The overall survival of SBRT for T2N0M0 NSCLC was 67.9% at 3 years and 40.8% at 5 years with low toxicity.
Keywords: T2N0M0 non-small cell lung cancer (NSCLC), stereotactic body radiotherapy, SBRT, late toxicity
Abstract
Purpose
A dose escalation study to determine the recommended dose with stereotactic body radiation therapy (SBRT) for peripheral T2N0M0 non-small cell carcinomas (JCOG0702) was conducted. The purpose of this paper is to report the survival and the late toxicities of JCOG0702.
Materials and methods
The continual reassessment method was used to determine the dose level that patients should be assigned to and to estimate the maximum tolerated dose. The starting dose was 40 Gy in four fractions at D95 of PTV.
Results
Twenty-eight patients were enrolled. Ten patients were treated with 40 Gy at D95 of PTV, four patients with 45 Gy, eight patients with 50 Gy, one patient with 55 Gy and five patients with 60 Gy. Ten patients were alive at the last follow-up. Overall survival (OS) for all patients was 67.9% (95% CI 47.3–81.8%) at 3 years and 40.8% (95% CI 22.4–58.5%) at 5 years. No Grade 3 or higher toxicity was observed after 181 days from the beginning of the SBRT. Compared to the toxicities up to 180 days, chest wall related toxicities were more frequent after 181 days.
Conclusions
The 5-year OS of 40.8% indicates the possibility that SBRT for peripheral T2N0M0 non-small cell lung cancer is superior to conventional radiotherapy. The effect of the SBRT dose escalation on OS is unclear and further studies are warranted.
Introduction
Stereotactic Body Radiation Therapy (SBRT) shows better clinical results than those of conventional radiotherapy for early stage non-small cell lung cancers (NSCLC) (1–10). However, some investigators have reported that the clinical outcomes of SBRT for T2N0M0 NSCLC appear to be poorer than those of T1N0M0 NSCLC (11–13). Dose escalation is an attractive method to improve clinical outcomes of SBRT for T2N0M0 NSCLC (14), considering that almost all candidates for SBRT suffer from comorbidities that may make it difficult to undergo adjuvant chemotherapy. The dose escalation study (JCOG0702) was planned to determine recommended doses (RD) for T2N0M0 NSCLC with the dose limiting toxicities (DLT) of Grade 2 or higher radiation pneumonitis (RP). The enrolled patients were stratified into two subgroups: those with PTV < 100 cc (Bin 1) and those with PTV ≥ 100 cc (Bin 2), to assess the toxicities accurately according to the irradiated volume of organ at risk (OAR). The RD is 55 Gy for the group with PTV < 100 cc and 50 Gy for the group with PTV ≥ 100 cc, respectively. These results have already been reported (15,16).
Many reports of SBRT include patients with T1N0M0 and T2N0M0 NSCLC simultaneously (3,4,8,9). The optimal treatment strategy for each stage may be different because there may be dose-response relationships so that a larger dose is required to control larger tumors (10,17,18) and the frequency of latent mediastinal lymph node metastasis may be different for different tumor sizes (19). Therefore, it is meaningful to report the efficacy outcomes of SBRT for only T2N0M0 NSCLC. Adding to that, severe late toxicities were reported in some Phase I or II studies (20–22). This points to the importance of investigating late toxicities in clinical trials, especially in the field of radiation oncology where late toxicities are sometimes lethal (23).
The purpose of this paper is to report the efficacy outcomes and late toxicities of JCOG0702, considering PTV volume.
Materials and Methods
The eligibility and exclusion criteria are the same as reported in the previous reports (15,16). The radiotherapy methods and the study design are also described in detail in the previous reports (15,16).
Patients
The major eligibility criteria were as follows: pathologically or cytologically proven NSCLC; peripheral T2N0M0 more than 3 cm in diameter (UICC 6th ed., 2002); either ‘age ≥ 20 years and unfit for lobectomy as determined by the surgeon’ or ‘age ≥ 70 years and refusing surgery’; no dyspnea on exertion that require stopping when ascending one flight of stairs or walking one city block (0.1 km); PaO2 ≥ 60 torr and FEV1.0 ≥ 700 mL; and written informed consent. The main exclusion criteria were as follows: apparent interstitial pneumonitis or pulmonary fibrosis diagnosed on chest X-rays; active infectious diseases; continuous systemic steroid administration; intermittent or continual oxygenation; fever above 38°C; and uncontrolled cough without narcotics.
Radiotherapy
A slice thickness of 1–3 mm was mandatory for the planning CT around the primary tumor. The gross tumor volume (GTV) was equal to the primary tumor. The clinical target volume (CTV) was the same as the GTV. A sufficient internal margin was added to the CTV to create the internal target volume (ITV). The planning target volume (PTV) was created from the ITV by adding a setup margin of 5 mm. No modification of the PTV was permitted to fulfill the dose constraints of the organ at risk (OAR) and a multileaf collimator was circumscribed around the PTV with a 5-mm distance, in principle.
The heterogeneity correction algorithm should be equivalent to superposition algorithms. The dose was prescribed at D95 of the PTV. The fraction number was fixed at 4. The dose constraints of the OAR are shown in Table 1.
Table 1.
Dose constraints
| Organ at risk | Dose |
|---|---|
| Spinal cord | Dmax < 25 Gy |
| Esophagus/Pulmonary artery | D1 cc < 40 Gy |
| D10 cc < 35 Gy | |
|
D10 cc < 36 Gy |
| D100 cc < 30 Gy | |
| Trachea/Bronchus | D10 cc < 40 Gy |
| Other organ excluding rib, chest wall, liver, spleen | D1 cc < 48 Gy |
| D10 cc < 40 Gy | |
| Skin | Dmax ≤ 40 Gy (if possible) |
| Lung | V20Gy ≤ 37% |
DXcc the highest dose irradiated X cc or smaller. V20 Gy the volume irradiated 20 Gy or greater.
Four to 6 MV X-rays were used, and it had to be verified that setup errors should be less than 5 mm before each irradiation delivery.
Study design
The continual reassessment method (CRM) (24) was used to determine the dose level that patients should be assigned to and to estimate the maximum tolerance dose (MTD). Precise details of the CRM have been described in the previous reports (15,16). Toxicities including the dose limiting toxicity (DLT) were assessed based on the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) ver. 3.0. The DLT was Grade 3 or higher radiation pneumonitis (RP) within 180 days of the start of the SBRT, and Grade 2 or higher RP was used as a surrogate DLT because the frequency of Grade 3 or higher RP was expected to be as low as 5%.
The starting dose was 40 Gy in four fractions at the D95 of the PTV, and the dose was increased in 5 Gy steps till 65 Gy. The 40 Gy starting dose was determined based on the estimate that 40 Gy in four fractions at the D95 of the PTV corresponds to 48 Gy in four fractions at the isocenter, a dose which is common practice in Japan; and the safety has been confirmed in a Phase I/II study (25). The maximum dose level was determined as 65 Gy in four fractions at the D95 of the PTV before starting the trial. The dose level which patients were assigned to was calculated monthly using the CRM except with the first five patients who were assigned to the fixed starting dose, 40 Gy in four fractions.
The study protocol was approved by the Japan Clinical Oncology Group (JCOG) Protocol Review Committee and the institutional review board of each participating institution. This study was registered at the UMIN Clinical Trials Registry [http://umin.ac.jp/ctr/] as UMIN000001459.
Follow-up
Patients were seen at 1, 2, 3, 4, 5, 6, 9, 12, 15, 18, 21 and 24 months after the start of the treatment. Chest X-rays were taken at 1, 3 and 5 months and chest CT at 2, 4, 6, 9, 12, 18 and 24 months after the start of the treatment. When a patient showed Grade 2 or higher RP, the information about the grade of RP of the patient and the patient accrual was shared among the participating institutions.
Statistical analysis
The posterior distribution by the CRM was updated by the JCOG Data Center. Overall survival (OS) was defined as the number of days from the registration of the patient in the study till death from any cause, and it was censored at the last follow-up date when the patient was alive. Progression-free survival (PFS) was defined as the number of days from the registration till death, local progression, or distant metastasis. Local progression-free survival (LPFS) was defined as the number of days from registration till death or local progression. Event-free survival (EFS) was defined as the number of days from the registration till death, local progression, distant metastasis or secondary lung cancer. The survival curve was estimated using the Kaplan–Meier method. All of the statistical analyses were carried out using the software program SAS, release 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics
Twenty-eight patients from the seven institutions were enrolled from October 2008 to April 2014. Fifteen patients were enrolled in the PTV < 100 cc subgroup (Bin 1) and 13 patients were in the PTV ≥ 100 cc subgroup (Bin 2). The ages were from 71 to 88 years (median 81 years) for the Bin 1, and from 76 to 86 years (median 80 years) for the Bin 2 patients. The tumor sizes were from 31 to 39 mm (median 32 mm) for the Bin 1, and from 30 to 48 mm (median 36 mm) for the Bin 2. Other characteristics are shown in Table 2.
Table 2.
Patient characteristics
| Bin 1 (N = 15) | Bin 2 (N = 13) | |||
|---|---|---|---|---|
| Age | Median 81 y.o., Range 71–88 | Median 80 y.o., Range 76–86 | ||
| Male/Female | 8/7 | 8/5 | ||
| Tumor size | Median 32 mm, Range 31–39 mm | Median 36 mm, Range 30–48 mm | ||
| Histology | Adenocarcinoma | 10 | Adenocarcinoma | 8 |
| SqCC | 4 | SqCC | 3 | |
| LCC | 1 | NSCLC not specified | 2 | |
| PS 0/1/2 | 8/5/2 | 5/7/1 | ||
| Tumor Location | Right upper lobe | 8 | Right upper lobe | 3 |
| Right middle lobe | 1 | Right middle lobe | 3 | |
| Right lower lobe | 0 | Right lower lobe | 3 | |
| Left upper lobe | 5 | Left upper lobe | 1 | |
| Left lower lobe | 1 | Left lower lobe | 3 | |
SqCC, Squamous cell carcinoma; LCC, Large cell carcinoma.
The numbers of patients treated with 40 Gy, 45 Gy, 50 Gy, 55 Gy and 60 Gy were 5, 1, 3, 1 and 5 in Bin 1, and 5, 3, 5, 0 and 0 in Bin 2, respectively.
The mean lung dose (MLD) was from 3.0 to 7.0 Gy for the Bin 1 and from 2.9 to 8.5 Gy for the Bin 2 patients. The absolute V20 of the lung was from 141.7 to 367.7 cc with a median of 201.2 cc for Bin 1, and from 182.9 to 559.0 cc with a median of 291.0 cc for Bin 2. The relative V20 of the lung was not available in this study.
The follow-up period for all patients ranged from 0.9 to 7.2 years with a median of 3.9 years.
Survival
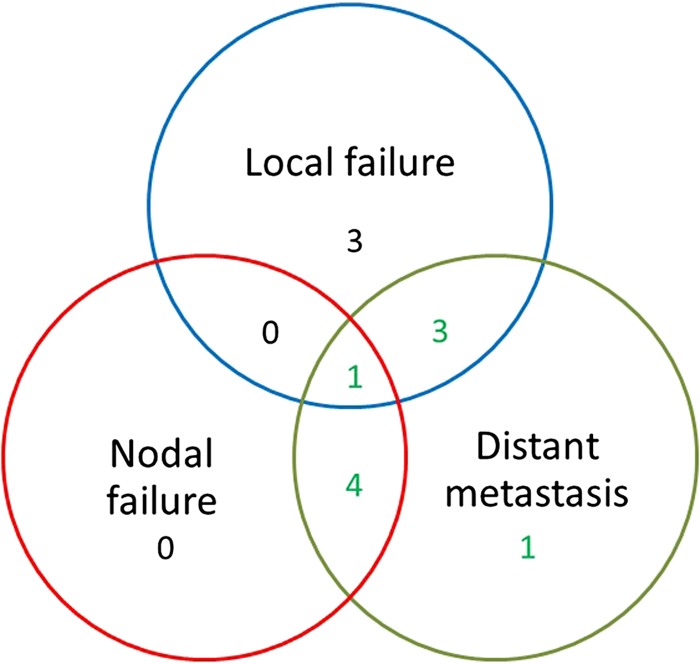
Ten patients were alive at last follow-up. Local progression occurred in seven patients, and four of these seven patients also showed metastasis. Figure 1 shows the pattern of failure. All of the seven patients with local progression died. Four of the seven patients with local progression had been treated with 40 Gy. Five patients without local progression died with metastasis. Six patients died for reasons other than cancer. Table 3 shows the causes of deaths.
Figure 1.
The pattern of failure.
Table 3.
The causes of deaths
| Local Failure | 3 |
| Local Failure and Distant Metastasis | 4 |
| Distant Metastasis | 5 |
| Death without NSCLC | 6 |
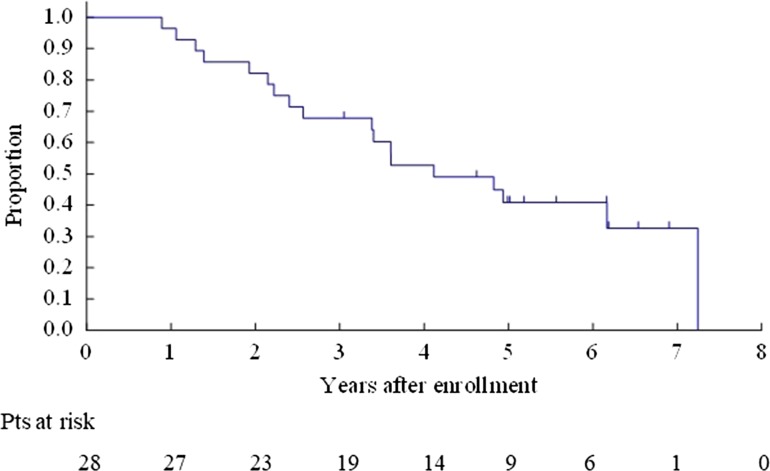
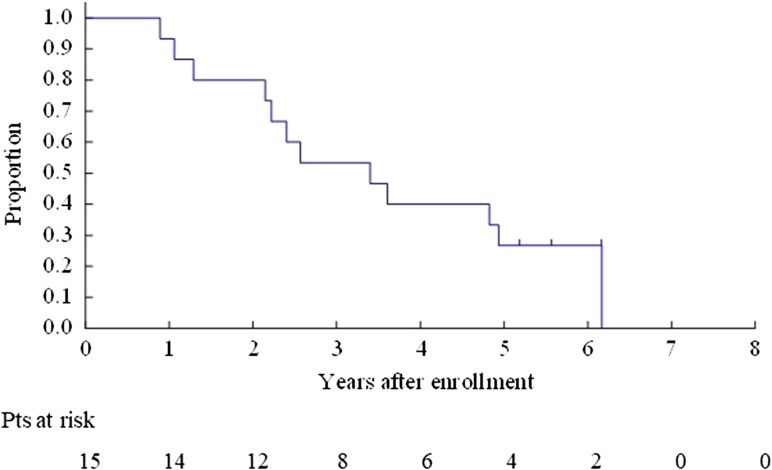
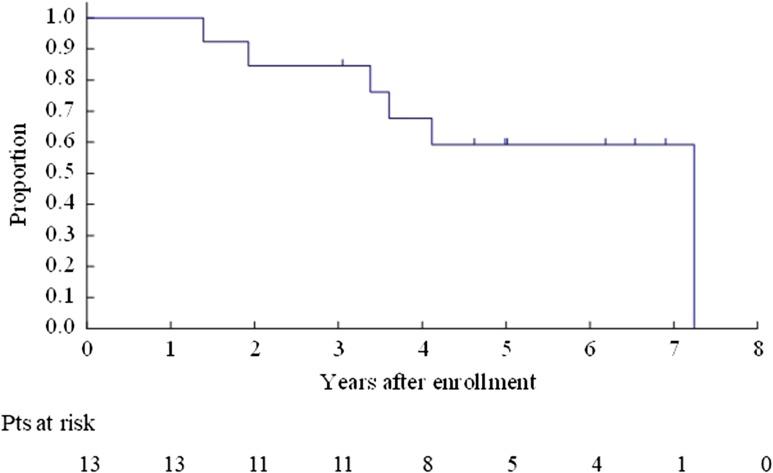
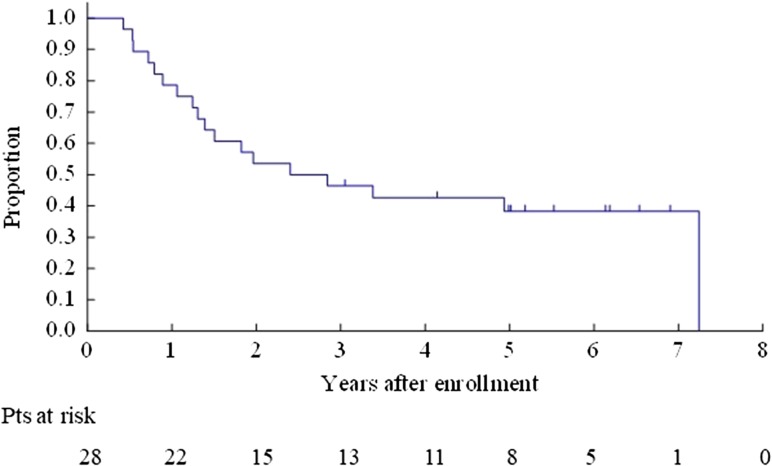
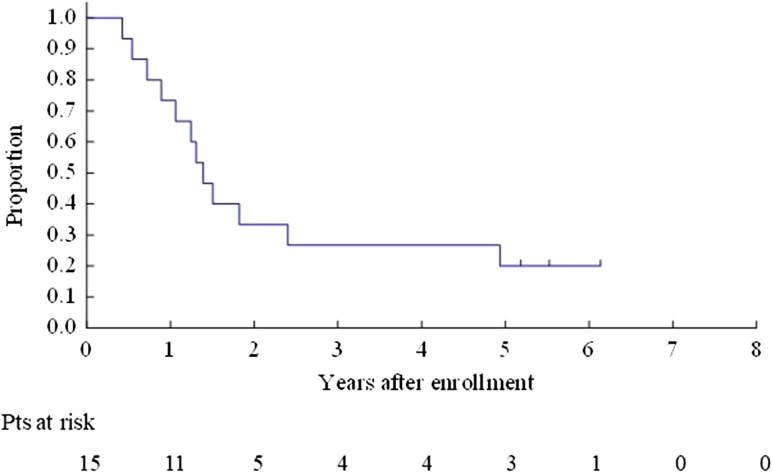
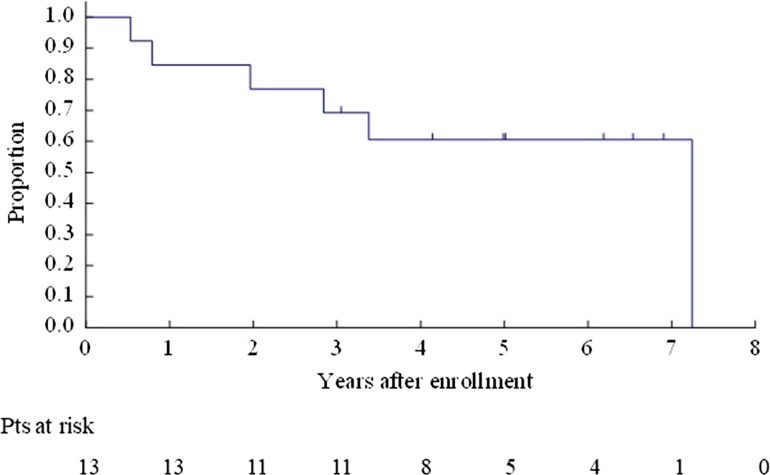
The OS for all enrolled patients was 67.9% (95% CI 47.3–81.8%) at 3 years and 40.8% (95% CI 22.4–58.5%) at 5 years (Fig. 2). The OS for Bin 1 was 53.3% (95% CI 26.3–74.4%) at 3 years and 26.7% (95% CI 8.3–49.6%) at 5 years (Fig. 3). The OS for Bin 2 was 84.6% (95% CI 51.2–95.9%) at 3 years and 59.2% (95% CI 27.9–80.7%) at 5 years (Fig. 4).
Figure 2.
Overall survival for all enrolled patients.
Figure 3.
Overall survival for Bin 1 patients.
Figure 4.
Overall survival for Bin 2 patients.
The PFS for all enrolled patients was 46.4% (95% CI 27.6–63.3%) at 3 years and 38.3% (95% CI 20.6–55.9%) at 5 years (Fig. 5). The PFS for Bin 1 was 26.7% (95% CI 8.3–49.6%) at 3 years and 20.0% (95% CI 4.9–42.4%) at 5 years (Fig. 6). The PFS for Bin 2 was 69.2% (95% CI 37.3–87.2%) at 3 years and 60.6% (95% CI 29.4–81.4%) at 5 years (Fig. 7).
Figure 5.
Progression-free survival for all enrolled patients.
Figure 6.
Progression-free survival for Bin 1 patients.
Figure 7.
Progression-free survival for Bin 2 patients.
The LPFS for all enrolled patients was 57.1% (95% CI 37.1–72.9%) at 3 years and 41.6% (95% CI 23.1–59.1%) at 5 years. The LPFS for Bin 1 was 46.7% (95% CI 21.2–68.7%) at 3 years and 26.7% (95% CI 8.3–49.6%) at 5 years. The LPFS for Bin 2 was 69.2% (95% CI 37.3–87.2%) at 3 years and 60.6% (95% CI 29.4–81.4%) at 5 years.
The EFS for all enrolled patients was 46.4% (95% CI 27.6–63.3%) at 3 years and 34.0% (95% CI 17.1–51.8%) at 5 years. The EFS for Bin 1 was 26.7% (95% CI 8.3–49.6%) at 3 years and 20.0% (95% CI 4.9–42.4%) at 5 years. The EFS for Bin 2 was 69.2% (95% CI 37.3–87.2%) at 3 years and 50.5% (95% CI 20.6–74.4%) at 5 years.
Toxicities
Within 56 days
Grade 2 dyspnea occurred in one of the five patients treated with 40 Gy in Bin 2 within 56 days of the beginning of radiotherapy. There was no other toxicity greater or equal to Grade 2.
57–180 Days
Grade 4 dyspnea and hypoxia occurred in one of the five patients treated with 60 Gy in Bin 1 from 57 to 180 days from the beginning of radiotherapy. Grade 2 Radiation Pneumonitis (RP) occurred in one of the five patients treated with 60 Gy in Bin 1 and in two of the five patients treated with 50 Gy in Bin 2.
After 181 days
No Grade 3 or higher toxicity was observed.
The most frequent Grade 2 AE were dyspnea and bone fracture (seven events) but various other Grade 2 AE were observed. Table 4 shows the details of toxicities by Bin and dose.
Table 4.
Toxicities (≥ Grade 2)
| Number of events | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Event | Bin 1 | Bin 2 | ||||||
| 40 Gy (N = 5) | 45 Gy (N = 1) | 50 Gy (N = 3) | 55 Gy (N = 1) | 60 Gy (N = 5) | 40 Gy (N = 5) | 45 Gy (N = 3) | 50 Gy (N = 5) | |
| within 56 days from the start of SBRT | ||||||||
| Radiation Pneumonitis | 0 | 0 | 0 | 0 | 0 | 1(G2) | 0 | 0 |
| from 57 to 180 days from the start of SBRT | ||||||||
| Radiation Pneumonitis | 0 | 0 | 0 | 0 | 1(G2) | 0 | 0 | 2(G2) |
| Dyspnea | 0 | 0 | 0 | 0 | 1(G4) | 0 | 0 | 0 |
| Hypoxia | 0 | 0 | 0 | 0 | 1(G4) | 0 | 0 | 0 |
| after 181 days from the start of SBRT | ||||||||
| Radiation Pneumonitis | 0 | 0 | 0 | 0 | 1(G2) | 1(G2) | 0 | 2(G2) |
| Cough | 1(G2) | 0 | 1(G2) | 0 | 0 | 0 | 0 | 1(G2) |
| Dyspnea | 2(G2) | 0 | 1(G2) | 0 | 1(G2) | 0 | 1(G2) | 2(G2) |
| Hypoxia | 1(G2) | 0 | 0 | 0 | 1(G2) | 0 | 1(G2) | 0 |
| Bone Fracture | 0 | 0 | 1(G2) | 0 | 3(G2) | 0 | 1(G2) | 2(G2) |
| Brachial Plexopathy | 0 | 0 | 1(G2) | 0 | 0 | 0 | 0 | 0 |
| Chest Pain | 0 | 0 | 0 | 0 | 2(G2) | 1(G2) | 0 | 2(G2) |
| Radiation Dermatitis | 0 | 0 | 0 | 0 | 0 | 1(G2) | 0 | 0 |
| Induration | 0 | 0 | 0 | 0 | 0 | 1(G2) | 0 | 0 |
| Fibrosis | 0 | 0 | 0 | 0 | 0 | 1(G2) | 0 | 0 |
| Neuropathy: Sensory | 0 | 0 | 0 | 0 | 0 | 0 | 1(G2) | 0 |
Toxicities were assessed based on CTCAE ver 3.0. G2, Grade 2; G4, Grade 4.
Discussion
The OS of all patients was 67.9% at 3 years and 40.8% at 5 years. The PFS for all patients was 46.4% at 3 years and 38.3% at 5 years, results suggesting the possibility that SBRT for peripheral T2N0M0 non-small cell lung cancer may be superior to conventional radiotherapy. Grade 4 dyspnea and hypoxia occurred in one of five patients at 57–180 days from the beginning of the radiotherapy. No further Grade 3 or higher toxicity was observed after 181 days from the start of the SBRT. Thus, SBRT for peripheral NSCLC may be considered safe using the dose constraints of this study.
The OS at 5 years of conventional radiotherapy for stage I NSCLC was reported as 21% by Qiao et al. (6) and 22.2% by Morita et al. (1). The OS at 5 years of this study was 40.8%, and SBRT would seem to be beneficial for patients with T2N0M0 NSCLC, as other investigators also have indicated (3,7,26). However, caution should be shown when comparing the results with historical data because the number of patients in this study is small and the results may reflect recent advances in NSCLC treatment and diagnostic imaging.
The starting dose of 40 Gy in four fractions at D95% of PTV in this study was estimated to correspond to 48 Gy in four fractions at the isocenter (15). Forty eight Gy in four fractions corresponds to a biologically effective dose (BED10) of 105.6 Gy10. This dose is thought to be sufficient based on the report by Onishi et al. (10), in which patients treated with a dose of BED10 at the isocenter ≥100 Gy10 show better local control and OS compared with patients treated with the dose of BED10 at the isocenter <100 Gy10 (10). Several investigators have recommended higher doses, above 100 Gy10, for improved local control of SBRT for NSCLC (10,17,18,27,28). Guckenberger et al. reported that BED10 to achieve 90% tumor control probability is 176 Gy10 (95% credible intervals 151–223 Gy10) (17). Kestin et al. reported that the optimal PTV mean BED10 is above 125 Gy10 (18). However, neither of these two reports referred to effects of dose escalation on OS.
Generally speaking, a larger dose is required to control the tumor as tumor volumes increase (29). Therefore, it is likely that larger irradiated doses are required to control T2 tumors than T1 tumors. Supporting this, over half of local progression cases (four of seven progressions) occurred in patients treated with 40 Gy in this study: 4 out of the 10 patients receiving 40 Gy and 3 out of the 18 patients receiving more than 40 Gy showed progression. Although detailed data are not available, a high local control rate is associated with longer survival, it may be suggested that dose escalation above 40 Gy is beneficial to improve the local control of SBRT for T2N0M0 NSCLC.
Careful assessment for the effect of dose escalation on OS is needed. Onishi et al. reported that local control and OS is better in patients treated with doses of BED10 at the isocenter ≥100 Gy10 than those treated with <100 Gy10 (10). However, different from the report by Onishi et al. (10), Stephans et al. reported that the higher dose is not associated with a better OS. They reported that higher BED10 (150–180 Gy10) may show better local control with a slight increase in toxicity but the OS is not prolonged significantly (27). The OS is affected by tumor characteristics as well as by comorbidities and therapies after the recurrence, so careful evaluation considering the various factors that could be involved is required.
The SBRT is a treatment for primary tumors, so occult lymph node metastasis may worsen the survival of patients with T2 NSCLC. In addition, we expected that it is theoretically plausible that larger tumors would result in a poorer OS and LPFS, and this had made us expect that the OS and LPFS of Bin 1 patients would be superior to those of Bin 2. However, the OS of the Bin 2 patients was 59.2% at 5 years, which was better than that of Bin 1 (26.7% at 5 years). The LPFS of Bin 2 was also superior to that of Bin 1 (26.7% vs. 60.6% at 5 years). The reason why the results were inconsistent with our expectations is unclear. One explanation could be that the median maximum diameter of the two groups was not so different (32 mm vs. 36 mm). Another explanation is that this counterintuitive result may be caused by chance due to the small number of patients in this study.
No Grade 3 or higher toxicity was observed after 181 days from the start of SBRT. However, various Grade 2 toxicities were observed after 181 days and these seemed to be characterized by chest wall related toxicities like bone fracture, chest pain, brachial plexopathy, and neuropathy:sensory. The grade of the toxicities was not severe, however, the risk of toxicities related to the chest wall may theoretically be higher in T2 tumors than in T1 tumors because of the large irradiated normal tissue volume surrounding T2 tumors. The irradiated volume of the rib and chest wall as well as the irradiated dose may need to be a constraint in future studies in order to avoid rib fracture and peripheral neurological toxicities.
Although particle therapy is thought to be one of the promising modality to treat early stage NSCLC, clinical benefit of particle therapy seems to be unclear. Chi et al. conducted systematic review and reported that the statistical difference was not shown in the OS between SBRT and particle therapy considering operability, although local control at 3 years were better in particle therapy group (30). Iwata et al. reported the results of particle therapy for T2a-T2bN0M0 NSCLC treated during April 2003 to Dec 2009 with various dose fractionation (31). They reported that OS at 4 years was 58% and PFS at 4 years was 46%. The results seem to be comparable to our study. Nantavithya et al. reported the randomized Phase 2 study to compare SBRT and stereotactic body proton therapy (SBPT) (32). The study closed early and the number of analyzed patients were 19 (9 SBRT, 10 SBPT). All enrolled patients had central tumor, which was excluded from our study. The OS at 3 years was 90% for SBPT group and 27.8% for SBRT group, and the local control at 3 years was 90.0% for SBPT group and 87.5% for SBRT group. Although they discussed that the performance status in SBRT group seems to be worse than SBPT group and this might be a bias, it is possible that SBPT may be beneficial to central tumors due to reducing the dose to OAR like esophagus and bronchus.
Recent advances in radiation oncology like image guided radiotherapy (IGRT) have been made since this study was conducted and the results of this study should be adapted with currently accepted knowledge. For example, Kimura et al. are conducting a randomized control trial (JCOG1408; UMIN000021029) for patients with tumors of 3 cm or less comparing Japanese standard and higher doses, based on the RD of this study (33). The conformity index in JCOG1408 is higher than that of this study because of recent advances of IGRT and SBRT techniques. It is expected that the results of JCOG0702 with an appropriate interpretation would be useful to plan future studies.
In conclusion, the results of this Phase I study suggests the potential of SBRT for peripheral T2N0M0 NSCLC as superior to conventional radiotherapy in terms of OS, and it is shown to be safe using the dose constraints of this study. The effect of dose escalation on OS is unclear making further studies warranted.
Acknowledgements
Data Center: Hidenobu Yamada and Chikako Aibara for data management, and Dr Haruhiko Fukuda for study oversight. Operations Office: Dr Kenichi Miyamoto, Dr Tomonori Mizutani, Taro Shibata, Dr Junko Eba and Dr Kenichi Nakamura for support in the drafting of the manuscript. Supported in part by the National Cancer Center Research and Development Fund (grants 23-A-16, 23-A-21 and 26-A-4), Grants-in-Aid for Cancer Research (20S-5 and 20S-6) and a Health and Labour Sciences Research Grant for Clinical Research (020) from the Ministry of Health, Labour and Welfare of Japan.
Conflict of interest statement
None declared.
References
- 1. Morita K, Fuwa N, Suzuki Y, et al. Radical radiotherapy for medically inoperable non-small cell lung cancer in clinical stage I: a retrospective analysis of 149 patients. Radiother Oncol 1997;42:31–6. [DOI] [PubMed] [Google Scholar]
- 2. Sibley GS. Radiotherapy for patients with medically inoperable Stage I nonsmall cell lung carcinoma: smaller volumes and higher doses—a review. Cancer 1998;82:433–8. [PubMed] [Google Scholar]
- 3. Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153–9. [DOI] [PubMed] [Google Scholar]
- 4. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989–96. [DOI] [PubMed] [Google Scholar]
- 6. Qiao X, Tullgren O, Lax I, Sirzen F, Lewensohn R. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer 2003;41:1–11. [DOI] [PubMed] [Google Scholar]
- 7. Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290–6. [DOI] [PubMed] [Google Scholar]
- 8. Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer 2010;68:72–7. [DOI] [PubMed] [Google Scholar]
- 9. Zimmermann FB, Geinitz H, Schill S, et al. Stereotactic hypofractionated radiation therapy for stage I non-small cell lung cancer. Lung Cancer 2005;48:107–14. [DOI] [PubMed] [Google Scholar]
- 10. Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623–31. [DOI] [PubMed] [Google Scholar]
- 11. Shirata Y, Jingu K, Koto M, et al. Prognostic factors for local control of stage I non-small cell lung cancer in stereotactic radiotherapy: a retrospective analysis. Radiat Oncol 2012;7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eriguchi T, Takeda A, Sanuki N, et al. Stereotactic body radiotherapy for operable early-stage non-small cell lung cancer. Lung Cancer 2017;109:62–7. [DOI] [PubMed] [Google Scholar]
- 13. Onimaru R, Fujino M, Yamazaki K, et al. Steep dose-response relationship for stage I non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:374–81. [DOI] [PubMed] [Google Scholar]
- 14. Koshy M, Malik R, Weichselbaum R, Sher D. Increasing radiation therapy dose is associated with improved survival in patients undergoing stereotactic body radiation therapy for Stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2015;91:344–50. [DOI] [PubMed] [Google Scholar]
- 15. Onimaru R, Shirato H, Shibata T, et al. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer with PTV<100cc using a continual reassessment method (JCOG0702). Radiother Oncol 2015;116:276–80. [DOI] [PubMed] [Google Scholar]
- 16. Onimaru R, Onishi H, Shibata T, et al. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer (JCOG0702): results for the group with PTV100cc. Radiother Oncol 2017;122:281–5. [DOI] [PubMed] [Google Scholar]
- 17. Guckenberger M, Klement RJ, Allgauer M, et al. Local tumor control probability modeling of primary and secondary lung tumors in stereotactic body radiotherapy. Radiother Oncol 2016;118:485–91. [DOI] [PubMed] [Google Scholar]
- 18. Kestin L, Grills I, Guckenberger M, et al. Dose-response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance. Radiother Oncol 2014;110:499–504. [DOI] [PubMed] [Google Scholar]
- 19. Li L, Ren S, Zhang Y, et al. Risk factors for predicting the occult nodal metastasis in T1-2N0M0 NSCLC patients staged by PET/CT: potential value in the clinic. Lung Cancer 2013;81:213–7. [DOI] [PubMed] [Google Scholar]
- 20. Gomez DR, Gillin M, Liao Z, et al. Phase 1 study of dose escalation in hypofractionated proton beam therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;86:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheung P, Faria S, Ahmed S, et al. Phase II study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage T1-3 N0 M0 non-small cell lung cancer: NCIC CTG BR.25. J Natl Cancer Inst 2014;106:dju164. [DOI] [PubMed] [Google Scholar]
- 22. Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833–9. [DOI] [PubMed] [Google Scholar]
- 23. Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med 2012;366:2327–9. [DOI] [PubMed] [Google Scholar]
- 24. Ishizuka N, Morita S. Practical implementation of the continual reassessment method In: Crowley J, Hoering A, Ankerst DP, editors. Handbook of Statistics in Clinical Oncology. New York, NW: Chapman & Hall, 2006;97–116. [Google Scholar]
- 25. Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427–31. [DOI] [PubMed] [Google Scholar]
- 26. Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic body radiotherapy for early-stage non-small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol 2018;36:710–19. [DOI] [PubMed] [Google Scholar]
- 27. Stephans KL, Woody NM, Reddy CA, et al. Tumor control and toxicity for common stereotactic body radiation therapy dose-fractionation regimens in Stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2018;100:462–9. [DOI] [PubMed] [Google Scholar]
- 28. Janssen S, Kaesmann L, Rudat V, Rades D. Stereotactic Body Radiotherapy (SBRT) with lower doses for selected patients with Stage I Non-small-cell Lung Cancer (NSCLC). Lung 2016;194:291–4. [DOI] [PubMed] [Google Scholar]
- 29. Rwigema JC, Heron DE, Ferris RL, et al. The impact of tumor volume and radiotherapy dose on outcome in previously irradiated recurrent squamous cell carcinoma of the head and neck treated with stereotactic body radiation therapy. Am J Clin Oncol 2011;34:372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang JY, Zhang W, Komaki R, et al. Long-term outcome of phase I/II prospective study of dose-escalated proton therapy for early-stage non-small cell lung cancer. Radiother Oncol 2017;122:274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iwata H, Demizu Y, Fujii O, et al. Long-term outcome of proton therapy and carbon-ion therapy for large (T2a-T2bN0M0) non-small-cell lung cancer. J Thorac Oncol 2013;8:726–35. [DOI] [PubMed] [Google Scholar]
- 32. Nantavithya C, Gomez DR, Wei X, et al. Phase 2 study of stereotactic body radiation therapy and stereotactic body proton therapy for high-risk, medically inoperable, early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2018;101:558–63. [DOI] [PubMed] [Google Scholar]
- 33. Kimura T, Nagata Y, Eba J, et al. A randomized Phase III trial of comparing two dose-fractionations stereotactic body radiotherapy (SBRT) for medically inoperable Stage IA non-small cell lung cancer or small lung lesions clinically diagnosed as primary lung cancer: Japan Clinical Oncology Group Study JCOG1408 (J-SBRT trial). Jpn J Clin Oncol 2017;47:277–81. [DOI] [PubMed] [Google Scholar]