acknowledged.
Footnotes
Sample Availability: Samples of the compounds 4e-h are available from the authors.
References and Notes
- 1.Coates A.R.M., Hu Y. Novel approaches to developing new antibiotics for bacterial infections. British J. Pharm. 2007;152:1147–1154. doi: 10.1038/sj.bjp.0707432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yacoby I., Benhar I. Antibacterial nanomedicine. Nanomedicine. 2008;3:329–341. doi: 10.2217/17435889.3.3.329. [DOI] [PubMed] [Google Scholar]
- 3.Boas U., Heegaard P.M.H. Dendrimers in drug research. Chem. Soc. Rev. 2004;33:43–63. doi: 10.1039/b309043b. [DOI] [PubMed] [Google Scholar]
- 4.Tomalia D.A., Frechet J.M.J. Introduction to dendrimers and dendritic polymers. Prog. Polym. Sci. 2005;30:217–219. doi: 10.1016/j.progpolymsci.2005.03.003. [DOI] [Google Scholar]
- 5.Tomalia D.A., Frechet J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. A Polym. Chem. 2002;40:2719–2728. doi: 10.1002/pola.10301. [DOI] [Google Scholar]
- 6.Stiriba S.E., Frey H., Haag R. Dendritic polymers in biomedical applications: From potential to clinical use in diagnostics and therapy. Angew. Chem. Int. Ed. 2002;41:1329–1334. doi: 10.1002/1521-3773(20020415)41:8<1329::AID-ANIE1329>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Goodman M., Cai W.B., Kinberger G.A. The new science of protein mimetics. Macromol. Symposia. 2003;201:223–236. doi: 10.1002/masy.200351125. [DOI] [Google Scholar]
- 8.Gong E., Matthews B., McCarthy T., Chu J.H., Holan G., Raff J., Sacks S. Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses. Antivir. Res. 2005;68:139–146. doi: 10.1016/j.antiviral.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Landers J.J., Cao Z.Y., Lee I., Piehler L.T., Myc P.P., Myc A., Hamouda T., Galecki A.T., Baker J.R. Prevention of influenza pneumonitis by sialic acid-conjugated dendritic polymer. J. Infect. Dis. 2002;186:1222–1230. doi: 10.1086/344316. [DOI] [PubMed] [Google Scholar]
- 10.Chaves F., Calvo J.C., Carvajal C., Rivera Z., Ramirez L., Pinto M., Trujillo M., Guzman F., Patarroyo M.E. Synthesis, isolation and characterization of Plasmodium falciparum antigenic tetrabranched peptide dendrimers obtained by thiazolidine linkage. J. Pept. Res. 2001;58:307–316. doi: 10.1034/j.1399-3011.2001.00921.x. [DOI] [PubMed] [Google Scholar]
- 11.Sadler K.J., Tam P. Peptide dendrimers: Applications and synthesis. Rev. Mol. Biotechnol. 2001;90:195–229. doi: 10.1016/S1389-0352(01)00061-7. [DOI] [PubMed] [Google Scholar]
- 12.Svenson S., Tomalia D.A. Dendrimers in biomedical applications—reflections on the field. Adv. Drug Deliv. Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Dufes C., Uchegbu I.F.A., Schatzlein G. Dendrimers in Gene Delivery. Adv. Drug Deliv. Rev. 2005;57:2177–2202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Braun C.S., Vetro J.A., Tomalia D.A., Koe G.S., Koe J.G., Middaugh C.R. Structure/function relationships of polyamidoamine/DNA dendrimers as gene delivery vehicles. J. Pharm. Sci. 2005;94:423–436. doi: 10.1002/jps.20251. [DOI] [PubMed] [Google Scholar]
- 15.Calabretta M.K., Kumar A., McDermott A.M., Cai C.Z. Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups. Biomacromolecules. 2007;8:1807–1811. doi: 10.1021/bm0701088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czen C.Z., Cooper S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23:3359–3368. doi: 10.1016/S0142-9612(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 17.Ortega P., Copa-Patiño J.L., Muñoz-Fernandez M.A., Soliveri J., Gomez R., de la Mata F.J. Amine and ammonium functionalized chloromethylsilane-ended dendrimers. Antimicrobial activity studies. Org. Biomol. Chem. 2008;6:3264–3269. doi: 10.1039/b809569h. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y.Y., Qu H., Ma M.L., Xu Z.H., Xu P., Fang Y.J., Xu T.W. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: An in vitro study. Eur. J. Med. Chem. 2007;42:1032–1038. doi: 10.1016/j.ejmech.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 19.Yung H., Lopina S.T. Penicillin V—Conjugated PEG-PAMAM star polymers. J. Biomat. Sci.-Polym. 2003;14:1043–1056. doi: 10.1163/156856203769231556. [DOI] [PubMed] [Google Scholar]
- 20.Balogh L., Swanson D.R., Tomalia D.A., Hagnauer G.L;. McManus., A.T. Dendrimer-silver complexes and nanocomposites as antimicrobial agents. Nano Lett. 2001;1:18–21. doi: 10.1021/nl005502p. [DOI] [Google Scholar]
- 21.Hancock R.E.W., Patrzykat A. Clinical development of cationic antimicrobial peptides: From natural to novel antibiotics. Curr. Drug Targets Infect. Disord. 2002;2:79–83. doi: 10.2174/1568005024605855. [DOI] [PubMed] [Google Scholar]
- 22.Marr A.K., Gooderham W.J., Hancock R.E.W. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharm. 2006;6:468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Tam J.P., Lu Y.A., Yang J.L. Antimicrobial dendrimeric peptides. Eur. J. Biochem. 2002;269:923–932. doi: 10.1046/j.0014-2956.2001.02728.x. [DOI] [PubMed] [Google Scholar]
- 24.Rojo J., Delgado R. Dendrimers and dendritic polymers as anti-infective agents: New antimicrobial strategies for therapeutic drugs. Antiinf. Agents Med. Chem. 2007;6:151–174. [Google Scholar]
- 25.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 26.Janiszewska J., Swieton J., Misicka A., Lipkowski A.W., Urbanczyk-Lipkowska Z. Small peptide dendrimers with antimicrobial properties. Peptides. 2002:568–567. [Google Scholar]
- 27.Janiszewska J., Urbanczyk-Lipkowska Z. Amphiphilic dendrimeric peptides as model non-sequential pharmacophores with antimicrobial properties. J. Mol. Microbiol. Biotechn. 2007;13:220–225. doi: 10.1159/000104751. [DOI] [PubMed] [Google Scholar]
- 28.Janiszewska J., Swieton J., Lipkowski A.W., Urbanczyk-Lipkowska Z. Low molecular mass peptide dendrimers that express antimicrobial properties. Bioorg. Med. Chem. Lett. 2003;13:3711–3713. doi: 10.1016/j.bmcl.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Klajnert B., Janiszewska J., Urbanczyk-Lipkowska Z., Bryszewska M., Shcharbin D., Labieniec M. Biological properties of low molecular mass peptide dendrimers. Int. J. Pharm. 2006;309:208–217. doi: 10.1016/j.ijpharm.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 30.Klajnert B., Janiszewska J., Urbanczyk-Lipkowska Z., Bryszewska A., Epand R.M. DSC studies on interactions between low molecular mass peptide dendrimers and model lipid membranes. Int. J. Pharm. 2006;327:145–152. doi: 10.1016/j.ijpharm.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Buhleyer E., Wehner W., Voegtle F. Cascade “and non-skid-chain-like” syntheses of molecular cavity topologies. Synthesis. 1978:155–158. [Google Scholar]
- 32.McKinney L.L., Uhing E.H., Setzkorn E.A., Cowan J.C. Cyanoethylation of α-amino acids. I. Monocyanoethyl derivatives. J. Am. Chem. Soc. 1950;72:2599–2603. doi: 10.1021/ja01162a069. [DOI] [Google Scholar]
- 33.McKinney L.L., Uhing E.H., Setzkorn E.A., Cowan J.C. Cyanoethylation of alpha amino acids, II. Dicyanoethyl and tricyanoethyl derivatives. J. Am. Chem. Soc. 1951;73:1641–1652. [Google Scholar]
- 34.Riehm J.P., Scheraga H.A. Structural studies of ribonuclease. XX. Acrylonitrile. A reagent for blocking the amino groups of lysine residues in ribonuclease. Biochemistry. 1966;5:93–99. doi: 10.1021/bi00865a013. [DOI] [PubMed] [Google Scholar]
- 35.Rylander P.N. Hydrogenation Methods. Academic Press; London, UK: 1985. [Google Scholar]
- 36.de Brabander-van den Berg E.M.M., Meijer E.W. Poly(propylene imine) Dendrimers: Large-Scale Synthesis via Heterogeneously Catalyzed Hydrogenation. Angew. Chem. Int. Ed. Engl. 1993;9:1308–1311. doi: 10.1002/anie.199313081. [DOI] [Google Scholar]
- 37.Brown H.C., Choi Y.M., Narasimhan S. Selective reductions. 29. A simple technique to achieve an enhanced rate of reduction of representative organic compounds by borane-dimethyl sulfide. J.O.C. 1982;47:3153–3163. doi: 10.1021/jo00137a025. [DOI] [Google Scholar]
- 38.Hancock R.W.E. Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001;1:156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 39.Dawson R.M., Liu C.Q. Properties and applications of antimicrobial peptides in biodefense against biological warfare threat agents. Critical Rev. Microbiol. 2008;34:89–107. doi: 10.1080/10408410802143808. [DOI] [PubMed] [Google Scholar]
- 40.Conlon J.M., Al-Ghaferi N., Abraham B., Leprince J. Strategies for development of naturally occurring antimicrobial peptides into therapeutically valuable anti-infective agents. Methods. 2007;42:349–357. doi: 10.1016/j.ymeth.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Fisher D., Li Y. Ahlenmeyer, B., Krieglstein J., Kissel T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/S0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 42.Malik N., Wiwattanapatapee R., Klopsch R., Lorentz K., Frey. H., Weener J.W., Meijer E.W., Paulus W., Duncan R. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled PAMAM dendrimers in vivo. J. Control. Release. 2000;65:133–148. doi: 10.1016/S0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 43.Kuroda K., Caputo G.A., DeGrado W.F. The role of hydrophobicity in the antimicrobial and hemolytic activities of polymethacrylate derivatives. Chem. Eur. J. 2009;15:1123–1133. doi: 10.1002/chem.200801523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.CLSI. ReferenceMethod for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. 2nd. CLSI; Wayne, PA, USA: 2002. M27-A2. [Google Scholar]
- 45.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. CLSI; Wayne, Pa, USA: 2006. M07-A7, M100-S17. [Google Scholar]
- 46.Rao S.L.N. Chemical synthesis of N-β-oxalyl-l-α,β-diaminopropionic acid and optical specificity in its neurotoxic action. Biochemistry. 1975;14:23–31. doi: 10.1021/bi00694a031. [DOI] [PubMed] [Google Scholar]