Abstract
The leaf economics spectrum (LES) is an ecophysiological concept describing the trade-offs of leaf structural and physiological traits, and has been widely investigated on multiple scales. However, the effects of the breeding process on the LES in crops, as well as the mechanisms of the trait trade-offs underlying the LES, have not been thoroughly elucidated to date. In this study, a dataset that included leaf anatomical, biochemical, and functional traits was constructed to evaluate the trait covariations and trade-offs in domesticated species, namely rice (Oryza species). The slopes and intercepts of the major bivariate correlations of the leaf traits in rice were significantly different from the global LES dataset (Glopnet), which is based on multiple non-crop species in natural ecosystems, although the general patterns were similar. The photosynthetic traits responded differently to leaf structural and biochemical changes, and mesophyll conductance was the most sensitive to leaf nitrogen (N) status. A further analysis revealed that the relative limitation of mesophyll conductance declined with leaf N content; however, the limitation of the biochemistry increased relative to leaf N content. These findings indicate that breeding selection and high-resource agricultural environments lead crops to deviate from the leaf trait covariation in wild species, and future breeding to increase the photosynthesis of rice should primarily focus on improvement of the efficiency of photosynthetic enzymes.
Keywords: Leaf economics spectrum, mesophyll conductance, mesophyll structure, nitrogen, photosynthesis, photosynthetic limitation
Introduction
The global covariation of leaf anatomical, biochemical, and gas exchange traits along with resource availability gradients, which is known as the leaf economics spectrum (LES), has received widespread attention (Wright et al., 2004; Blonder et al., 2011; Sack et al., 2013, 2014; Niinemets, 2015; Onoda et al., 2017). In brief, the global LES describes a continuum leaf spectrum, ranging from fast-growing species combined with low structural investment, high nutrient investment, quick return, and generally highly photosynthesizing leaves, to slow-growing species combined with high structure investment, low nutrient investment, slow return, and stress-tolerant leaves. The global LES is defined by several core leaf traits, including leaf mass per area (LMA), nitrogen (N) concentration (Nm), the light-saturated photosynthetic rate per leaf mass (Am), and leaf lifespan (Wright et al., 2004). However, the LES trait network is typically based on multiple non-crop species in natural ecosystems, and it is unclear whether the trade-offs among the leaf traits of domesticated species in agroecosystems are constrained by similar principles to those of non-crops. Agricultural environments often present strong ecological contrasts with the natural environments of non-crop species. This is because agriculture has historically been undertaken in resource-rich and low-risk areas, since farmers supply sufficient resources, including water and nutrients, and protect their crops from herbivores and pathogens (Meyer et al., 2012; Zeder, 2015). A recent study confirmed that domestication has increased the leaf N and phosphorus (P) concentration by 57% in domesticated crops compared with their wild relatives (Delgado-Baquerizo et al., 2016). Hence, it is logical to expect that selection for desired agronomic traits and breeding in resource-rich and predictable environments potentially shifts leaf trait correlations in domesticated crops from the LES correlations, which are based on non-crop species in natural ecosystems. A detailed investigation is required to fill important gaps in our understanding of the LES.
Despite broad recognition of the LES on multiple scales, the fundamental constraints underlying the LES are still unclear (Blonder et al., 2011; Osnas et al., 2013; Sack et al., 2013; Niinemets, 2015; Onoda et al., 2017). Several previous studies suggest that the core trait relationships of the LES are likely to operate via other traits (Shipley et al., 2006; Sack et al., 2013; Onoda et al., 2017). For instance, Shipley et al. (2006) proposed that the ratio of the cell volume to cell wall volume was responsible for generating the LES. More recently, Onoda et al. (2017) highlighted the fundamental role of cell wall thickness and the proportion of N allocation to the cell wall in mediating the trait correlations in the LES. These studies basically focused on the role of N investment in the content of photosynthetic enzymes, especially Rubisco. However, in C3 plants, the area-based light-saturated photosynthetic rate (A) is limited by stomatal conductance (gs), mesophyll conductance to CO2 (gm), and/or the biochemistry of photosynthesis (Flexas, 2016). Under a given ambient condition, gs relates to the leaf water status, which is largely determined by plant hydraulic conductance. Indeed, the coupling of LES traits and leaf hydraulic traits, including vein density (vein length per unit area; VLA) and leaf hydraulic conductance (Kleaf), has been proposed (Blonder et al., 2011; Sack et al., 2013; Reich, 2014), while Li et al. (2015) observed that leaf vein traits are decoupled from LES traits. Recently, gm was identified as an important photosynthetic limiting factor and was related to leaf structure and biochemical traits (Flexas et al., 2012; Giuliani et al., 2013; Tomás et al., 2013; Xiong et al., 2017a). Chloroplast size, number, and arrangement, mesophyll cell wall thickness (Tcw), and the permeability of membranes are suggested as the major traits restricting gm. The biochemical limitations include the amount and activities of enzymes and metabolites involved in photosynthesis and the components of the thylakoid electron transport chains. Any change in N allocations within the leaf structure may potentially change the photosynthetic limitation processes and subsequently affect A.
Rice (Oryza sativa) is one of the most important crops worldwide, and enhancing A is considered a primary approach to improve grain yield (Long et al., 2006; Zhu et al., 2010; Long et al., 2015). One ambitious approach is to convert current rice from C3 to C4 photosynthesis by introducing the CO2 concentrating mechanism (CCM) because the radiation use efficiency in C4 plants is higher than in C3 plants. However, introducing the CCM pathway requires many changes in both leaf anatomy and biochemical enzymes. In consideration of the elusive mechanistic basis of some of the photosynthetic traits and the methodological bottlenecks in certain aspects of biotechnology, simultaneous alterations of all of the limiting factors of photosynthesis, achieved by manipulating multiple genes, are unlikely to be accomplished in the near future (Zhu et al., 2010; Flexas, 2016). Altering Rubisco kinetics is another approach for genetic engineering to improve the photosynthetic efficiency in crops. However, the complex assembly pathway of Rubisco and the apparent trade-offs in its kinetic parameters (Tcherkez et al., 2006) indicate that creating a ‘better Rubisco’ is not likely, at least in the near future (Whitney et al., 2011). Therefore, an efficient way to improve photosynthesis would be to exploit existing genetic variations in photosynthetic traits and the coordination among these traits in the existing genotypes. In fact, the genetic variations of leaf functional, anatomical, and biochemical traits have been observed in many crop species, including rice (Giuliani et al., 2013; Gu et al., 2014; Xiong et al., 2017a). However, the coordination and/or trade-offs of intra-species leaf functional, anatomical, and biochemical traits (i.e. LES) has not been fully revealed.
In this study, we constructed a database that included leaf functional, anatomical, and biochemical traits of the most important cereal crop species, rice, to elucidate: (i) whether the concept of the LES can be applied within a domesticated crop and (ii) the roles of gs and gm in LES trait correlations. Based on these analyses, we then discuss the implications for improving photosynthesis and source use efficiency of rice along the LES.
Materials and methods
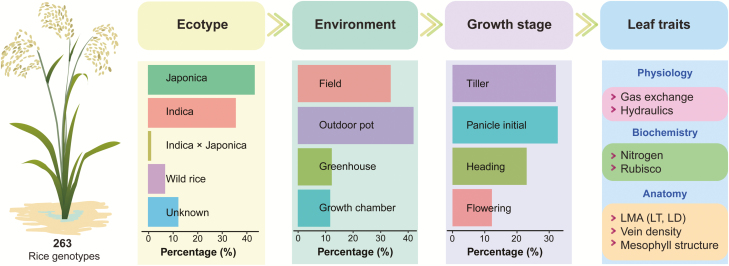
To test the correlations among the leaf traits, a database of rice leaf functional, biochemical, and structural traits of 263 genotypes growing in multiple conditions was compiled from the literature (see Supplementary data at JXB online). Because the aim of this study was to identify the potential effects of anatomical and biochemical traits on leaf function, the articles that reported one or more gas exchange traits and at least one structural or biochemical trait or leaf hydraulic conductance (Kleaf) were included in the database. Leaf traits vary among plant species, growth conditions, and genotypes, and to extend the rice leaf spectrum as widely as possible, we included field, outdoor pot, greenhouse, and growth chamber studies (Fig. 1). Studies of short-term treatments, including light, CO2, temperature, and vapour-pressure deficit (VPD), were excluded. However, studies of long-term nutrient treatments were included in the database. In this study, we considered gas exchange parameters, including A, gs, and gm, and leaf anatomical and structural traits including the leaf mass per leaf area (LMA), leaf vein length per area (VLA), leaf thickness (LT), leaf density (LD), volume fraction of intercellular air space (fIAS), cell wall thickness (Tcw), mesophyll surface area exposed to the intercellular air space per leaf area (Sm), and mesophyll cell surface area occupied by chloroplasts exposed to the intercellular air space per leaf area (Sc). The LD values were calculated as LMA/LT in the case of papers only reporting the LMA and LT values. The biochemical traits included the N content and Rubisco content. Kleaf was estimated using the evaporative flux method and its components, Kleaf inside the xylem (Kx) and outside the xylem (Kox), were measured using the cutting method (e.g. Stiller et al., 2003; Xiong et al., 2017a). All of the data were extracted directly from the tables, text, and supplementary information in the original papers or indirectly from the figures, and all of the data were later converted to their standard units. Other information, if available, such as temperature, the maximum rate of carboxylation (Vcmax), and the maximum rate of electron transport (Jmax), were also extracted for further analysis.
Fig. 1.
Summary of rice ecotypes, growth conditions (environment), plant developmental stage, and the leaf traits used for the analysis.
Many approaches have been developed to estimate gm. In the current database, three major common methods were used: (i) the online carbon isotope discrimination method (Evans et al., 1986), (ii) the combined chlorophyll fluorescence and gas exchange method (Harley et al., 1992), and (iii) the curve-fitting method (Ethier & Livingston, 2004). In all of the studies providing gm values from the curve-fitting method, parallel estimates were provided by using the combined chlorophyll fluorescence and gas exchange method, and the gm values from these two methods are quite similar in rice (Xiong et al., 2015b) as well as across other species (Carriquí et al., 2015). Thus, for these studies, only the gm values from the combined chlorophyll fluorescence and gas exchange method were used to analyse the relationship of gm with the other traits. Several studies have compared the gm values from the online carbon isotope discrimination method and from the combined chlorophyll fluorescence and gas exchange method, and noted a remarkable similarity in the values of gm from these two methods (Kodama et al., 2011).
The effect of environmental factors (i.e. light, CO2, and temperature) on the instantaneous gas exchange has been verified by many studies (Bernacchi et al., 2002; Yamori et al., 2011; Walker et al., 2013; Xiong et al., 2015a). In the current database, the gas exchange measurements were performed under light-saturated conditions, and the light was not considered to affect the inter-study comparisons. Although almost all of the studies claimed that gas exchange measurements were performed under ambient CO2 conditions, the actual CO2 concentrations ranged between 350 and 420 ppm with a mean of 379 ppm (Supplementary Fig. S1). Overall, the first quartile (Q1) of the CO2 concentration was 374 ppm and the third quartile (Q3) was 391 ppm. This result indicated that across all of the studies, the CO2 concentrations in most of the studies were very similar (~380 ppm). Leaf temperature is another important factor influencing photosynthesis, and in the current database, the leaf temperature ranged from 25.0 to 30.6 °C, with an average of 28.2 °C. This temperature range may potentially affect the rice gas exchange. However, previous studies show the variations in photosynthesis in this temperature range are quite small and the photosynthetic optical temperature of rice is around 30 °C (Yamori et al., 2011; Scafaro et al., 2012; von Caemmerer & Evans, 2015).
The relationships between the leaf N content (Na) and the physiological traits (A, gs, gm, Vcmax, and Jmax) were fitted by a logistic model (Sinclair & Horie, 1989; Rotundo & Cipriotti, 2017) as:
where y represents A, gs, gm, Vcmax, or Jmax, α is the asymptotic y at high leaf N content, β is the curvature of the response, and γ is the leaf N content at which y is zero. Next, the photosynthetic N use efficiency (PNUE) was calculated as:
A photosynthetic limitation analysis is a helpful tool to quantify the relative limitation of gs, gm, and the photosynthetic biochemistry on A (Grassi & Magnani, 2005; Buckley & Diaz-Espejo, 2015), and it has been widely used recently, especially under stress conditions (Flexas et al., 2009; Galle et al., 2009; Tosens et al., 2016; Wang et al., 2018). In this study, the photosynthetic limitations, including the relative stomatal (ls), mesophyll (lm), and biochemical (lb) limitations at different Na, were calculated using fitted A, gs, gm, and Vcmax values according to Grassi and Magnani (2005):
where gt is the total conductance, which is calculated as:
C c is the CO2 concentration in the chloroplasts, which is calculated as:
where Ca is the ambient CO2 concentration, and 400 µbar was used in this study.
The differences in the slope and intercept of the bivariate relationships between rice and the global dataset (Glopnet) were tested by using standardized major axis tests with the R package, SMATR 3v (Warton et al., 2012). All of the analyses in this study were performed in R v3.4.4 (R Core Team, 2018).
Results
Variation of leaf traits in rice
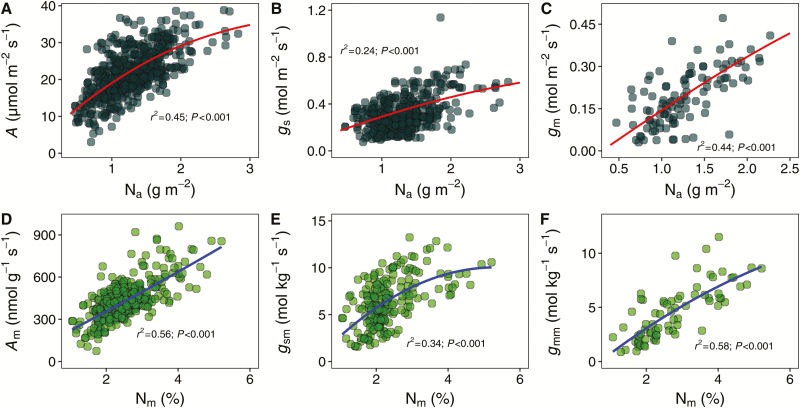
Most of the leaf functional, biochemical, and anatomical traits of rice in the current dataset showed considerable variability (Fig. 1). A and gs showed the widest variation, which was 59-fold between the highest and the lowest, and the cell wall thickness (Tcw) showed the narrowest variation, which was only 1.9-fold between the highest and the lowest. A varied from 0.66 to 38.8 μmol m−2 s−1 with a median of 20.8 μmol m−2 s−1, Na varied from 0.47 to 2.83 g m−2 with a median of 1.20 g m−2, and LMA varied from 24.6 to 73.8 g m−2 with a median of 48.7 g m−2. The variation in gm, the Rubisco concentration per leaf area, and the Rubisco concentration per leaf mass was more than 20-fold, and the variation in the mass-based light-saturated photosynthetic rate (Am), the mass-based stomatal conductance (gsm), the mass-based mesophyll conductance (gmm), and the leaf density was more than 10-fold.
Coordination of leaf traits
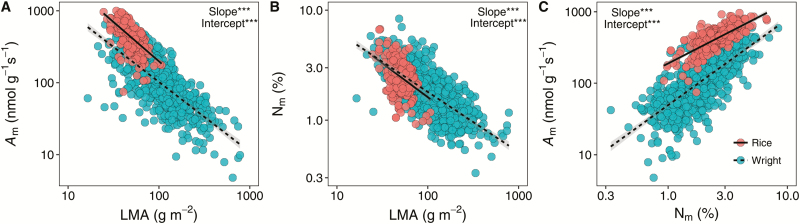
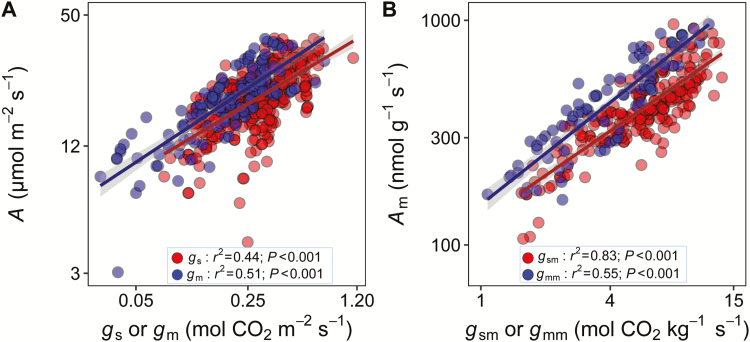
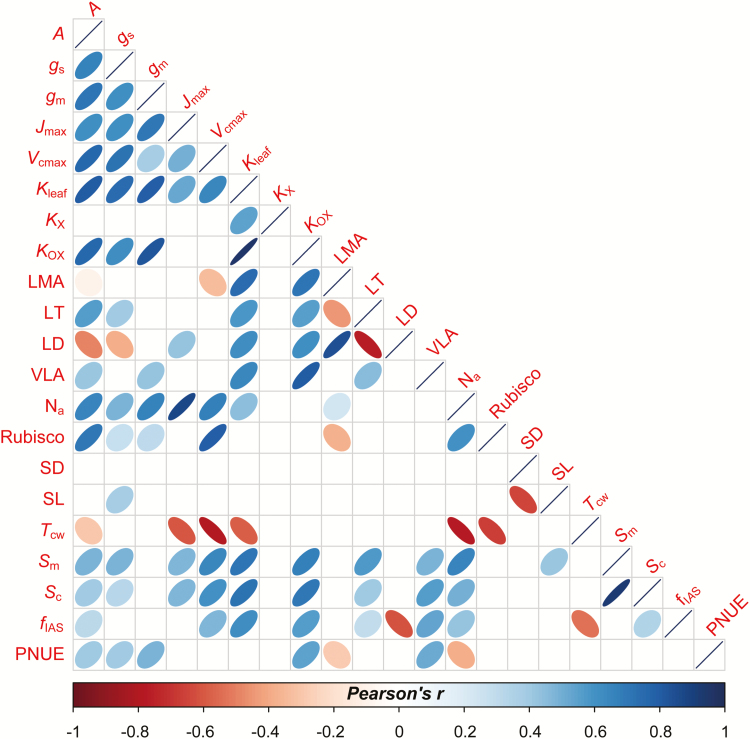
The leaf economic trait correlations in rice were observed in this study. As shown in Fig. 2, the bivariate correlations among LMA, Am, and Nm in the domesticated rice were generally consistent with the correlations for the natural species in Glopnet (Wright et al., 2004). However, we observed significant differences in the slopes and intercepts of those correlations between rice and Glopnet (Fig. 2). Compared with the global database, rice tended to have a higher value of Am at a given LMA or Nm, but the decline in Nm with increasing LMA in rice was large. In rice, A was positively correlated with both gs (r2=0.51, P<0.001) and gm (r2=0.44, P<0.001; Fig. 3A), and the correlations were stronger when expressed on a mass basis (Fig. 3B). However, A (r2=0.03, P=0.43), gs (r2=0.00, P=0.97), and gm (r2=0.04, P=0.053) were independent of LMA in rice (Fig. 4; Supplementary Fig. S2). Otherwise, A and gs increased with the leaf thickness, and decreased with leaf density. In contrast, gm was independent of leaf thickness, as well as leaf density (Fig. 4). In this study, we also observed significant effects of the leaf anatomical and mesophyll structural traits on the leaf physiological traits (Fig. 4). A was positively correlated with the mesophyll (Sm) and chloroplast (Sc) surface exposed to the intercellular airspace but was negatively correlated with the cell wall thickness (Tcw). We also found a significant effect of LMA, LT, and LD on leaf hydraulic conductance (Kleaf). All of the estimated functional traits were independent of the leaf vein length per area (Fig. 4). Moreover, we observed significant interactions among the leaf structural traits (Fig. 4). For instance, the intercellular air space proportion (fIAS), Sm, and Sc were positively correlated with LT, and fIAS was negatively correlated with LD. In addition, Sm and Sc were tightly correlated.
Fig. 2.
Relationships among the leaf nitrogen concentration (Nm), the leaf mass per area (LMA), and the light-saturated photosynthetic rate per mass (Am). The circles represent data from Glopnet (Wright et al., 2004) and rice as shown. The gray shaded area indicates the 95% confidence interval. Solid lines are standardized major axis (SMA) lines fitted to the rice dataset, and dashed lines SMA lines fitted to the Glopnet dataset. ***P < 0.001.
Fig. 3.
Correlations of the light-saturated photosynthetic rate (A) to the stomatal conductance (gs) and the mesophyll conductance (gm). (A) area-based correlations; (B) mass-based correlations. The gray shaded area indicates the 95% confidence interval.
Fig. 4.
Correlations between the leaf traits (area base). The full name and units of the traits are shown in Supplementary Table S1. The correlations were estimated by the linear model and the 95% confidence level was used to draw the ellipses. The significant correlations are shown (P<0.05).
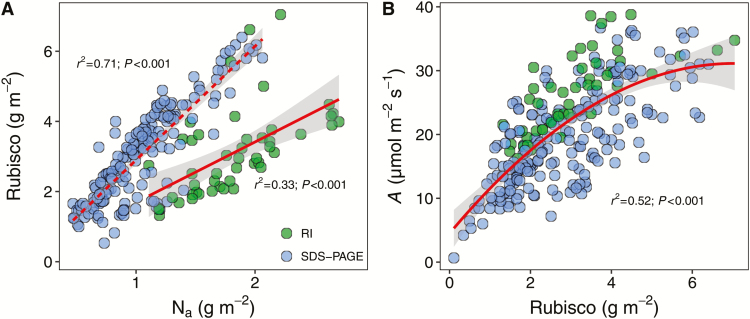
The leaf N content was correlated with almost all of the leaf structural, functional, and biochemical traits (Fig. 4). The light-saturated photosynthetic rate (area base: r2=0.43, P<0.001; mass base: r2=0.50, P<0.001) the stomatal conductance (area base: r2=0.24, P<0.001; mass base: r2=0.34, P<0.001) and the mesophyll conductance (area base: r2=0.44, P<0.001; mass base: r2=0.58, P<0.001) were strongly correlated with the leaf N concentration on the area and mass base (Fig. 5). Basically, Rubisco concentration was linearly correlated with leaf N concentration, although the correlation differed strongly depending on the Rubisco estimation method (Fig. 6A). Moreover, A increased linearly with increasing Rubisco concentration at relatively low Rubisco concentrations and later levelled off (Fig. 6B). Typically, A (r2=0.65; P<0.001), gs (r2=0.55; P<0.001), and gm (r2=0.63; P<0.001) linearly increased with Kleaf. However, the correlations were weaker for the mass than for the area base (Supplementary Fig. S3).
Fig. 5.
Effects of leaf nitrogen (N) concentration on the light-saturated photosynthetic rate (A) (A, D), the stomatal conductance (gs) (B, E), and the mesophyll conductance (gm) (C, F). (A–C) Area-based correlations; (D–F) mass-based correlations. The correlations were fitted by the logistic model as described in ‘Materials and methods’.
Fig. 6.
(A) Correlation between the N concentration per area (Na) and the Rubisco concentration, and (B) the correlation between the light-saturated photosynthetic rate (A) and the Rubisco concentration. RI, Rubisco concentration estimated by the radial immunodiffusion method; SDS-PAGE, Rubisco concentration estimated by SDS-PAGE. The gray shaded area indicates the 95% confidence interval.
Photosynthetic N use efficiency
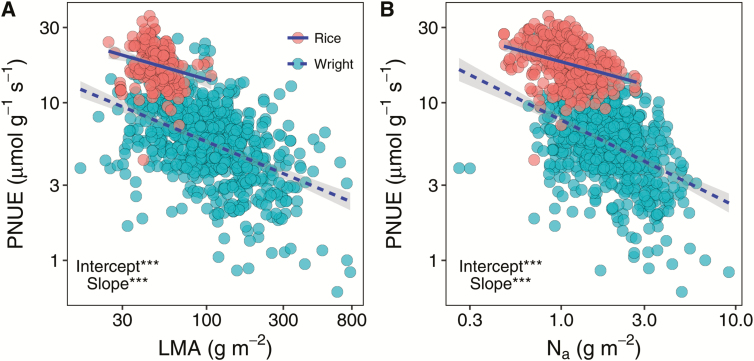
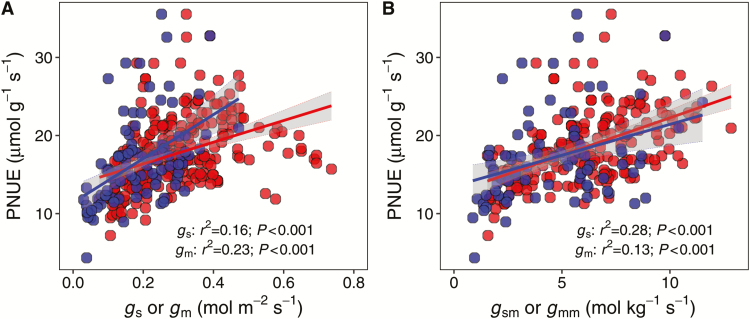
The photosynthetic N use efficiency (PNUE) varied 10-fold across the database (Table 1). The PNUE was strongly correlated with the Na, VLA, and LMA but not with the other leaf anatomical and biochemical traits (Fig. 4). As shown in Fig. 6 and Supplementary Fig. S4, PNUE increased with A (r2=0.16, P<0.001) and Am (r2=0.22, P<0.001) but decreased with LMA (r2=0.10, P<0.001) and Na (r2=0.76, P<0.001). Compared with the global species database, rice tended to have a high PNUE under a given LMA, Na, Nm, or Am (Fig. 7; Supplementary Figs S4, S5). Moreover, as shown in Fig. 8, PNUE increased with increasing gs (r2=0.16, P<0.001), gsm (r2=0.28, P<0.001), gm (r2=0.23, P<0.001), and gmm (r2=0.13, P<0.001).
Table 1.
Functional, biochemical, and anatomical trait variations in rice
| Trait | Min. | Q1 | Median | Mean | Q3 | Max. |
|---|---|---|---|---|---|---|
| Function | ||||||
| A | 0.66 | 16.3 | 20.8 | 21.2 | 25.4 | 38.8 |
| gs | 0.075 | 0.219 | 0.290 | 0.313 | 0.383 | 1.138 |
| gm | 0.03 | 0.129 | 0.210 | 0.218 | 0.295 | 0.75 |
| Kleaf | 3.31 | 4.99 | 7.20 | 7.77 | 10.72 | 13.46 |
| Vcmax | 41.2 | 84.0 | 100.0 | 103.1 | 118.8 | 163.0 |
| Jmax | 59.4 | 111.4 | 140.9 | 149.3 | 170.3 | 308.3 |
| Am | 74.77 | 337.7 | 417.7 | 456.8 | 543.6 | 998.5 |
| gsm | 1.57 | 4.45 | 6.27 | 6.39 | 8.14 | 19.75 |
| gmm | 0.87 | 2.66 | 4.38 | 4.67 | 6.17 | 11.51 |
| Kleafm | 0.122 | 0.135 | 0.199 | 0.199 | 0.263 | 0.307 |
| Biochemistry | ||||||
| Na | 0.47 | 0.97 | 1.20 | 1.25 | 1.49 | 2.83 |
| Nm | 0.92 | 1.94 | 2.24 | 2.47 | 2.81 | 6.73 |
| Rubisco | 0.11 | 1.74 | 2.68 | 2.90 | 3.74 | 7.05 |
| Rubiscom | 0.032 | 0.058 | 0.087 | 0.094 | 0.126 | 0.207 |
| Anatomy | ||||||
| LMA | 24.6 | 42.8 | 48.7 | 49.2 | 56.1 | 73.8 |
| VLA | 2.98 | 3.98 | 4.34 | 4.62 | 5.28 | 6.73 |
| VLAm | 0.073 | 0.095 | 0.112 | 0.118 | 0.140 | 0.192 |
| LT | 0.058 | 0.084 | 0.132 | 0.148 | 0.216 | 0.282 |
| LD | 0.039 | 0.098 | 0.136 | 0.210 | 0.213 | 0.654 |
| fIAS | 7.7 | 16.7 | 19.5 | 19.3 | 23.1 | 28.8 |
| Tcw | 0.125 | 0.155 | 0.167 | 0.168 | 0.183 | 0.235 |
| Sm | 6.27 | 11.24 | 15.72 | 16.48 | 19.26 | 31.20 |
| Sc | 6.43 | 11.54 | 15.72 | 15.81 | 18.04 | 30.59 |
| Efficiency | ||||||
| PNUE | 4.33 | 14.52 | 17.31 | 18.12 | 21.1 | 44.4 |
The full name and units of the traits are shown in Supplementary Table S1. Min, minimum value; Q1, the first quartile; Q3, the third quartile; Max., Maximum value.
Fig. 7.
Effects of leaf mass per area (LMA) (A) and N concentration per leaf area (Na) (B) on photosynthetic N use efficiency (PNUE). The gray shaded area indicates the 95% confidence interval. Solid lines are standardized major axis (SMA) lines fitted to the rice dataset, and dashed lines SMA lines fitted to the Glopnet dataset. ***P < 0.001.
Fig. 8.
Contributions of the area- (A) and mass-based (B) stomatal conductance and mesophyll conductance to photosynthetic N use efficiency (PNUE). The gray shaded area indicates the 95% confidence interval.
Relative photosynthetic limitation
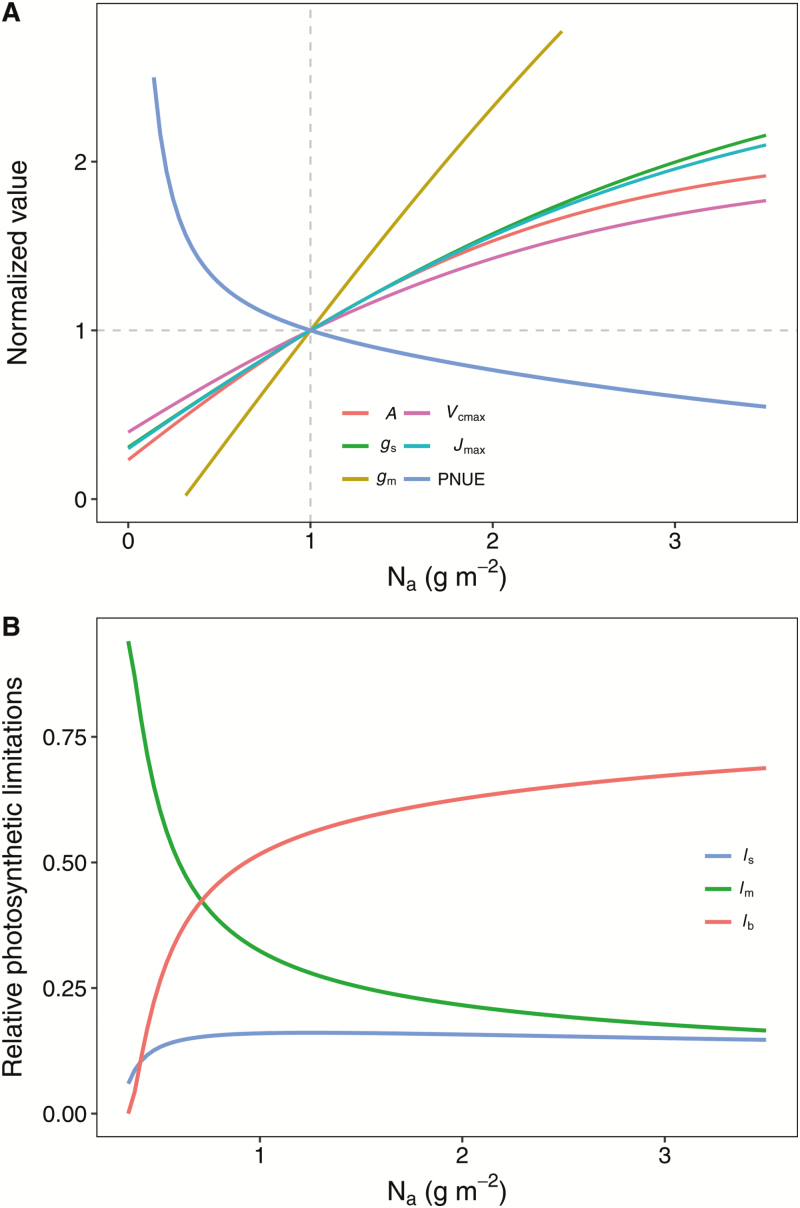
In this study, A, gs, gm, Vcamx, and Jmax were modeled using the logistic model with the input of the leaf N content per area (Na), and the outputs shown in Table 2. The curvature (β) of the relationship between A and Na was the highest, and the value of β of the relationship between gm and Na was the lowest. To estimate the sensitivity of the photosynthetic traits to Na, the values of A, gs, gm, Vcamx, and Jmax were normalized by their values at 1.0 g m−2 of Na (Fig. 9). The results showed that gm was the most N-sensitive trait, and Vcmax was the most N-insensitive trait. We analysed the relative photosynthetic limitation based on the modeled photosynthetic traits. The leaf N status had a strong influence on the relative stomatal (ls), mesophyll (lm), and biochemical (lb), limitations in rice (Fig. 9). Overall, the biochemical limitation was the major photosynthetic limiting factor, which contributed more than 60% of the relative photosynthetic limitations, while ls contributed than 10%. lm strongly declined with Na, but lb increased with Na.
Table 2.
Fitted parameters relating light-saturated photosynthetic rate (A), stomatal conductance (gs), mesophyll conductance (gm), maximum carboxylation rate (Vcmax), and maximum electron transport rate (Jmax) in responding to leaf N content per area (Na)
| Trait | Fitted parameters | Value at Na=1.0 | ||
|---|---|---|---|---|
| α | β | γ | ||
| A | 40.04 | 0.8117 | −0.2737 | 19.03 |
| g s | 0.8044 | 0.5350 | −0.4183 | 0.2913 |
| g m | 0.8623 | 0.4813 | 0.3003 | 0.1438 |
| V cmax | 182.6 | 0.7052 | −0.5763 | 92.17 |
| J max | 300.6 | 0.5600 | −0.3988 | 116.7 |
Fig. 9.
(A) Responses of light-saturated photosynthetic rate (A), stomatal conductance (gs), mesophyll conductance (gm), maximum carboxylation rate (Vcamx), maximum electron transport rate (Jmax), and photosynthetic N use efficiency (PNUE) to leaf N content per area (Na). (B) Changes in the relative photosynthetic limitation of stomatal conductance (ls), mesophyll conductance (lm), and biochemistry (lb) with Na. In (A), the parameters were normalized by dividing their values by 1.0 g m−2 of Na.
Discussion
Leaf economics spectrum
For a given species, leaf traits vary depending on (i) the genotype, (ii) the growth environment and crop management, and (iii) the leaf and plant age (Wright et al., 2004; Niinemets, 2015). In this study, 263 rice genotypes, which covered all of the ecotypes of rice growing under field, outdoor, greenhouse and/or growth chamber conditions, were included in our dataset (Fig. 1). Moreover, we also included multiple N management, growth stage, and leaf age variables. Considering that it would be almost impossible to estimate the leaf spectrum traits of all existing rice genotypes at all growth stages under all possible growth conditions, our dataset is a reasonable subset to represent the rice leaf spectrum.
Contrasting with two recent studies that indicated that the leaf trait correlations within a species may differ from the correlations among species (Anderegg et al., 2018; Osnas et al., 2018), the patterns of the leaf functional and anatomical trait correlations of rice were generally consistent with previously reported correlations in global (Wright et al., 2004) and intraspecies LESs (Gagliardi et al., 2015; Niinemets, 2015; Martin et al., 2017). The significant trait covariations observed in this study suggested that the fundamental ecophysiological trade-off applies not only to natural species but also to domesticated species. This raises another question of whether human selection for traits in domesticated species has modified the leaf trait trade-offs from the global economic spectrum.
The light-saturated photosynthetic rate of domesticated rice was significantly higher than that in natural species in the Glopnet dataset at a given leaf N level, which indicated that the PNUE was improved by human selection in rice. In fact, an enhancement in A during breeding progress was observed in previous studies (Fischer et al., 1998; Koester et al., 2016). Although more information is needed to reveal the mechanism of PNUE enhancement in rice, greater N allocation to Rubisco may be one. The Rubisco concentration in rice is almost twice as high as in the natural species under a given leaf N level (Supplementary Fig. S6). A further difference from the Glopnet dataset was that both Am and Nm decline faster with LMA in rice, suggesting that the influence of leaf structure on function were stronger in rice than in the native species in the Glopnet. Several recent studies demonstrated that correlations in the global LES arise, in part, by mathematical necessity (Lloyd et al., 2013; Osnas et al., 2013). However, these correlations reflect the physical effects of leaf structure on physiology (Sack et al., 2013). In the current study, the different trait correlations between the two datasets was caused by biological factors because the same mathematical method was used. Moreover, the area-based bivariate correlations also support that the effects of leaf structure on physiological processes are stronger in rice (Fig. 4).
Photosynthetic limitations in rice
The photosynthetic limitation factors in C3 plants have been widely investigated and three have been identified, namely gs, gm, and the biochemistry of photosynthesis, which includes the enzymes and metabolites involved in photosynthesis and the components of the thylakoid electron transport chain (Flexas, 2016). The positive relationships between photosynthetic rate and CO2 diffusion conductance, including the stomatal and mesophyll conductance, on both the area and mass base, indicated important limiting roles of gs and gm on rice photosynthesis (Fig. 3). Moreover, A was also tightly correlated with Vcmax and Jmax, which supports a biochemical limitation in photosynthesis. In fact, previous studies suggested that the major photosynthetic limiting factors in C3 plant leaves are modified by slight changes in the growing conditions (Yamori et al., 2011; Wang et al., 2018). Therefore, the LES trait trade-offs may relate to the photosynthetic limitations, shifting the cause to leaf anatomy and/or biochemical changes.
g s is dependent on both the stomatal features, namely density and size and stomatal opening status. In our dataset, gs of rice is independent of stomatal density and size (Fig. 4), which agreed with previous studies, suggesting that stomatal density and size mainly determine the maximum theoretical stomatal conductance, rather than the operational gs (Franks & Beerling, 2009; Bartlett et al., 2016; Xiong et al., 2017a). The regulation of stomatal opening is complex, and many factors, including leaf water potential and abscisic acid concentration, are involved. In addition, under a given ambient condition, stable gs is predominantly determined by the water supplement of the plant. In this study, the strong positive correlation between gs and Kleaf supported a mechanistic relationship between the carbon assimilation and plant hydraulics (Brodribb et al., 2005, 2007; Xiong et al., 2015b, 2017a; Scoffoni et al., 2016).
In C3 plants, photosynthesis and respiration primarily occur in the mesophyll; therefore, the trade-offs among the mesophyll structural, biochemical, and physiological traits are largely represented the LES (Onoda et al., 2017). As one of the photosynthetic limiting factors, gm is proposed to be related to both mesophyll structure and biochemistry (Evans et al., 2009; Flexas et al., 2012; Xiong et al., 2015a, 2017b). Contrary to several previous studies (Tomás et al., 2013; Tosens et al., 2016; Xiong et al., 2017a), gm was independent of mesophyll structural traits in the current study. However, our results are consistent with the results obtained by Giuliani et al. (2013), who observed no correlation between gm and mesophyll structural features across 24 genotypes of rice. Considering the important role of mesophyll structures in gm, the lack of correlation in this instance may be partly attributable to the narrow range of structural traits in the current dataset (Table 1). Our results indicated that gm in rice might be predominately regulated by biochemical factors. In fact, gm was positively correlated with Na, which is one of the most important leaf biochemical traits in determining leaf functions. However, a further analysis showed that the effects of Na on gm might be mediated by both biochemical factors and mesophyll structure. On one hand, a high Na may promote aquaporin gene expression (Hacke et al., 2010; Ding et al., 2016) and the subsequent accumulation of aquaporins would enhance gm by improving the permeability of biological membrane (Flexas et al., 2006; Uehlein et al., 2012; Mori et al., 2014). Conversely, there were tight correlations between Na and leaf structural traits, including Sc and Tcw (Fig. 4). A leaf with a high Na tends to have a large size and/or number of chloroplasts, and hence a large Sm and Sc (Xiong et al., 2015a). A negative relationship was observed between Na and Tcw and may indicate that chloroplasts in leaves with low photosynthetic capacity may require thick and/or flexible cell walls to avoid excess light energy absorption, and the cell wall is known to restrict the diffusion of CO2 in the mesophyll.
According to the Farquhar–Berry–von Caemmerer model (Farquhar et al., 1980), the biochemical limitation largely relates to the carboxylation capacity of Rubisco and the ribulose 1,5-bisphosphate regeneration rate, which is represented by Vcmax and Jmax, respectively. It is clear that Vcmax and Jmax are highly dependent on the Calvin–Benson cycle and the amounts and activities of electron transport proteins. The allocation of leaf N to those photosynthetic proteins, especially Rubisco, is suggested to be determined by the species position in the LES (Onoda et al., 2017). In this study, the Rubisco content per area increased linearly with Na in rice, indicating a relatively constant proportion of N allocation to Rubisco (Fig. 6). However, the non-linear correlation between Na and Vcmax indicated that Rubisco activity might vary with Na. Indeed, a reduction of Rubisco activation states (the ratio of the initial activity and the total activity) with an increase in Na has been observed in previous studies (Cheng & Fuchigami, 2000; Warren & Adams, 2001). This result suggests that a Vcmax estimate based only on the Rubisco content (e.g. Buckley & Warren, 2014) may need to be calibrated by the Rubisco activation states. The mechanism of the low Rubisco activation state in the high Na leaf is unclear, although the Rubisco activase activity, the ATP supplement capacity, and the storage function of Rubisco are suspected to cause the deactivation of Rubisco in high N leaves (Cheng & Fuchigami, 2000; Yamori et al., 2006, 2012).
The photosynthetic limitation factors are apparently influenced by the leaf N concentration due to the profound effects of N on both leaf structural and leaf biochemical traits (Fig. 3). Therefore, the responses of the photosynthetic limitation factors to Na may potentially exploit the mechanisms of the curvilinear correlation between the A and Na. In fact, gs, gm, and Vcmax responded differently to Na in rice, and gm was the most N-sensitive trait. The dramatic responses of gm to Na might be primarily caused by the enlargement of the chloroplasts and, to a lesser extent, the increasing Sc in the high Na leaf (Xiong et al., 2015a). More importantly, the relative limitation analysis suggested that gm contributed the major relative limitation in A in the low Na leaf. However, due to the dramatic increase in gm with Na, photosynthetic biochemistry (i.e. Vcmax and Jmax) contributed the largest relative photosynthetic limitation in the high Na leaf (Fig. 9). The results suggest that more attention should be paid to photosynthetic biochemistry for future A, as well as PNUE improvement in rice. However, we note that there are substantial variations in gs and gm, and also biochemical limitations across genotypes, and therefore the photosynthetic limiting factors are actually genotype specific.
In summary, this study focused on the leaf structural, biochemical, and physiological trait variations and trade-offs in domesticated rice. The major bivariate correlations, including Amvs. Nm, Amvs. LMA, and Nmvs. LMA, of the LES traits in rice were shifted in comparison with the global LES dataset. Am was higher in rice than in the natural species in the Glopnet at a given Na, and the breeding process in the past has therefore improved the PNUE of rice. The photosynthetic traits, including gs, gm, Vcmax, and Jmax, were sensitive to the leaf structural and biochemical traits, and all of these traits increased with Na in rice. Due to the asynchronous responses of the photosynthetic traits to the changes in Na, the major photosynthetic limitation steps were altered dramatically with Na, and biochemistry was the major limiting factor at an Na above 1.0 g m−2. The leaf trait trade-offs underlying the general LES should be considered for future photosynthetic improvement in crops.
Supplementary data
Supplementary data are available at JXB online.
Table S1. List of leaf traits in this study, and symbols and units adopted.
Fig. S1. Leaf temperature and the reference CO2 concentration inside the cuvette for gas exchange measurements.
Fig. S2. Influences of the leaf mass per area (LMA) on the light-saturated photosynthetic rate (A), the stomatal conductance (gs) and the mesophyll conductance (gm).
Fig. S3. Correlations of the leaf hydraulic conductance (Kleaf) to the light-saturated photosynthetic rate (A), the stomatal conductance (gs), and the mesophyll conductance (gm) in rice.
Fig. S4. Correlations between the area-based light-saturated photosynthetic rate (A) and the photosynthetic N use efficiency (PNUE), and between the mass-based light-saturated photosynthetic rate (Am) and the PNUE.
Fig. S5. Effects of the N concentration per leaf mass (Nm) and the mass-based light-saturated photosynthetic rate (Am) on the photosynthetic N use efficiency (PNUE).
Fig. S6. The correlation between Rubisco content and leaf N content within rice or among natural species (data from Onoda et al., 2017).
Data deposition
The data for the results presented here are available at the Dryad Digital Repository: https://doi.org/10.5061/dryad.6060q21 (Xiong and Flexas, 2018).
Acknowledgements
We would like to thank Xiaoxiao Wang and Tingting Du for their help in data collection. DX would like to thank Dr Thomas N. Buckley for providing the opportunity to carry out this study and Dr William T. Salter for his help in getting access to the literature databases in Camden. This work was supported by the China Postdoctoral Science Foundation (2017M620326).
Author contributions
DX planned and designed the research and collected data, and DX and JF analysed the data and wrote the manuscript.
References
- Anderegg LDL, Berner LT, Badgley G, Sethi ML, Law BE, HilleRisLambers J. 2018. Within-species patterns challenge our understanding of the leaf economics spectrum. Ecology Letters 21, 734–744. [DOI] [PubMed] [Google Scholar]
- Bartlett MK, Klein T, Jansen S, Choat B, Sack L. 2016. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proceedings of the National Academy of Sciences, USA 113, 13098–13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. 2002. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiology 130, 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonder B, Violle C, Bentley LP, Enquist BJ. 2011. Venation networks and the origin of the leaf economics spectrum. Ecology Letters 14, 91–100. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ. 2007. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM, Zwieniecki MA, Palma B. 2005. Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima. New Phytologist 165, 839–846. [DOI] [PubMed] [Google Scholar]
- Buckley TN, Diaz-Espejo A. 2015. Partitioning changes in photosynthetic rate into contributions from different variables. Plant, Cell & Environment 38, 1200–1211. [DOI] [PubMed] [Google Scholar]
- Buckley TN, Warren CR. 2014. The role of mesophyll conductance in the economics of nitrogen and water use in photosynthesis. Photosynthesis Research 119, 77–88. [DOI] [PubMed] [Google Scholar]
- Carriquí M, Cabrera HM, Conesa MÀ, et al. 2015. Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant, Cell & Environment 38, 448–460. [DOI] [PubMed] [Google Scholar]
- Cheng L, Fuchigami LH. 2000. Rubisco activation state decreases with increasing nitrogen content in apple leaves. Journal of Experimental Botany 51, 1687–1694. [DOI] [PubMed] [Google Scholar]
- Delgado-Baquerizo M, Reich PB, García-Palacios P, Milla R. 2016. Biogeographic bases for a shift in crop C: N: P stoichiometries during domestication. Ecology Letters 19, 564–575. [DOI] [PubMed] [Google Scholar]
- Ding L, Gao L, Liu W, Wang M, Gu M, Ren B, Xu G, Shen Q, Guo S. 2016. Aquaporin plays an important role in mediating chloroplastic CO2 concentration under high-N supply in rice (Oryza sativa) plants. Physiologia Plantarum 156, 215–226. [DOI] [PubMed] [Google Scholar]
- Ethier GJ, Livingston NJ. 2004. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant, Cell & Environment 27, 137–153. [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. 2009. Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany 60, 2235–2248. [DOI] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. 1986. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Functional Plant Biology 13, 281–292. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Fischer RA, Rees D, Sayre KD, Lu ZM, Condon AG, Saavedra AL. 1998. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Science 38, 1467–1475. [Google Scholar]
- Flexas J. 2016. Genetic improvement of leaf photosynthesis and intrinsic water use efficiency in C3 plants: Why so much little success?Plant Science 251, 155–161. [DOI] [PubMed] [Google Scholar]
- Flexas J, Barbour MM, Brendel O, et al. 2012. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Science 193–194, 70–84. [DOI] [PubMed] [Google Scholar]
- Flexas J, Barón M, Bota J, et al. 2009. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri×V. rupestris). Journal of Experimental Botany 60, 2361–2377. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R. 2006. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. The Plant Journal 48, 427–439. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Beerling DJ. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences, USA 106, 10343–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi S, Martin AR, Filho EDMV, Rapidel B, Isaac ME. 2015. Intraspecific leaf economic trait variation partially explains coffee performance across agroforestry management regimes. Agriculture, Ecosystems & Environment 200, 151–160. [Google Scholar]
- Galle A, Florez-Sarasa I, Tomas M, Pou A, Medrano H, Ribas-Carbo M, Flexas J. 2009. The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): acclimation or limitation?Journal of Experimental Botany 60, 2379–2390. [DOI] [PubMed] [Google Scholar]
- Giuliani R, Koteyeva N, Voznesenskaya E, Evans MA, Cousins AB, Edwards GE. 2013. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (genus Oryza). Plant Physiology 162, 1632–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Magnani F. 2005. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell & Environment 28, 834–849. [Google Scholar]
- Gu J, Yin X, Stomph TJ, Struik PC. 2014. Can exploiting natural genetic variation in leaf photosynthesis contribute to increasing rice productivity? A simulation analysis. Plant, Cell & Environment 37, 22–34. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Plavcová L, Almeida-Rodriguez A, King-Jones S, Zhou W, Cooke JE. 2010. Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar. Tree Physiology 30, 1016–1025. [DOI] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. 1992. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology 98, 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama N, Cousins A, Tu KP, Barbour MM. 2011. Spatial variation in photosynthetic CO2 carbon and oxygen isotope discrimination along leaves of the monocot triticale (Triticum× Secale) relates to mesophyll conductance and the Péclet effect. Plant, Cell & Environment 34, 1548–1562. [DOI] [PubMed] [Google Scholar]
- Koester RP, Nohl BM, Diers BW, Ainsworth EA. 2016. Has photosynthetic capacity increased with 80 years of soybean breeding? An examination of historical soybean cultivars. Plant, Cell & Environment 39, 1058–1067. [DOI] [PubMed] [Google Scholar]
- Li L, McCormack ML, Ma C, Kong D, Zhang Q, Chen X, Zeng H, Niinemets Ü, Guo D. 2015. Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecology Letters 18, 899–906. [DOI] [PubMed] [Google Scholar]
- Lloyd J, Bloomfield K, Domingues TF, Farquhar GD. 2013. Photosynthetically relevant foliar traits correlating better on a mass vs an area basis: of ecophysiological relevance or just a case of mathematical imperatives and statistical quicksand?New Phytologist 199, 311–321. [DOI] [PubMed] [Google Scholar]
- Long SP, Marshall-Colon A, Zhu XG. 2015. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66. [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR. 2006. Can improvement in photosynthesis increase crop yields?Plant, Cell & Environment 29, 315–330. [DOI] [PubMed] [Google Scholar]
- Martin AR, Rapidel B, Roupsard O, Van den Meersche K, de Melo Virginio Filho E, Barrios M, Isaac ME, Barton K. 2017. Intraspecific trait variation across multiple scales: the leaf economics spectrum in coffee. Functional Ecology 31, 604–612. [Google Scholar]
- Meyer RS, DuVal AE, Jensen HR. 2012. Patterns and processes in crop domestication: an historical review and quantitative analysis of 203 global food crops. New Phytologist 196, 29–48. [DOI] [PubMed] [Google Scholar]
- Mori IC, Rhee J, Shibasaka M, Sasano S, Kaneko T, Horie T, Katsuhara M. 2014. CO2 transport by PIP2 aquaporins of barley. Plant & Cell Physiology 55, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü. 2015. Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytologist 205, 79–96. [DOI] [PubMed] [Google Scholar]
- Onoda Y, Wright IJ, Evans JR, Hikosaka K, Kitajima K, Niinemets Ü, Poorter H, Tosens T, Westoby M. 2017. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytologist 214, 1447–1463. [DOI] [PubMed] [Google Scholar]
- Osnas JLD, Katabuchi M, Kitajima K, Wright SJ, Reich PB, Van Bael SA, Kraft NJB, Samaniego MJ, Pacala SW, Lichstein JW. 2018. Divergent drivers of leaf trait variation within species, among species, and among functional groups. Proceedings of the National Academy of Sciences, USA 115, 5480–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osnas JLD, Lichstein JW, Reich PB, Pacala SW. 2013. Global leaf trait relationships: mass, area, and the leaf economics spectrum. Science 340, 741–744. [DOI] [PubMed] [Google Scholar]
- R Core Team 2018. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102, 275–301. [Google Scholar]
- Rotundo JL, Cipriotti PA. 2017. Biological limits on nitrogen use for plant photosynthesis: a quantitative revision comparing cultivated and wild species. New Phytologist 214, 120–131. [DOI] [PubMed] [Google Scholar]
- Sack L, Scoffoni C, John GP, Poorter H, Mason CM, Mendez-Alonzo R, Donovan LA. 2013. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. Journal of Experimental Botany 64, 4053–4080. [DOI] [PubMed] [Google Scholar]
- Sack L, Scoffoni C, John GP, Poorter H, Mason CM, Mendez-Alonzo R, Donovan LA. 2014. Leaf mass per area is independent of vein length per area: avoiding pitfalls when modelling phenotypic integration (reply to Blonder et al. 2014). Journal of Experimental Botany 65, 5115–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafaro AP, Yamori W, Carmo-Silva AE, Salvucci ME, von Caemmerer S, Atwell BJ. 2012. Rubisco activity is associated with photosynthetic thermotolerance in a wild rice (Oryza meridionalis). Physiologia Plantarum 146, 99–109. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Chatelet DS, Pasquet-Kok J, Rawls M, Donoghue MJ, Edwards EJ, Sack L. 2016. Hydraulic basis for the evolution of photosynthetic productivity. Nature Plants 2, 16072. [DOI] [PubMed] [Google Scholar]
- Shipley B, Lechowicz MJ, Wright I, Reich PB. 2006. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 87, 535–541. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Horie T. 1989. Leaf nitrogen, photosynthesis, and crop radiation use efficiency: a review. Crop Science 29, 90–98. [Google Scholar]
- Stiller V, Lafitte HR, Sperry JS. 2003. Hydraulic properties of rice and the response of gas exchange to water stress. Plant Physiology 132, 1698–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez GG, Farquhar GD, Andrews TJ. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proceedings of the National Academy of Sciences, USA 103, 7246–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Ribas-Carbó M, Tosens T, Vislap V, Niinemets Ü. 2013. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. Journal of Experimental Botany 64, 2269–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T, Nishida K, Gago J, et al. 2016. The photosynthetic capacity in 35 ferns and fern allies: mesophyll CO2 diffusion as a key trait. New Phytologist 209, 1576–1590. [DOI] [PubMed] [Google Scholar]
- Uehlein N, Sperling H, Heckwolf M, Kaldenhoff R. 2012. The Arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant, Cell & Environment 35, 1077–1083. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR. 2015. Temperature responses of mesophyll conductance differ greatly between species. Plant, Cell & Environment 38, 629–637. [DOI] [PubMed] [Google Scholar]
- Walker B, Ariza LS, Kaines S, Badger MR, Cousins AB. 2013. Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant, Cell & Environment 36, 2108–2119. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang W, Huang J, Peng S, Xiong D. 2018. Diffusional conductance to CO2 is the key limitation to photosynthesis in salt-stressed leaves of rice (Oryza sativa). Physiologia Plantarum 163, 45–58. [DOI] [PubMed] [Google Scholar]
- Warren CR, Adams MA. 2001. Distribution of N, Rubisco and photosynthesis in Pinus pinaster and acclimation to light. Plant, Cell & Environment 24, 597–609. [Google Scholar]
- Warton DI, Duursma RA, Falster DS, Taskinen S. 2012. smatr 3 – an R package for estimation and inference about allometric lines. Methods in Ecology and Evolution 3, 257–259. [Google Scholar]
- Whitney SM, Houtz RL, Alonso H. 2011. Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiology 155, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428, 821–827. [DOI] [PubMed] [Google Scholar]
- Xiong D, Flexas J. 2018. Data from: Leaf economics spectrum in rice: leaf anatomical, biochemical and physiological trait trade-offs. Dryad Digital Repository, 10.5061/dryad.6060q21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D, Flexas J, Yu T, Peng S, Huang J. 2017a. Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 in Oryza. New Phytologist 213, 572–583. [DOI] [PubMed] [Google Scholar]
- Xiong D, Huang J, Peng S, Li Y. 2017b. A few enlarged chloroplasts are less efficient in photosynthesis than a large population of small chloroplasts in Arabidopsis thaliana. Scientific Reports 7, 5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D, Liu X, Liu L, Douthe C, Li Y, Peng S, Huang J. 2015a. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant, Cell & Environment 38, 2541–2550. [DOI] [PubMed] [Google Scholar]
- Xiong D, Yu T, Zhang T, Li Y, Peng S, Huang J. 2015b. Leaf hydraulic conductance is coordinated with leaf morpho-anatomical traits and nitrogen status in the genus Oryza. Journal of Experimental Botany 66, 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W, Masumoto C, Fukayama H, Makino A. 2012. Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. The Plant Journal 71, 871–880. [DOI] [PubMed] [Google Scholar]
- Yamori W, Nagai T, Makino A. 2011. The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant, Cell & Environment 34, 764–777. [DOI] [PubMed] [Google Scholar]
- Yamori W, Suzuki K, Noguchi K, Nakai M, Terashima I. 2006. Effects of Rubisco kinetics and Rubisco activation state on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant, Cell & Environment 29, 1659–1670. [DOI] [PubMed] [Google Scholar]
- Zeder MA. 2015. Core questions in domestication research. Proceedings of the National Academy of Sciences, USA 112, 3191–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. 2010. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology 61, 235–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.