Unmanaged consumption and climate change are profoundly affecting water resources at a global level. It is therefore vital to understand plant responses to drought and flooding, and to breed crops with greater water use efficiency and resilience. Root system architecture is critically important in this, but understanding how architecture and root function should be manipulated to increase resource capture is complex and must take account of the phenology of crop growth and water availability. This virtual issue brings together fundamental research that is helping drive this agenda forward and sets out key areas of development that are needed.
Fresh water is a relatively rare resource that is fundamental to our survival. While 70% of the earth is covered in water, only 2.5% of that is freshwater, of which a mere 1% is easily accessible. Yet water is often misused. Moreover, in recent years, the effects of climate change have translated into increasing incidences of drought and flooding, and deteriorating water security (Dai et al., 2004; Kron and Berz, 2007). In an important paper, NASA recently highlighted how unmanaged human consumption and climate change have deeply affected water resources at a global level over just 15 years (Rodell et al., 2018). For example, in California’s Central Valley, where intensive agricultural use has been accompanied by persistent drought, groundwater levels have fallen dramatically. A similar trend due to agricultural pressure was observed in Saudi Arabia. Extremes of water availability will put increasing strain on global agriculture that will be felt most acutely in the Global South (especially Africa, Latin America and developing countries in Asia), where a lack of resources and infrastructure makes responding to harsh environmental conditions all the more difficult (Miyan, 2015).
In this context it is of pivotal importance to understand plant responses to drought and flooding, and to breed crops with greater water use efficiency and resilience in the face of more severe environmental conditions. Root architecture is critical for the uptake of both water and nutrients (Lynch, 2013), but understanding how the multiple parameters that define root system architecture and function contribute to effective resource capture is a complex topic that must take account of the phenology of crop growth and water availability (put simply, when it rains relative to when the crop grows) (Tron et al., 2015).
Development and physiology of different root types
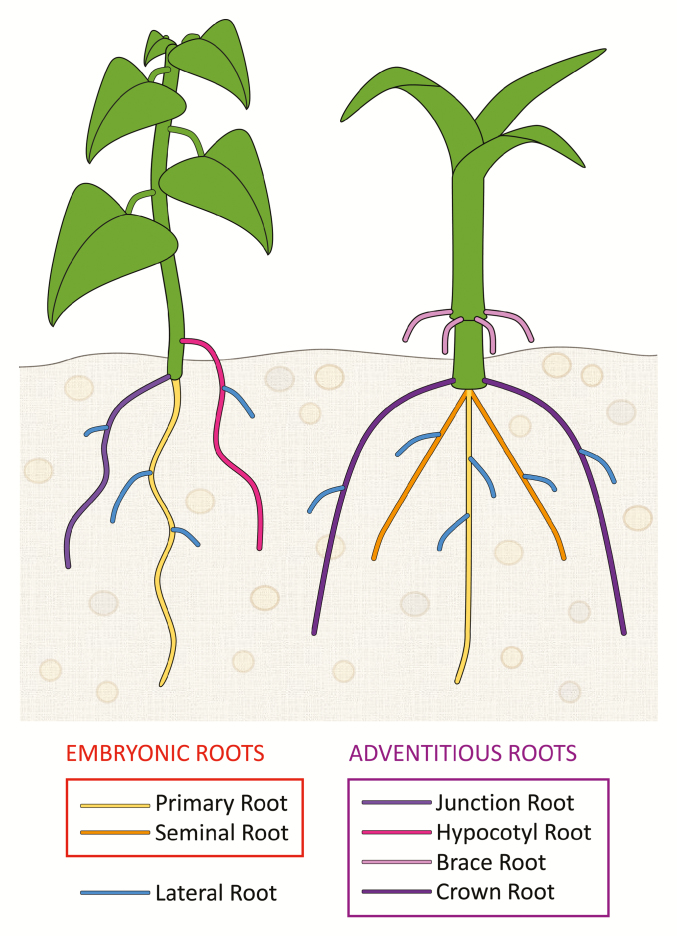
The root system is composed of diverse root types, which differ in origin, anatomy and function (Atkinson et al., 2014; Box 1). Generally speaking, the primary root is of embryonic origin and is the first to emerge upon germination, anchoring the new seedling to the ground and supplying initial nutrients. Some species, like wheat and rice, carry extra embryonic root primordia that give rise to the so-called seminal roots. The term adventitious root describes all plant roots that form from any non-root tissue, such as junction roots (at the root–shoot junction), crown roots (from nodes below ground), brace roots (from nodes above ground), stem roots (internodes), and hypocotyl roots (Steffens and Rasmussen, 2016). All these root types, including primary roots, can develop lateral roots. The number, length and growth angle of these root classes constitute what is normally called root architecture.
Box 1. Different types of plant root
Primary and seminal roots are of embryonic origin. Adventitious roots form from any non-root tissue, such as junction roots (root–shoot junction), hypocotyl roots, brace roots (node above ground), and crown roots (node below ground). All of these root types can form lateral roots.
Great effort has gone into deciphering the molecular mechanisms regulating the development of the different root types and their physiological roles. The plant hormone auxin has been shown to regulate the formation of primary, seminal, lateral and adventitious roots [Kircher and Schopfer, 2016 (see also the Insight article by Scheres and Laskowski, 2016); Roberts et al., 2016 (see also the Insight article by Taleski et al., 2016); Herrbach et al., 2017; Du and Scheres, 2018]. Other signalling pathways, involving cytokinin, ethylene, nutrients and stress, interact with auxin to finely regulate root branching and growth (Murphy et al., 2016; Veloccia et al., 2016; Qu et al., 2017). Auxin is also a major regulator of root growth angle (Roychoudhry and Kepinski, 2015; Wang et al., 2018), which is a critical determinant of the distribution of root mass within the soil. Different root types vary in their contribution to structural and absorbance functions, and in terms of the stage of plant growth. Primary and seminal roots are mostly important in the first stages of plant growth. In monocots, seminal and primary roots do not persist at later stages, and the crown and brace roots constitute the majority of the root system (Hochholdinger et al., 2004a, b). In contrast, in dicotyledonous species, lateral roots form the bulk of the root system once the primary root has stopped growing. Transcriptomic work in maize has revealed a possible functional specialization of seminal roots, compared to primary and crown roots, in response to stress (Tai et al., 2016; see also Price, 2016). Interestingly, the presence in maize of mutants lacking only adventitious and seminal roots indicates a common regulatory origin of these two root types (Tai et al., 2017; see also the Insight article by Salvi, 2017). The above-ground brace roots play an important role in structural support of the plant. If they penetrate the soil, brace roots form laterals and help further anchor the plant, while absorbing water and nutrients (Atkinson et al., 2014).
Environmental modification of root architecture
There is much evidence that water availability can regulate root architecture. Water deficiency in the upper soil suppresses lateral root growth and root growth angle in Arabidopsis (Rellán-Álvarez et al., 2015) and crown root growth in Setaria viridis (Sebastian et al., 2016). Flooding, on the other hand, promotes adventitious root formation in rice and elongation in Arabidopsis (Lin and Sauter, 2018). The genetic responses to drought and flooding are also very complex and involve variations in both the transcriptome (Janiak et al., 2016; Kwasniewski et al., 2016; Opitz et al., 2016) and the methylome (Chwialkowska et al., 2016), representing changes in gene expression over both short- and longer timescales. Different cell types respond differently to water status (Opitz et al., 2016), although root hairs seem to be the prime site of water availability perception (Kwasniewski et al., 2016). Responses to variations in water availability involve auxin (Ma et al., 2017; Nakajima et al., 2017), cytokinin (Xu et al., 2016), H2O2 (Giuliani et al., 2005; Ma et al., 2017), ABA (Kong et al., 2016) and ethylene (Ali and Kim, 2018). Flooding and drought can affect different crop species in distinct ways (Striker and Colmer, 2017; Pavlović et al., 2018). In particular, structural differences, such as number of xylem bundles (Prince et al., 2017; see also Considine et al., 2017) or crown roots (Gao and Lynch, 2016; see also the Insight article by Hochholdinger, 2016) for drought, and root porosity for flooding (Striker and Colmer, 2017), are major components of such variation. From a molecular point of view, auxin has been revealed to be required for hydrotropism in pea and rice, but not in Lotus japonica (Nakajima et al., 2017).
Crop improvement
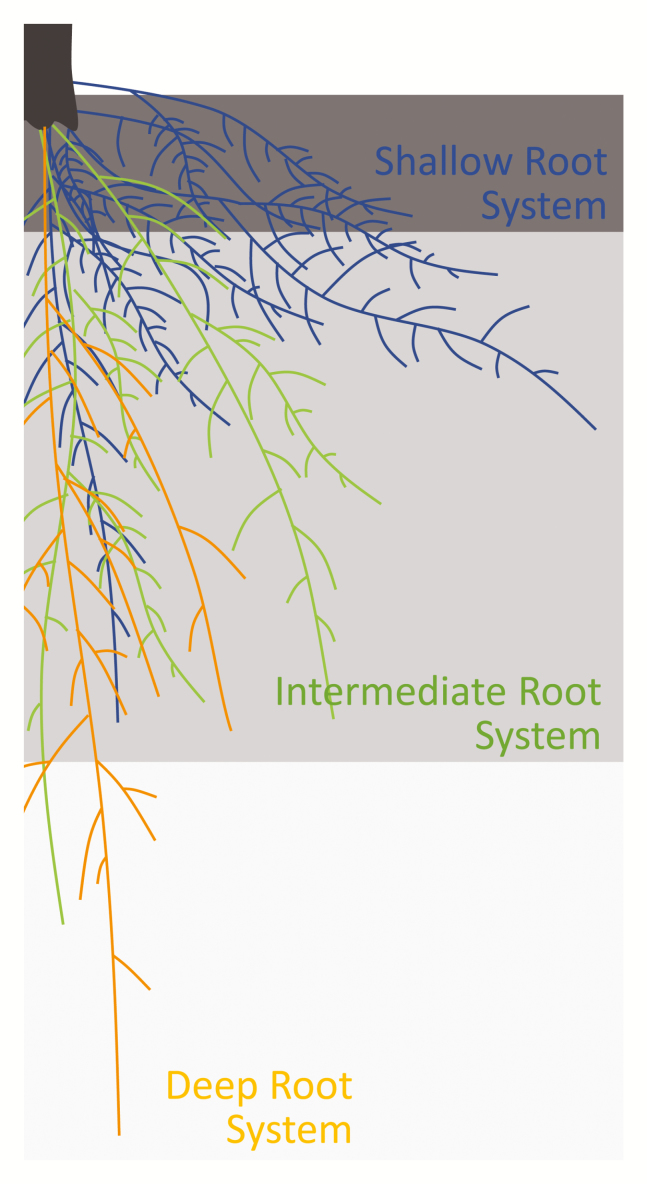
The central importance of root systems in the acquisition of water and nutrients by plants has meant that they have become a focus of plant breeders and crop improvement programmes (Box 2). In particular, traits such as root length, branching and growth angle determine the distribution of root surface area within the soil profile where nutrients and water are unevenly distributed (Lynch, 2011). Based on a comprehensive experimental analysis, an ideotype for enhanced water and nitrate acquisition has been proposed comprising of a deep-growing primary root with few laterals, steep-angled seminal roots with few long laterals, and crown roots with many laterals (Lynch, 2013). Steeper roots have already been shown to improve water uptake in a number of species including wheat (Manschadi et al., 2006), and nitrogen uptake in maize (Lynch, 2018; see also Varshney et al., 2018). Deeper rooting has also been shown to enhance the yield of rice grown under drought conditions (Uga et al., 2013).
Box 2. Plant root idiotypes
External factors, such as water and nutrient availability, can regulate the formation of lateral and adventitious roots, and differential root growth to favour a shallower or deeper root phenotype. While nutrients are often more abundant in the superficial layers of the soil, water can often only be found in the deeper layers, especially during drought.
Plant breeding relies on germplasm collections for each crop. These have been described in various species (see also Rebetzke, 2016), including wheat (Lilley and Kirkegaard, 2016; Kenobi et al., 2017), soybean (Prince et al., 2017; Valliyodan et al., 2017), chickpea (Chen et al., 2017), Lupinus angustifolius (Chen et al., 2016) and rice (Mori et al., 2016). Phenotyping is a key challenge for plant breeding and technological advances are crucial. In this context, computational approaches represent a valuable means to process, analyze and understand the complexity of root architecture and rhizosphere processes. Researchers have developed a non-invasive platform for maize root and shoot phenotyping able to predict drought tolerance under field conditions by measuring growth parameters at the seedling stage (Avramova et al., 2016). A density-based model, developed in Brassica rapa, is capable of reconstructing the growth parameters without the need to perform time-course experiments (Kalogiros et al., 2016). Further, a computational model has been developed in pea to predict mature root characteristics by linking them to genotypes (Zhao et al., 2017). Such approaches represent potentially powerful tools to dissect the developmental biology of root system architecture across a range of species.
Much more field data will be required to tune computational models for plants grown in the field. Fortunately, an advanced imaging system has been developed for the high-throughput phenotyping of root architecture in soil-grown wheat (Wasson et al., 2016). All high-throughput experiments, both in vitro and on soil, would benefit from multivariate analysis tools like the one developed for wheat. Phenotyping in combination with the analysis of quantitative trait loci (QTL) has been successful in identifying novel regulators of root architecture in both maize (Salvi et al., 2016) and wheat (Maccaferri et al., 2016). Furthermore, genome editing has opened up the possibility to quickly translate the knowledge gained from fundamental research into improved pre-breeding material and, hence, crops.
Perspectives
Humans have been selecting crops in some form since the beginnings of plant domestication, some 10,000 years ago. In that time, breeding has completely transformed crops like corn, wheat and banana, and created multiple varieties from wild-type ancestors. Crops have been bred for their productivity or to enhance certain traits like colour or root storage. Importantly, much of the breeding over the last 70 years has been for performance at relatively high levels of agricultural input, including fertilizer application and irrigation (Lynch, 2007). As well as being costly for farmers in financial terms, these inputs are also often associated with a high carbon footprint, something that is incompatible with the concept of the sustainable intensification of agriculture. To meet the challenge of producing more food while minimizing the damage to the very environmental systems within which that food is produced has meant that crop improvement programmes are now heavily focused on new ways to enable crops to perform well with lower levels of inputs and to withstand harsh conditions such as prolonged drought and flooding (Ashraf, 2010; Mustroph, 2018). With this in mind, plant scientists of every kind, from those researching fundamental aspects of plant growth and development to those finding innovative ways to exploit new discoveries in maximising agricultural productivity and minimising environmental harm, are playing their part in giving the planet, and all of its human inhabitants, the greatest chance of making it through the coming century in the best shape possible.
References
- Ali S, Kim WC. 2018. Plant growth promotion under water: decrease of waterlogging-induced ACC and ethylene levels by ACC deaminase-producing bacteria. Frontiers in Microbiology 9, 1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M. 2010. Inducing drought tolerance in plants: recent advances. Biotechnology advances 28, 169–183. [DOI] [PubMed] [Google Scholar]
- Atkinson JA, Rasmussen A, Traini R, Voß U, Sturrock C, Mooney SJ, Wells DM, Bennett MJ. 2014. Branching out in roots: uncovering form, function, and regulation. Plant Physiology 166, 538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramova V, Nagel KA, AbdElgawad H, Bustos D, DuPlessis M, Fiorani F, Beemster GT. 2016. Screening for drought tolerance of maize hybrids by multi-scale analysis of root and shoot traits at the seedling stage. Journal of Experimental Botany 67, 2453–2466. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ghanem ME, Siddique KH. 2017. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. Journal of Experimental Botany 68, 1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Shan F, Nelson MN, Siddique KH, Rengel Z. 2016. Root trait diversity, molecular marker diversity, and trait-marker associations in a core collection of Lupinus angustifolius. Journal of Experimental Botany 67, 3683–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwialkowska K, Nowakowska U, Mroziewicz A, Szarejko I, Kwasniewski M. 2016. Water-deficiency conditions differently modulate the methylome of roots and leaves in barley (Hordeum vulgare L.). Journal of Experimental Botany 67, 1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine MJ, Siddique KHM, Foyer CH. 2017. Nature’s pulse power: legumes, food security and climate change. Journal of Experimental Botany 68, 1815–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai A, Trenberth KE, Qian T. 2004. A global dataset of Palmer Drought Severity Index for 1870–2002: relationship with soil moisture and effects of surface warming. Journal of Hydrometeorology 5, 1117–1130. [Google Scholar]
- Du Y, Scheres B. 2018. Lateral root formation and the multiple roles of auxin. Journal of Experimental Botany 69, 155–167. [DOI] [PubMed] [Google Scholar]
- Gao Y, Lynch JP. 2016. Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). Journal of Experimental Botany 67, 4545–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani S, Sanguineti MC, Tuberosa R, Bellotti M, Salvi S, Landi P. 2005. Root-ABA1, a major constitutive QTL, affects maize root architecture and leaf ABA concentration at different water regimes. Journal of Experimental Botany 56, 3061–3070. [DOI] [PubMed] [Google Scholar]
- Herrbach V, Chirinos X, Rengel D, et al. 2017. Nod factors potentiate auxin signaling for transcriptional regulation and lateral root formation in Medicago truncatula. Journal of Experimental Botany 68, 569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F, Park WJ, Sauer M, Woll K. 2004. From weeds to crops: genetic analysis of root development in cereals. Trends in Plant Science 9, 42–48. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Woll K, Sauer M, Dembinsky D. 2004. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Annals of Botany 93, 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F. 2016. Untapping root system architecture for crop improvement. Journal of Experimental Botany 67, 4431–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak A, Kwaśniewski M, Szarejko I. 2016. Gene expression regulation in roots under drought. Journal of Experimental Botany 67, 1003–1014. [DOI] [PubMed] [Google Scholar]
- Kalogiros DI, Adu MO, White PJ, Broadley MR, Draye X, Ptashnyk M, Bengough AG, Dupuy LX. 2016. Analysis of root growth from a phenotyping data set using a density-based model. Journal of Experimental Botany 67, 1045–1058. [DOI] [PubMed] [Google Scholar]
- Kenobi K, Atkinson JA, Wells DM, Gaju O, De Silva JG, Foulkes MJ, Dryden IL, Wood ATA, Bennett MJ. 2017. Linear discriminant analysis reveals differences in root architecture in wheat seedlings related to nitrogen uptake efficiency. Journal of Experimental Botany 68, 4969–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S, Schopfer P. 2016. Priming and positioning of lateral roots in Arabidopsis. An approach for an integrating concept. Journal of Experimental Botany 67, 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Luo Z, Dong H, Eneji AE, Li W. 2016. H2O2 and ABA signaling are responsible for the increased Na+ efflux and water uptake in Gossypium hirsutum L. roots in the non-saline side under non-uniform root zone salinity. Journal of Experimental Botany 67, 2247–2261. [DOI] [PubMed] [Google Scholar]
- Kron W, Berz G. 2007. Flood disasters and climate change: trends and options – a (re-)insurer’s view. In: Lozán JL, Grassl H, Hupfer P, Menzel L, Schönwiese C-D, eds. Global change: enough water for all? Hamburg: Wissenschaftliche Auswertungen, 268–273. [Google Scholar]
- Kwasniewski M, Daszkowska-Golec A, Janiak A, Chwialkowska K, Nowakowska U, Sablok G, Szarejko I. 2016. Transcriptome analysis reveals the role of the root hairs as environmental sensors to maintain plant functions under water-deficiency conditions. Journal of Experimental Botany 67, 1079–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley JM, Kirkegaard JA. 2016. Farming system context drives the value of deep wheat roots in semi-arid environments. Journal of Experimental Botany 67, 3665–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Sauter M. 2018. Control of adventitious root architecture in rice by darkness, light, and gravity. Plant Physiology 176, 1352–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany 55, 493–512. [Google Scholar]
- Lynch JP. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology 156, 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2018. Rightsizing root phenotypes for drought resistance. Journal of Experimental Botany 69, 3279–3292. [DOI] [PubMed] [Google Scholar]
- Ma Y, Yang C, He Y, Tian Z, Li J. 2017. Rice OVATE family protein 6 regulates plant development and confers resistance to drought and cold stresses. Journal of Experimental Botany 68, 4885–4898. [DOI] [PubMed] [Google Scholar]
- Maccaferri M, El-Feki W, Nazemi G, Salvi S, Canè MA, Colalongo MC, Stefanelli S, Tuberosa R. 2016. Prioritizing quantitative trait loci for root system architecture in tetraploid wheat. Journal of Experimental Botany 67, 1161–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschadi AM, Christopher J, deVoil P, Hammer GL. 2006. The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33, 823–837. [DOI] [PubMed] [Google Scholar]
- Miyan MA. 2015. Droughts in asian least developed countries: vulnerability and sustainability. Weather & Climate Extremes 7, 8–23. [Google Scholar]
- Mori A, Fukuda T, Vejchasarn P, Nestler J, Pariasca-Tanaka J, Wissuwa M. 2016. The role of root size versus root efficiency in phosphorus acquisition in rice. Journal of Experimental Botany 67, 1179–1189. [DOI] [PubMed] [Google Scholar]
- Murphy E, Vu LD, Van den Broeck L, et al. 2016. RALFL34 regulates formative cell divisions in Arabidopsis pericycle during lateral root initiation. Journal of Experimental Botany 67, 4863–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A. 2018. Improving flooding tolerance of crop plants. Agronomy 8, 160. [Google Scholar]
- Nakajima Y, Nara Y, Kobayashi A, Sugita T, Miyazawa Y, Fujii N, Takahashi H. 2017. Auxin transport and response requirements for root hydrotropism differ between plant species. Journal of Experimental Botany 68, 3441–3456. [DOI] [PubMed] [Google Scholar]
- Opitz N, Marcon C, Paschold A, Malik WA, Lithio A, Brandt R, Piepho HP, Nettleton D, Hochholdinger F. 2016. Extensive tissue-specific transcriptomic plasticity in maize primary roots upon water deficit. Journal of Experimental Botany 67, 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlović I, Petřík I, Tarkowská D, Lepeduš H, Vujčić Bok V, Radić Brkanac S, Novák O, Salopek-Sondi B. 2018. Correlations between phytohormones and drought tolerance in selected Brassica crops: Chinese cabbage, white cabbage and kale. International Journal of Molecular Sciences 19 doi: 10.3390/ijms19102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. 2016. Plant roots: new challenges in a changing world. Journal of Experimental Botany 67, 991–993. [Google Scholar]
- Prince SJ, Murphy M, Mutava RN, Durnell LA, Valliyodan B, Shannon JG, Nguyen HT. 2017. Root xylem plasticity to improve water use and yield in water-stressed soybean. Journal of Experimental Botany 68, 2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Wang Q, Guo J, Wang P, Song P, Jia Q, Zhang X, Kudla J, Zhang W, Zhang Q. 2017. Peroxisomal CuAOζ and its product H2O2 regulate the distribution of auxin and IBA-dependent lateral root development in Arabidopsis. Journal of Experimental Botany 68, 4851–4867. [DOI] [PubMed] [Google Scholar]
- Rebetzke G. 2016. From inspiration to impact: delievering value from global root research. Journal of Experimental Botany 67, 3601–3603. [Google Scholar]
- Rellán-Álvarez R, Lobet G, Lindner H, et al. 2015. GLO-Roots: an imaging platform enabling multidimensional characterization of soil-grown root systems. Elife 4 doi: 10.7554/eLife.07597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I, Smith S, Stes E, et al. 2016. CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. Journal of Experimental Botany 67, 4889–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodell M, Famiglietti JS, Wiese DN, Reager JT, Beaudoing HK, Landerer FW, Lo MH. 2018. Emerging trends in global freshwater availability. Nature 557, 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhry S, Kepinski S. 2015. Shoot and root branch growth angle control-the wonderfulness of lateralness. Current Opinion in Plant Biology 23, 124–131. [DOI] [PubMed] [Google Scholar]
- Salvi S. 2017. An evo-devo perspective on root genetic variation in cereals. Journal of Experimental Botany 68, 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S, Giuliani S, Ricciolini C, Carraro N, Maccaferri M, Presterl T, Ouzunova M, Tuberosa R. 2016. Two major quantitative trait loci controlling the number of seminal roots in maize co-map with the root developmental genes rtcs and rum1. Journal of Experimental Botany 67, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B, Laskowski M. 2016. Root patterning: it takes two to tangle. Journal of Experimental Botany 67, 1201–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian J, Yee MC, Goudinho Viana W, et al. 2016. Grasses suppress shoot-borne roots to conserve water during drought. Proceedings of the National Academy of Sciences, USA 113, 8861–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Rasmussen A. 2016. The Physiology of adventitious roots. Plant Physiology 170, 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker GG, Colmer TD. 2017. Flooding tolerance of forage legumes. Journal of Experimental Botany 68, 1851–1872. [DOI] [PubMed] [Google Scholar]
- Tai H, Lu X, Opitz N, Marcon C, Paschold A, Lithio A, Nettleton D, Hochholdinger F. 2016. Transcriptomic and anatomical complexity of primary, seminal, and crown roots highlight root type-specific functional diversity in maize (Zea mays L.). Journal of Experimental Botany 67, 1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai H, Opitz N, Lithio A, Lu X, Nettleton D, Hochholdinger F. 2017. Non-syntenic genes drive RTCS-dependent regulation of the embryo transcriptome during formation of seminal root primordia in maize (Zea mays L.). Journal of Experimental Botany 68, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleski M, Imin N, Djordjevic MA. 2016. New role for a CEP peptide and its receptor: complex control of lateral roots. Journal of Experimental Botany 67, 4797–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron S, Bodner G, Laio F, Ridolfi L, Leitner D. 2015. Can diversity in root architecture explain plant water use efficiency? A modeling study. Ecological Modelling 312, 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, et al. 2013. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics 45, 1097–1102. [DOI] [PubMed] [Google Scholar]
- Varshney RK, Tuberosa R, Tardieu F. 2018. Progress in understanding drought tolerance: from alleles to cropping systems. Journal of Experimental Botany 69, 3175–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valliyodan B, Ye H, Song L, Murphy M, Shannon JG, Nguyen HT. 2017. Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. Journal of Experimental Botany 68, 1835–1849. [DOI] [PubMed] [Google Scholar]
- Veloccia A, Fattorini L, Della Rovere F, Sofo A, D’Angeli S, Betti C, Falasca G, Altamura MM. 2016. Ethylene and auxin interaction in the control of adventitious rooting in Arabidopsis thaliana. Journal of Experimental Botany 67, 6445–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Guo M, Li Y, et al. 2018. LARGE ROOT ANGLE1, encoding OsPIN2, is involved in root system architecture in rice. Journal of Experimental Botany 69, 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson A, Bischof L, Zwart A, Watt M. 2016. A portable fluorescence spectroscopy imaging system for automated root phenotyping in soil cores in the field. Journal of Experimental Botany 67, 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Burgess P, Zhang X, Huang B. 2016. Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. Journal of Experimental Botany 67, 1979–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bodner G, Rewald B, Leitner D, Nagel KA, Nakhforoosh A. 2017. Root architecture simulation improves the inference from seedling root phenotyping towards mature root systems. Journal of Experimental Botany 68, 965–982. [DOI] [PMC free article] [PubMed] [Google Scholar]