Abstract
A large variety of different interactions between the chalcogen atoms, Q, occur in the solid state structures of polyselenides and polytellurides, including both molecular and infinite units. The simplest motifs are classical Q22– dumbbells and nonlinear Qn2– chains (n = 3, 4, 5, ..), e.g. found in alkali metal polychalcogenides. In addition, nonclassical so-called hypervalent motifs exist in the form of linear Q34– units or within larger units such as Q44– and Q54–. Infinitely extended Q units include zigzag, cis/trans and linear chains, as well as planar and slightly puckered layers. Several of those are susceptible to Peierls distortions, leading to the formation of both commensurate and incommensurate superstructures and anomalies in transport properties, including metal-nonmetal transitions.
Keywords: selenium, tellurium, bonding, hypervalent
1. Introduction
Solid state materials based on chalcogenides, i.e., sulfides, selenides and tellurides, play a large role in today’s society. Examples include semiconductor devices, e.g. in solid state electronics [1], fast-ion conductors [2,3], rechargeable batteries [4], data storage including phase-change materials [5,6,7], chalcogenide glasses [8], and the thermoelectric energy conversion [9,10,11,12]. Polychalcogenides, like the potential thermoelectric material HfTe5, are materials that comprise homonuclear bonds between negatively charged chalcogen atoms, e.g. a Se–Se bond within Se22– pairs of Rb2Se2 [13]. These bonds occur in chalcogen-rich materials, where the chalcogen atom cannot be reduced to attain the closed-shell formation as in Q2–. For example, Se carries a charge of –1 in Rb2Se2, like O in SrO2, and therefore forms one Se–Se bond. As such, Rb2Se2 is a typical Zintl phase. Zintl phases AXx consist of an electropositive element A (here: Rb) and a more electronegative element X of the later (post transition) main groups (here: Se). Assuming a complete charge transfer of all of A’s valence-electrons to X, the cation Az+ possesses a full octet, and the (formal) anion Xz/x- attains the octet by forming homonuclear X–X bonds in addition to the reduction by A. In most cases, these bonds are classical single bonds (two-center two-electron, 2c-2e), i.e. exactly one bond is formed for each electron missing to complete the octet of X [14,15,16].
The rich structural chemistry in particular of the polyselenides and polytellurides is the scope of this review, going beyond the common 2c-2e bonds: hypervalent interactions as found in XeF2 [17] or SF6 [18] are often observed in various fragments of these materials [19]. In addition, weaker fractional bonds or cohesive interactions of different lengths, as in elemental tellurium, render the Se/Te substructures intriguingly complex [20,21], which in turn may be beneficial for the thermoelectric energy conversion [22]. Four reviews about polychalcogenides from the years 1995 to 2000 underline the importance of this field [20,23,24,25].
2. Results and Discussion
2.1. Molecular units in polyselenides and polytellurides
2.1.1. Oligomeric Qn2– motifs
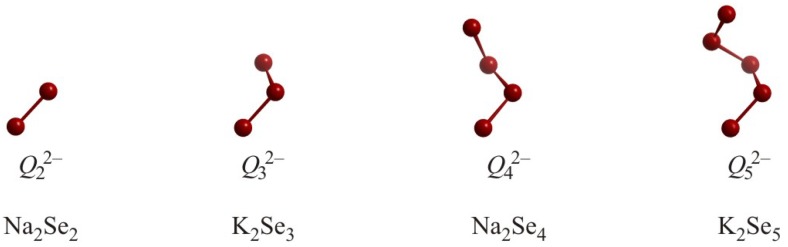
The simplest polychalcogenide motif is the dumbbell unit Q22–, occurring for example in Na2Se2 [26], Rb2Se2 and Rb2Te2 with Se–Se and Te–Te distances of 2.38 Å, 2.47 Å and 2.78 Å, respectively (Figure 1). These dumbbells are also found in organic compounds with similar bond lengths, e.g. in [N(CH3)4]2Te2 (dTe–Te = 2.74 Å) [27] and [Na(CH3OH)3]2Te2 [28]. Assigning charges is straightforward in these examples, e.g. (Na+)2Se22–, with Se22– being isoelectronic to Br2 and I2, with 14 valence-electrons for the dumbbells, which are therefore held together by one 2c-2e bond. While generally these distances compare well with the sum of the single bond radii, rSe = 1.17 Å and rTe = 1.37 Å [29], the influence of the cations is evident, for the Se–Se bond in Rb2Se2 is approximately 0.1 Å longer than in Na2Se2.
Figure 1.
From left to right: Oligomeric Qn2– motifs in Na2Se2, K2Se3, Na2Se4, and K2Se5.
The dumbbells can formally be extended to oligomeric chain-like units Qn2– (with n = 3, 4, 5, 6, 12, 13) by adding neutral Q atoms to the chain, wherein the terminal Q atoms remain negatively charged. Each neutral Q atom participates in two 2c-2e bonds, comparable to the elemental structures of selenium and tellurium. Se32– groups (with one central neutral Se atom) occur for example in, K2Se3 [30], Sr2SnSe5 [31] and Ba2SnSe5 [32], with bond lengths of approximately 2.4 Å, and Te32– groups in K2Te3 with dTe–Te = 2.80 Å [33] and in Ba7Au2Te14 with dTe–Te = 2.89 Å [34]. It is evident that the lengths of regular single (2c-2e) bonds may vary substantially, noting that these bonds are significantly elongated compared to 2× rSe = 2.34 Å and 2× rTe = 2.74 Å, respectively.
The Q32– anions are bent, ideally exhibiting C2v symmetry, with typical Q–Q–Q bond angles of 105° - 110°, in accordance with the VSEPR concept, which suggests tetrahedral arrangement of the two bonds and two free electron pairs around the central Q atom. The packing of the three-dimensional structure may cause a severe distortion of the angle as well, e.g. down to 92° in Ba7Au2Te14.
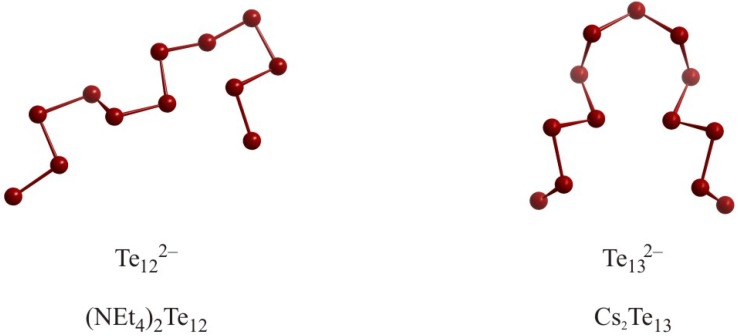
Anions with larger n, such as Se42– in Na2Se4 (dSe–Se = 2.35 Å and 2.36 Å) [35], Te42– in (Ph4P)2Te4∙2CH3OH (dTe–Te = 2.72 Å and 2.76 Å) [36], Se52– in K2Se5 (2.34 Å ≤ dSe–Se ≤ 2.37 Å) [37], and Se62– in [Me3N(CH2)13CH3]Se6 (2.27 Å ≤ dSe–Se ≤ 2.35 Å) [38] are best described as oligomeric zigzag or helical chain fragments. Larger chain-like polychalcogenide anions, though extremely rare, do exist, like Te122– in [N(C2H5)4]2Te12 [39] and Te132– in Cs2Te13 [40] (Figure 2), and they are often - as in these two examples - interconnected via longer interchain interactions (here: 3.14 Å and 3.18 Å). These interactions are much shorter than twice the van der Waals radius and will be discussed later in this review.
Figure 2.
Oligomeric Ten2– motifs in (NEt4)2Te12 (left) and Cs2Te13 (right).
2.1.2. Oligomeric Qn4– motifs
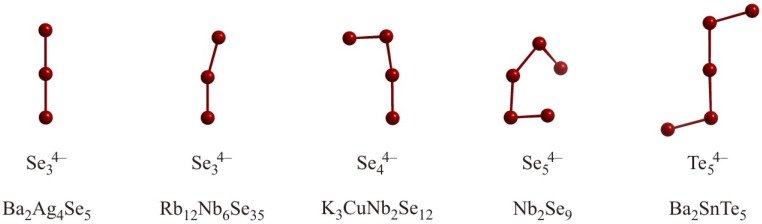
Since the Qn4– fragments comprise two more valence-electrons than their Qn2– counterparts, they cannot contain the same 2c-2e bonds. n = 2 is a hypothetical case only, as it would correspond to two isolated closed-shell Q2– anions. n = 3 is realized in Se34– units of Ba2Ag4Se5 [41], and Rb12Nb6Se35 [42]. In the former, Se34– exhibits the highest symmetry possible, namely linearity and equidistant interactions, thus point group D∞h, while Rb12Nb6Se35 is comprised of two differently distorted, almost linear Se34– units of C2v and C1 symmetry, respectively. In these two compounds, the Se–Se–Se bond angle of Se34– varies between 180° and 164°, and the Se–Se bond lengths between 2.59 Å and 2.77 Å (Figure 3).
Figure 3.
From left to right: Se34– (D∞h), Se34– (C2v), Se44–, cis-Se54– and trans-Te54–.
Ba2Ag4Se5 contains two isolated Se2– anions and one Se34– per formula unit, according to the ionic formulation (Ba2+)2(Ag+)4Se34–(Se2–)2. Electronic structure calculations and electrical conductivity measurements confirmed the semiconducting, hence electron precise character of this compound [41]. Rb12Nb6Se35 is a special case, containing both Se32– and Se34– units, according to the formula (Rb+)12(Nb5+)6(Se32–)2(Se22–)7(Se34–)3(Se2–)6. Since diffuse reflectance measurements supported the semiconducting and thus closed-shell character of Rb12Nb6Se35 [42], this treatment of Rb12Nb6Se35 within the Zintl concept is justified.
With 22 valence-electrons, Se34– is isoelectronic with hypervalent linear XeF2 and I3–. Several examples are known that contain the linear I3– motif, including CsI3 [43] and [Ph4As]I3 [44]. Ideally the two I–I distances are equivalent, with a bond angle of 180° as in [Ph4As]I3 (dI–I = 2.90 Å), but deviations from the centrosymmetric arrangement are often found with smaller cations, like in CsI3 with I–I bonds of 2.84 Å and 3.04 Å and a bond angle of 178°. The linear arrangement is well understood based on Rundle’s model [45], which treats the s orbitals as well as the p orbitals of π symmetry as lone pairs. Then the frontier orbital set consists of one filled σ bonding, one filled nonbonding, and one empty σ antibonding molecular orbital, formed by the pz orbitals when z corresponds to the molecular axis. The nonbonding orbital contains a nodal plane at the center of the triatomic unit, resulting in a three-center-four-electron (3c-4e) bond. The validity of Rundle’s model waswas - in principle - confirmed for Se34– via Gaussian calculations using the B3LYP functional [41].
Since the 3c-4e bonds are electron deficient, containing only one bonding molecular orbital for two bonds (which is why they are often called "half" bonds), they are longer than the regular 2c-2e bonds. Correspondingly, the I–I single bond in I2 of 2.76 Å is much shorter than the above-mentioned I–I bonds (2.84 Å and 3.04 Å), and Se–Se single bonds (2.34 Å) are shorter than the Se–Se bonds of Se34– (2× 2.77 Å in Ba2Ag4Se5 and 2.59 Å - 2.65 Å in Rb12Nb6Se35). In Rb12Nb6Se35, the bond angles of approximately 164° deviate substantially from linearity, which is likely caused by the packing effect. Despite the distortion, the Se34– unit of Rb12Nb6Se35 can easily be distinguished from the Se32– unit occurring in the same structure, which shows bond distances of 2.39 Å and a bond angle of 94°.
While no isolated, linear hypervalent Te34– anion has been reported so far, the isoelectronic linear units P37–, As37–, Sb37–, and Bi37– all occur in the numerous representatives of the Ca14AlSb11 type [46], including Ca14-xEuxMnSb11, which exhibits colossal magnetoresistance [47], and the high temperature thermoelectric Yb14MnSb11 [48].
The only Q44– representative known to date is Se44– in K3CuNb2Se12 [49], which is basically an almost linear Se34– unit (bond angle: 166°) with an additional Se atom attached via a single bond to one end. Therefore, its ideal valence-electron number is 28, namely 22 for the Se34– fragment plus six for the additional Se atom. Within this model, we interpret the two bonds of the linear part as 3c-4e bonds and the exo bond as 2c-2e. This is in accord with the 2.73 Å and 2.54 Å distances for the (pseudo) collinear bonds, and the short 2.39 Å bond to the fourth Se atom with a bond angle of 93°. K3CuNb2Se12 is another example for a complex selenide with three topologically different Se units, namely tetrameric Se44–, Se22– pairs, and isolated Se2–, according to (K+)3Cu+(Nb5+)2Se44–(Se22–)3(Se2–)2.
Representatives for Qn4– with n = 5 exist both among selenides and tellurides. In all cases, the Q54– units contain a central (almost) linear hypervalent unit, with the two remaining Q atoms attached via a single bond to both ends of the linear part, like the fourth atom in Se44–. By analogy, we conclude an ideal valence-electron number of 34, namely 22 for the Q34– fragment plus 2× six for the two additional Q atoms, hence Q54–: 5× 6 valence-electrons (from the neutral Q atoms) plus four for the negative charges equals 34.
The two terminal Q atoms may be in cis or trans conformation. The cis variant is realized in the heavily distorted Se54– of Nb2Se9, wherein the hypervalent bonds of 2.64 Å and 2.66 Å exhibit a bond angle of only 143°, and the terminal single bonds of 2.36 Å/2.37 Å are both connected with a 83° bond angle. Considering the presence of a 2.90 Å Nb–Nb bond, Nb is most likely in the 4+ state, and noting the presence of two Se22– pairs per formula unit, (Nb4+)2Se54–(Se22–)2 is also a closed-shell material [50].
The trans conformation is only known within the tellurides, occurring in NaTe ((Na+)6Te54–Te2–) [51] and Ba2SnTe5 ((Ba2+)6(Sn4+)3Te54–(Te2–)10) [52]. An isoelectronic cationic variant is I5+ in I5AsF6 [53], while analogous pnictides have not been reported yet.
The largest representative of the series Qn4– currently known is Te74–, coordinated to an Ag+ or Hg2+ cation via the central Te and the two terminal Te atoms [54,55]. The Te74– anion can formally be constructed from adding two neutral Te atoms to a cis-Te54–. In case of the Ag compound, the central Te3 unit contains hypervalent bonds of 2.87 Å and 3.23 Å with a bond angle of 174°, and the additional Te atoms form regular 2c-2e bonds of 2.71 Å to 2.76 Å.
Two interpenetrating Te34– units forming an almost square planar Te56– unit with a central four-bonded Te atom are found in K2SnTe5 with distances of 3.02 Å and 3.06 Å [56]. These units can polymerize [57], the products of which will be discussed later in this manuscript. Adding two more Te atoms via single bonds, for example, results in bicyclic Te72– [58].
2.2. Infinite motifs in polyselenides and polytellurides
2.2.1. One-dimensional motifs: chains
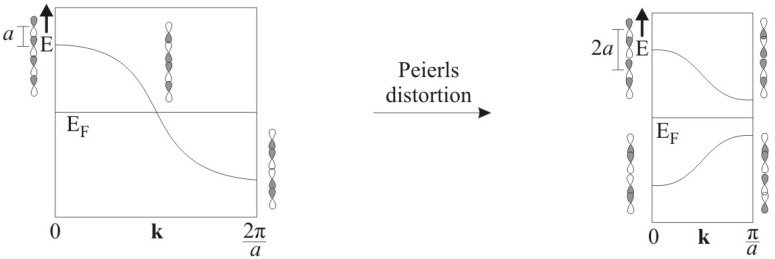
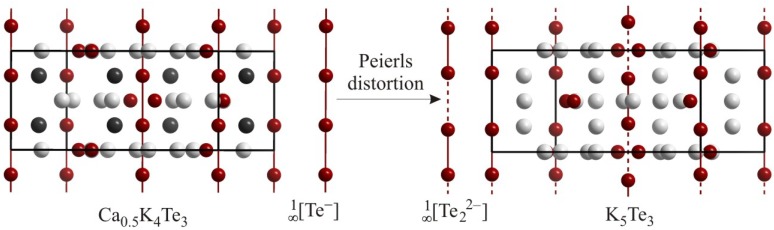
The above-mentioned fragments Qn2– and Qn4– can form higher dimensional arrays such as infinite chains, ribbons or layers. At least two bonds are required per Q atom in infinite chains, but the Q atoms may only participate in two 2c-2e bonds, when their oxidation state is 0. Such neutral chains exist in the elements selenium and tellurium. On the other hand, Te is especially well known for its ability to form electron deficient multicenter bonds, thereby producing linear Te–] chains. These chains occur in CuTe [59], UTe2 [60] and Ca0.66K4Te3 [61] with typical intrachain distances between 3.0 Å and 3.1 Å. These interactions are delocalized 2c-1e (“half”) bonds, wherein one p orbital is half-filled, indicating a one-dimensional metal.
Such linear equidistant chains may undergo a Peierls distortion, i.e. exhibit alternating short and long distances, occurring with a metal-insulator transition (Figure 5) [62]. There are also examples of distorted linear chains of Te atoms with a formal charge of –1 such as in Cs5Te3 [63] or K5Te3 [64]. In these compounds, the Te atom chain features two different Te–Te distances, one of around 2.8 Å and the other larger than 3.5 Å. Thus, a description as Te22–] is more appropriate for these compounds, wherein van der Waals forces connect the pairs to linear chains.
Figure 5.
Peierls distortion of a linear Te–] chain.
–] chain.
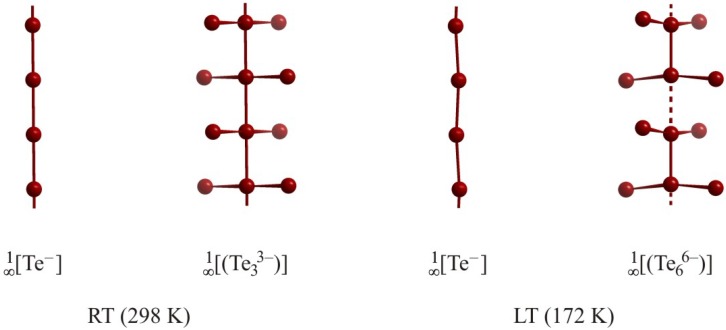
The dimorph TlTe is a nice example of a material exhibiting different Te atom chains [65]. The room temperature (RT) modification of TlTe represents an equidistant Te–] chain with Te–Te distances of 3.08 Å. A parallel running, second linear equidistant Te atom chain within the same structure is more complex, as two additional Te atoms are connected to each chain atom via hypervalent bonds of 3.01 Å, yielding a Te33–] chain (Figure 6). Thus, the RT modification may be written as (Tl+)4Te33–Te–. In the low temperature (LT, 172 K) modification, both chains are distorted. Every second Te33–] chain is not equidistant with alternation distances of 2.86 Å and 3.30 Å, while the other Te33–] remain undistorted. Moreover, the linear equidistant Te–] chain of the RT form is slightly bent in the LT form. Overall, the LT phase may be viewed as (Tl+)16Te66–(Te33–)2(Te–)4.
Figure 6.
Various Te atom chains of TlTe.
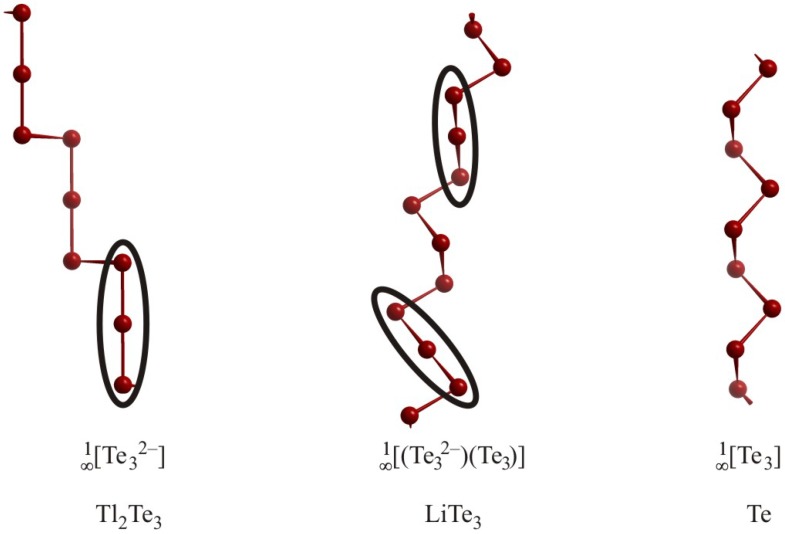
By reducing the number of valence electrons from 7 per atom in such a one-dimensional unit, the conditions become more and more complicated. The Te atoms in Tl2Te3, for example, have a valence electron concentration (VEC(Te)) of 6⅔, forming an infinite Te chain that can be described as polymerized linear Te34– fragments (Figure 7). The bonds between the Te34– units decrease the negative charges, leading to the description Te32–] [66]. Two different distances were found in this chain due to different bonding situations. The short distance of 2.83 Å, a typical length for a 2c-2e Te–Te bond, is found between the Te atoms of charge –½, the second distance (3.02 Å) occurs between Te1– and Te½–, indicative of a 3c-4e bond.
Figure 7.
Infinite Te atom chains with different VEC(Te) in Tl2Te3 (left), LiTe3 (center) and Te (right).
In LiTe3 [67], the VEC(Te) within the chain is lowered to 6⅓. Consequently, this chain exhibits parts known from the Te atom chains in Tl2Te3 as well as from elemental Te [68], and can therefore be viewed as (Te3)(Te32–)]. The distances within this chain are 2.85 Å for the 2c-2e bonds in the neutral Te3 part of the chain, 3.02 Å for the 3c-4e bond in the Te32– unit and 2.91 Å for the bond connecting these fragments.
2.2.2. One-dimensional motifs: Ribbons
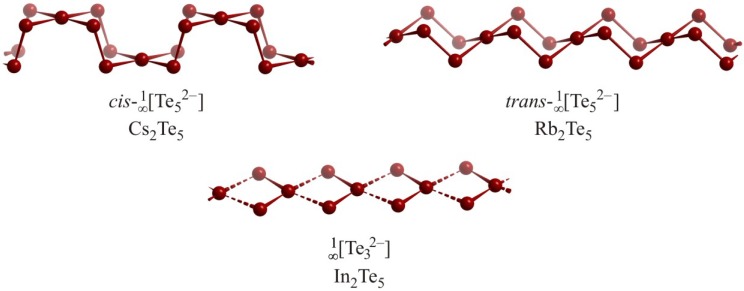
Only very few polychalcogenides exist that contain one-dimensionally extended Q atom substructures with more than two Q–Q bonds per Q atom. The dialkali pentatellurides show two different forms of intercondensation of above-mentioned Te56– squares to yield Te52–] ribbons with VEC(Te) = 6.4, namely the cis conformation in Cs2Te5 [69] and the trans conformation in Rb2Te5 [70] (Figure 8).
Figure 8.
Infinite Te ribbons with different modifications in Cs2Te5 (top left), Rb2Te5 (top right) and In2Te5 (bottom center).
The distances in these ribbons range from 2.78 Å in Rb2Te5 and 2.77 Å in Cs2Te5 for the 2c-2e bond between the Te5 units to 3.04 Å in Rb2Te5 and 3.05 Å in Cs2Te5 for the 3c-4e bond within the Te5 units. In2Te5 [71] possesses a similar unit with a higher VEC(Te) of 6⅔, which causes a distortion, namely an alternation of short and long Te–Te distances of 2.83 Å (solid lines) and 3.36 Å (dashed lines). Therefore it is best described as a one-dimensional arrangement of Te32– fragments.
2.2.3. Two-dimensional motifs: Layers
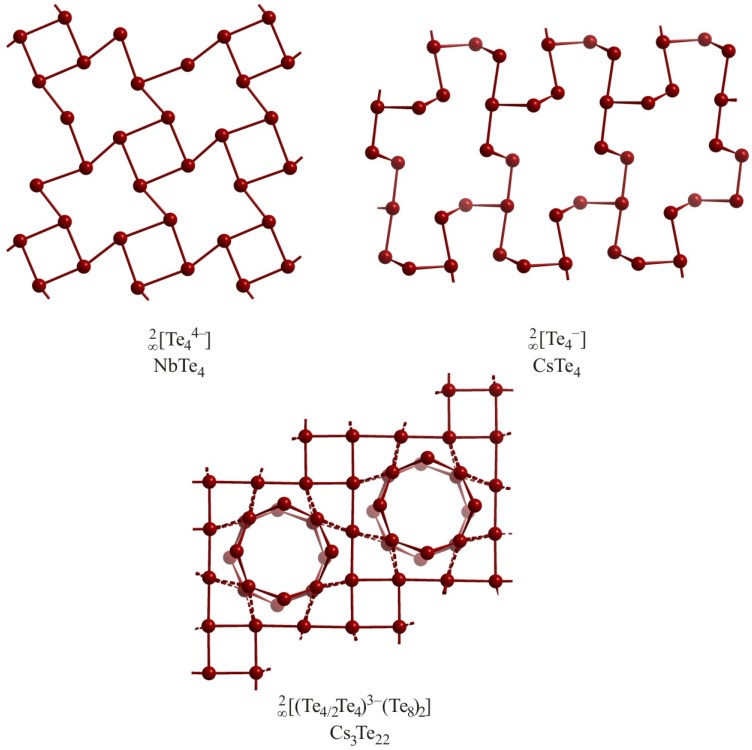
Compounds with hypervalently bonded Te atoms that are arranged in planar or puckered layers are often dominated by T-shaped fragments. These building blocks are then either directly connected to each other or bridged via other Te atoms. An overview of (schematic) T nets was published in the year 2004 [72]. One of the simplest examples of a T network containing Te atoms can be found in the planar layers of NbTe4 [73]. Each Te atom in this layer is surrounded by three other Te atoms in form of a heavily distorted T. Four-membered rings, exhibiting Te–Te distances of 3.30 Å, are connected to surrounding four-membered rings via shorter bonds of 2.88 Å (Figure 9). This hole-style arrangement in NbTe4 is subject to a distortion, driven by a charge density wave along the Nb atom chains perpendicular to the layer of interest. Considering the large difference of 0.4 Å between these distances, one could view this layer as loosely connected Te22– units. This is in contrast to the Sb atom layer of Hf5Sb9 [74], wherein all bonds are between 2.99 Å and 3.03 Å, i.e. all bonds of that T net are electron deficient multicenter bonds [75].
Figure 9.
Different T nets in NbTe4 (top left), CsTe4 (top right) and Cs3Te22 (bottom center).
CsTe4 [76] also features T-shaped Te atom units forming a layer, which is comprised of a polymerized Te44– anion. This anion loses, due to this polymerization, three charges and builds a puckered layer that could be described as Te4–]. The distances in the original T-shaped fragment in this layer are 2.92 Å and 3.14 Å for the collinear bonds, and 2.84 Å for the perpendicular bond. The connection between two (similar or different) fragments is slightly shorter (2.76 Å). The majority of the Te atoms are twofold coordinated and provide bonding angles between 96° and 103°. Therefore, the bonds within the Te44– fragments could be considered as asymmetric 3c-4e bonds and the remaining bonds as 2c-2e bonds.
Cs3Te22 provides an example of an electron-poor layer of Te atoms [77]. Cs3Te22 contains also neutral eight-membered Te rings, and can be described as (Cs+)3(Te8)2(Te4Te4/2)3–. Its planar Te4Te4/23–] layer consists of two- and threefold connected Te atoms, with the former being linearly coordinated and the latter T-shaped. The linearly bonded atoms interconnect the Te4 squares comprising the T connected Te atoms. All Te–Te distances are between 3.00 Å and 3.07 Å. Band structure calculations indicate that this layer would be semiconducting with a charge of –4, but its actual charge of –3 renders it metallic [78].
2.2.4. Two-dimensional motifs: Chains connected to layers
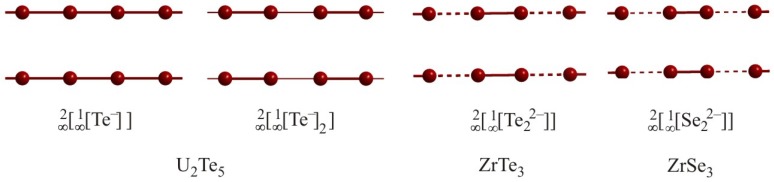
The previously discussed lower dimensional fragments can be connected to two-dimensional layers in several ways. The binary chalcogenides UTe2 [79], U2Te5 [80], α-UTe3 [81] and ZrQ3 [82,83] all contain linear chains aligned to form planar layers (Figure 10).
Figure 10.
Planar layers of linear chains in U2Te5 (left), ZrTe3 (center) and ZrSe3 (right).
The structure of U2Te5 contains two different kinds of planar Te atom layers, i.e. Te–]] and Te–)2]]. The Te– layer is sandwiched between two puckered [UTe] double layers, while two Te–)2]] layers are separated by a van der Waals gap and sandwiched between puckered double slabs. Both layers are comprised of linear chains with interchain distances of 4.18 Å. Within the Te–]] layer, almost equidistant intrachain bond lengths of 3.03 Å and 3.05 Å occur, while the Te–)2]] layer exhibits Te22– pairs (2.90 Å) interconnected by longer interactions of 3.18 Å to a linear chain.
A very similar almost equidistant chain is found in UTe2 as well (3.05 Å and 3.08 Å), while the distortion in α-UTe3 is more severe with alternating distances of 2.75 Å and 3.35 Å. Similarly, the isostructural ZrTe3 contains Te chains with alternating intrachain distances of 2.79 Å and 3.11 Å and interchain distances of 3.93 Å. The most severe distortion is present in the analogous ZrSe3 with intrachain distances of 2.34 Å and 3.06 Å and an interchain distance of 3.77 Å.
Furthermore, many more complex variants, mostly tellurides, are known in this class, including UTe5 [84], incommensurately modulated ALn3Te8 (A = K, Rb, Cs; Ln = La - Nd) [85], and LnSeTe2 (Ln = La - Nd, Sm) [86], which exhibit corrugated chains interconnected to planar or slightly puckered layers. A detailed discussion of all these materials would go beyond the scope of this review; square nets and their distortions were featured in a review published in 2002 [21].
2.2.5. Two-dimensional motifs: oligomeric units connected to layers
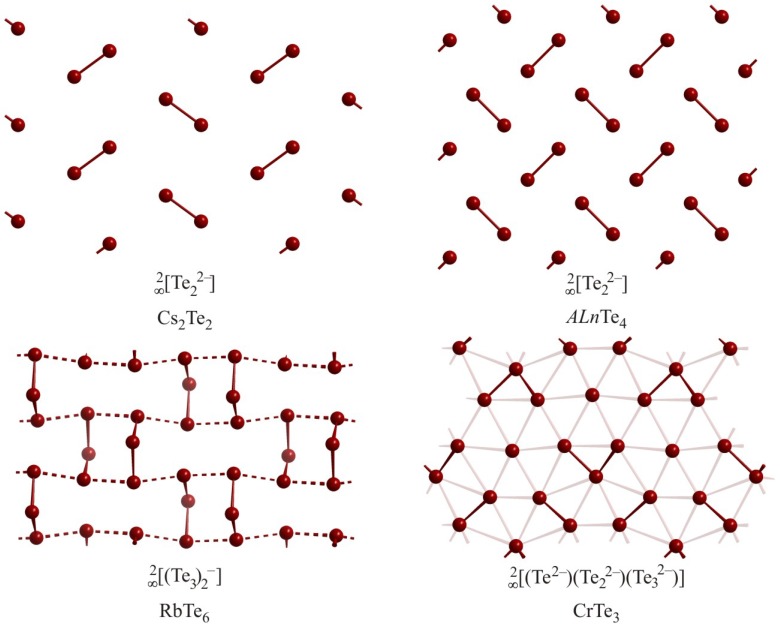
An enormous variety of compounds contain fragments arranged to form layers, e.g. the Q22– and Q23– units. The structure of Cs2Te2 [87] features Te22– dumbbells with a Te–Te distance of 2.78 Å, arranged in a herringbone-like pattern (Figure 11), and stacked between layers of cesium atoms. The distances between these dumbbells of 4.71 Å and 5.01 Å are too long for bonding interactions. Another planar layer formation only consisting of Te22– dumbbells (dTe–Te = 2.78 Å) occurs in the crystal structure of ALnTe4 [88,89] with an interpair distance of 3.50 Å, which is shorter than a van der Waals contact.
Figure 11.
Layers of oligomeric Te atom fragments in Cs2Te2 (top left), ALnTe4 (top right), RbTe6 (bottom left), and CrTe3 (bottom right).
The structure of RbTe6 [90] features a Te6– layer that is puckered because of its bent Te3 units, where the central Te atom takes a position either beneath or above the layer plane. Within these bent Te3 units, the Te–Te distances are 2.78 Å and 2.79 Å, respectively, with a bond angle of 102°, while the distances between these units are 3.20 Å and 3.21 Å. The charge of the Te6– layer cannot be readily understood; ignoring the 3.2 Å distances would indicate a charge of –2 for each Te3 unit, while treating them as hypervalent half bonds would ideally result in neutral Te3 units.
The structure of CrTe3 contains Te atoms in three different oxidation states in a nearly planar layer [91]. The Te33–] layer of this compound contains Te2– ions and Te22– and Te32– units, with typical Te–Te distances of 2.82 Å. The assignment of charges is straightforward and yields a balanced formula for Cr in its +3 state: (Cr3+)2Te32–Te22–Te2–.
2.2.6. Three-dimensional motifs
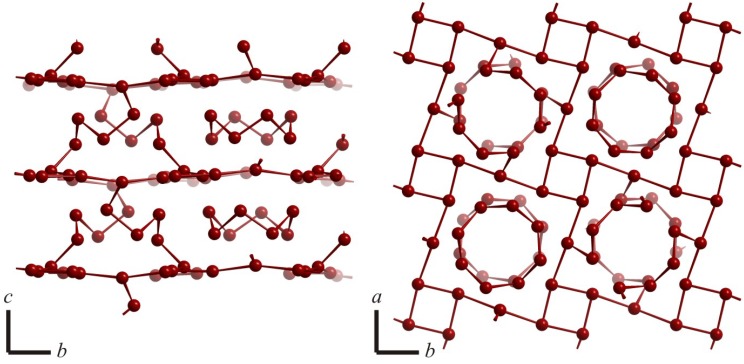
The only known compound that is comprised of a three-dimensional, covalently bonded network of Te atoms is Cs4Te28 [40]. The Te atom network is similar to the one in Cs3Te22, but in this case, half of the Te8 rings are broken into Te4 units that connect to one of the former linearly bonded atoms (Figure 12), which then assumes an oxidation state of zero.
Figure 12.
Three-dimensional network of Te atoms in Cs4Te28.
This covalently bonded three-dimensional network could be considered as (Cs+)4Te8[(Te4)4/2(Te4Te4/2)24–], wherein the neutral Te8 ring is comprised of typical 2c-2e bonds (2.79 Å - 2.83 Å) like elemental sulfur. The Te–Te bonds of the connecting (also neutral) Te4 fragments are comparable (2.77 Å and 2.79 Å), as are the connections of these Te4 units of 2.80 Å with the layer of Te atoms. As a result of the connection with the Te4 units, all Te atoms of the layer are connected in a T-like shape exhibiting hypervalent bonds >2.9 Å, in contrast to the twofold linearly connected Te atoms in the structure Cs3Te22.
3. Conclusions
An overview of the variety of Se–Se and Te–Te interactions occurring in the solid state of both inorganic and organic polychalcogenides was presented. In contrast to polysulfides, the Se and Te atoms are capable of forming electron deficient multicenter (hypervalent) bonds. This adds significantly to the connectivity possibilities, e.g. the formation of T-shaped Te motifs or linear Se/Te fragments, which in turn increases the complexity of these polychalcogenides, a desired feature for, e.g., thermoelectric materials.
The tendency of Te towards higher coordination numbers is reflected in the higher abundance of complex Te atom layers, compared to Se. However, within the oligomeric units, the selenides and tellurides are quite comparable, so that more polyselenides with related two-dimensional motifs are likely to be uncovered in the near future as well.
Figure 4.
Infinite Te1– chains in Ca0.5K4Te3 (left) and K5Te3 (right).
Acknowledgements
Financial support from NSERC, CFI, OIT (Ontario Distinguished Researcher Award for H.K.), and the Canada Research Chair program (CRC for H.K.) is appreciated. Acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund for partial support of this research.
Footnotes
Sample Availability: Not available.
References and Notes
- 1.Kosbar L.L., Murray C.E., Copel M., Afzali A., Mitzi D.B. High-mobility ultrathin semiconducting films prepared by spin coating. Nature. 2004;428:299–303. doi: 10.1038/nature02389. [DOI] [PubMed] [Google Scholar]
- 2.Lange S., Nilges T. Ag10Te4Br3: A new silver(I) (poly)chalcogenide halide solid electrolyte. Chem. Mater. 2006;18:2538–2544. doi: 10.1021/cm060226m. [DOI] [Google Scholar]
- 3.Zheng N., Bu X., Feng P. Synthetic design of crystalline inorganic chalcogenides exhibiting fast-ion conductivity. Nature. 2003;426:428–432. doi: 10.1038/nature02159. [DOI] [PubMed] [Google Scholar]
- 4.Tarascon J.-M., Armand M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001;414:359–367. doi: 10.1038/35104644. [DOI] [PubMed] [Google Scholar]
- 5.Atwood G. Phase-change materials for electronic memories. Science. 2008;321:210–211. doi: 10.1126/science.1160231. [DOI] [PubMed] [Google Scholar]
- 6.Lencer D., Salinga M., Grabowski B., Hickel T., Neugebauer J., Wuttig M. A map for phase-change materials. Nat. Mater. 2008;7:972–977. doi: 10.1038/nmat2330. [DOI] [PubMed] [Google Scholar]
- 7.Yamada N., Wuttig M. Phase-change materials for rewriteable data storage. Nat. Mater. 2007;6:824–832. doi: 10.1038/nmat2009. [DOI] [PubMed] [Google Scholar]
- 8.Zakery A., Elliott S.R. Optical properties and applications of chalcogenide glasses: A review. J. Non-Cryst. Sol. 2003;330:1–12. doi: 10.1016/j.jnoncrysol.2003.08.064. [DOI] [Google Scholar]
- 9.Lowhorn N.D., Tritt T.M., Abbott E.E., Kolis J.W. Enhancement of the power factor of the transition metal pentatelluride HfTe5 by rare-earth doping. Appl. Phys. Lett. 2006;88:022101:1–022101:3. doi: 10.1063/1.2162703. [DOI] [Google Scholar]
- 10.Rowe D.M. Thermoelectrics Handbook: Macro to Nano. CRC Press, Taylor & Francis Group; Boca Raton, FL, USA: 2006. [Google Scholar]
- 11.Sootsman J.R., Kong H., Uher C., D’Angelo J.J., Wu C.-I., Hogan T.P., Caillat T., Kanatzidis M.G. Large enhancements in the thermoelectric power factor of bulk PbTe at high temperature by synergistic nanostructuring. Angew. Chem. Int. Ed. 2008;47:8618–8622. doi: 10.1002/anie.200803934. [DOI] [PubMed] [Google Scholar]
- 12.Xu H., Kleinke K.M., Holgate T., Zhang H., Su Z., Tritt T.M., Kleinke H. Thermoelectric performance of NiyMo3Sb7-xTex (y ≤ 0.1, 1.5 ≤ x ≤ 1.7) J. Appl. Phys. 2009;105:053703:1–053703:5. doi: 10.1063/1.3086641. [DOI] [Google Scholar]
- 13.Böttcher P., Getzschmann J., Keller R. Zur Kenntnis der Dialkalimetalldichalkogenide β-Na2S2, K2S2, α-Rb2S2, β-Rb2S2, K2Se2, Rb2Se2, α-K2Te2, β-K2Te2 und Rb2Te2. Z. Anorg. Allg. Chem. 1993;619:476–478. doi: 10.1002/zaac.19936190309. [DOI] [Google Scholar]
- 14.Schäfer H., Eisenmann B., Müller W. Zintl Phases: Transitions between metallic and ionic bonding. Angew. Chem. Int. Ed. Engl. 1973;12:694–712. doi: 10.1002/anie.197306941. [DOI] [Google Scholar]
- 15.Nesper R. Zintl-phases containing Li. Prog. Solid State Chem. 1990;20:1–45. doi: 10.1016/0079-6786(90)90006-2. [DOI] [Google Scholar]
- 16.Kauzlarich S.M. Chemistry, Structure, and Bonding of Zintl Phases and Ions. VCH; New York, NY, USA: 1996. [Google Scholar]
- 17.Siegel S.G. Crystallographic studies of XeF2 and XeF4. J. Am. Chem. Soc. 1963;85:240–240. doi: 10.1021/ja00885a035. [DOI] [Google Scholar]
- 18.Curnow O.J. A Simple qualitative molecular-orbital/valence-bond description for the bonding in main group "hypervalent" molecules. J. Chem. Educ. 1998;75:910–915. doi: 10.1021/ed075p910. [DOI] [Google Scholar]
- 19.Papoian G. A., Hoffmann R. Hypervalent bonding in one, two, and three dimensions: Extending the Zintl-Klemm concept to nonclassical electron-rich networks. Angew. Chem. Int. Ed. 2000;39:2408–2448. doi: 10.1002/1521-3773(20000717)39:14<2408::AID-ANIE2408>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 20.Böttcher P. Tellurium-Rich Tellurides. Angew. Chem. Int. Ed. Engl. 1988;27:759–772. doi: 10.1002/anie.198807593. [DOI] [Google Scholar]
- 21.Patschke R., Kanatzidis M.G. Polytelluride compounds containing distorted nets of tellurium. Phys. Chem. Chem. Phys. 2002;4:3266–3281. doi: 10.1039/b201162j. [DOI] [Google Scholar]
- 22.Xu J., Kleinke H. Unusual Sb–Sb bonding in high temperature thermoelectric materials. J. Comput. Chem. 2008;29:2134–2143. doi: 10.1002/jcc.20950. [DOI] [PubMed] [Google Scholar]
- 23.Kanatzidis M.G. From cyclo-Te8 to Texn- Sheets: Are Nonclassical Polytellurides More Classical than We Thought? Angew. Chem. Int. Ed. Engl. 1995;34:2109–2111. doi: 10.1002/anie.199521091. [DOI] [Google Scholar]
- 24.Böttcher P., Doert T. Chalcogen-rich chalcogenides: from the first ideas to a still growing field of research. Phosphorus, Sulfur, Silicon. 1998;136-138:255–282. doi: 10.1080/10426509808545950. [DOI] [Google Scholar]
- 25.Smith D.M., Ibers J.A. Syntheses and solid-state structural chemistry of polytelluride anions. Coord. Chem. Rev. 2000;200-202:187–205. doi: 10.1016/S0010-8545(00)00256-3. [DOI] [Google Scholar]
- 26.Föppl H., Busmann E., Frorath F.K. Die Kristallstrukturen von α-Na2S2 und K2S2, β-Na2S2 und Na2Se2. Z. Anorg. Allg. Chem. 1962;314:12–29. doi: 10.1002/zaac.19623140104. [DOI] [Google Scholar]
- 27.Batchelor R.J., Einstein F.W.B., Gay I.D., Jones C.H.W., Sharma R.D. Syntheses and solid-state NMR of tetrabutylammonium hydrogen telluride, tetramethylammonium hydrogen selenide and bis(tetramethylammonium) ditelluride and x-ray crystal structures of Me4NSeH and (Me4N)2Te2. Inorg. Chem. 1993;32:4378–4383. doi: 10.1021/ic00072a036. [DOI] [Google Scholar]
- 28.Thiele K.-H., Steinicke A., Dümichen U., Neumüller B. Darstellung und Reaktionen von Natriumtellurid, Na2Te - Kristallstruktur von [Na(CH3OH)3]2Te2. Z. Anorg. Allg. Chem. 1996;622:231–234. doi: 10.1002/zaac.19966220205. [DOI] [Google Scholar]
- 29.Pauling L. The Nature of the Chemical Bond. 3rd ed. Cornell University Press; Ithaca, NY, USA: 1948. [Google Scholar]
- 30.Böttcher P. Die Kristallstruktur von K2S3 und K2Se3. Z. Anorg. Allg. Chem. 1977;432:167–172. doi: 10.1002/zaac.19774320122. [DOI] [Google Scholar]
- 31.Assoud A., Soheilnia N., Kleinke H. Band gap tuning in new strontium seleno-stannates. Chem. Mater. 2004;16:2215–2221. doi: 10.1021/cm0499242. [DOI] [Google Scholar]
- 32.Assoud A., Soheilnia N., Kleinke H. The new semiconducting polychalcogenide Ba2SnSe5 exhibiting Se32- units and distorted SnSe6 octahedra. J. Solid State Chem. 2005;178:1087–1093. doi: 10.1016/j.jssc.2005.01.011. [DOI] [Google Scholar]
- 33.Eisenmann B., Schäfer H. K2Te3: The first binary alkali-metal polytelluride with Te32- ions. Angew. Chem. Int. Ed. Engl. 1978;17:684. doi: 10.1002/anie.197806841. [DOI] [Google Scholar]
- 34.Cui Y., Assoud A., Xu J., Kleinke H. Structures and Physical Properties of new Semiconducting gold and copper polytellurides: Ba7Au2Te14 and Ba6.76Cu2.42Te14. Inorg. Chem. 2007;46:1215–1221. doi: 10.1021/ic061616d. [DOI] [PubMed] [Google Scholar]
- 35.Getzschmann J.R., Rönsch E., Böttcher P. Crystal structure of dinatriumtetraselenide, Na2Se4. Z. Kristallogr. -NCS. 1997;212:87. [Google Scholar]
- 36.Huffman J.C., Haushalter R.C. Preparation and crystal structure of (Ph4P)2Te4·2CH3OH. Z. Anorg. Allg. Chem. 1984;518:203–209. doi: 10.1002/zaac.19845181121. [DOI] [Google Scholar]
- 37.Müller V., Frenzen G., Dehnicke K., Fenske D. Synthese, FIR-Spektren und Kristallstrukturen der Pentaselenide K2Se5 und (Na(15-Krone-5))2Se5. Z. Naturforsch. B. 1992;47:205–210. doi: 10.1515/znb-1992-0211. [DOI] [Google Scholar]
- 38.Weller F., Adel J., Dehnicke K. Polyselenide mit langkettigen Tetraalkylammoniumionen. Die Kristallstruktur von Trimethyl-tetradecyl-ammonium-hexaselenid. Z. Anorg. Allg. Chem. 1987;548:125–132. doi: 10.1002/zaac.19875480514. [DOI] [Google Scholar]
- 39.Warren C.J., Haushalter R.C., Bocarsly A.B. Electrochemical synthesis of a pseudo-two-dimensional polytelluride containing Te122- anions: Structure of [(C2H5)4N]2Te12. J. Alloys Compd. 1996;233:23–29. doi: 10.1016/0925-8388(95)01950-2. [DOI] [Google Scholar]
- 40.Sheldrick W.S., Wachhold M. Synthesis and structure of Cs2Te13 and Cs4Te28, tellurium-rich tellurides on the methanolothermal route to Cs3Te22. Chem. Commun. 1996:607–608. doi: 10.1039/cc9960000607. [DOI] [Google Scholar]
- 41.Assoud A., Xu J., Kleinke H. Structures and physical properties of new semiconducting polyselenides Ba2CuδAg4-δSe5 with unprecedented linear Se34- units. Inorg. Chem. 2007;46:9906–9911. doi: 10.1021/ic701278b. [DOI] [PubMed] [Google Scholar]
- 42.Dürichen P., Bolte M., Bensch W. Synthesis, crystal structure, and properties of polymeric Rb12Nb6Se35, a novel ternary niobium selenide consisting of infinite anionic chains built up by Nb2Se11 units containing an uncommon Se34--fragment. J. Solid State Chem. 1998;140:97–102. doi: 10.1006/jssc.1998.7869. [DOI] [Google Scholar]
- 43.Tasman H.A., Boswijk K.H. Reinvestigation of the crystal structure of CsI3. Acta Crystallogr. 1955;8:59–60. doi: 10.1107/S0365110X55000170. [DOI] [Google Scholar]
- 44.Mooney-Slater R.C.L. The triiodide ion in tetraphenylarsonium triiodide. Acta Crystallogr. 1959;12:187–196. doi: 10.1107/S0365110X5900055X. [DOI] [Google Scholar]
- 45.Rundle R.E. On the Problem Structure of XeF4 and XeF2. J. Am. Chem. Soc. 1963;85:112–113. doi: 10.1021/ja00884a026. [DOI] [Google Scholar]
- 46.Cordier G., Schäfer H., Stelter M. Darstellung und Struktur der Verbindung Ca14AlSb11. Z. Anorg. Allg. Chem. 1984;519:183–188. doi: 10.1002/zaac.19845191219. [DOI] [Google Scholar]
- 47.Kim H., Olmstead M.M., Klavins P., Webb D.J., Kauzlarich S.M. Structure, magnetism, and colossal magnetoresistance (CMR) of the ternary transition metal solid solution Ca14-xEuxMnSb11 (0 < x <14) Chem. Mater. 2002;14:3382–3390. [Google Scholar]
- 48.Brown S.R., Kauzlarich S.M., Gascoin F., Snyder G.J. Yb14MnSb11: New high efficiency thermoelectric material for power generation. Chem. Mater. 2006;18:1873–1877. doi: 10.1021/cm060261t. [DOI] [Google Scholar]
- 49.Lu Y., Ibers J.A. Synthesis and characterization of the new quaternary one-dimensional chain materials, potassium copper niobium selenides, K2CuNbSe4 and K3CuNb2Se12. Inorg. Chem. 1991;30:3317–3320. doi: 10.1021/ic00017a018. [DOI] [Google Scholar]
- 50.Sunshine S.A., Ibers J.A. Redetermination of the structures of CuTaS3 and Nb2Se9. Acta Crystallogr. C. 1987;43:1019–1022. doi: 10.1107/S0108270187093168. [DOI] [Google Scholar]
- 51.Böttcher P., Keller R. The crystal structure of NaTe and its relationship to tellurium-rich tellurides. J. Less-Common Met. 1985;109:311–321. doi: 10.1016/0022-5088(85)90062-1. [DOI] [Google Scholar]
- 52.Assoud A., Derakhshan S., Soheilnia N., Kleinke H. Electronic structure and physical properties of the semiconducting polytelluride Ba2SnTe5 with a unique Te54- unit. Chem. Mater. 2004;16:4193–4198. doi: 10.1021/cm049155m. [DOI] [Google Scholar]
- 53.Apblett A., Grein F., Johnson J.P., Passmore J., White P.S. Preparation and X-ray crystal structure of [I5+][AsF6-], an electronic structure of the I5+ cation. Inorg. Chem. 1986;25:422–426. doi: 10.1021/ic00224a009. [DOI] [Google Scholar]
- 54.McConnachie J.M., Ansari M.A., Bollinger J.C., Salm R.J., Ibers J.A. Synthesis and structural characterization of the telluroargentate [PPh4]2[NEt4][AgTe7] and telluromercurate [PPh4]2[HgTe7] compounds containing the unprecedented η3-Te74- polytelluride anion. Inorg. Chem. 1993;32:3201–3202. doi: 10.1021/ic00067a001. [DOI] [Google Scholar]
- 55.Smith D.M., Roof L.C., Ansari M.A., McConnachie J.M., Bollinger J.C., Pell M.A., Salm R.J., Ibers J.A. Synthesis, reactivity, and structural characterization of the nonclassical [MTe7]n- Anions (M = Ag, Au, n = 3; M = Hg, n = 2) Inorg. Chem. 1996;35:4999–5006. doi: 10.1021/ic951668v. [DOI] [PubMed] [Google Scholar]
- 56.Eisenmann B., Schwerer H., Schäfer H. Plane, zu Ketten verknüpfte Te56--Anionen im K2SnTe5. Mat. Res. Bull. 1983;18:383–387. doi: 10.1016/0025-5408(83)90128-9. [DOI] [Google Scholar]
- 57.Bernstein J., Hoffmann R. Hypervalent Tellurium in One-Dimensional Extended Structures Containing Te5n- Units. Inorg. Chem. 1985;24:4100–4108. doi: 10.1021/ic00218a028. [DOI] [Google Scholar]
- 58.Harbrecht B., Selmer A. Rhenium selenide tellurides Re2SexTe5-x: The structure of Re6Se8Te7. Z. Anorg. Allg. Chem. 1994;620:1861–1866. doi: 10.1002/zaac.19946201105. [DOI] [Google Scholar]
- 59.Anderko K., Schubert K. Untersuchungen im System Kupfer-Tellur. Z. Metallk. 1954;45:371–378. [Google Scholar]
- 60.Klein-Haneveld A.J., Jellinek F. The crystal structure of stoichiometric uranium ditelluride. J. Less-Common Met. 1970;21:45–49. doi: 10.1016/0022-5088(70)90163-3. [DOI] [Google Scholar]
- 61.Schewe-Miller I., Böttcher P. Ternäre Telluride mit W5Si3-Typ-Struktur: MxK4Te3 (M=Ca, Sr) J. Alloys Compd. 1992;183:98–108. doi: 10.1016/0925-8388(92)90734-Q. [DOI] [Google Scholar]
- 62.Peierls R.E. Quantum Theory of Solids. Clarendon Press; Oxford, UK: 1955. [Google Scholar]
- 63.Schewe-Miller I., Böttcher P. Synthesis and crystal structures of K5Se3, Cs5Te3 and Cs2Te. Z. Kristallogr. 1991;196:137–151. doi: 10.1524/zkri.1991.196.1-4.137. [DOI] [Google Scholar]
- 64.Schewe-Miller I., Böttcher P. Darstellung und Kristallstruktur des K5Te3. Z. Naturforsch. B. 1990;45:417–422. doi: 10.1515/znb-1990-0402. [DOI] [Google Scholar]
- 65.Stöwe K. The Phase Transition of TlTe: Crystal Structure. J. Solid State Chem. 2000;149:123–132. doi: 10.1006/jssc.1999.8509. [DOI] [Google Scholar]
- 66.Doert T., Cardoso Gil R.H., Böttcher P. The crystal structure of Tl2Te3 - a reinvestigation. Z. Anorg. Allg. Chem. 1999;625:2160–2163. doi: 10.1002/(SICI)1521-3749(199912)625:12<2160::AID-ZAAC2160>3.0.CO;2-J. [DOI] [Google Scholar]
- 67.Valentine D.Y., Cavin O.B., Yakel H.L., Jr. On the crystal structure of LiTe3. Acta Crystallogr. B. 1977;33:1389–1396. doi: 10.1107/S0567740877006141. [DOI] [Google Scholar]
- 68.Bradley A.J. The crystal structure of Te. Philos. Mag. 1924;48:477–496. doi: 10.1080/14786442408634511. [DOI] [Google Scholar]
- 69.Böttcher P., Kretschmann U. Darstellung und Kristallstruktur von Dicaesiumpentatellurid Cs2Te5. Z. Anorg. Allg. Chem. 1982;491:39–46. doi: 10.1002/zaac.19824910106. [DOI] [Google Scholar]
- 70.Böttcher P., Kretschmann U. Darstellung und Kristallstruktur von Dirubidiumpentatellurid, Rb2Te5. J. Less-Common Met. 1983;95:81–91. doi: 10.1016/0022-5088(83)90386-7. [DOI] [Google Scholar]
- 71.Sutherland H.H., Hogg J.H.C., Walton P.D. Indium polytelluride In2Te5. Acta Crystallogr. B. 1976;32:2539–2541. doi: 10.1107/S0567740876008182. [DOI] [Google Scholar]
- 72.Ienco A., Proserpio D. M., Hoffmann R. Main group element nets to a T. Inorg. Chem. 2004;43:2526–2540. doi: 10.1021/ic0353211. [DOI] [PubMed] [Google Scholar]
- 73.Böhm H., von Schnering H.G. The modulated structure of niobium tetratelluride NbTe4. Z. Kristallogr. 1985;171:41–64. [Google Scholar]
- 74.Assoud A., Kleinke K.M., Soheilnia N., Kleinke H. T-shaped nets of Sb atoms in the binary antimonide Hf5Sb9. Angew. Chem. Int. Ed. 2004;43:5260–5262. doi: 10.1002/anie.200460488. [DOI] [PubMed] [Google Scholar]
- 75.Xu J., Kleinke K.M., Kleinke H. electronic structure and physical properties of Hf5Sb9 containing a unique T net of Sb atoms. Z. Anorg. Allg. Chem. 2008;634:2367–2372. doi: 10.1002/zaac.200800288. [DOI] [Google Scholar]
- 76.Böttcher P., Kretschmann U. Darstellung und Kristallstruktur von CsTe4. Z. Anorg. Allg. Chem. 1985;523:145–152. doi: 10.1002/zaac.19855230418. [DOI] [Google Scholar]
- 77.Sheldrick W.S., Wachhold M. Discrete crown-shaped Te8 rings in Cs3Te22. Angew. Chem. Int. Ed. Engl. 1995;34:450–451. doi: 10.1002/anie.199504501. [DOI] [Google Scholar]
- 78.Liu Q., Goldberg N., Hoffmann R. A 2,3-connected tellurium net and the Cs3Te22 phase. Chem. Eur. J. 1996;2:390–7. doi: 10.1002/chem.19960020407. [DOI] [Google Scholar]
- 79.Stöwe K. Contributions to the crystal chemistry of uranium tellurides. III. Temperature-dependent structural investigations on uranium ditelluride. J. Solid State Chem. 1996;127:202–210. doi: 10.1006/jssc.1996.0376. [DOI] [Google Scholar]
- 80.Stöwe K. Beiträge zur Kristallchemie der Urantelluride. II. Die Kristallstruktur des Diuranpentatellurids U2Te5. Z. Anorg. Allg. Chem. 1996;622:1423–1427. doi: 10.1002/zaac.19966220825. [DOI] [Google Scholar]
- 81.Stöwe K. Beiträge zur Kristallchemie der Urantelluride. I. Die Kristallstruktur des α-Urantritellurids. Z. Anorg. Allg. Chem. 1996;622:1419–1422. doi: 10.1002/zaac.19966220824. [DOI] [Google Scholar]
- 82.Krönert W., Plieth K. Die Struktur des Zirkontriselenids ZrSe3. Z. Anorg. Allg. Chem. 1965;336:207–218. doi: 10.1002/zaac.19653360311. [DOI] [Google Scholar]
- 83.Felser C., Finckh E.W., Kleinke H., Rocker F., Tremel W. Electronic properties of ZrTe3. J. Mater. Chem. 1998;8:1787–1798. doi: 10.1039/a802948b. [DOI] [Google Scholar]
- 84.Noel H. Crystal structure of the low-dimensional uranium pentatulluride: UTe5. Inorg. Chim. Acta. 1985;109:205–207. doi: 10.1016/S0020-1693(00)81771-5. [DOI] [Google Scholar]
- 85.Patschke R., Heising J., Schindler J. L., Kannewurf C. R., Kanatzidis M. Site occupancy wave and unprecedented infinite zigzag (Te22-)n chains in the flat Te nets of the new ternary rare earth telluride family. J. Solid State Chem. 1998;135:111–115. doi: 10.1006/jssc.1997.7606. [DOI] [Google Scholar]
- 86.Fokwa B.P.T., Doert T. The ternary rare-earth polychalcogenides LaSeTe2, CeSeTe2, PrSeTe2, NdSeTe2, and SmSeTe2: Syntheses, crystal structures, electronic properties, and charge-density-wave-transitions. Solid State Sci. 2005;7:573–587. doi: 10.1016/j.solidstatesciences.2005.02.006. [DOI] [Google Scholar]
- 87.Getzschmann J., Böttcher P., Kaluza W. Darstellung und Kristallstrukturen von β-Rb2Te2 und Cs2Te2 sowie die Verfeinerung der Strukturen von Ca2P2 und Sr2As2. Z. Kristallogr. 1996;211:90–95. doi: 10.1524/zkri.1996.211.2.90. [DOI] [Google Scholar]
- 88.Dürichen P., Bensch W. Cesium gadolinium tetratelluride. Acta Crystallogr. C. 1997;53:267–269. [Google Scholar]
- 89.Stöwe K. Syntheses and crystal structures of KPrTe4, KGdTe4 and RbGdTe4. Solid State Sci. 2003;5:765–769. doi: 10.1016/S1293-2558(03)00095-5. [DOI] [Google Scholar]
- 90.Sheldrick W.S., Schaaf B. RbTe6, ein Polytellurid mit Schichtstruktur [Te6-] Z. Naturforsch. B. 1994;49:993–996. doi: 10.1515/znb-1994-0726. [DOI] [Google Scholar]
- 91.Klepp K.O., Ipser H. CrTe3 - A novel transition-Metal polytelluride. Angew. Chem. Int. Ed. Engl. 1982;21:911. doi: 10.1002/anie.198209111. [DOI] [Google Scholar]