Functional analysis of the HMA4 protein C-terminal extension reveals a similar and key function of high affinity zinc binding by di-Cys motifs in both Arabidopsis thaliana and A. halleri.
Keywords: Arabidopsis, HMA4, hyperaccumulation, in vivo imaging, metal binding, metal–protein interaction, P-type ATPase, zinc
Abstract
The PIB ATPase heavy metal ATPase 4 (HMA4) has a central role in the zinc homeostasis network of Arabidopsis thaliana. This membrane protein loads metal from the pericycle cells into the xylem in roots, thereby allowing root to shoot metal translocation. Moreover, HMA4 is key for zinc hyperaccumulation as well as zinc and cadmium hypertolerance in the pseudometallophyte Arabidopsis halleri. The plant-specific cytosolic C-terminal extension of HMA4 is rich in putative metal-binding residues and has substantially diverged between A. thaliana and A. halleri. To clarify the function of the domain in both species, protein variants with truncated C-terminal extension, as well as with mutated di-Cys motifs and/or a His-stretch, were functionally characterized. We show that di-Cys motifs, but not the His-stretch, contribute to high affinity zinc binding and function in planta. We suggest that the HMA4 C-terminal extension is at least partly responsible for protein targeting to the plasma membrane. Finally, we reveal that the C-terminal extensions of both A. thaliana and A. halleri HMA4 proteins share similar function, despite marginally different zinc-binding capacity.
Introduction
Zinc is fundamental for all forms of life, including plants (Broadley et al., 2007; Hänsch and Mendel, 2009). This transition metal is widely used in proteins where it has structural or catalytic roles (Auld, 2001; Andreini et al., 2008). It is estimated that the percentage of the genome encoding Zn2+-binding proteins ranges from 4% in bacteria to 10% in eukaryotes (Andreini et al., 2006). However, high concentrations of zinc and other essential transition metals (e.g. copper and iron), as well as some metals known as non-essential (e.g. cadmium, lead, and mercury), are toxic for cells (Goyer, 1997; Nzengue et al., 2011; Lemire et al., 2013; Clemens and Ma, 2016). Plants have therefore developed a metal homeostasis network allowing a tight control of metal availability at both the organism and the cellular level, consisting of uptake, chelation, compartmentalization, and efflux mechanisms (Krämer and Clemens, 2005; Palmer and Guerinot, 2009).
Heavy metal ATPase 4 (HMA4) is an essential component of the zinc homeostasis network in Arabidopsis thaliana (Mills et al., 2003; Hussain et al., 2004; Verret et al., 2004; Wong and Cobbett, 2009; Cun et al., 2014). In this species, eight HMA proteins have been identified (Axelsen and Palmgren, 2001; Williams and Mills, 2005; Pedersen et al., 2012; Hanikenne and Baurain, 2014). HMA5–8 are monovalent cation pumps involved in copper homeostasis (Woeste and Kieber, 2000; Shikanai et al., 2003; Abdel-Ghany et al., 2005; Andrés-Colás et al., 2006; Kobayashi et al., 2008; Binder et al., 2010). HMA1 is a broad-specificity divalent cation transporter (Ca2+, Cd2+, Zn2+, Cu2+, Co2+) located in the chloroplast (Seigneurin-Berny et al., 2006; Moreno et al., 2008; Kim et al., 2009; Boutigny et al., 2014), whereas HMA3 localizes to the vacuolar membrane and is involved in zinc/cadmium sequestration (Gravot et al., 2004; Morel et al., 2009). Finally, HMA2 and HMA4 are both found in the plasma membrane and are expressed in root pericycle and in shoot cells bordering the xylem (Hussain et al., 2004; Verret et al., 2004; Sinclair et al., 2007; Wong et al., 2009; Siemianowski et al., 2013). Together, HMA2 and HMA4 are responsible for zinc and cadmium loading in root xylem, thereby allowing their translocation from root to shoot (Hussain et al., 2004; Verret et al., 2004; Wong and Cobbett, 2009; Cun et al., 2014). They are also essential for zinc loading in seeds (Olsen et al., 2016). An hma2hma4 double mutant has a stunted growth phenotype, resulting from severe zinc deficiency in shoots (Hussain et al., 2004). In addition, HMA4 plays a key role in zinc and cadmium hyperaccumulation and hypertolerance in the pseudometallophyte Arabidopsis halleri (Courbot et al., 2007; Willems et al., 2007; Hanikenne et al., 2008; Verbruggen et al., 2009; Krämer, 2010; Hanikenne and Nouet, 2011). In this species, HMA4 is overexpressed thanks to cis-activation and triplication of the gene, which triggers higher rates of root-to-shoot metal translocation compared with A. thaliana (Talke et al., 2006; Hanikenne et al., 2008).
HMA proteins are PIB ATPases whose protein architecture consists of a transmembrane domain (TM domain) and two cytoplasmic catalytic domains, the actuator domain (A domain) and the ATP-binding domain (ATP domain). The latter is divided into a nucleotide-binding domain (N domain) and a phosphorylation domain (P domain). In addition, most of them possess N-, as well as occasionally C-, terminal cytosolic extensions (Williams and Mills, 2005; Argüello et al., 2007; Rosenzweig and Argüello, 2012). PIB ATPases are a subfamily of the larger P-type ATPase family that are membrane proteins coupling ATP hydrolysis to their transport of substrate, following the E1/E2 Post–Albers cycle (Albers et al., 1963; Post and Sen, 1965). During this cycle, the phosphorylation and dephosphorylation of an invariant Asp residue located in the P domain, as well as ion binding in the TM domain, trigger conformational changes allowing ion transport across the membrane (Kühlbrandt, 2004; Palmgren and Nissen, 2011; Rosenzweig and Argüello, 2012; Sitsel et al., 2015). The TM domain of PIB ATPases is involved in the metal specificity of the transporter (Argüello, 2003; Williams and Mills, 2005; Hanikenne and Baurain, 2014; Smith et al., 2014). A recent study combining homology modeling of the A. thaliana HMA4 TM region to functional analysis in vivo delineated a zinc permeation pathway across the membrane (G. Lekeux et al., unpublished results). Similar to HMA2 (Eren et al., 2007; Wong et al., 2009), the CCxxE metal binding motif present in the HMA4 N-terminal extension binds Zn2+ with nanomolar affinity and this interaction is essential for the function of the protein in planta (Zimmermann et al., 2009; Laurent et al., 2016). The HMA4 N-terminal metal binding domain might achieve its function through interaction with a docking platform positioned at the membrane interface of the TM domain (G. Lekeux et al., unpublished results) as suggested for its prokaryotic homolog ZntA of Shigella sonnei (Wang et al., 2014a).
In contrast to their bacterial homologs, the well-known ZntA and CadA efflux pumps (Nucifora et al., 1989; Rensing et al., 1997), plant zinc/cadmium PIB ATPases exhibit a cytosolic C-terminal extension that is rich in putative metal-binding amino acids such as Cys, His, Asp, and Glu residues (Williams and Mills, 2005; Argüello et al., 2007; Rosenzweig and Argüello, 2012). Interestingly, they all possess di-Cys motifs ranging from two in AtHMA3 to 13 in AtHMA4 (Williams and Mills, 2005). The AtHMA2 C-terminal extension (HMA2c), with six di-Cys motifs (Supplementary Fig. S1 at JXB online), was shown to bind three Zn2+ ions with high affinity. The truncation of HMA2c decreased the ATPase activity of AtHMA2, but enabled almost total complementation of the zinc deficiency phenotype of A. thaliana hma2hma4 plants, despite partial mislocalization of the protein in the pericycle, suggesting that HMA2c is not essential for function (Eren et al., 2006; Wong et al., 2009). The function of the HMA4 C-terminal extension (HMA4c) remains more elusive, and contradictory results have been reported. In comparison with AtHMA2c, AtHMA4c is longer (470 amino acids) and contains approximately two times more Asp, Glu, and Cys residues (including 13 di-Cys motifs) and an 11 His-stretch at the C-terminal extremity (Supplementary Fig. S1). Consistently, it was estimated to interact with more Zn2+ ions (10) compared with AtHMA2c (Baekgaard et al., 2010). The functional importance of AtHMA4c was tested in complementation experiments in yeast. A C-terminal truncated form of AtHMA4 was able to rescue the zinc sensitivity of a yeast mutant (Mills et al., 2005), whereas in contradiction a His-stretch-deleted version was not (Verret et al., 2005). Moreover, gradual deletion of AtHMA4c revealed a progressive increase in the ability of the protein to confer zinc tolerance to sensitive yeast (Baekgaard et al., 2010). This is in contrast to a simultaneous study showing that a C-terminal-truncated AtHMA4 protein failed to complement the hma2hma4 zinc deficiency phenotype (Mills et al., 2010). Similar discrepancies were observed when analysing the effect of the C-terminal extension truncation of Oryza sativa HMA2 and HMA3 upon expression in yeast or in plants (Satoh-Nagasawa et al., 2012; Kumagai et al., 2014). Note that these rice mutant proteins localized properly in onion epidermal cells (Satoh-Nagasawa et al., 2012; Kumagai et al., 2014).
Interestingly, the HMA4c might account for functional differences between A. thaliana and A. halleri HMA4 proteins. Indeed, compared with AtHMA4c, the AhHMA4c exhibits 11 di-Cys motifs and a 10 His residue His-stretch. The amino acid sequences of the AtHMA4c and AhHMA4c display 64% of identity only, in contrast with other parts of the proteins sharing 96% amino acid sequence identity (Supplementary Fig. S1). These differences might arise from directional positive selection that operated on AhHMA4c during the evolutionary history of A. halleri and directly contribute to zinc/cadmium hyperaccumulation (Mano and Innan, 2008; Hanikenne et al., 2013).
Here, the function in vivo and Zn2+ binding properties in vitro of the C-terminal extensions of the AtHMA4 and AhHMA4 were compared to examine potential functional divergence. Moreover, to alleviate conflicting results reported in the literature, a series of mutations were introduced in both HMA4c allowing assessment of the functional relevance of the whole domain, and of the di-Cys motif and His-stretch. We show that the C-terminal extensions of both proteins share a similar function and that they control, at least in part, the intracellular localization. We further reveal the key contribution of the di-Cys motifs, but not the His-stretch, to high affinity Zn2+ binding and function in planta. Finally, Zn2+ binding studies in vitro suggest that HMA4c is both a strong zinc chelator and a zinc donor.
Materials and methods
Plant material, growth condition, and transformation
Arabidopsis thaliana L. Heynhold (accession Columbia, Col-0) and hma2hma4 double mutant A. thaliana plants (Col-0 background) (Hussain et al., 2004) were used in all experiments. Prior to transformation, plants were grown on soil supplied with 1 mM ZnSO4 in a short-day growth chamber (22 °C and 8 h day−1 photoperiod) for 7 weeks. Plants were then transferred to long days (16 h day−1 photoperiod) and supplied with 3 mM ZnSO4 for 5 weeks to allow flowering. The plants were transformed using Agrobacterium tumefaciens by floral dipping (Clough and Bent, 1998).
For phenotyping and metal accumulation analysis on soil, third generation (T3) homozygous transgenic seeds were germinated in short days on 1/2 Murashige and Skoog (MS) agar medium containing 1% sucrose. After 14 d, seedlings were transferred to soil (potting mix, Brill TYPical, Tonerde 1/100 l) watered with tap water and grown for 6 weeks in long days prior to imaging and sample harvesting. For metal accumulation analysis in hydroponic conditions, the 2-week-old seedlings were instead transferred to hydroponic trays (Araponics, Belgium; Tocquin et al., 2003) with modified Hoagland medium (Talke et al., 2006; Charlier et al., 2015; Nouet et al., 2015) containing 1 µM ZnSO4 (control condition) and grown for 3 weeks in short days. The treatment was then initiated: plants were grown in the presence of 0.2 µM ZnSO4 (Nouet et al., 2015). Nutrient solutions were changed weekly. After 3 weeks of treatment, root and shoot samples were harvested separately before processing for inductively coupled plasma atomic emission spectroscopy (ICP-AES) analyses or RNA extraction.
Cloning
To generate the pAtHMA4::AtHMA4 cassette, the full length coding sequence of the A. thaliana HMA4 (AtHMA4) was cloned into the pAtHMA4::AhHMA4 pBluescript II KS+ vector (pBKS) (Laurent et al., 2016) in replacement of the full length coding sequence of the A. halleri HMA4 (AhHMA4). The pAtHMA4::AtAhHMA4 cassette was obtained by replacing in the same vector a fragment encoding residues 1–702 of AhHMA4 by the corresponding AtHMA4 fragment using the In-Fusion HD cloning kit (Takara). The pAtHMA4::AhAtHMA4 cassette was obtained by replacing in the pAtHMA4::AtHMA4 pBKS a fragment encoding residues 1–702 of AtHMA4 by the corresponding AhHMA4 fragment using the In-Fusion HD cloning kit. Synthetic genes encoding C-terminal fragments of AtHMA4 (residues 742–1172) with 11 His-stretch→11 Ala-stretch (HA variant) and 13 di-Cys→13 di-Ala motifs (CCAA variant) were obtained from GenScript (USA). The fragments were subsequently cloned into pAtHMA4::AtHMA4 pBKS, using HpaI/PacI restriction sites to generate pAtHMA4::AtHMA4HA pBKS and pAtHMA4::AtHMA4CCAA pBKS, respectively. A fragment of the pAtHMA4::AtHMA4CCAA cassette, encoding the 1–1156 residues, was then cloned into pAtHMA4::AtHMA4HA pBKS in replacement of the corresponding region to obtain pAtHMA4::AtHMA4CHA pBKS (CHA variant, combining His-stretch and di-Cys mutations). For AhHMA4, synthetic genes encoding C-terminal fragments with 10 His-stretch→10 Ala-stretch (residues 1083–1163, HA variant) and 13 di-Cys→13 di-Ala motifs (residues 742–1163, CCAA variant) were obtained from GenScript. The fragments were subsequently cloned into pAtHMA4::AhHMA4 pBKS (Laurent et al., 2016), using SpeI/PacI and HpaI/PacI restriction sites to generate pAtHMA4::AhHMA4HA pBKS and pAtHMA4::AhHMA4CCAA pBKS, respectively. A fragment of the pAtHMA4::AhHMA4CCAA cassette, encoding the residues 1–1148 was then cloned into pAtHMA4::AhHMA4HA pBKS in replacement of the corresponding region to obtain pAtHMA4::AhHMA4CHA pBKS (CHA variant). The pAtHMA4::AhHMA4Ctrunc cassette was obtained by cloning a fragment encoding the 1–702 residues of AhHMA4 directly followed by a stop codon into pAtHMA4::AhHMA4 pBKS (Laurent et al., 2016) in replacement of AhHMA4, using the In-Fusion HD cloning kit. To create binary vectors by plant transformation, all promoter::cDNA cassettes were finally cloned in a promoter-less variant of the pMDC32 vector (Curtis and Grossniklaus, 2003; Hanikenne et al., 2008) after AscI/PacI-excision from the pBKS vectors. In contrast, a fragment encoding the AtHMA4 residues 1–702 was amplified by PCR with primers allowing the addition of an AscI restriction site in 5′ and a stop codon followed by a PacI restriction site in 3′ and subsequently cloned into the promoter-less variant of pMDC32 to generate pAtHMA4::AtHMA4Ctrunc pMDC32.
For localization experiments, the fragments encoding the AxHMA4CCAA, AxHMA4CHA, and AxHMA4Ctrunc variants were cloned into pAhHMA4-2::AhHMA4::GFP pBKS (Nouet et al., 2015) in replacement of the AhHMA4 coding sequence, using the In-Fusion HD cloning kit. All six promoter::cDNA::GFP cassettes were then cloned in a promoter-less variant of the pMDC32 vector (Curtis and Grossniklaus, 2003; Hanikenne et al., 2008) after AscI/PacI-excision from pBKS. The pAhHMA4-2::AtHMA4::GFP pBKS vector used as control was already available from G. Lekeux et al., unpublished results. Note that the pAhHMA4-2 promoter was preferred to the pAtHMA4 promoter as it supported higher expression levels facilitating green fluorescent protein (GFP) imaging (Nouet et al. 2015; Laurent et al. 2016).
For production in E. coli, synthetic genes with optimized codon usage encoding C-terminal fragments of AtHMA4 (residues 703–1172), the corresponding AtHMA4CCAA mutant, and AhHMA4 (residues 703–1163) were obtained from GenScript. The fragments were cloned into the pMalC2x vector (NEB), using EcoRI/HindIII restriction sites to allow expression in N-terminal fusion with maltose binding protein (MBP) generating the MBP::AtHMA4c, MBP::AtHMA4CCAAc, and MBP::AhHMA4c cassettes. Site-directed mutagenesis, using the QuikChange Site-Directed Mutagenesis method (Agilent Technologies) was then performed on the newly constructed MBP::AtHMA4c pMalC2x to insert a stop codon at the 3′ extremity of the sequence linking the sequence encoding MBP and AtHMA4c, allowing expression of the MBP protein alone.
All final constructions were verified by sequencing.
Metal accumulation analyses
Shoot tissues were cleaned with milliQ water, while root tissues were desorbed as described (Talke et al., 2006) and dried at 60 °C for 3 d. Shoot samples (10–50 mg of tissues) were then acid-digested in DigiPrep tubes with 3 ml of ≥65% HNO3 (Sigma-Aldrich) on a DigiPrep Graphite Block Digestion System (SCP Science) as follows: 15 min at 45 °C, 15 min at 65 °C, and 90 min at 105 °C. After cooling, sample volumes were adjusted to 10 ml with milliQ water, and 200 µl ≥65% HNO3 was added. Metal concentrations were determined using ICP-AES with a Vista-AX instrument (Varian, Melbourne, Australia) as described (Nouet et al., 2015).
Gene expression analyses
Total RNAs were extracted from root and shoot tissues separately using the RNeasy Plant Mini kit with on-column DNAse treatment (Qiagen). cDNAs were then synthesized from 1 µg of total RNA with the RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) using Oligo(dT). Quantitative RT-PCR reactions were performed in 384-well plates with a QuantStudio 5 Real-Time PCR system (Thermo Fisher Scientific) using Takyon™ Low Rox SYBR® MasterMix dTTP blue (Eurogentec). Three technical replicates were performed for each sample/primer pair (Supplementary Table S1). The reactions were performed in a total volume of 10 µl including 5 µl of Takyon™ Low Rox SYBR® MasterMix, 2.5 pmol of forward and reverse primers (Supplementary Table S1), and 4 µl of cDNA diluted 50×. The thermal profile used was: 2 min at 50 °C, 2 min at 95 °C, 40 repeats of 15 s at 95 °C, and 1 min at 60 °C, and a final stage of 15 s at 95 °C, 1 min at 60 °C, and 15 s at 95 °C to determine dissociation curves of the amplified products. The quality of the PCRs was checked visually through analysis of dissociation and amplification curves, and reaction efficiencies were determined for each PCR using the LinRegPCR software v2013 (Ruijter et al., 2009). For each primer pair, mean reaction efficiencies were calculated from all reactions (Supplementary Table S1) and were then used to quantify relative gene expression levels by normalization using two reference genes, At1g18050 and EF1α (Czechowski et al., 2005), with the qBase software (Biogazelle; Hellemans et al., 2007). The adequacy of the reference genes to normalize gene expression in the experimental conditions was checked using geNorm software in qBase (gene stability measure M=0.262, pairwise variation CV=0.091) (Vandesompele et al., 2002).
Confocal microscopy
T1 seeds of A. thaliana plants expressing the variant HMA4 proteins fused to GFP were germinated on 1/2 MS agar medium containing 1% sucrose and hygromycin B (20 µg ml−1) in short days. After 14 d, seedlings were transferred on the same medium without antibiotic. After 3 d, roots of three to six independent lines per construct were analysed. Images were collected at a 1024 × 1024-pixel resolution using a TCS SP5 inverted confocal laser microscope (Leica Microsystems) with a water-immersion PlanApochromat ×63 1.20 objective (Leica Microsystems) as previously described (Rausin et al., 2010). An argon-ion laser (488 nm) was used for GFP excitation and the emission light was dispersed and recorded between 500 and 540 nm. Within one experiment, all images were acquired with the same excitation and detection settings (photomultiplier tube (PMT) gain, offset, ...) for all genotypes, with a PMT gain ensuring detection of GFP fluorescence only and excluding autofluoresence. To estimate HMA4 protein expression levels in root cells (Tillemans et al. 2005; Dubeaux et al. 2018), GFP fluorescence intensities were measured from confocal microscope images using ImageJ (https://imagej.nih.gov/ij/; accessed Sept 03, 2018) and plot profile analysis. Briefly, in pericycle cells expressing HMA4 fused to GFP, 10 optical sections were drawn across the transverse plasma membranes. GFP fluorescence intensity values (n=20) were then used to calculate a mean fluorescence intensity for each independent mutant line.
Protein production and purification
Escherichia coli cells [strain BL21 (DE3)] transformed with the MBP::AtHMA4c pMalC2x, MBP::AtHMA4CCAAc pMalC2x, or MBP::AhHMA4c pMalC2x expression vector were grown at 37 °C in 500 ml terrific broth (TB) medium containing 50 µM ZnCl2, 100 µg ml−1 ampicillin and 2 g l−1 glucose. At an OD600 of ~0.6, the production was directly induced with 1 mM isopropyl β-D-thiogalactopyranoside. The culture was then incubated for 18 h at 18 °C. The cells, collected by centrifugation, were resuspended in 100 ml of 20 mM Tris/HCl (pH 7.5) with 200 mM NaCl, 2.5 mM tris(2-carboxyethyl)phosphine (TCEP) and 50 µM ZnCl2. A protease inhibitor cocktail (mini complete EDTA-free, Roche) and benzonase (Merck) were added. Cells were subsequently lysed using an EmulsiFlex-C3 cell disrupter (Avestin). The cellular extracts were clarified by centrifugation at 48000 g for 40 min at 4 °C. The soluble fraction was then loaded onto a 5 ml amylose resin high flow column (NEB) equilibrated in 20 mM Tris/HCl (pH 7.5) with 200 mM NaCl, 2.5 mM TCEP, 50 µM ZnCl2, and 1 mM phenylmethylsulfonyl fluoride (buffer A). The bound proteins were eluted with buffer A in the presence of 10 mM maltose. The fractions containing AtHMA4c, AtHMA4CCAAc, or AhHMA4c were pooled and directly loaded onto a HisTrap 5 ml column (GE Healthcare) equilibrated in buffer A. The bound proteins were washed with buffer A in the presence of 50 mM imidazole. They were then eluted in buffer A in the presence of 500 mM imidazole. The fractions containing AtHMA4c, AtHMA4CCAAc, or AhHMA4c were pooled and dialysed against 20 mM Tris/HCl (pH 7.5) with 200 mM NaCl, 1 mM TCEP and 100 µM ZnCl2. The MBP was similarly expressed in E. coli and purified on the amylose column only before being dialysed. The protein purity was assessed by SDS/PAGE.
Zinc binding assay
To ensure metal removal and reductive conditions, proteins were incubated with an excess EDTA and TCEP for 3 h, followed by desalting in 50 mM MOPS (pH 7.3) with 100 mM NaCl, under anaerobic condition in a glovebox ([O2]<2 ppm). The protein concentration was estimated via a thiol assay with Ellman’s reagent, 5,5-dithiobis(2-nitrobenzoic acid), which reacts quantitatively with free cysteine thiols releasing the chromophore 5-mercapto-2-nitrobenzoate (λmax=418 nm; extinction coefficient ε=13600 M−1 cm−1) (Zimmermann et al., 2009). This concentration estimation was warranted by (i) high detection sensitivity due to the high Cys contents in each protein target, (ii) unchanged Cys contents with/without addition of EDTA/SDS, demonstrating that there was neither metal-blocking of Cys thiols nor inaccessible thiols, and (iii) identical Zn2+ binding in the presence and absence of TCEP, demonstrating the absence of oxidized disulfide bonds.
Zn2+ binding assays were performed in 50 mM Mops (pH 7.3) with 100 mM NaCl and 1 mM TCEP; 1.0 µM AtHMA4c, AtHMA4CCAAc, or AhHMA4c and 22 or 111 µM of the zinc binding chromophoric ligand 4-(2-pyridylazo)resorcinol (Par) were used. Zn2+ and Par working solutions were prepared as described in Zimmermann et al. (2009). UV-visible spectra were recorded on a Varian Cary 300 spectrophotometer in dual-beam mode with quartz cuvettes with a path length of 1 cm.
The experiment was conducted based on following several relationships:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
Titration of a limited amount of Zn2+ into a solution containing the metal indicator Par and purified protein P (i.e. AtHMA4c, AtHMA4CCAAc, AhHMA4c, or MBP) induces competitive binding for the added Zn2+ ions between the Par ligand and the protein P according to an overall equilibrium described by Eq. (1). The Cys/His-rich C-terminal extension contains multiple Zn2+-binding sites with different affinities and can bind various numbers of Zn2+ ions. Consequently, the Zn2+-containing HMA4c species are a mixture of ZnIIi-HMA4c (i=1, 2, … n) with the distribution of the number i depending on the Zn2+ availability. The total Zn2+ bound in is equal to the difference between total Zn2+ added to the system and total Zn2+ bound by the Par metal indicator according to Eq. (2), and then the average number of zinc ions bound by the protein P under a specific condition is estimated by Eq. (3).
The Zn2+ availability in the system is estimated from the chromophoric response of Par upon Zn2+ binding according to Eqs (4) and (5) while Zn2+ binding to various protein sites may be described by Eqs (6) and (7). Since in the same experimental solution, the free Zn2+ concentration in Eqs (4) and (5) is identical to the free Zn2+ concentration in Eqs (6) and (7), the Zn2+ binding KD defined by Eqs (6) and (7) can be monitored and estimated by the Par probe from the free [Zn2+] estimated from Eqs (4) and (5). The effective formation constants β′2 (=1/KDeff) for the complex ZnII(Par)2 have been estimated at a range of pH values where logβ′2=12.02 at pH 7.3 (Kocyła et al., 2015). The equilibrium concentration of ZnII(Par)2 in the system was estimated directly from the characteristic solution absorbance at 500 nm with a reported pH-dependent extinction coefficients that is 70.1 mM−1 cm−1 at pH 7.3 (Kocyła et al., 2015). The total free [Par] that does not bind Zn2+ was obtained by the relationship [Par]=[Par]tot−2[ZnII(Par)2] and free [Zn2+] by Eq. (5).
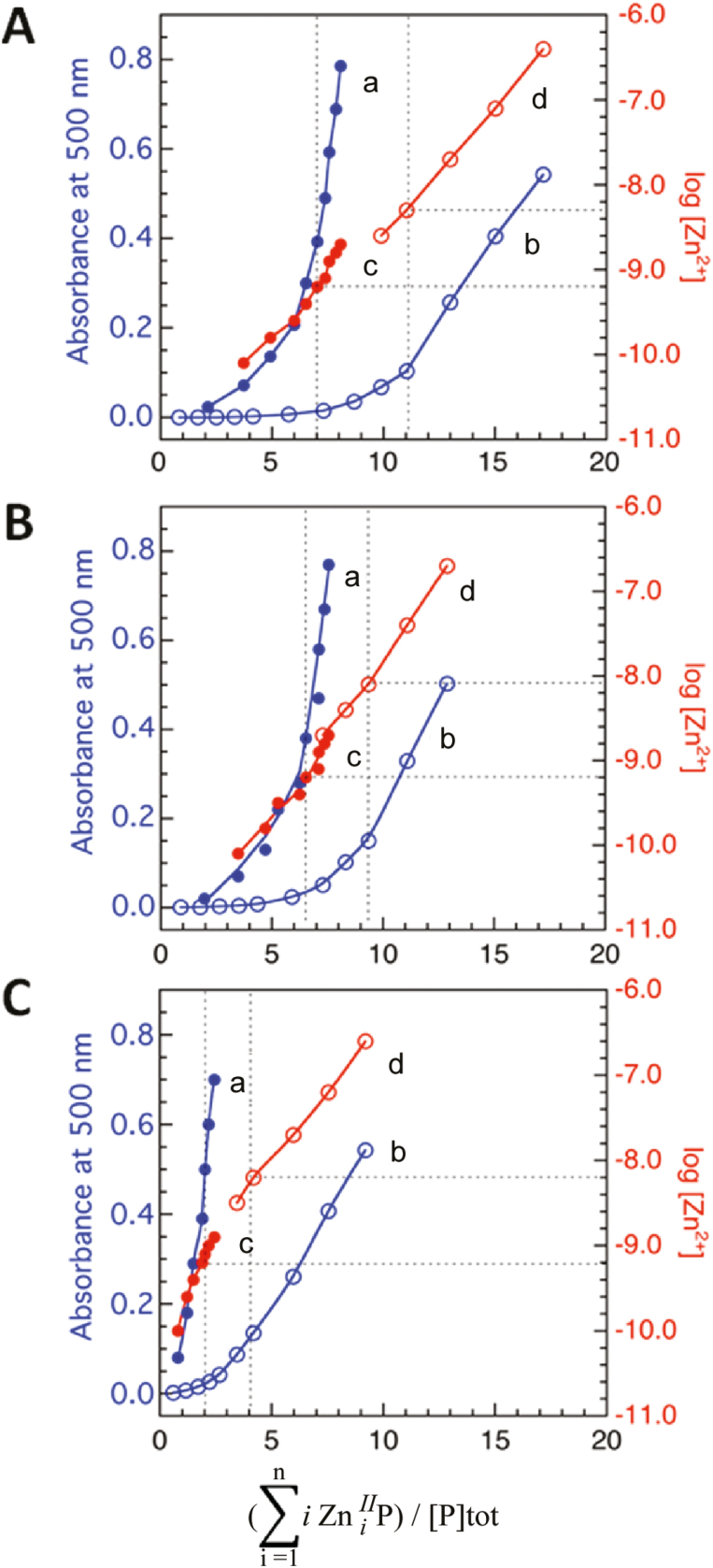
Stepwise titration of Zn2+ into the solution containing Par and HMA4c will increase free [Zn2+] concentration in the solution and this will in turn increase the Zn2+ occupations on HMA4c [Eq. (3)] and/or Par [Eq. (4); detected by A500], depending not only on the affinity of HMA4c for Zn2+, but also on the total Par concentration in the solution. These relationships may be analysed graphically by plotting an average Zn2+ occupation number n [defined by Eq. (3)] on HMA4c versus ZnII(HPar)2 formation (via A500) and log[Zn2+] [via Eq. (5)], as shown in Fig. 6.
Fig. 6.
Quantification of Zn2+ binding affinity and stoichiometry of isolated proteins AtHMA4c (A), AhHMA4c (B), and AtHMA4CCAAc (C) with Par probe at either 22 µM (open circles) or 111 µM (solid circles). (a, b) Variation of A500 with average zinc binding stoichiometry of proteins; (c, d) Variation of log [Zn2+] with average zinc binding stoichiometry of proteins. The experiments were conducted by titration of each protein (1.0 µM) in MOPS buffer (50 mM, pH 7.3) containing NaCl (100 mM) and TCEP (1 mM) in the presence of excess Par ligand.
Results
Function of the HMA4 C-terminal extension in vivo
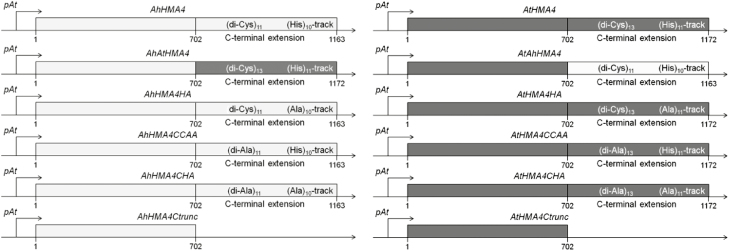
To assess and compare the function of the C-terminal domains of the A. thaliana and A. halleri HMA4 proteins, a series of mutants were generated as summarized in Fig. 1. First, chimeras consisting of the sequence coding for the AtHMA4 N-terminal and TM region followed by the coding sequence of AhHMA4c (AtAhHMA4) and vice versa (AhAtHMA4) were generated to assess whether the C-terminal extension is responsible for functional differences between the two proteins. Second, to tackle the specific importance of the HMA4c di-Cys motifs and His-stretch separately, they were respectively replaced by di-Ala motifs and a poly-Ala-stretch to create respectively the AxHMA4CCAA and AxHMA4HA mutant proteins (with x being t for thaliana or h for halleri). Third, these mutations were combined in the AxHMA4CHA proteins. Finally, the importance of the C-terminal extensions themselves was reassessed, truncating the C-terminal extension of both proteins (AxHMA4Ctrunc). Native and mutant forms of both genes were expressed under the control of the endogenous AtHMA4 promoter (pAtHMA4) in the loss-of-function hma2hma4 A. thaliana mutant (Hussain et al., 2004). Four to eight independent homozygous lines (T3 generation) were obtained for each construct.
Fig. 1.
Overview of the HMA4 variant constructs for complementation experiments in plants. The expression cassettes are schematically represented. The nucleotide sequences encoding A. thaliana (dark gray) or A. halleri (light gray) native or mutant HMA4 proteins are preceded by the A. thaliana HMA4 promoter (pAt, 2595 bp). Numbers correspond to the amino acid position in the HMA4 protein. Ah, A. halleri; At, A. thaliana; AtAhHMA4 and AhAtHMA4, swapped C-terminal extensions; HA: His- → Ala-stretch; CCAA: di-Cys → di-Ala motifs; CHA: HA and CCAA mutations combined; Ctrunc: fully truncated C-terminal extension.
Both native AxHMA4 proteins complemented the hma2hma4 phenotype and allowed plants to develop normally until seed setting, when grown on soil watered with tap water (Fig. 2). The hma2hma4 plants expressing the AtAhHMA4, AhAtHMA4, and AxHMA4HA variants grew similarly to those expressing the native proteins. In contrast, the expression of the AxHMA4Ctrunc variants completely failed in complementing the zinc deficiency phenotype of the hma2hma4 mutant, as reported previously for AtHMA4 (Mills et al., 2010). Indeed, plants expressing these C-truncation variants exhibited a stunted growth and chlorotic aspect identical to the hma2hma4 mutant plants (Fig. 2). An intermediate complementation was observed in plants expressing the AxHMA4CCAA and AxHMA4CHA variants. Although their growth was strongly impaired, with chlorosis and a bushy aspect, they were not as dramatically affected as the hma2hma4 mutant. Some of them were able to flower, yet they could not set functional seeds (Fig. 2).
Fig. 2.
Complementation of the A. thaliana hma2hma4 zinc deficiency phenotype. HMA4 variants were expressed in hma2hma4 plants under the control of the AtHMA4 promoter. The plant phenotypes are shown after 6 weeks of growth on soil without zinc supplementation. Non-transformed hma2hma4 plants or expressing the native HMA4 proteins were respectively used as negative and positive controls. Images are representative of multiple observations of four to eight independent homozygous T3 lines for each genotype. Ah, A. halleri; At, A. thaliana; AtAhHMA4 and AhAtHMA4, swapped C-terminal extensions; HA: His- → Ala-stretch; CCAA: di-Cys → di-Ala motifs; CHA: HA and CCAA mutations combined; Ctrunc: fully truncated C-terminal extension.
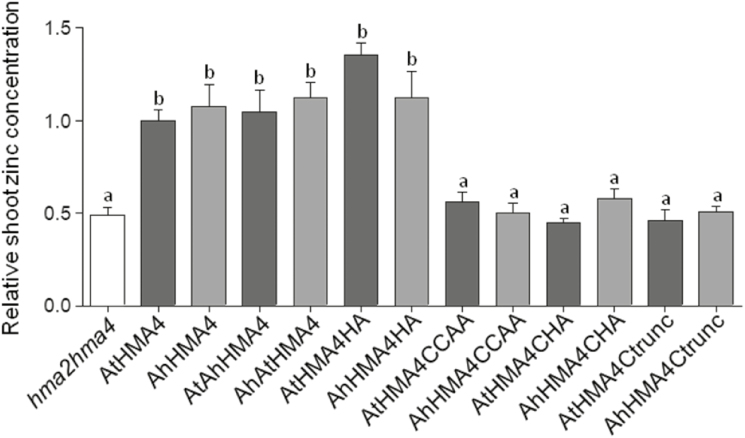
Zinc accumulation in rosette leaves was then determined for all genotypes grown on soil watered with tap water. Expression of the native proteins increased shoot zinc accumulation by 2-fold compared with the hma2hma4 mutant. No differences were observed between the ability of AtHMA4 and AhHMA4 to increase shoot zinc levels (Fig. 3). Consistent with their restored visual phenotype, plants expressing the AtAhHMA4, AhAtHMA4, and AxHMA4HA variants displayed zinc levels in shoots similar to those expressing the native proteins. In contrast, plants expressing the remaining variants (AxHMA4CCAA, AxHMA4CHA, and AxHMA4Ctrunc) exhibited shoot zinc contents identical to the hma2hma4 mutant, again in agreement with their visual phenotype (Fig. 3).
Fig. 3.
Zinc accumulation in complemented plants grown on soil. Non-transformed hma2hma4 mutant (white) and expressing the native or mutant A. thaliana (dark gray) or A. halleri (light gray) HMA4 proteins under the control of the AtHMA4 promoter were grown for 6 weeks on soil without zinc supplementation. Zinc concentrations were measured in shoot tissues collected from two plants per line. Values are relative to lines expressing the native AtHMA4 proteins and are means±SEM of four to eight homozygous T3 independent lines for each genotype. The data were analysed with one-way ANOVA followed by Tukey’s multiple comparison test. Statistically significant differences (P<0.05) between means are indicated by different letters. Ah, A. halleri; At, A. thaliana; AtAhHMA4 and AhAtHMA4, swapped C-terminal extensions; HA: His- → Ala-stretch; CCAA: di-Cys → di-Ala motifs; CHA: HA and CCAA mutations combined; Ctrunc: fully truncated C-terminal extension.
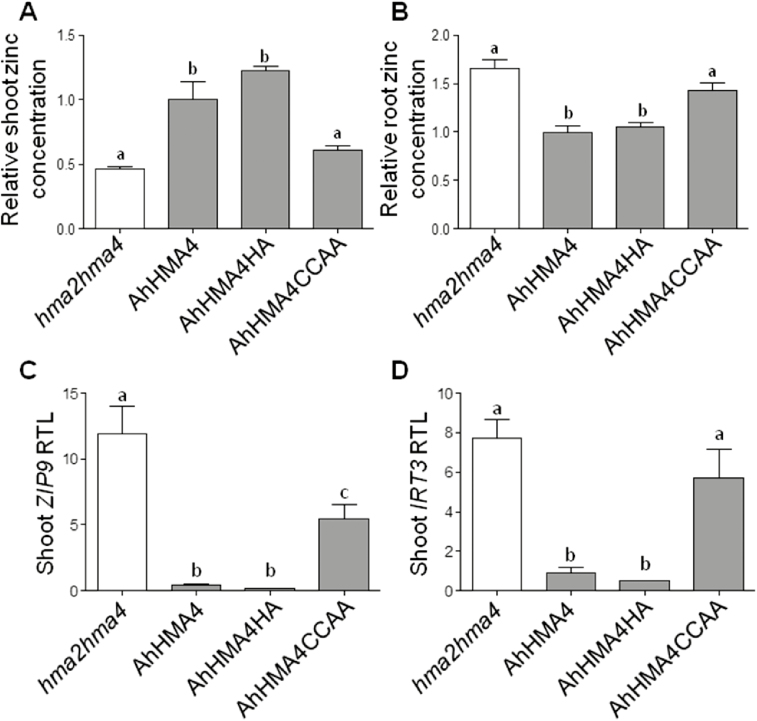
Zinc distribution in plant tissues was further detailed for a subset of constructs (AhHMA4, AhHMA4HA, and AhHMA4CCAA) upon growth in hydroponic medium (0.2 µM zinc) (Nouet et al., 2015). Plants expressing the native protein accumulated respectively about 2-fold higher and 1.5-fold lower zinc levels in shoots and roots compared with the hma2hma4 mutant, respectively (Fig. 4A, B). In agreement with the soil experiment, AhHMA4HA plants displayed zinc levels similar to AhHMA4 plants in both roots and shoots. Finally, zinc levels in AhHMA4CCAA plants were similar to levels found in the hma2hma4 mutant with, although not significant, slightly higher and lower zinc levels in shoots and roots compared with the mutant, respectively (Fig. 4A, B). Note that no other major changes were observed in the ionome of the plants (Supplementary Table S2). As with the hma2hma4 mutant, the AhHMA4CCAA plants, however, displayed slightly lower manganese, calcium, and magnesium levels in shoots compared with complemented plants (AhHMA4 and AhHMA4HA).
Fig. 4.
Zinc accumulation and expression of zinc deficiency response genes in complemented plants grown in hydroponic conditions. Non-transformed hma2hma4 mutant (white) and expressing the native or mutant A. halleri HMA4 proteins (medium gray) under the control of AtHMA4 promoter were grown for the last 3 weeks before harvest in Hoagland hydroponic medium containing 0.2 µM zinc. Zinc concentrations were measured in shoot (A) and root (B) tissues collected from two plants per line. Values are relative to lines expressing the native AhHMA4 proteins and are means±SEM of two independent lines from three biological replicates. Transcript levels were quantified from plant tissues collected from two plants per line. Relative transcript levels (RTL) of IRT3 (C) and ZIP9 (D) in shoots are mean±SEM of two independent lines from two biological replicates. The data were analysed with one-way ANOVA followed by Tukey’s multiple comparison test. Statistically significant differences (P<0.05) between means within each figure panel are indicated by different letters. Ah: A. halleri; HA: His-stretch→Ala-stretch; CCAA: di-Cys→di-Ala motifs.
To further assess the zinc status in the plants, the expression levels of ZIP9 and IRT3, genes whose expression is induced upon zinc deficiency and repressed upon zinc excess (Talke et al., 2006), were assessed in shoot tissues, which strongly respond to zinc deficiency in the hma2hma4 mutant (Nouet et al., 2015). The IRT3 and ZIP9 shoot transcript levels were respectively about 8- and 30-fold higher in the hma2hma4 mutant than in AhHMA4 and AhHMA4HA plants. In contrast, ZIP9, and although not significantly IRT3, transcript levels in AhHMA4CCAA shoots were intermediate between the hma2hma4 mutant and AhHMA4 or AhHMA4HA plants (Fig. 4C, D). Thus, both shoot and root zinc accumulation as well as shoot expression of zinc status marker genes displayed subtle differences between the hma2hma4 and AhHMA4CCAA plants (Fig. 4), possibly explaining the slightly improved growth of AhHMA4CCAA plants compared with the hma2hma4 mutant (Fig. 2).
Function of the HMA4 C-terminal extension in protein localization
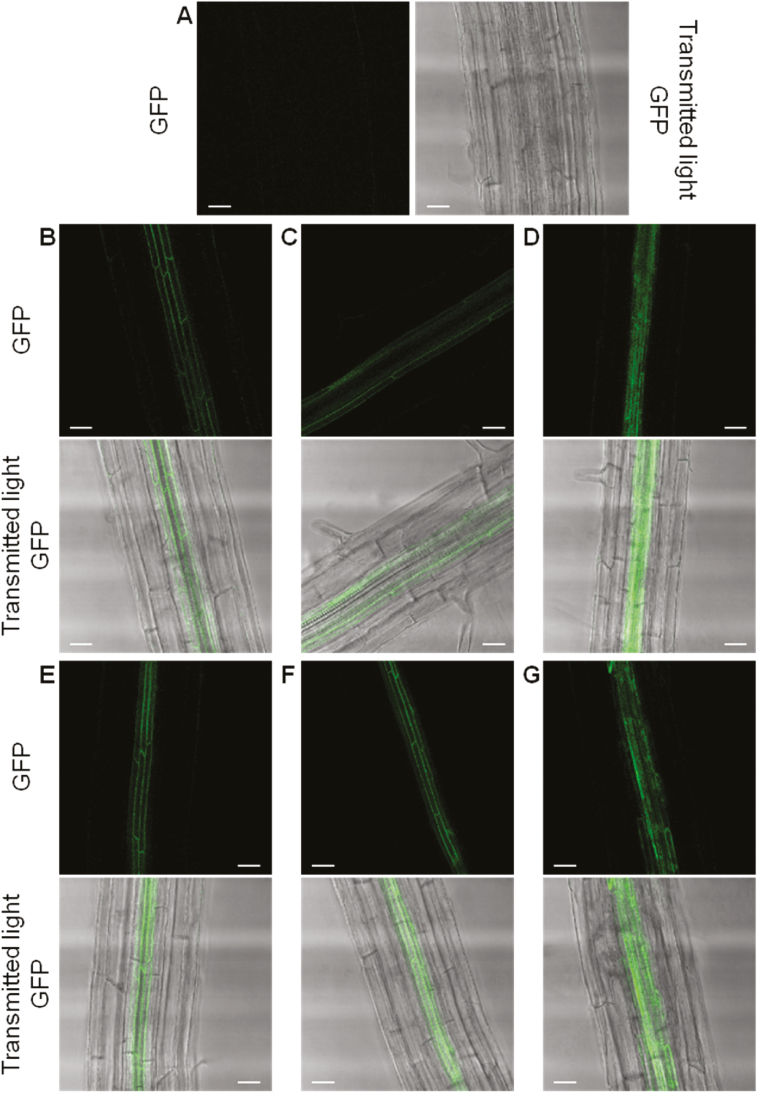
Whether the localization and/or the protein expression level of the variants unable to complement the hma2hma4 mutant were altered was next examined. The AxHMA4CCAA, AxHMA4CHA, and AxHMA4Ctrunc variants were expressed in fusion to GFP, under the control of the A. halleri HMA4 promoter 2 (pAhHMA4-2) in the Col-0 genetic background (Nouet et al., 2015). Roots of 18-day-old seedlings were examined by confocal microscopy. Non-transformed hma2hma4 seedlings did not emit any fluorescence (Fig. 5A). The native AtHMA4 and AhHM4 proteins were previously shown to localize to the plasma membrane of root pericycle cells in A. thaliana when expressed under the control of pAhHMA4-2 (Nouet et al., 2015; G. Lekeux et al., unpublished results), which was also observed for the AxHMA4CCAA and AxHMA4CHA, with no evidence of GFP aggregation in cells (Fig. 5B, C, E, F). Protein expression levels in cells were estimated for the four variants through GFP quantification and were similar to the expression level of the native AtHMA4 protein (Supplementary Fig. S2). The inability of the AxHMA4CCAA and AxHMA4CHA variants to complement the hma2hma4 phenotype therefore likely results from an impaired function of the protein in plants. In contrast, the AxHMA4Ctrunc variants were also detected in root pericycle cells, yet the GFP signal in the plasma membrane was not well defined and a high proportion of diffuse and dotted signal was detected inside the cells, suggesting protein retention in the membrane of an intracellular organelle (Fig. 5D, G; Supplementary Fig. S3). This altered localization likely accounted for the inability of the Ctrunc variants to complement the hma2hma4 mutant.
Fig. 5.
HMA4 variant localization in A. thaliana. The AtHMA4CCAA (B), AtHMA4CHA (C), AtHMA4Ctrunc (D), AhHMA4CCAA (E), AhHMA4CHA (F), and AhHMA4Ctrunc (G) variants fused to GFP and expressed under control of the copy 2 AhHMA4 promoter in Col-0 were imaged by confocal microscopy in roots of 18-day-old T1 seedlings. Non-transformed Col-0 seedlings (A) were used as negative controls. The images are, for each genotype, representative of three to six independent lines. Scale bars 25 µm. Ah, A. halleri; At, A. thaliana; CCAA: di-Cys→di-Ala motifs; CHA: HA: His- → Ala-stretch; CCAA: di-Cys → di-Ala motifs; CHA: HA and CCAA mutations combined; Ctrunc: fully truncated C-terminal extension.
Quantification of Zn2+ binding properties of isolated native C-terminal sequences and a CCAA variant
To provide molecular insights into the observed consequences of replacement of the C-terminal di-Cys motifs by di-Ala motifs on the HMA4 functions in vivo, the native AxHMA4 C-terminal extensions (AxHMA4c) and a corresponding protein variant, AtHMA4CCAAc, were expressed in E. coli and purified for zinc binding assay in vitro. To overcome a protein solubility problem and to facilitate the protein purification, the nucleotide sequences encoding AxHMA4c and AtHMA4CCAAc were fused to the C-terminal gene sequence of a maltose binding protein (MBP) and the target proteins expressed and purified as MBP-fusion proteins. The zinc binding assay was conducted by titrations of the purified protein samples with Zn2+ in the presence of a zinc binding chromophoric ligand, 4-(2-pyridylazo)resorcinol (Par) (Zimmermann et al., 2009; Kocyła et al., 2015). Here the ligand Par acts as a metal buffer controlling free Zn2+ concentrations in solution and as a metal speciation indicator monitoring zinc binding to the protein under various conditions (see ‘Materials and methods’ section for details).
Titration of Zn2+ into a MOPS buffer (pH 7.3) containing Par (111 µM) and AtHMA4c (1.0 µM) allowed monitoring of Zn2+ binding to the protein at free Zn2+ concentrations in the subnanomolar range (Fig. 6A, c). In such a solution, AtHMA4c was detected to bind about 7 equiv of zinc with dissociation constants KD<1 nM (Fig. 6A, a); see Eqs 6 and 7 in the ‘Materials and methods’ section for the definition of KD and its relationship to the free Zn2+ concentrations in solution). Equivalent titration but with lower total Par concentration (22 µM) increased the sensitive detection range of zinc binding to protein by ~1000-fold to free Zn2+ concentrations <1 µM (Fig. 6A, d). This titration confirmed the above-mentioned subnanomolar affinity zinc binding sites for the seven equivalents of zinc and detected further binding of ~4 equivalents of Zn2+ with KD in the range of 1–10 nM and of more than six equivalents of weak zinc binding with KD in the range of 10–1000 nM (Fig. 6A, b). However, such weak Zn2+ binding at KD>10 nM might be of little biological significance in nature. Control experiments carried out in the same conditions confirmed that the MBP carrier protein alone did not compete with Par for Zn2+, and therefore lacked Zn2+ binding sites.
Likewise, the native sequence of AhHMA4c was detected to possess comparable Zn2+-binding capacity under the same conditions: ~6 equivalents of zinc with KD<1 nM, ~3 equivalents with KD=1–10 nM and >3 equivalents with KD=10–1000 nM, but the overall Zn2+ binding number was reduced (Fig. 6B). This may be attributed to the reduced number of di-Cys motifs in AhHMA4c (Supplementary Fig. S1).
In contrast, the equivalent experiments on the protein variant AtHMA4CCAAc detected that the protein can bind ~2 equivalents of zinc with KD<1 nM and another two with KD=1–10 nM and more than five with KD=10–1000 nM (Fig. 6C). This demonstrated that a replacement of the 13 di-Cys motifs by the di-Ala motifs compromised the high affinity Zn2+ binding sites (with KD<10 nM) specifically. The variant retained comparable capacity for weak Zn2+ binding (KD>10 nM), which, again, might be of little biological significance.
Discussion
This comparative analysis of HMA4 variant proteins allowed a deeper understanding of the function of the C-terminal extension of the protein, and provides plausible interpretations for conflicting results from previous reports on this function (see below). The study highlighted the particular importance of the di-Cys motifs. It further emphasized that studying a protein in vivo in its endogenous context is key for proper functional characterization. Here, the A. thaliana HMA4 promoter was used to express HMA4 variants in the hma2hma4 mutant background. In addition, point mutations replacing native residues by Ala residues rather than truncations was favored to address the importance of specific amino acids.
The His-stretch in the HMA4 C-terminal extension is not required for function
In this study, replacing the AxHMA4 His-stretch by an Ala-stretch (Fig. 1) did not impair the ability of the protein to complement the A. thaliana hma2hma4 zinc deficiency phenotype. The visual phenotype, and root and shoot zinc accumulation, as well as the expression of zinc deficiency marker genes, were restored (Figs 2–4), suggesting that the His-stretch is not essential for the protein function in vivo. In contrast, truncating the A. thaliana HMA4 C-terminal extremity to delete the His-stretch was shown to disable the ability of the protein to rescue the zinc sensitivity of a mutant yeast (Verret et al., 2005). This contradiction suggests that one of the amino acid residues downstream of the His-stretch might be important for the protein function. Interestingly, a Ser residue is present in the penultimate position of the HMA4 protein. As reported for the penultimate Thr residue of the H+-ATPase C-terminal extension, the phosphorylation of this Ser residue might regulate the protein function (Fuglsang et al., 1999; Svennelid et al., 1999; Ottmann et al., 2007; Ekberg et al., 2010).
Deleting the HMA4 C-terminal extension results in altered localization and loss of function
Conflicting results were also reported regarding the impact of C-terminal truncations of A. thaliana HMA4, depending on the complementation assay set-up (yeast or A. thaliana). An HMA4 version with fully truncated C-terminal extension was still able to complement zinc sensitivity of a mutant yeast (Mills et al., 2005). Moreover, sequential deletion of the di-Cys motifs of HMA4 revealed a progressive increase in zinc tolerance when the deleted versions were expressed in a zinc sensitive yeast strain (Baekgaard et al., 2010). On the other hand, full truncation of the HMA4 C-terminal extension led to a complete lack of complementation when the protein was expressed in A. thaliana hma2hma4 plants (Mills et al., 2010). However, in the latter study, this observation may stem from ectopic and constitutive expression resulting in protein overexpression and mislocalization. In the present study, an identical phenotype was observed when the Ctrunc form of AxHMA4 was expressed under the control of the endogenous pAtHMA4 in the hma2hm4 mutant (Figs 2, 3), and the truncated proteins showed altered localization (Fig. 5D, G; Supplementary Fig. S3). The C-terminal truncation might trigger the retention of the protein in the endoplasmic reticulum, as the protein signal roughly delineated a membrane system and surrounded the nucleus (Supplementary Fig. S3) (Baekgaard et al., 2010). This mislocalization of the HMA4 version with fully truncated C-terminal extension likely accounts for its inability to complement the hma2hma4 in Mills et al. (2010) as well as in our study and might also explain the discrepancy with the results obtained in yeast (Mills et al., 2005; Baekgaard et al., 2010). It also prevents determination of the function of HMA4c as a whole, as the effect on function of (i) the mis-localization and (ii) the lack of amino acid residues required for metal binding can be confounded. However, we show here the key contribution of di-motifs of HMA4c (see below for discussion).
The truncation of the A. thaliana HMA2c also led to protein mislocalization, with a diffuse distribution in the cytoplasm (Wong et al., 2009). In contrast, the truncation of the HMA2 N-terminal domain, mutations in the CCTSE motif in the N-terminal domain of HMA4, as well as mutation of the residues forming the zinc permeation pathway of HMA4 did not alter their intracellular localization (Wong et al., 2009; Laurent et al., 2016; G. Lekeux et al., unpublished results). It is thus tempting to hypothesize that the signal targeting HMA4 to the plasma membrane might stand in the C-terminal extension. In particular, di-Leu motifs, whose mutations were previously shown to alter membrane protein trafficking, are present in the HMA4 C-terminal extension (Petris et al., 1998; Petris and Mercer, 1999; Wang et al., 2014b). In addition, HMA4c is rich in Ser and Thr residues. The phosphorylation of such residues has been proposed to be related to the regulation of ATP7B cellular trafficking (Lutsenko, 2016). Alternatively, it is not excluded that the C-terminal truncation might disrupt proper protein folding and thereby cause its retention in the ER (Lefebvre et al., 2012). Such misfolding might trigger protein aggregation, explaining the presence of GFP fluorescence clusters in cells expressing the C-terminal-truncated AxHMA4 protein (Fig. 5D, G; Supplementary Fig. S3). However, the C-terminal extension might not be as important for the localization of some other HMA proteins, as the truncation of OsHMA2 and OsHMA3 C-terminal extensions did not alter their localization in onion epidermal cells (Satoh-Nagasawa et al., 2012; Kumagai et al., 2014). Note that the C-terminal extensions of these proteins do not exhibit any di-Leu motifs.
The di-Cys motifs in the HMA4 C-terminal extension are required for function and confer nanomolar affinity for multiple zinc ions
To assess their function, all di-Cys motifs present in the C-terminal extension of AxHMA4 were replaced by di-Ala motifs (Fig. 1). The expression of this variant partially complemented the zinc deficiency phenotype of the hma2hma4 mutant (Figs 2–4), despite proper expression at the transcript and protein levels (Supplementary Figs S2, S4) and proper cellular localization (Fig. 5B, E; Supplementary Fig. S3), thus highlighting the key contribution of these di-Cys motifs to the HMA4 protein function. The same phenotype was observed when di-Cys→di-Ala and His-stretch→Ala-stretch mutations were combined (Figs 1–4), again confirming the low importance of the His-stretch (Figs 2–4).
Following production and purification in E. coli, the Zn2+ binding capability of both native AxHMA4c proteins and a selected variant, AtHMA4CCAAc, were examined in vitro. Both native proteins were shown to be able to bind multiple zinc ions (>10) with a continuous spectrum of different affinities from subnanomolar to micromolar, but it was not possible to define individual binding sites and their affinity (Fig. 6). High binding capacity was anticipitated from their protein sequences that are rich in putative metal-binding ligands (Cys, His, Asp, and Glu residues) including multiple di-Cys motifs (Supplementary Fig. S1). Nevertheless, we were able to detect that AtHMA4c can bind ~11 Zn2+ ions with KD<10 nM, in excellent agreement with a previous observation that an AtHMA4c protein form containing ~10 equivalents of Zn2+ could be isolated by size-exclusion chromatography (Baekgaard et al., 2010). It is possible that only those Zn2+ ions bound by the protein with nanomolar affinity or below can survive the size-exclusion chromatography elution. It is also likely that only such high affinity Zn2+ binding sites are biologically significant.
AhHMA4c was shown here to possess comparable binding affinities for all types of Zn2+ binding but with some marginal decrease in binding capacity, i.e. lower number of bound Zn2+ ions. This is likely a consequence of a decreased number of di-Cys motifs in AhHMA4c relative to that in AtHMA4c (11 vs 13) although their total Cys numbers are comparable (42 vs 44) (Supplementary Fig. S1). Nevertheless, these differences did not account for functional differences, as AtHMA4 and AhHMA4 identically complemented the hma2hma4 mutant (Figs 2–4), demonstrating that both C-terminal extensions possess more than enough Zn2+ binding capacity for their cellular functions.
In contrast, a replacement of all 13 di-Cys motifs by di-Ala in AtHMA4CCAAc reduced the number of high affinity Zn2+ binding sites specifically (from ~11 to ~4 with KD<10 nM) while the weak binding sites (KD>10 nM) were largely retained (see Fig. 6). A di-Cys motif in the N-terminus of AhHMA4 was recently shown to constitute a Zn2+ site with KD=6 nM, but a contribution of a nearby Glu as a co-ligand enhanced the affinity by 25-fold to a KD=0.25 nM (Laurent et al., 2016). These experiments suggest that di-Cys motifs contribute dominantly to the high affinity Zn2+ binding sites but other co-ligands are required for the observed high Zn2+ binding affinity at subnanomolar levels. The severe functional impairment of both AxHMA4CCAA proteins in complementation of the hma2hma4 mutant (Figs 2–4) highlights the functional importance of Zn2+ binding sites with KD<10 nM and the biological irrelevance of those weak sites with KD>10 nM. Notably, AtHMA4CCAAc still retained limit binding sites for four Zn2+ ions with KD<10 nM. These sites may be either contributed originally from other non-di-Cys ligands such as single Cys or numerous His, Asp, and Glu residues also present in HMA4c (Supplementary Fig. S1), or from structural coordination rearrangement induced upon removal of di-Cys motifs, creating new high affinity binding sites. Nevertheless, limited high affinity Zn2+ binding in AxHMA4CCAA is not sufficient for the proper cellular functions, but may account for the observed residual activity in complementing the function of the hma2hma4 mutant (Figs 2–4).
In agreement with the putative importance of the C-terminal extension through high affinity Zn2+ binding, the truncation of A. thaliana HMA2c, which binds three Zn2+ with high affinity, was shown to intermediately decrease the activity of the protein in vitro (Eren et al., 2006). A tendency towards partial or complete loss of function was also observed when truncating the HMA2 N-terminal extension (Wong et al., 2009) or mutating the high affinity Zn2+ binding motif of both HMA2 and HMA4 N-terminal extensions (Eren et al., 2007; Zimmermann et al., 2009; Laurent et al., 2016). This is, however, in contrast to the previous report showing that the progressive deletion of the di-Cys motifs gradually increased the ability to confer zinc resistance to a sensitive yeast (Baekgaard et al., 2010). This contradiction might be explained by the lack of a plant component (such as a putative interactor) when the experiments are carried out in yeast.
The presence of multiple metal binding sites with different affinities is a common feature for Cys-rich proteins and may be important for scavenging metal ions with high affinity in metal-limiting conditions and to bind excess metal ions for export when the metal supply is too high. For example, Cys-rich human metallothionein possesses three types of Zn2+ binding sites with different affinities and can act as both a strong Zn2+ binder and a donor (Krężel and Maret, 2007, 2017). The Cys-rich C-terminus of HMA4 proteins may assume similar functions. Moreover, as has already been proposed for cytosolic extensions found in zinc/cadmium PIB ATPases, HMA4c might regulate the protein activity, possibly by interacting with other domains of the protein (Mitra and Sharma, 2001; Eren et al., 2006, 2007; Liu et al., 2006; Wong et al., 2009; Wang et al., 2014a; Laurent et al., 2016; G. Lekeux et al., unpublished results). A putative interaction with another cytosolic protein typical of plants should not be neglected too.
Sequence divergence among AtHMA4c and AhHMA4c does not result in functional divergence
Finally, the A. thaliana and A. halleri HMA4 proteins, as well as chimeric proteins with swapped C-terminal extensions, were equally able to complement the hma2hma4 mutant, restoring similar growth on soil and shoot zinc accumulation (Figs 2–3). The C-terminal domains of the two proteins displayed consistent Zn2+ binding properties in vitro (Fig. 6), whereas the CCAA and CHA mutations of the two proteins resulted in the same phenotypes when assessed in vivo (Figs 2, 3). High sequence divergence between these two domains does not seem to support essential functional differences. The contribution of HMA4 to higher zinc translocation from root to shoot in A. halleri compared with A. thaliana thus appears to exclusively result from higher expression of HMA4 in A. halleri (Talke et al., 2006; Courbot et al., 2007; Hanikenne et al., 2008). At a biochemical level, it suggests a flexible organization of the C-terminal extensions of the AtHMA4 and AhHMA4 proteins, which despite a high sequence divergence, fulfill the same functions in vivo.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Amino acid sequence alignment of plant PIB-2 ATPase C-terminal extensions.
Fig. S2. HMA4 native and variant protein expression level in A. thaliana.
Fig. S3. Closer views of HMA4 variant localization in A. thaliana.
Fig. S4. AhHMA4 native and variant gene expression levels in complemented plants grown in hydroponic conditions.
Table S1. Sequences and reaction efficiencies of quantitative RT-PCR primer pairs.
Table S2. Ionome profile of complemented plants grown in hydroponic conditions.
Acknowledgements
We thank Gianmarco Mastrosanti, Arnaud Degueldre and Marie Schloesser for technical support. We also thank Dr Ute Krämer for helpful discussions. Funding was provided by the “Fonds de la Recherche Scientifique – FNRS” (FRFC-2.4583.08, PDR-T.0206.13; MH, MG), the University of Liège (SFRD-12/03; MH) and the Belgian Program on Interuniversity Attraction Poles (IAP no. P7/44; MG, MH). MH is Research Associate of the FNRS. GL thanks the FRIA for his PhD fellowship.
References
- Abdel-Ghany SE, Müller-Moulé P, Niyogi KK, Pilon M, Shikanai T. 2005. Two P-type ATPases are required for copper delivery in Arabidopsis thaliana chloroplasts. The Plant Cell 17, 1233–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers RW, Fahn S, Koval GJ. 1963. The role of sodium ions in the activation of electrophorus electric organ adenosine triphosphatase. Proceedings of the National Academy of Sciences, USA 50, 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. 2006. Zinc through the three domains of life. Journal of Proteome Research 5, 3173–3178. [DOI] [PubMed] [Google Scholar]
- Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. 2008. Metal ions in biological catalysis: from enzyme databases to general principles. Journal of Biological Inorganic Chemistry 13, 1205–1218. [DOI] [PubMed] [Google Scholar]
- Andrés-Colás N, Sancenón V, Rodríguez-Navarro S, Mayo S, Thiele DJ, Ecker JR, Puig S, Peñarrubia L. 2006. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. The Plant Journal 45, 225–236. [DOI] [PubMed] [Google Scholar]
- Argüello JM. 2003. Identification of ion-selectivity determinants in heavy-metal transport P1B-type ATPases. The Journal of Membrane Biology 195, 93–108. [DOI] [PubMed] [Google Scholar]
- Argüello JM, Eren E, González-Guerrero M. 2007. The structure and function of heavy metal transport P1B-ATPases. Biometals 20, 233–248. [DOI] [PubMed] [Google Scholar]
- Auld DS. 2001. Zinc coordination sphere in biochemical zinc sites. Biometals 14, 271–313. [DOI] [PubMed] [Google Scholar]
- Axelsen KB, Palmgren MG. 2001. Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiology 126, 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekgaard L, Mikkelsen MD, Sørensen DM, et al. . 2010. A combined zinc/cadmium sensor and zinc/cadmium export regulator in a heavy metal pump. The Journal of Biological Chemistry 285, 31243–31252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, Rodríguez FI, Bleecker AB. 2010. The copper transporter RAN1 is essential for biogenesis of ethylene receptors in Arabidopsis. The Journal of Biological Chemistry 285, 37263–37270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutigny S, Sautron E, Finazzi G, Rivasseau C, Frelet-Barrand A, Pilon M, Rolland N, Seigneurin-Berny D. 2014. HMA1 and PAA1, two chloroplast-envelope PIB-ATPases, play distinct roles in chloroplast copper homeostasis. Journal of Experimental Botany 65, 1529–1540. [DOI] [PubMed] [Google Scholar]
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. 2007. Zinc in plants. New Phytologist 173, 677–702. [DOI] [PubMed] [Google Scholar]
- Charlier JB, Polese C, Nouet C, Carnol M, Bosman B, Krämer U, Motte P, Hanikenne M. 2015. Zinc triggers a complex transcriptional and post-transcriptional regulation of the metal homeostasis gene FRD3 in Arabidopsis relatives. Journal of Experimental Botany 66, 3865–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Ma JF. 2016. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annual Review of Plant Biology 67, 489–512. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Courbot M, Willems G, Motte P, Arvidsson S, Roosens N, Saumitou-Laprade P, Verbruggen N. 2007. A major quantitative trait locus for cadmium tolerance in Arabidopsis halleri colocalizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiology 144, 1052–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cun P, Sarrobert C, Richaud P, Chevalier A, Soreau P, Auroy P, Gravot A, Baltz A, Leonhardt N, Vavasseur A. 2014. Modulation of Zn/Cd P1B2-ATPase activities in Arabidopsis impacts differently on Zn and Cd contents in shoots and seeds. Metallomics 6, 2109–2116. [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. 2005. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology 139, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubeaux G, Neveu J, Zelazny E, Vert G. 2018. Metal sensing by the IRT1 transporter-receptor orchestrates its own degradation and plant metal nutrition. Molecular Cell 69, 953–964.e5. [DOI] [PubMed] [Google Scholar]
- Ekberg K, Palmgren MG, Veierskov B, Buch-Pedersen MJ. 2010. A novel mechanism of P-type ATPase autoinhibition involving both termini of the protein. The Journal of Biological Chemistry 285, 7344–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren E, González-Guerrero M, Kaufman BM, Argüello JM. 2007. Novel Zn2+ coordination by the regulatory N-terminus metal binding domain of Arabidopsis thaliana Zn2+-ATPase HMA2. Biochemistry 46, 7754–7764. [DOI] [PubMed] [Google Scholar]
- Eren E, Kennedy DC, Maroney MJ, Argüello JM. 2006. A novel regulatory metal binding domain is present in the C terminus of Arabidopsis Zn2+-ATPase HMA2. The Journal of Biological Chemistry 281, 33881–33891. [DOI] [PubMed] [Google Scholar]
- Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, Jensen ON, Aducci P, Palmgren MG. 1999. Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr946-Thr-Val and requires phosphorylation of Thr947. The Journal of Biological Chemistry 274, 36774–36780. [DOI] [PubMed] [Google Scholar]
- Goyer RA. 1997. Toxic and essential metal interactions. Annual Review of Nutrition 17, 37–50. [DOI] [PubMed] [Google Scholar]
- Gravot A, Lieutaud A, Verret F, Auroy P, Vavasseur A, Richaud P. 2004. AtHMA3, a plant P1B-ATPase, functions as a Cd/Pb transporter in yeast. FEBS Letters 561, 22–28. [DOI] [PubMed] [Google Scholar]
- Hanikenne M, Baurain D. 2014. Origin and evolution of metal P-type ATPases in Plantae (Archaeplastida). Frontiers in Plant Science 4, 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikenne M, Kroymann J, Trampczynska A, Bernal M, Motte P, Clemens S, Krämer U. 2013. Hard selective sweep and ectopic gene conversion in a gene cluster affording environmental adaptation. PLoS Genetics 9, e1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikenne M, Nouet C. 2011. Metal hyperaccumulation and hypertolerance: a model for plant evolutionary genomics. Current Opinion in Plant Biology 14, 252–259. [DOI] [PubMed] [Google Scholar]
- Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Krämer U. 2008. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453, 391–395. [DOI] [PubMed] [Google Scholar]
- Hänsch R, Mendel RR. 2009. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Current Opinion in Plant Biology 12, 259–266. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biology 8, R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain D, Haydon MJ, Wang Y, Wong E, Sherson SM, Young J, Camakaris J, Harper JF, Cobbett CS. 2004. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. The Plant Cell 16, 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Choi H, Segami S, Cho HT, Martinoia E, Maeshima M, Lee Y. 2009. AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. The Plant Journal 58, 737–753. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kuroda K, Kimura K, Southron-Francis JL, Furuzawa A, Kimura K, Iuchi S, Kobayashi M, Taylor GJ, Koyama H. 2008. Amino acid polymorphisms in strictly conserved domains of a P-type ATPase HMA5 are involved in the mechanism of copper tolerance variation in Arabidopsis. Plant Physiology 148, 969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocyła A, Pomorski A, Krężel A. 2015. Molar absorption coefficients and stability constants of metal complexes of 4-(2-pyridylazo)resorcinol (PAR): Revisiting common chelating probe for the study of metalloproteins. Journal of Inorganic Biochemistry 152, 82–92. [DOI] [PubMed] [Google Scholar]
- Krämer U. 2010. Metal hyperaccumulation in plants. Annual Review of Plant Biology 61, 517–534. [DOI] [PubMed] [Google Scholar]
- Krämer U, Clemens S. 2005. Functions and homeostasis of zinc, copper, and nickel in plants. In: Tamas MJ, Martinoia E, eds. Molecular biology of metal homeostasis and detoxification. Berlin, Heidelberg:Springer-Verlag, 215–271. [Google Scholar]
- Krężel A, Maret W. 2007. Dual nanomolar and picomolar Zn(II) binding properties of metallothionein. Journal of the American Chemical Society 129, 10911–10921. [DOI] [PubMed] [Google Scholar]
- Krężel A, Maret W. 2017. The functions of metamorphic metallothioneins in zinc and copper metabolism. International Journal of Molecular Sciences 18, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W. 2004. Biology, structure and mechanism of P-type ATPases. Nature Reviews. Molecular Cell Biology 5, 282–295. [DOI] [PubMed] [Google Scholar]
- Kumagai S, Suzuki T, Tezuka K, Satoh-Nagasawa N, Takahashi H, Sakurai K, Watanabe A, Fujimura T, Akagi H. 2014. Functional analysis of the C-terminal region of the vacuolar cadmium-transporting rice OsHMA3. FEBS Letters 588, 789–794. [DOI] [PubMed] [Google Scholar]
- Laurent C, Lekeux G, Ukuwela AA, et al. . 2016. Metal binding to the N-terminal cytoplasmic domain of the PIB ATPase HMA4 is required for metal transport in Arabidopsis. Plant Molecular Biology 90, 453–466. [DOI] [PubMed] [Google Scholar]
- Lefebvre B, Klaus-Heisen D, Pietraszewska-Bogiel A, Hervé C, Camut S, Auriac MC, Gasciolli V, Nurisso A, Gadella TW, Cullimore J. 2012. Role of N-glycosylation sites and CXC motifs in trafficking of Medicago truncatula Nod factor perception protein to plasma membrane. The Journal of Biological Chemistry 287, 10812–10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire JA, Harrison JJ, Turner RJ. 2013. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nature Reviews. Microbiology 11, 371–384. [DOI] [PubMed] [Google Scholar]
- Liu J, Dutta SJ, Stemmler AJ, Mitra B. 2006. Metal-binding affinity of the transmembrane site in ZntA: implications for metal selectivity. Biochemistry 45, 763–772. [DOI] [PubMed] [Google Scholar]
- Lutsenko S. 2016. Copper trafficking to the secretory pathway. Metallomics 8, 840–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano S, Innan H. 2008. The evolutionary rate of duplicated genes under concerted evolution. Genetics 180, 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RF, Francini A, Ferreira da Rocha PS, Baccarini PJ, Aylett M, Krijger GC, Williams LE. 2005. The plant P1B-type ATPase AtHMA4 transports Zn and Cd and plays a role in detoxification of transition metals supplied at elevated levels. FEBS Letters 579, 783–791. [DOI] [PubMed] [Google Scholar]
- Mills RF, Krijger GC, Baccarini PJ, Hall JL, Williams LE. 2003. Functional expression of AtHMA4, a P1B-type ATPase of the Zn/Co/Cd/Pb subclass. The Plant Journal 35, 164–176. [DOI] [PubMed] [Google Scholar]
- Mills RF, Valdes B, Duke M, Peaston KA, Lahner B, Salt DE, Williams LE. 2010. Functional significance of AtHMA4 C-terminal domain in planta. PLoS One 5, e13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra B, Sharma R. 2001. The cysteine-rich amino-terminal domain of ZntA, a Pb(II)/Zn(II)/Cd(II)-translocating ATPase from Escherichia coli, is not essential for its function. Biochemistry 40, 7694–7699. [DOI] [PubMed] [Google Scholar]
- Morel M, Crouzet J, Gravot A, Auroy P, Leonhardt N, Vavasseur A, Richaud P. 2009. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiology 149, 894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno I, Norambuena L, Maturana D, Toro M, Vergara C, Orellana A, Zurita-Silva A, Ordenes VR. 2008. AtHMA1 is a thapsigargin-sensitive Ca2+/heavy metal pump. The Journal of Biological Chemistry 283, 9633–9641. [DOI] [PubMed] [Google Scholar]
- Nouet C, Charlier JB, Carnol M, Bosman B, Farnir F, Motte P, Hanikenne M. 2015. Functional analysis of the three HMA4 copies of the metal hyperaccumulator Arabidopsis halleri. Journal of Experimental Botany 66, 5783–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G, Chu L, Misra TK, Silver S. 1989. Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium-efflux ATPase. Proceedings of the National Academy of Sciences, USA 86, 3544–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzengue Y, Candéias SM, Sauvaigo S, Douki T, Favier A, Rachidi W, Guiraud P. 2011. The toxicity redox mechanisms of cadmium alone or together with copper and zinc homeostasis alteration: Its redox biomarkers. Journal of Trace Elements in Medicine and Biology 25, 171–180. [DOI] [PubMed] [Google Scholar]
- Olsen LI, Hansen TH, Larue C, et al. . 2016. Mother-plant-mediated pumping of zinc into the developing seed. Nature Plants 2, 16036. [DOI] [PubMed] [Google Scholar]
- Ottmann C, Marco S, Jaspert N, et al. . 2007. Structure of a 14-3-3 coordinated hexamer of the plant plasma membrane H+-ATPase by combining X-ray crystallography and electron cryomicroscopy. Molecular Cell 25, 427–440. [DOI] [PubMed] [Google Scholar]
- Palmer CM, Guerinot ML. 2009. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nature Chemical Biology 5, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG, Nissen P. 2011. P-type ATPases. Annual Review of Biophysics 40, 243–266. [DOI] [PubMed] [Google Scholar]
- Pedersen CN, Axelsen KB, Harper JF, Palmgren MG. 2012. Evolution of plant p-type ATPases. Frontiers in Plant Science 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petris MJ, Camakaris J, Greenough M, LaFontaine S, Mercer JF. 1998. A C-terminal di-leucine is required for localization of the Menkes protein in the trans-Golgi network. Human Molecular Genetics 7, 2063–2071. [DOI] [PubMed] [Google Scholar]
- Petris MJ, Mercer JF. 1999. The Menkes protein (ATP7A; MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C-terminal di-leucine endocytic signal. Human Molecular Genetics 8, 2107–2115. [DOI] [PubMed] [Google Scholar]
- Post RL, Sen AK. 1965. An enzymatic mechanism of active sodium and potassium transport. Journal of Histochemistry and Cytochemistry 13, 105–112. [DOI] [PubMed] [Google Scholar]
- Rausin G, Tillemans V, Stankovic N, Hanikenne M, Motte P. 2010. Dynamic nucleocytoplasmic shuttling of an Arabidopsis SR splicing factor: role of the RNA-binding domains. Plant physiology 153, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing C, Mitra B, Rosen BP. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proceedings of the National Academy of Sciences, USA 94, 14326–14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig AC, Argüello JM. 2012. Toward a molecular understanding of metal transport by P1B-type ATPases. Current Topics in Membranes 69, 113–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. 2009. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Research 37, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Nagasawa N, Mori M, Nakazawa N, Kawamoto T, Nagato Y, Sakurai K, Takahashi H, Watanabe A, Akagi H. 2012. Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant & Cell Physiology 53, 213–224. [DOI] [PubMed] [Google Scholar]
- Seigneurin-Berny D, Gravot A, Auroy P, et al. . 2006. HMA1, a new Cu-ATPase of the chloroplast envelope, is essential for growth under adverse light conditions. The Journal of Biological Chemistry 281, 2882–2892. [DOI] [PubMed] [Google Scholar]
- Shikanai T, Müller-Moulé P, Munekage Y, Niyogi KK, Pilon M. 2003. PAA1, a P-type ATPase of Arabidopsis, functions in copper transport in chloroplasts. The Plant Cell 15, 1333–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemianowski O, Barabasz A, Weremczuk A, Ruszczyńska A, Bulska E, Williams LE, Antosiewicz DM. 2013. Development of Zn-related necrosis in tobacco is enhanced by expressing AtHMA4 and depends on the apoplastic Zn levels. Plant, Cell & Environment 36, 1093–1104. [DOI] [PubMed] [Google Scholar]
- Sinclair SA, Sherson SM, Jarvis R, Camakaris J, Cobbett CS. 2007. The use of the zinc-fluorophore, Zinpyr-1, in the study of zinc homeostasis in Arabidopsis roots. New Phytologist 174, 39–45. [DOI] [PubMed] [Google Scholar]
- Sitsel O, Grønberg C, Autzen HE, Wang K, Meloni G, Nissen P, Gourdon P. 2015. Structure and function of Cu(I)- and Zn(II)-ATPases. Biochemistry 54, 5673–5683. [DOI] [PubMed] [Google Scholar]
- Smith AT, Smith KP, Rosenzweig AC. 2014. Diversity of the metal-transporting P1B-type ATPases. Journal of Biological Inorganic Chemistry 19, 947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennelid F, Olsson A, Piotrowski M, Rosenquist M, Ottman C, Larsson C, Oecking C, Sommarin M. 1999. Phosphorylation of Thr-948 at the C terminus of the plasma membrane H+-ATPase creates a binding site for the regulatory 14-3-3 protein. The Plant Cell 11, 2379–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talke IN, Hanikenne M, Krämer U. 2006. Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiology 142, 148–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillemans V, Dispa L, Remacle C, Collinge M, Motte P. 2005. Functional distribution and dynamics of Arabidopsis SR splicing factors in living plant cells. The Plant Journal 41, 567–582. [DOI] [PubMed] [Google Scholar]
- Tocquin P, Corbesier L, Havelange A, Pieltain A, Kurtem E, Bernier G, Périlleux C. 2003. A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biology 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3, RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C, Schat H. 2009. Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist 181, 759–776. [DOI] [PubMed] [Google Scholar]
- Verret F, Gravot A, Auroy P, Leonhardt N, David P, Nussaume L, Vavasseur A, Richaud P. 2004. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Letters 576, 306–312. [DOI] [PubMed] [Google Scholar]
- Verret F, Gravot A, Auroy P, Preveral S, Forestier C, Vavasseur A, Richaud P. 2005. Heavy metal transport by AtHMA4 involves the N-terminal degenerated metal binding domain and the C-terminal His11 stretch. FEBS Letters 579, 1515–1522. [DOI] [PubMed] [Google Scholar]
- Wang K, Sitsel O, Meloni G, Autzen HE, Andersson M, Klymchuk T, Nielsen AM, Rees DC, Nissen P, Gourdon P. 2014a. Structure and mechanism of Zn2+-transporting P-type ATPases. Nature 514, 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cai Y, Wang H, Zeng Y, Zhuang X, Li B, Jiang L. 2014b. Trans-Golgi network-located AP1 gamma adaptins mediate dileucine motif-directed vacuolar targeting in Arabidopsis. The Plant Cell 26, 4102–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems G, Dräger DB, Courbot M, Godé C, Verbruggen N, Saumitou-Laprade P. 2007. The genetic basis of zinc tolerance in the metallophyte Arabidopsis halleri ssp. halleri (Brassicaceae): an analysis of quantitative trait loci. Genetics 176, 659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Mills RF. 2005. P1B-ATPases – an ancient family of transition metal pumps with diverse functions in plants. Trends in Plant Science 10, 491–502. [DOI] [PubMed] [Google Scholar]
- Woeste KE, Kieber JJ. 2000. A strong loss-of-function mutation in RAN1 results in constitutive activation of the ethylene response pathway as well as a rosette-lethal phenotype. The Plant Cell 12, 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CK, Cobbett CS. 2009. HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytologist 181, 71–78. [DOI] [PubMed] [Google Scholar]
- Wong CK, Jarvis RS, Sherson SM, Cobbett CS. 2009. Functional analysis of the heavy metal binding domains of the Zn/Cd-transporting ATPase, HMA2, in Arabidopsis thaliana. New Phytologist 181, 79–88. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Clarke O, Gulbis JM, Keizer DW, Jarvis RS, Cobbett CS, Hinds MG, Xiao Z, Wedd AG. 2009. Metal binding affinities of Arabidopsis zinc and copper transporters: selectivities match the relative, but not the absolute, affinities of their amino-terminal domains. Biochemistry 48, 11640–11654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.