Abstract
CuO/AB was found to be a simple and efficient catalyst for the N-arylation of a variety of nitrogen-containing heterocycles, giving the products in excellent yields.
Keywords: N-arylation, copper, heterocycles, heterogeneous catalyst, acetylene black
1. Introduction
The copper-mediated C-heteroatom (C-N, C-O, C-S, C-P, C-Se), C-C, and C-metal bonds formations are pivotal transformations that have been developed to include a wide range of substrates. Specifically, the N-arylation of nitrogen-containing heterocycles is of particular interest as the resulting products represent important structural motifs of numerous natural products and biologically active compounds. N-Arylimidazoles, N-arylpyrroles, N-arylpyrazoles, N-arylindoles, and N-aryl-triazoles have received substantial attention in a variety of fields throughout the chemical, pharmaceutical, and material sciences [1,2,3,4,5,6]. Despite the significant progress made in the development of copper catalyzed coupling reactions of this type, there still exists a need for new methods that involve cheap and `environmentally sound catalysts [7,8,9]. Classical Ullmann chemistry, along with closely related methods, have been known for a full century. This copper-mediated synthesis of biaryl is known as the Ullmann condensation (synthesis of diaryl ethers, diaryl amines, or diaryl thioethers). (Scheme 1). During the first 70 years of the 20th century, copper was nearly the only metal usable for aryl-aryl bond formation, finding initial in the reductive symmetrical coupling of aryl halides, corresponding aromatic compounds, aryl halides, diaryl ether, N-containing reactants, phenols, and related nucleophilic agents [10,11,12,13,14,15,16,17,18]. Relatively mild and highly efficient CuI-catalyzed N-arylation procedures for nitrogen-containing heterocycles (imidazoles, benzimidazoles, pyrroles, pyrazoles, indoles, triazoles) with aryl and heteroaryl halides have also been developed [19,20,21,22]. In this paper, the CuO hollow nanospheres were used to catalyze the N-arylation of nitrogen-containing heterocycles with aryl halides.
Scheme 1.
Ullmann condensation.
2. Results and Discussion
In the present study, an approach for gram-scale synthesis of uniform Cu2O nanocubes by a one-pot polyol process was developed [23]. The CuO hollow nanospheres were prepared by adding an aqueous ammonia solution to the Cu2O nanocube colloidal solution. The CuO hollow nanospheres were then immobilized onto acetylene black (AB) or charcoal. Thus, these immobilized CuO hollow nanospheres overcome the issue of reuse [24].
2.1. Catalyst characterization
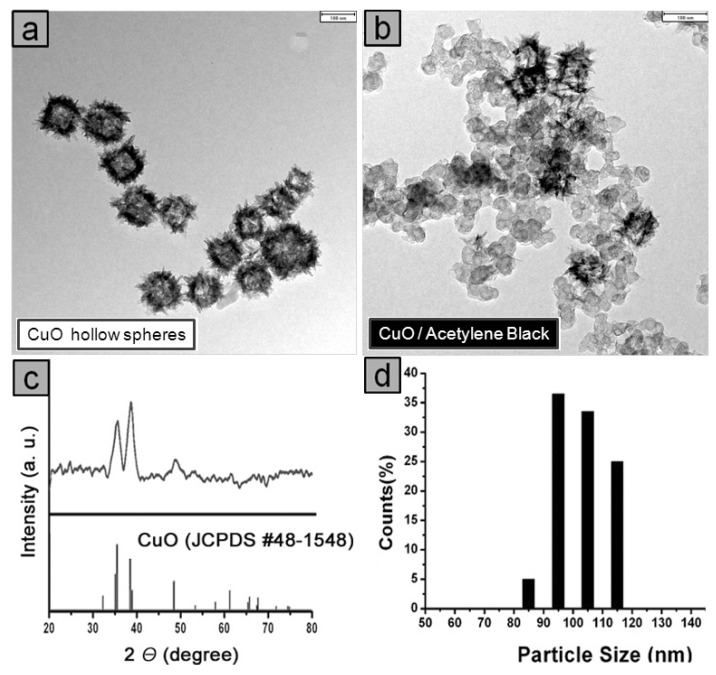
The Cu2O nanocubes prepared by a polyol process were transformed into CuO hollow nanospheres by a controlled oxidation reaction using an aqueous ammonia solution. Addition of ammonia solution (2.0 mL, 3.7 M) into a Cu2O colloidal solution gave the CuO hollow nanospheres. The transmission electron microscopy (TEM) image in Figure 1a shows the regular hollow shape of the CuO particles. The CuO hollow spheres were obtained as (103 ± 8)-nm-sized, highly monodisperse nanoparticles (Figure 1d). The crystalline features of the hollow spheres are represented in the XRD data (Figure 1c). The main peaks at θ = 35o and 39o were assigned to the reflections of the (002)/(11–1) and (111)/(200) planes in the CuO phase (JCPDS No. 48-1548). The CuO hollow particles were immobilized on acetylene carbon black through a simple sonication method at room temperature. The TEM image in Figure 1b shows that the immobilized CuO hollow spheres were well dispersed, isolated, and maintained their original size and structure with an average diameter of approximately 100 nm. The absolute amount of copper metal on the acetylene black was determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). The CuO hollow spheres on the acetylene black showed excellent activity towards a wide range of azides and acetylenes.
Figure 1.
(a) TEM image, c) XRD pattern, d) size distribution diagrams of the CuO hollow nanospheres, and (b) TEM image of the CuO hollow nanospheres on acetylene black (CuO/AB). The scale bars represent 100 nm.
2.2. Reaction test
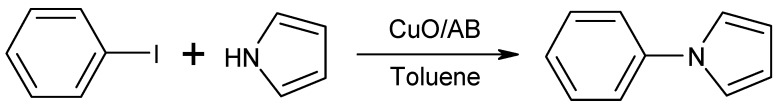
Prior research studies that have tested catalyst effectiveness have employed iodobenzene and pyrrole as benchmark substrates (Scheme 2).
Scheme 2.
Test reaction.
The N-arylation of iodobenzene (0.17 mL, 1.5 mmol) and pyrrole (0.15 mL, 2.25 mmol) with CuO/AB in toluene (7.0 mL), afforded 1-phenyl-1H-pyrrole. As shown in Table 1, 3.0 mol% CuO/AB in toluene at 180 °C for 8 h gave 1-phenyl-1H-pyrrole in 51% yield (entry 1, Table 1). When the reaction time was increased to 18 h, an 81% conversion was achieved (entry 2, Table 1). The reaction temperature was increased to 180 °C through use of a stainless steel reactor. Furthermore, when 5.0 mol% of the catalyst was used, a 22% yield was achieved at 150 °C for, 8 h (entry 3, Table 1). In general, it was found that increasing reaction temperature and time were effective means of increasing the conversion (59% for 180 °C, 8 h, entry 4, Table 1). Increasing the reaction time and changing the base to KOH, K2CO3, and KOtBu, gave respective, varied yields of 9, 71 and 72% (entries 5, 6, and 7, Table 1). After that, in order to exploit the thermally stable properties of the CuO/AB catalyst, reaction temperature was increased. Placing the reaction in a closed system (stainless steel reactor) at 180 °C and reacting for 18 h achieved a 92% conversion (entry 8, Table 1). Finally, the optimum reaction conditions were achieved: N-arylation of iodobenzene (0.17 mL, 1.5 mmol) and pyrrole (0.15 mL, 2.25 mmol) with CuO/AB (70 mg, 5.0 mol%) in Toluene (7.0 mL) at 180 °C for 18 h to afford 1-phenyl-1H-pyrrole. In addition, under optimum reaction conditions, no reaction occurred without a catalyst. It was also observed via NMR, TLC and column chromatography that no side products, such as Ar-Ar coupling products, were formed. Remarkably, after the reaction, the CuO on AB as separated by centrifugation and could be reused ten times under the same reaction conditions, without any loss of catalytic activity; the maximum reusability has not yet been tested. These results confirm that the catalytic system presented herein satisfies the conditions for heterogeneous catalysts of ease of separation, recyclability, and persistence.
Table 1.
Screening reaction condition for N-arylation of iodobenzene with pyrrole.
| Entry | Cat (mol %) | Temp (°C) | Time (h) | Base | Conv. (%)a |
|---|---|---|---|---|---|
| 1 | 3 mol% | 180 | 8 | KOtBu | 51 |
| 2 | 3 mol% | 180 | 18 | KOtBu | 81 |
| 3 | 5 mol% | 150 | 8 | KOtBu | 22 |
| 4 | 5 mol% | 180 | 8 | KOtBu | 59 |
| 5 | 5 mol% | 180 | 12 | K2CO3 | 9 |
| 6 | 5 mol% | 180 | 12 | KOH | 71 |
| 7 | 5 mol% | 180 | 12 | KOtBu | 72 |
| 8 | 5 mol% | 180 | 18 | KOtBu | 96 |
| 9 | recovered from # 8 | 180 | 18 | KOtBu | 94 |
| 10 | recovered from # 9 | 180 | 18 | KOtBu | 92 |
| 11 | recovered from # 10 | 180 | 18 | KOtBu | 92 |
| 12 | recovered from # 11 | 180 | 18 | KOtBu | 90 |
| 13 | recovered from # 12 | 180 | 18 | KOtBu | 96 |
| 14 | recovered from # 13 | 180 | 18 | KOtBu | 96 |
| 15 | recovered from # 14 | 180 | 18 | KOtBu | 94 |
| 16 | recovered from # 15 | 180 | 18 | KOtBu | 96 |
| 17 | recovered from # 16 | 180 | 18 | KOtBu | 87 |
a Determined by 1H-NMR. Yields are based on the amount of iodobenzene used.
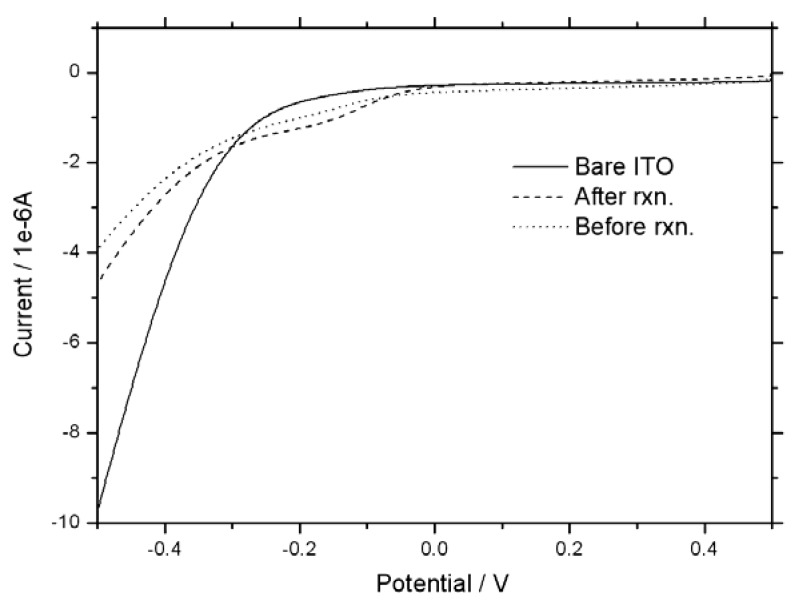
As transmission electron microscope (TEM) and cyclic voltammograms (CVs) studies prove, the structure of the CuO hollow nanospheres on acetylene black (CuO/AB) remained unchanged after the reaction, which is demonstrating catalyst recyclability (Figure 2). CV were measured by using CHI 405A (CH Instruments). 70 μL of a CuO in ethanol was dropped to ITO electrode. After 3 h, the signal of the reduced CuO on ITO was measured via linear sweep voltammetry. The linear sweep was voltamogram obtained at CuO ITO electrode in 10 mM tris buffer (pH 7.4) at scan rate of 50 mV/s.
Figure 2.
Cyclic voltammograms (CVs) of CuO hollow nanospheres on acetylene black, before (Dot line) and after the two cycle. (Dash line).
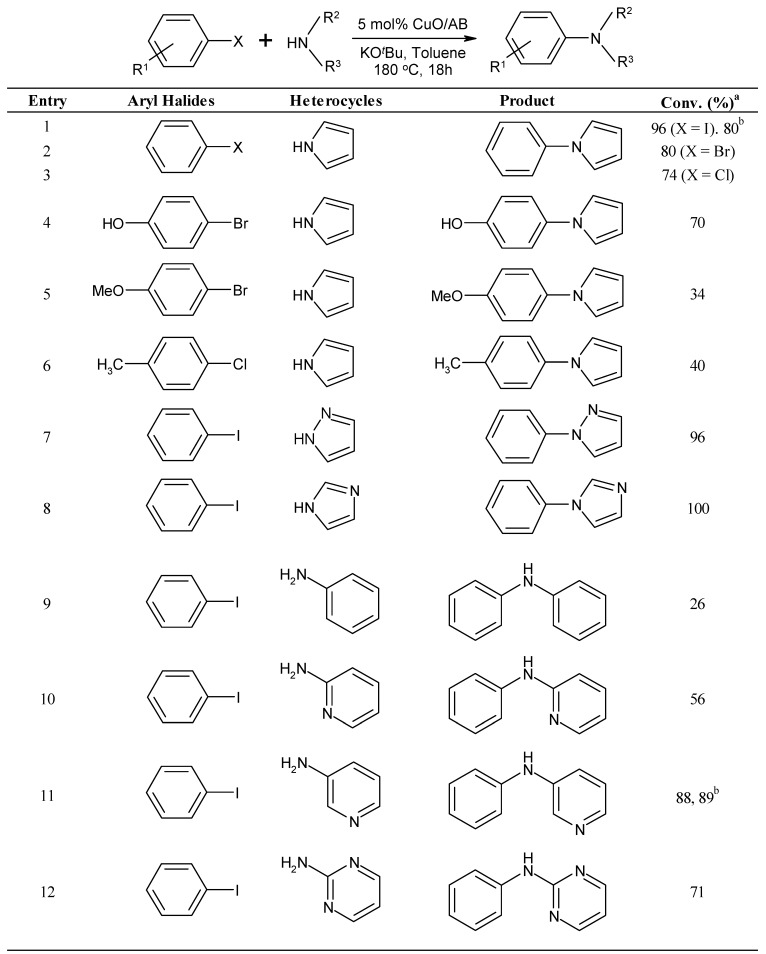
Furthermore, in the case of using various nitrogencontaining heterocycles, good results were achieved (Table 2). A wide variety of substrates with C-I, C-Br, and C-Cl bonds, as well as nucleophiles, have been examined. Relative reactivities of halogen-substituted substrates toward the halophilic attack by a carboanion were also investigated. The following order of relative reactivities toward the halophilic attack was ascertained: R-I, R-Br or R-Cl, with a bond reactivity order of: C-I > C-Br > C-Cl (entries 1~3, Table 2). This new catalytic system was also suitable for other electron-rich nitrogen heterocycles. Experiments on substituted aryl halides were conducted with OH-, OMe-, and CH3-containing substrates. The results were showing 70% conversion yield in case of 4-bromo-phenol while only a low yield was obtained in case of 1-bromo-4-methoxybenzene. In one case, the use of 1-chloro-4-methylbenzene resulted in lower obtained yields (entry 6, Table 2). Unfortunately, no reaction occurred in case of p-chloroacetophenone. It is worth noting that these optimized conditions could be applied to the N-arylation of other imidazole derivatives. (pyrroles, pyrazoles, imidazoles; Table 2). For example, the N-arylation of 1H-pyrazole with iodobenzene afforded the corresponding 1-phenyl-1H-pyrazole in 96% conversion yield (entry 7, Table 2). In addition, the coupling of 1H-imidazole with iodobenzene affords the 1-phenyl-1H-imidazole. Further investigation of the reaction of iodobenzene with other heteroarylamines, as well as aniline, under the optimized conditions was also undertaken. Pyridin-3-amine, and pyrimidin-2-amine heteroarylamines gave the expected diphenylamine, N-phenylpyridin-3-amine, and N-phenylpyrimidin-2-amine with good conversion (entries 11 and 12, Table 2). Such a result shows a similar yield albeit with shorter reaction time (24 h–40 h), compared with known literature [25,26,27,28].
Table 2.
CuO/AB catalyzed N-arylation of various Nitrogen-containing Heterocycles with Aryl Halides.
a Determined by 1H-NMR. Yields are based on the amount of aryl halides used; b Yields are isolated yields after column chromatography on silica.
3. Experimental
3.1. General remarks
Reagents were purchased from Aldrich Chemical Co. and Strem Chemical Co. and used as received. Reaction products were analyzed by 1H-NMR. 1H-NMR spectroscopy obtained on a Varian Mercury Plus (300 MHz). Chemical shift values were recorded as parts per million relative to a tetramethylsilane internal standard, unless otherwise indicated, and coupling constants in Hertz. Reaction products were assigned by comparison with the literature value of known compounds. The CuO and CuO nanoparticles immobilized on acetylene black were characterized by TEM (Philips F20 Tecnai operated at 200 kV, KAIST). Samples were prepared by placing a few drops of the corresponding colloidal solution on carbon-coated copper grids (Ted Pellar, Inc.). The X-ray powder diffraction (XRD) patterns were recorded on a Rigaku D/MAX-RB (12 kW) diffractometer. The copper loading amounts were measured by inductively coupled plasma-atomic emission spectrometry (ICP-AES).
3.2. General procedure for catalytic N-arylation of nitrogen-containing heterocycles with aryl halides
In a 25 mL stainless steel reactor, CuO hollow nanospheres on acetylene black (CuO/AB) (70 mg, 5.0 mol% with respect to the substrate concentration), iodobenzene (0.17 mL, 1.5 mmol), pyrrole (0.15 mL, 2.25 mmol), KOtBu (0.34 g, 3.0 mmol), and toluene (7.0 mL) were added. The mixture was stirred for 18 h at 180 °C. After the reaction, the nanoparticles were separated from the clean solution by centrifugation and the clean solution analyzed by 300 MHz NMR.
3.3. Synthesis of CuO hollow nanospheres
The CuO hollow nanospheres were synthesized by a controlled oxidation reaction of Cu2O nanocubes. Typically, Cu2O nanocubes were prepared by a polyol process in 1,5-pentanediol (PD, Aldrich, 96%) in the presence of poly(vinyl pyrrolidone) (PVP, Aldrich, Mw = 55,000). The PVP (5.3 g) dissolved in 45.0 mL of 1,5-pentanediol (PD, Aldrich, 96%), was slowly heated to 240 °C under a nitrogen atmosphere. Then, 4.0 mmol of Cu(acac)2 (Strem, 98%), dissolved in 15 mL of PD, was injected into the hot PVP solution at 240 °C and the mixture allowed to stir for 15 min at the same temperature. The yellowish colloidal dispersion was cooled to room temperature and precipitated by adding acetone followed by centrifugation at 8,000 rpm for 20 min. The precipitated Cu2O particles were washed with ethanol several times and re-dispersed in ethanol. To obtain the CuO hollow nanospheres, an aqueous ammonia solution (2.0 mL, 3.7 M) was added into 25.0 mL of the Cu2O cube dispersion in ethanol (16.0 mM with respect to the precursor concentration). The mixture was then stirred at room temperature for 2 h. After the reaction, the final products were collected by centrifugation at 6,000 rpm for 20 min.
3.4. Immobilization of CuO hollow nanospheres on acetylene carbon black (CuO/AB) and charcoal (CuO/C)
The acetylene carbon black (Strem, 99.99%, 1.2 g) was mixed with the CuO hollow nanosphere dispersion in ethanol (100 mL, 17.0 mM), and the reaction mixture sonicated for 1 h at room temperature. After 1 h, the product CuO/AB was washed with ethanol several times and vacuum dried at room temperature. For the synthesis of CuO/C, the mixture solution of charcoal (0.8 g) and CuO hollow nanosphere dispersion in ethanol (50.0 mL, 50.0 mM) was refluxed for 4 h. After 4 h, the black suspension was cooled to room temperature and precipitated by centrifugation. The product CuO/C was washed with ethanol thoroughly and dried in a vacuum oven at room temperature.
4. Conclusions
In conclusion, an environmentally sound process for CuO/AB catalyzed N-arylation has been developed, for a variety of nitrogen-containing heterocycles with aryl halides. In addition, the CuO/AB was readily separated by centrifugation and could be reused ten times under the present reaction conditions without any loss of catalytic activity. Transition metals loaded on acetylene black were useful reagents for a wide variety of organic transformations. Moreover, these heterogeneous systems are promising industrial catalysts with the concept of chemical economy. Further studies to expand the applications of the catalytic system are underway in this laboratory.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0070926) and by the Korean Research Foundation Grant (KRF-2006-312-C00565).
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Craig P.N. In: Comprehensive Medicinal Chemistry. Drayton C.J., editor. Vol. 8 Pergamon Press; New York, NY, USA: 1991. [Google Scholar]
- 2.Cozzi P., Carganico G., Fusar D., Grossoni M., Menichincheri M., Pinciroli V., Tonani R., Vaghi F., Salvati P. Imidazol-1-yl and pyridin-3-yl derivatives of 4-phenyl-1,4-dihydropyridines combining Ca2+ antagonism and thromboxane A2 synthase inhibition. J. Med. Chem. 1993;36:2964–2972. doi: 10.1021/jm00072a017. [DOI] [PubMed] [Google Scholar]
- 3.Negwer M. Organic-Chemical Drugs and Their SYNONYMS: An International Survey. 7th ed. Akademie Verlag GmbH; Berlin, Germany: 1994. [Google Scholar]
- 4.Kundu N.G., Mahanty J.S., Chowdhurry C., Dasgupta S.K., Das B., Spears C.P., Balzarini J., De Clercq E. Studies on uracil derivatives and analogs. 25. 5-(acylethynyl)uracils, 5-(acylethynyl)-2’-deoxyuridines and 5-(acylethynyl)-1-(2-hydroxyethoxy)methyluracils. their synthesis, antiviral and cytotoxic activities. Eur. J. Med. Chem. 1999;34:389–398. doi: 10.1016/S0223-5234(99)80088-9. [DOI] [Google Scholar]
- 5.Kunz K., Scholtz U., Ganzer D. Renaissance of Ullmann and Goldberg reactions—Progress in copper catalyzed C-N-, C-O- and C-S-coupling. Synlett. 2003;15:2428–2439. doi: 10.1055/s-2003-42473. [DOI] [Google Scholar]
- 6.Herrmann W.A. N-heterocyclic carbenes. Part 31. N-heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002;41:1290–1309. doi: 10.1002/1521-3773(20020415)41:8<1290::aid-anie1290>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Ley S.V., Thomas A.W. Modern Synthetic Methods for Copper-Mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S Bond Formation. Angew. Chem. Int. Ed. 2003;42:5400–5449. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]
- 8.Beletskaya I.P., Cheprakov A.V. Copper in cross-coupling reactions. Coord. Chem. Rev. 2004;248:2337–2364. doi: 10.1016/j.ccr.2004.09.014. [DOI] [Google Scholar]
- 9.Corbet J.P., Mignani G. Selected Patented Cross-Coupling Reaction Technologies. Chem. Rev. 2006;106:2651–2710. doi: 10.1021/cr0505268. [DOI] [PubMed] [Google Scholar]
- 10.Altman R.A., Shafir A., Lichtor P.A., Buchwald S.L. An Improved Cu-Based Catalyst System for the Reactions of Alcohols with Aryl Halides. J. Org. Chem. 2008;73:284–286. doi: 10.1021/jo702024p. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y.J., Chen H.H. 1,1,1-Tris(hydroxymethyl)ethane as a new, efficient, and versatile tripod ligand for copper-catalyzed cross-coupling reactions of aryl iodides with amides, thiols, and phenols. Org. Lett. 2006;8:5609–5612. doi: 10.1021/ol062339h. [DOI] [PubMed] [Google Scholar]
- 12.Ma D., Cai Q. N,N-Dimethyl glycine-promoted Ullmann coupling reaction of phenols and aryl halides. Org. Lett. 2003;5:3799–3802. doi: 10.1021/ol0350947. [DOI] [PubMed] [Google Scholar]
- 13.Cristau H.J., Cellier P.P., Hamada S., Spindler J.F., Taillefer M. A general and mild ullmann-type synthesis of diaryl ethers. Org. Lett. 2004;6:913–916. doi: 10.1021/ol036290g. [DOI] [PubMed] [Google Scholar]
- 14.Kwong F.Y., Klapars A., Buchwald S.L. Copper-catalyzed coupling of alkylamines and aryl iodides: An efficient system even in an air atmosphere. Org. Lett. 2002;4:581–584. doi: 10.1021/ol0171867. [DOI] [PubMed] [Google Scholar]
- 15.Wolf C., Liu S., Mei X., August A.T., Casimir M.D. Regioselective copper-catalyzed amination of bromobenzoic acids using aliphatic and aromatic amines. J. Org. Chem. 2006;71:3270–3273. doi: 10.1021/jo060034a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan Y., Lu H., Fang Y., Fang X., Chen L., Qian J., Wang J., Li C. Synthesis of pyrroles via copper-catalyzed coupling of amines with bromoenones. Synthesis. 2007:1242–1246. doi: 10.1002/chin.200734115. [DOI] [Google Scholar]
- 17.Fang Y., Li C. Preference of 4-exo Ring closure in copper-catalyzed intramolecular coupling of vinyl bromides with alcohols. J. Am. Chem. Soc. 2007;129:8092–8093. doi: 10.1021/ja072793w. [DOI] [PubMed] [Google Scholar]
- 18.Lipshutz B.H., Unger J.B., Taft B.R. Copper-in-charcoal (cu/c) promoted diaryl ether formation. Org. Lett. 2007;9:1089–1092. doi: 10.1021/ol0700409. [DOI] [PubMed] [Google Scholar]
- 19.Goodbrand H.B., Hu N.X. Ligand-Accelerated catalysis of the ullmann condensation: Application to hole conducting triarylamines. J. Org. Chem. 1999;64:670–674. doi: 10.1021/jo981804o. [DOI] [Google Scholar]
- 20.Fagan P.J., Hauptman E., Shapiro R., Casalnuovo A.J. Using intelligent/random library screening to design focused libraries for the optimization of homogeneous catalysts: Ullmann ether formation. J. Am. Chem. Soc. 2000;122:5043–5051. doi: 10.1021/ja000094c. [DOI] [Google Scholar]
- 21.Hassan J., Sevifnon M., Gozzi C., Schulz E., Lemaire M. Aryl−aryl bond formation one century after the discovery of the ullmann reaction. Chem. Rev. 2002;102:1359–1470. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]
- 22.Ley S.V., Thomas A.W. Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation. Angew. Chem. Int. Ed. 2003;42:5400–5449. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]
- 23.Park J.C., Kim J., Kwon H., Song H. Gram-scale synthesis of Cu2O nanocubes and subsequent oxidation to CuO hollow nanostructures for lithium-ion battery anode materials. Adv. Mater. 2009;21:803–807. doi: 10.1002/adma.200800596. [DOI] [Google Scholar]
- 24.Kim J.Y., Park J.C., Kang H., Song H., Park K.H. CuO Hollow Nanostructures catalyzed [3+2] cycloaddition of azides with terminal alkynes. Chem. Commun. :2010. doi: 10.1039/b917781g. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z., Mao J., Zhu D., Wu F., Chen H., Wan B. Highly efficient copper-catalyzed N-arylation of alkylamines with aryl iodides using phosphoramidite as ligand. Catalysis Commun. 2005;6:784–787. doi: 10.1016/j.catcom.2005.07.016. [DOI] [Google Scholar]
- 26.Guo X., Rao H., Fu H., Jiang Y., Zhaoa Y. An inexpensive and efficient copper catalyst for N-arylation of amines, amides and nitrogen-containing heterocycles. Adv. Synth. Catal. 2006;348:2197–2202. doi: 10.1002/adsc.200606198. [DOI] [Google Scholar]
- 27.Liu Y., Bai Y., Zhang J., Li Y., Jiao J., Qi X. Optimization of the conditions for Copper-Mediated N-Arylation of Heteroarylamines. Eur. J. Org. Chem. 2007:6084–6088. doi: 10.1002/ejoc.200700577. [DOI] [Google Scholar]
- 28.Zhu L., Guo P., Li G., Lan J., Xie R., You J. Simple copper Salt-Catalyzed N-Arylation of Nitrogen-Containing Heterocycles with Aryl and Heteroaryl Halides. J. Org. Chem. 2007;72:8535–8538. doi: 10.1021/jo0712289. [DOI] [PubMed] [Google Scholar]