Abstract
Radix Angelicae Sinensisis (RAS) is one of the most popular traditional Chinese herbal medicines. In the present study, six RAS extracts (i.e., phenolic extract PE, petroleum ether extract PEE, ethyl acetate extract EAE, absolute ethanol extract AEE, 95% ethanol extract 95 EE, and water extract WE) were prepared and their antioxidant activities measured by DPPH (1,1-diphenyl-2-picrylhydrazyl radical), ABTS [2,2′-azino-bis(3- ethylbenzothiazoline-6-sulfonic acid diammonium salt)], Reducing power, •O2– and lipid peroxidation assays. In general, PE, PEE and EAE had relatively high antioxidant activity, followed by AEE with moderate activity, as compared with 95 EE and WE that had low activity. Their phenolic contents (including total phenolic, ferulic acid, caffeic acid, same as below) were then determined by HPLC or spectrophotometry. The sequence of phenolic contents was roughly identical with that of antioxidant activity. When the values of 1/IC50 of various antioxidant assays were used to evaluate the level of antioxidant of the RAS extracts, (plot between 1/IC50 values and phenolic contents), the correlation coefficient (R) ranged from 0.642 to 0.941, with an average value of 0.839. Significant positive correlations demonstrated that the antioxidant effects of RAS might generally be considered a result of the presence of the phenolic compounds, especially ferulic acid and caffeic acid.
Keywords: Radix Angelicae Sinensis, antioxidant, phenolic, correlation
1. Introduction
Radix Angelicae Sinensisis (RAS, named Danggui in Chinese) is the dried root of Angelica sinensis (Oliv.) Diels. As a Chinese herbal medicine, RAS was originally described in an ancient Traditional Chinese Medicine (TCM) classic named Shennong’s Classic of Herbology, in which it is classified as top grade (superior). In TCM, it is mainly used to nourish blood, regulate menstruation, promote blood circulation, relieve pain and constipation [1]. As for the menstrual cycle and treatment of menopausal symptoms caused by the hormonal changes, it can produce favorable effects, so it is also known as “female ginseng” in Europe. Even though it is good for women, it also helps to treat the heart, spleen, liver and kidneys of both men and women. RAS, together with other Chinese herbal medicines can be used for treating various diseases, such as anemia [2], hepatitis [3], ulcerative colitis [4], dermatosis [5], neuropathy [6], cancer [7], diabetes and nephroses [8].
It has been well established that, a number of biochemical reactions involve the generation of reactive oxygen species (ROS) in our body. Under normal conditions, the balance between the generation and diminution of ROS is controlled by the antioxidant defense system, but under certain pathological conditions, when ROS are not effectively eliminated by the antioxidant defense system, the dynamic balance between the generation and diminution of ROS is broken. Excessive ROS attack lipids, carbohydrates, proteins, DNA, and result in oxidative stress, that leads to various disorders and diseases [9]. There are several studies which reported the antioxidant properties of RAS [10,11,12]. The purpose of this work was therefore to systematically assess the antioxidant activity and its correlation with the phenolic compounds found in RSA.
2. Results and discussion
2.1. DPPH· and ABTS·+ scavenging activity
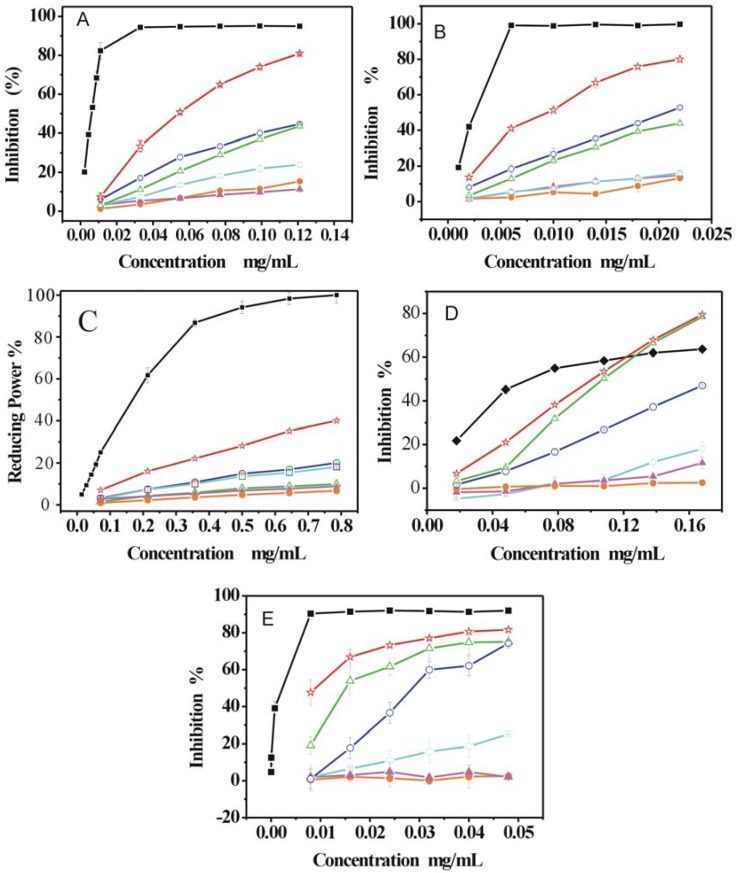
The DPPH (1,1-Diphenyl-2-picrylhydrazyl radical) and ABTS [2,2′-azino-bis(3-ethylbenzo- thiazoline-6-sulfonic acid diammonium salt)] assays have been widely used to determine the free radical-scavenging activity of various plants and pure compounds. Both DPPH and ABTS are stable free radicals which dissolve in methanol or ethanol, and their colors show characteristic absorptions at 519 nm or 734 nm, respectively. When an antioxidant scavenges the free radicals by hydrogen donation, the colors in the DPPH and ABTS assay solutions become lighter. As presented in Figure 1A and B, both the DPPH and ABTS inhibition percentage values were dose dependent, whereby they increased in the range of the tested concentrations, for the RAS extracts and the positive control (Trolox). Both the DPPH and ABTS radicals inhibition decreased in the order Trolox > PE > EAE > PEE > AEE > WE ≈ 95 EE. The DPPH and ABTS IC50 values (IC50 value is the concentration of the sample required to inhibit 50% of radical) of the RAS extracts and Trolox were calculated and are listed in Table 1.
Figure 1.
Effect of different concentration of extracts of Radix Angelicae Sinensis in free radical scavenging tests: (A) DPPH assay, (B)ABTS assay, (C) Reducing power assay,(D) Superoxide anion assay, (E) Lipid peroxidation assay.
☆ = PE (phenolic extract); △= PEE (petroleum ether extract); ○ = EAE (ethyl acetate extract); □ = AEE (absolute ethanol extract);▲ = 95 EE (95% ethanol extract); ● = WE (water extract); ■ = Trolox (positive control ); ◆ = GSH (positive control ); Each value is expressed as mean ± standard deviation, n = 3.
Table 1.
The values of IC50 and 1/IC50 of the extracts of Radix Angelicae Sinensis.
| DPPH | ABTS | Reducing power | •O2– | LPO | ||||||
| IC50 | 1/IC50 | IC50 | 1/IC50 | IC50 | 1/IC50 | IC50 | 1/IC50 | IC50 | 1/IC50 | |
| PE | 0.055 | 18.18 | 0.008 | 125 | 1.00 | 1.00 | 0.10 | 10 | 0.0083 | 120.48 |
| PEE | 0.14 | 7.14 | 0.023 | 43.48 | 3.87 | 0.26 | 0.11 | 9.09 | 0.015 | 66.67 |
| EAE | 0.13 | 7.69 | 0.022 | 45.45 | 2.03 | 0.49 | 0.18 | 5.56 | 0.028 | 35.71 |
| AEE | 0.24 | 4.17 | 0.070 | 14.29 | 2.38 | 0.42 | 0.39 | 2.56 | 0.093 | 10.75 |
| 95 EE | 0.66 | 1.51 | 0.15 | 6.67 | 5.78 | 0.17 | 0.64 | 1.56 | 0.28 | 3.57 |
| WE | 0.39 | 2.56 | 0.093 | 10.75 | 5.62 | 0.18 | 2.77 | 0.36 | 1.00 | 1.00 |
| Control | 0.0061* | 163.93* | 0.0025* | 400* | 0.017* | 58.82* | 0.061** | 16.39** | 0.0025* | 400* |
PE = phenolic extract; PEE = petroleum ether extract; EAE = ethyl acetate extract; AEE = absolute ethanol extract; 95 EE = 95% ethanol extract; WE= water extract; * Trolox; ** GSH.
2.2. Reducing power assay
The reducing power of an extract may serve as a significant indicator of its potential antioxidant activity. It can be seen that the reducing power percentage values of all RAS extracts and the positive control (Trolox) were concentration related and increased with the increase in sample concentration in the range of the tested concentrations (Figure 1C). Their relative reducing powers were as follows: Trolox > PE > EAE > AEE > PEE > 95EE > WE. The IC50 values of the RAS extracts and Trolox were calculated and are listed in Table 1.
2.3. Superoxide anion (·O2 -) radical-scavenging activity
The superoxide radical was generated by the pyrogallol system at pH 8.2. Figure 1D shows the superoxide radical activity of the RAS extracts compared with the positive control (GSH). The positive control (GSH) and all RAS extracts demonstrated an ability to inhibit superoxide anion in a dose-dependent manner. At low concentrations (0.00–0.12 mg/mL), GSH had the highest inhibition percentage value, but it was exceeded by PE and PEE at high concentration (>0.12mg/mL). According to the IC50 values (Table 1), the superoxide radical-scavenging activity of all samples decreased in the following order: GSH > PE > PEE > EAE > AEE > 95 EE > WE.
2.4. Lipid peroxidation in a linoleic acid emulsion model system
Lipid peroxidation (LPO) was used because of the formation of cytotoxic products such as MDA and 4-hydroxynonenal, which can influence cell function and the course of major human diseases [13]. As shown in Figure 1E, except for AEE, 95 EE and WE, the other extracts and Trolox showed a good inhibitory activity against lipid peroxidation. Obviously, the activity of all samples occurred in the order Trolox > PE > PEE > EAE > AEE > 95 EE > WE. The LPO IC50 values were calculated and are listed in Table 1.
2.5. Total phenolic content
The total phenolic contents of RAS extracts were determined and are presented in Table 2. The phenolic contents were calculated using pyrogallol. Analysis of the phenolic content in all of the extracts using the Folin-Ciocalteu method revealed that PE contained the maximum phenolic content (30.20 ± 0.18 mg/g) in terms of pyragallol equivalents (PYR.), followed by EAE (29.57 ± 0.37 mg/g), PEE (28.83 ± 0.64 mg/g), AEE (15.65 ± 0.68 mg/g), 95EE (5.310.69 mg/g), and WE(2.26 ± 0.55 mg/g).
Table 2.
The phenolic contents (including total phenolic,ferulic acid, caffeic acid) of the RAS extracts.
| Phenolic* mg PYR/g |
Ferulic acid* μg /g |
Caffeic acid* μg /g |
Ferulic acid plus Caffeic acid μg /g |
|
| PE | 30.20 ± 0.18 | 510.00 ± 7.00 | 160.00 ± 2.30 | 670.00 |
| PEE | 28.83 ± 0.64 | 6.40 ± 0.14 | 8.00 ± 0.20 | 14.00 |
| EAE | 29.57 ± 0.37 | 41.00 ± 1.70 | 4.60 ± 0.12 | 46.00 |
| AEE | 15.65 ± 0.68 | 5.20 ± 0.18 | 5.90 ± 0.31 | 11.00 |
| 95 EE | 5.31 ± 0.69 | 8.80 ± 0.74 | 0.00 | 8.80 |
| WE | 2.26 ± 0.55 | 0.81 ± 0.013 | 1.00 ± 0.11 | 1.80 |
* Each value is expressed as mean±standard deviation, n = 3.
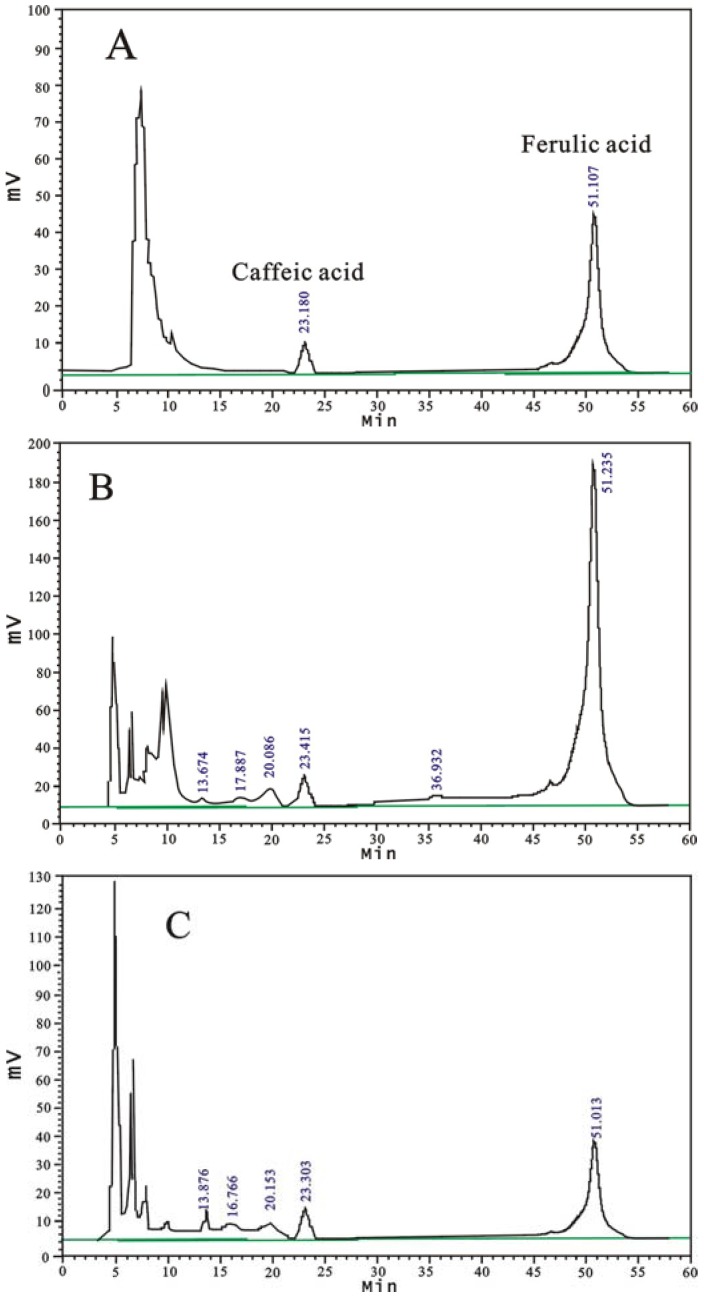
2.6. Determination of ferulic acid and caffeic acid by HPLC
As the common active phenolic acid compounds, ferulic acid and caffeic acid had been earlier found in RAS and their antioxidant effects have also been reported in previous studies [14,15]. In the present study, the RAS extracts were assayed by chromatographic analysis, using the HPLC-UV, to determine the contents of ferulic acid and caffeic acid. Typical chromatograms of the standard compounds and EAE, WE are shown in Figure 2. The calibration curves of ferulic acid and caffeic acid also showed good linearity (R = 0.9997 and 0.9998, respectively) (Table 3). As shown in Table 2, the results indicated that the ferulic acid content of PE (510.00 ± 7.00 μg/g) was highest among the RAS extracts, followed by EAE (41.00 ± 1.70 μg/g) as compared with 95 EE (8.80 ± 0.74 μg/g), PEE (6.40 ± 0.14 μg/g), AEE (5.20 ± 0.18 μg/g) and WE (0.81±0.013 μg/g), respectively. Moreover, PE also had the highest caffeic acid content (160.00 ± 2.30 μg/g) on comparing it with PEE (8.00 ± 0.20 μg/g), AEE (5.90 ± 0.31 μg/g), EAE (4.60 ± 0.12 μg/g), WE (1.00 ± 0.11 μg/g) and 95 EE, which showed complete absence of caffeic acid.
Figure 2.
HPLC chromatograms of: (A) caffeic acid and ferulic acid (the standards), (B) ethyl acetate extract (EAE) and (C) water extract (WE).
Table 3.
The calibration curve and R values of caffeic acid and ferulic acid.
| Standard | Regression equation | R |
| Ferulic acid | y = 645622568x - 223872 | 0.9997 |
| Caffeic acid | y = 294445216x -1399648 | 0.9998 |
Each value is expressed as mean±standard deviation, n = 3.
3. Experimental
3.1. Plants material
Radix Angelicae Sinensis was purchased from the Yanghe Pharmacy of Guangzhou University of Chinese Medicine (Guangzhou, China), and authenticated by Professor Shuhui Tan. A voucher specimen was deposited in our laboratory.
3.2. Chemicals
1,1-Diphenyl-2-picrylhydrazyl radical (DPPH·), pyrogallol, linoleic acid, Trolox [(±)-6- hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid], Folin & Ciocalteu’s phenol reagent were purchased from Sigma Co. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt) (ABTS) and glutathione (GSH) were obtained from Amresco Co.; ferulic acid (NO.110773 -200611) and caffeic acid (NO.110885-200102) were obtained from NICPBP (National Institute for the Control of Pharmaceutical and Biological Products, China); acetonitrile and water were of HPLC grade. All other reagents were of analytical grade.
3.3. Preparation of different extracts of Radix Angelicae Sinensis
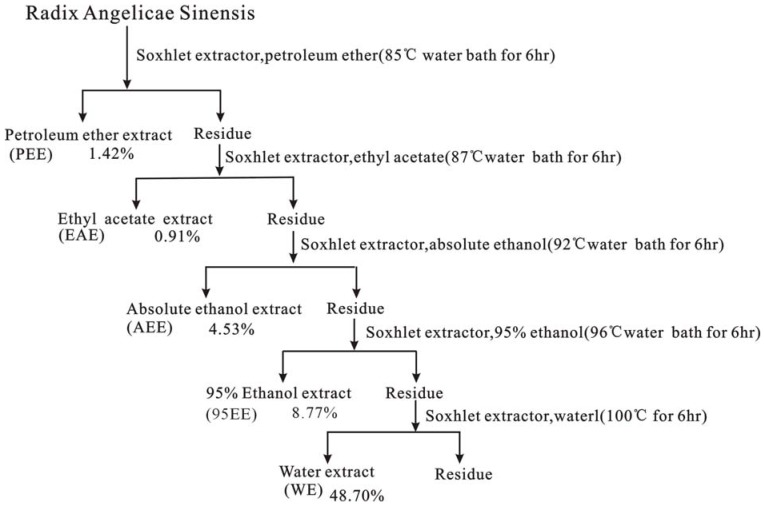
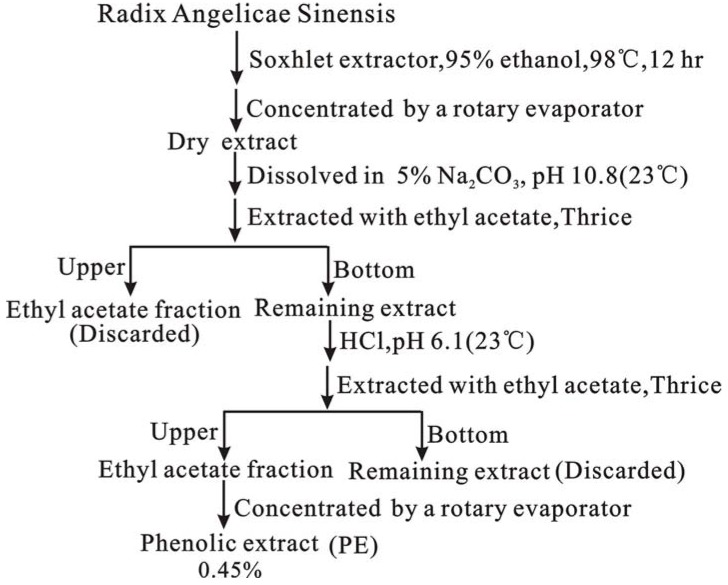
Radix Angelicae Sinensis (RAS) was ground to a coarse powder. Then the powdered sample was first extracted sequentially in the order of petroleum ether (b.p.60–90 ºC), ethyl acetate, absolute ethanol, 95% ethanol, and water to obtain the petroleum ether extract (PEE), ethyl acetate extract (EAE), absolute ethanol extract (AEE), 95% ethanol extract (95 EE), and water extract (WE), respectively (Figure 3). In addition, a phenolic extract (PE) of Radix Angelicae Sinensis was prepared using the pH adjustment method (Figure 4).
Figure 3.
Extraction of Radix Angelicae Sinensis powder by increasing order solvent polarity.
Figure 4.
The preparation of phenolic extract (PE) of Radix Angelicae Sinensis using a pH adjustment method.
3.4. DPPH radical-scavenging activity
DPPH radical-scavenging activity was determined as described [16,17]. Briefly, DPPH·solution (1 mL, 0.1 mmol/L) was mixed with various concentrations of samples (0.5 mL) dissolved in suitable solvents (absolute or 95% ethanol). The mixture was kept at room temperature for 30 min, and then the absorbance at 519 nm was measured on a spectrophotometer (UNICO 2100, Shanghai, China) using 95% ethanol as the blank. GSH was used as the positive control, and the percentage DPPH· inhibition of the test samples was calculated as:
| Inhibition % = (1 – A/A0) × 100 |
where A0 is the absorbance at 519 nm of DPPH without sample, and A is the absorbance at 519 nm of the reaction mixture containing DPPH and sample.
3.5. ABTS·+ radical cation scavenging activity
The scavenging activity of ABTS·+ was measured as described [18] with some modifications [19]. The ABTS·+ was produced by mixing ABTS diammonium salt (0.35 mL, 7.4 mmol/L) with of potassium persulfate (0.35 mL, 2.6 mmol/L). The mixture was kept in the dark at room temperature for 12 h to allow completion of radical generation, and then diluted with 95% ethanol (about 1:50) so that its absorbance at 734 nm was 0.70 ± 0.02 measured on a spectrophotometer (UNICO 2100 Spectrophotometer; Shanghai, China). To determine the scavenging activity, ABTS·+ reagent (1.2 mL) was mixed with sample or negative control (95% ethanol) (0.3 mL) and the absorbance at 734 nm was measured 6 min after the initial mixing, using 95% ethanol as the blank. The percentage inhibition of the samples was calculated as:
| Inhibition % = (1 – A/A0) ×100 |
where A0 is the absorbance at 734 nm of the negative control, A is the absorbance at 734 nm of the mixture with sample. Trolox, with a final concentration range of 0.002–0.022mg/mL, was prepared as a standard.
3.6. Reducing power assay
Reducing power was determined by the method of Oyaizu [20]. Samples (x mL) of each extract at various concentrations were mixed with Na2HPO4/KH2PO4 buffer (3.5-x mL, 0.2 mol/L, pH 6.6) and K3Fe(CN)6 (2.5 mL, 1 g/100 mL). The mixture was incubated at 50 ºC for 20 min, TCA (2.5 mL, 10 g/100 mL) was added, and the mixture was centrifuged at 2,500 g for 10 min. The supernatant (2.5 mL) was recovered, mixed with distilled water (2.5 mL) and FeCl3 (2.5 mL, 0.1 g/100 mL) and placed immediately into the spectrophotometer (UNICO 2100, Shanghai, China), and the timer was started. The absorbance at 700 nm was measured at 90 s. Samples were analyzed in groups of three, and when the analysis of one group has finished, the next group of three samples were mixed with FeCl3 to avoid oxidization by air. GSH was used as the positive control, and an increased absorbance reading indicated increased reducing power. The percentage reduction of the sample as compared to standard, i.e., GSH was calculated by using the formula (1−AS /AC) × 100. Here, AC = absorbance of standard at maximum concentration tested and AS = absorbance of sample.
3.7. Superoxide anion (•O2–) radical-scavenging activity for auto-oxidation of pyrogallol
The scavenging ability at pH 8.2 of all test samples was determined by the method of Marklund and Marklund [21], as described by Wang [22]. Briefly, samples were dissolved in suitable solvents (absolute or 95% ethanol) at a concentration of 2 mg/mL. The sample solution (x μL, where x = 0, 25, 50, 100, 150, 200, or 250 μL) was mixed with Tris-HCl buffer (1 475 - x μL, 0.05 mol/L, pH 8.2) containing EDTA (1 mmol/L) and pyrogallol (25 μL, 6 mmol/L), then shaken rapidly at room temperature. The absorbance at 325 nm of the mixture was measured (UNICO 2100, Shanghai, China) against the Tris-HCl buffer every 30 s for 5 min. The slope of the correlation of absorbance with time was calculated. The reaction mixture without added sample was used as the control. The •O2– scavenging ability was calculated as:
| (1 – Slope of sample/Slope of control) × 100 % |
3.8. Lipid peroxidation in a linoleic acid emulsion model system
Lipid peroxidation (LPO) in a linoleic acid emulsion model system was applied to produce peroxyl radical (LOO·). The scavenging ability of the RAS extracts on LPO was assessed by a method using ammonium thiocyanate [23,24]. A linoleic acid emulsion was made by vortexing a mixture of linoleic acid (312.6 mg) with Tween20 (78.2 mg) in 30% (v/v) ethanol (20 mL). The reaction mixture consisted of linoleic acid emulsion (1.5 mL) and sample solution (0.1 mL). The total volume was adjusted to 2.0 mL with distilled water, and the reaction mixture was incubated at room temperature in an open vitreous bottle for 72 h. Samples (0.15 mL) were withdrawn from the incubated mixture and tested for lipid peroxidation. The assay consisted of sequential addition of 75% (v/v) ethanol (3.65 mL), ammonium thiocyanate (0.1 mL, 30 g/100 mL), and ferrous sulfate (0.1 mL, 0.02 mol/L in 3.6%, v/v, HCl). After the mixture was rested for 3 min, the color development was determined as the difference of absorbance at 500 nm against that of 75% ethanol in the reference cuvette (UNICO 2100, Shanghai, China). The scavenging ability on LPO was calculated as:
| Inhibition % = (1 – A500nm(sample)/A500nm(reference)) × 100 |
3.9. Determination of total phenolic content (TP)
Total phenolic (TP) contents of the RAS extracts were analyzed by the Folin-Ciocalteu method [25]. In brief, samples of the extract were taken into a test-tube and made to a volume of 0.5 mL with 95% ethanol, the Folin-Ciocalteu reagent (0.5 mL, 0.25 mol/L) and the Na2CO3 reagent (1.0 mL, 150 g/L) were added, and the mixture was incubated at room temperature for 30 min. The absorbance at 760 nm of the mixture was measured (UNICO 2100 spectrophotometer, Shanghai, China), and the amount of total phenol was calculated as pyrogallol equivalents (mg PYR/g) from the calibration curve.
3.10. HPLC analysis for ferulic acid and caffeic acid
HPLC analysis of the RAS extracts for ferulic acid, caffeic acid was carried out on a LC2000 system equipped with a UV detector (Techcomp Ltd., China) and a L-2200 autosampler (Hitachi, Japan). The column was a C18 reversed-phase column (Diamonsil 5 μm film thickness, 250 mm × 4.6 mm, Dikma Ltd., China). The separation was performed isocratically with a mobile phase consisting of 0.1% (v/v) water acetic acid and acetonitrile (83:17). The injection volume was 50 μL. The flow rate was 0.5 mL/min and detection was at 325 nm wavelength. The standard curves using ferulic acid (0.01–0.08 mg/mL) and caffeic acid (0.07−0.112 mg/mL) were constructed and quantifications were done on the basis of the standard curves of ferulic acid and caffeic acid, respectively.
3.11. Statistical analysis
Data are given as the mean ± SD of three measurements. The IC50 values were calculated by linear regression analysis. All linear regression in this paper was analyzed by Origin 6.0 professional software.
4. Conclusions
Six extracts of Radix Angelicae Sinensisis dose-dependently increased the radical inhibition (or reducing power) values, suggesting that RAS possesses antioxidant activity. Therefore, RAS’s various pharmacological activities and curative effects maybe closely correlated with its antioxidant activities. However, the antioxidant activities of six RAS extracts were different within the tested concentration ranges. In general, PE, PEE and EAE had relatively high antioxidant activity, followed by AEE with moderate activity, as compared with 95 EE and WE which displayed low activity. This sequence of antioxidant activity was roughly identical to that of phenolic (including total phenolic, ferulic acid, caffeic acid) contents (Table 2). Thus, the antioxidant activity of RAS may likely be attributed to the phenolic content in each extract. This conclusion is expected, as similar observations have been reported in a large number of previous researches.
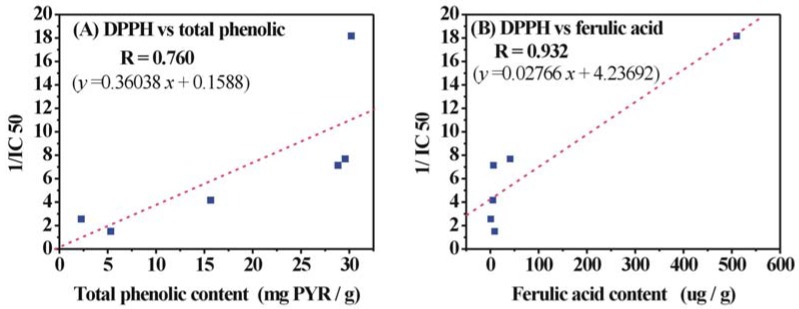
Furthermore, quantitative analysis was also used for investigating the correlation between antioxidant activities and phenolic contents in different extracts of RAS. As the 1/IC50 (not IC50) value showed parallelism with antioxidant activity, it was therefore calculated (Table 1) and used for evaluating antioxidant activity. In the present study, twenty correlation graphs were plotted between 1/IC50 values (including of DPPH, ABTS, reducing power, •O2– and LPO) and phenolic contents (including total phenolic, ferulic acid, caffeic acid and ferulic acid plus caffeic acid). Two typical correlation graphs (i.e., DPPH vs total phenolic, DPPH vs ferulic acid) are shown in Figure 5, while Table 4 shows the correlation coefficient (R) of the various assays and phenolic contents.
Figure 5.
Correlation graphs for DPPH 1 /IC50 values and (A) total phenolic contents, (B) ferulic acid contents in the six RAS extracts.
Table 4.
The R values (correlation coefficients) between antioxidant activities (1/IC50) and phenolic contents.
| DPPH | ABTS | Reducing power | •O2– | LPO | Average | |
| Total phenolic | 0.760 | 0.733 | 0.659 | 0.914 | 0.795 | 0.772 |
| Ferulic acid | 0.933 | 0.941 | 0.929 | 0.642 | 0.856 | 0.860 |
| Caffeic acid | 0.929 | 0.938 | 0.920 | 0.655 | 0.867 | 0.862 |
| Ferulic acid plus Caffeic acid | 0.931 | 0.941 | 0.928 | 0.645 | 0.859 | 0.861 |
| Average | 0.888 | 0.888 | 0.859 | 0.714 | 0.844 |
As shown in Figure 5 and Table 4, significant positive correlations (R = 0.659-0.911, the average of R was 0.772) were observed between total phenolic content and 1/IC50 values for DPPH, ABTS, Reducing power, •O2− and LPO assay, indicating the significant contribution of phenolics to these antioxidant assays. As the common phenolic acid compounds, ferulic acid (the average of R was 0.860) and caffeic acid (the average of R was 0.862) exhibit higher R values between antioxidant assays than total phenol (the average of R was 0.772). Therefore, it is indicated that ferulic acid and caffeic acid exert more antioxidant capacity than other phenolic compounds in the RAS extracts. In fact, ferulic acid has been reported to possess excellent inhibitory effect on •O2–, ·OH and NO [26], and to serve an important antioxidant function in preserving physiological integrity of cells exposed to both air and impinging UV radiation [14]. Caffeic acid was also proved to be a more efficient antiradical compound than coumaric acid [17]
However, among the five antioxidant assays (i.e., DPPH, ABTS, reducing power, •O2− and LPO), the the R value of the •O2− assay appears abnormal to some extent. Ferulic acid (R 0.642) and caffeic acid (R 0.655) exhibit lower (not higher) R values than total phenol (R 0.914). This result suggested that ferulic acid and caffeic acid exert less inhibitory effect on •O2− than other phenolic compounds in the RAS extracts.
Nevertheless, twenty R values ranged from 0.642 to 0.941, and the average value was 0.839, so these high levels suggest that the antioxidant activity of Radix Angelicae Sinensis might me in large part the result of the contributions of the phenolic compounds that it contained, especially ferulic acid and caffeic acid.
Footnotes
Sample Availability: Available from the authors.
References
- 1.China Pharmacopoeia Committee . Pharmacopoeia of the People’s Republic of China. China Chemical Industry Press; Beijing, China: 2005. p. 89. [Google Scholar]
- 2.Tong Y.Q., Hou H.M. Progress in mechanism of Danggui Buxue Tang therapy for anaemia (in Chinese) Jiangxi J. TCM. 2006;2:62–63. [Google Scholar]
- 3.You A.L. Adds. Danggui Buxue Tang Therapy for Virus Hepatitis (in Chinese) Modern TCM. 1991;6:44. [Google Scholar]
- 4.Bai L.P., Liu X.F. Thirty Cases of Danggui Buxue Tang therapy for ulcerative colitis (in Chinese) Shaanxi J. TCM. 2004;9:796–797. [Google Scholar]
- 5.Liu Q. The Clinical application of Danggui Sini Tang in department of dermatology (in Chinese) Shaanxi J. TCM. 1990;8:361–363. [Google Scholar]
- 6.Yan G.H., Hou Q.Z., Guo G.B. Observation on effect of Adds. Danggui Sini Tang therapy for peripheral neuropathy causing by diabetes on 30 patients (in Chinese) New J. TCM. 2006;7:53–54. [Google Scholar]
- 7.Yang K.Z., Sun X.F. Observation on recent effect of Danggui Huoxue Tang I combined with chemical therapy treat metaphase-late malignant tumor (in Chinese) J. Shandong Med. 2004;7:41–42. [Google Scholar]
- 8.Zhou Y.L. Danggui Liuhuang Tang therapy for early diabetes and nephrosis on 33 patients (in Chinese) TCM Res. 2008;5:37–38. [Google Scholar]
- 9.Zhao X.Y., Sun H.D., Hou A.J., Zhao Q.S., Wei T.T., Xin W.J. Antioxidant properties of two gallotannins isolated from the leaves of Pistacia weinmannifolia. BBA Gen. Subj. 2005;1725:103–110. doi: 10.1016/j.bbagen.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Huang S.H., Chen C.C., Lin C.M., Chiang B.H. Antioxidant and flavor properties of Angelica sinensis extracts as affected by processing. J. Food Compos. Anal. 2008;21:402–409. doi: 10.1016/j.jfca.2008.02.005. [DOI] [Google Scholar]
- 11.Li S.Y., Yu Y., Li S.P. Identification of antioxidants in essential oil of radix angelicae sinensis using HPLC coupled with DAD-MS and ABTS-based assay. J. Agr. Food Chem. 2007;55:3358–3362. doi: 10.1021/jf070140t. [DOI] [PubMed] [Google Scholar]
- 12.Yang X.B., Zhao Y., Zhou Y.J., Mao J.L., Zhao P. Component and antioxidant properties of polysaccharide fractions isolated from Angelica sinensis (Oliv.) diels. Biol. Pharm. Bull. 2007;30:1884–1890. doi: 10.1248/bpb.30.1884. [DOI] [PubMed] [Google Scholar]
- 13.Sevanian A., Ursini F. Lipid peroxidation in membranes and lowdensity lipoproteins: Similarities and differences. Free Radical Biol. Med. 2000;29:306–311. doi: 10.1016/S0891-5849(00)00342-7. [DOI] [PubMed] [Google Scholar]
- 14.Gülçin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217:213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Ernst G. Antioxidant potential of ferulic acid. Free Radical Bio. Med. 1992;13:435–448. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- 16.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 17.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci.Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 18.Re R., Pellegrini N., Proteggente A., Pannala A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 19.Thaipong K., Boonprakob U., Crosby K. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 20.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 21.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z.J., Luo D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohyd. Polym. 2007;68:54–58. doi: 10.1016/j.carbpol.2006.07.022. [DOI] [Google Scholar]
- 23.Siddhuraju P. Antioxidant activity of polyphenolic compounds extracted from defatted raw and dry heated Tamarindus indica seed coat. LWT Food Sci.Technol. 2007;40:982–990. doi: 10.1016/j.lwt.2006.07.010. [DOI] [Google Scholar]
- 24.Kitts D.D., Arosha N.W., Chun H. Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem. 2000;203:1–10. doi: 10.1023/A:1007078414639. [DOI] [PubMed] [Google Scholar]
- 25.Wua S.J., Ng L.T. Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in Taiwan. LWT Food Sci.Technol. 2008;41:323–330. doi: 10.1016/j.lwt.2007.03.003. [DOI] [Google Scholar]
- 26.Ogiwara T., Satoh K., Kadoma Y., Murakami Y., Unten S., Atsumi T., Sakagami H., Fujisawa S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002;22:2711–2717. [PubMed] [Google Scholar]