Abstract
Introduction
To report on SBRT as a bridge to OLT for patients with HCC and Child-Pugh ≥8 cirrhosis.
Methods
Retrospective review of 15 patients, treated from 2010-2017. Three patients excluded secondary to delisting from prohibitive substance. Twelve patients (17 lesions) included for final analysis. Hepatic SPECT functional treatment planning utilized.
Results
The median age of 60 years with a median CP 9 and MELD 14. The median SBRT dose was 40 Gy in 5 fractions, and median tumor size was 2.3cm (1.2-5.3cm). Median follow-up and survival was 40-months and 46-months, respectively. One patient succumbed to renal/hepatic failure before OLT. Radiographic response was 80%. pCR at explant was 46%. No grade ≥ 3 acute toxicities. Median time to progression of CP ≥ 2 was 9.7-months and MELD progression was not met before OLT.
Conclusion
SBRT with functional treatment planning can be used safely as a bridge to OLT in select patients with CP ≥8 cirrhosis.
Keywords: stereotactic body radiation treatment, hepatocellular carcinoma, bridge to transplant, downsizing, liver transplantation, hepatic cirrhosis
Introduction
Liver cirrhosis predisposes to the development of hepatocellular carcinoma (HCC), with 80-90% of HCC cases occurring in cirrhotic livers. (1) The gold standard treatment of HCC is liver transplant, which addresses both the underlying cirrhosis and the HCC. In the United States the Organ Procurement and Transplantation Network/United Network of Organ Sharing (OPTN/UNOS) is the organization that manages the allocation of the limited donor livers to patients on the transplant list. (2) A seminal publication in 1996 by Mazzaferro et al. from the University of Milan defined a restrictive selection or “Milan criteria”, of a solitary HCC ≤ 5 cm or up to 3 HCCs ≤ 3 cm, without vascular invasion or metastasis based on pathological review of the explanted livers which resulted in a 4-year survival of 85% and a recurrence-free survival of 92%, much better than prior experiences with liver transplantation. (3-5)
The waitlist process is based on medical urgency, with factors in the risk of death from liver dysfunction and HCC progression incorporated into the priority of liver transplant allocation. The median waiting time for all patients for a liver transplant in 2016 was 11.3 months, however there are geographical disparities in organ allocation. (2, 6) During this wait time the progression of tumor is unpredictable, resulting in a dropout rate of approximately 25% at 12 months. (7, 8)
Given the above, local therapy for HCC has been investigated as a bridge to liver transplant in order to decrease tumor progression and the dropout rate. The dropout rate of patients within the Milan criteria for transplantation is estimated to decrease to 0-10% when bridging therapy is utilized. (9) The American Association for the Study of Liver disease guidelines that locoregional therapies should be considered as a bridge to transplant if the anticipated wait time is above 6 months and if they are an appropriate candidate based on hepatic dysfunction. (10) In addition, patients marginally outside of the UNOS criteria can be evaluated for tumor downsizing treatment in order to meet Milan criteria and if successful orthotopic liver transplant (OLT) can be considered. (11) Many local and locoregional therapies have been investigated as a bridge to transplant, including radiofrequency ablation, intra-arterially directed therapies, resection, as well as ever-growing data on stereotactic body radiotherapy (SBRT). (11-16) However, in the setting of progressive cirrhosis it is critical that treatment is balanced against worsening a patient’s liver function. In patients with HCC and CP scores ≥8 the role of SBRT as a bridge to OLT has not been established. Thus, we evaluated clinical outcomes and toxicity of using SBRT as a bridging therapy or for tumor downsizing prior to OLT in selected patients with CP scores ≥8.
Materials and Methods
After obtaining Institutional Review Board approval, a retrospective review was performed on 15 patients with CP scores ≥8 treated with SBRT (≤6 fractions) as a bridge or tumor downsizing to OLT from 2010 to 2017 at a single institution. We excluded 3 patients who were treated as a bridge to OLT, however were delisted secondary to prohibitive substance. Out of the 12 patients used for final analysis, 11 had successful OLT with one dying prior to receiving OLT. All the cases were discussed at a multidisciplinary liver tumor board, where radiation oncologists, hepatologists, transplant surgeons, medical oncologists, radiologists, and interventional radiologists were present. Table 1 and Table 2 summarize the patient characteristics and treatment details at the time of SBRT, respectively.
Table 1.
Clinical details for study patients at time of SBRT, N = 12
| Variable | No. | % |
| ECOG status | ||
| 0 | 6 | 50 |
| 1 | 3 | 25 |
| 2 | 3 | 25 |
| Sex | ||
| Male | 11 | 91.66 |
| Female | 1 | 8.33 |
| HCC lesions treated | ||
| 1 | 7 | 58.33 |
| 2 | 5 | 41.66 |
| Milan Criteria | ||
| within | 9 | 75 |
| outside | 3 | 25 |
| Cause of Cirrhosis | ||
| Hepatitis C | 8 | 66.66 |
| NASH | 2 | 16.66 |
| Alcoholic Liver disease | 1 | 8.33 |
| Iron Overload | 1 | 8.33 |
| Child Pugh Score | ||
| 8 | 5 | 41.66 |
| 9 | 2 | 16.66 |
| 10 | 4 | 33.33 |
| 11 | 1 | 8.33 |
| MELD-Na score | ||
| <10 | 1 | 8.33 |
| 10-19 | 10 | 83.33 |
| 20-29 | 1 | 8.33 |
| >30 | 0 | 0 |
| Previous TACE X1 | ||
| No | 10 | 83.33 |
| Yes | 2 | 16.66 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NASH, Non-alcoholic Steatohepatitis; MELD-Na, Model for end-stage liver disease; TACE, Transarterial chemoembolization.
Table 2.
Treatment details at time of SBRT
| Variable | Median | Range |
| Tumor dimension (cm) | 2.3 | 1.3 – 5.2 |
| Total prescribed dose (Gray) | 40 | 30 50 |
| Planned target volume (cc) | 37.5 | 9-164 |
| Time from SBRT to OLT (months) | 5 | 2-10 |
The specifics of the radiotherapy planning have been previously published. (17) Briefly, each patient was positioned on a custom-molded vacuum cushion (Bionix, Toledo, OH) and a treatment planning CT was obtained to define the gross tumor volume (GTV). This was immediately followed by a 4D-CT to delineate the internal target volume accounting for respiratory-induced tumor motion. A planning target volume (PTV) was constructed by adding an additional 0.5cm margin to the internal target volume to account for set-up error. SBRT dose was prescribed to the isodose line encompassing the planning target volume (generally ≥90%) allowing up to 120% point dose to the target volume. Image guided radiation therapy (IGRT) using cone-beam CT was performed before each daily session to reduce set-up uncertainties. Implanted fiducial markers were not used.
Ten of the patients in this study underwent hepatic 3D-CT/SPECT with 99mTc-Sulfur colloid for identification and subsequent avoidance of well-perfused, functionally active hepatic parenchyma during SBRT (Figure 1a and 1b). Details of SPECT/CT co-registration and treatment planning methodology have been previously reported for liver SBRT in cirrhotic HCC patients. (17, 18) Liver dose constraints were imposed exclusively on residual functional liver volumes defined on 3D-CT/SPECT with calculation of predicted functional liver volume (pFLV) from an equation used in transplant surgery and 90Y radioembolization dosimetry; (predicted functional liver volume = 794.41 +1268.28 x body surface area). (19, 20) Next, we specified that at least 35% of predicted functional liver volume from treatment-planning 3D-CT/SPECT should receive no more than 16 Gy (4 fraction SBRT), or 18 Gy (5-6 Fraction SBRT). Thirty-five percent of residual functional liver to be avoided from threshold irradiation corresponds to a conservative estimate of normal liver volume to be spared from hepatic resection. (17) Additional constraints included stomach V25 <10 cc (maximum < 30 Gy); and bowel V20 <20 cc (maximum < 30 Gy) where V25 and V20 are the corresponding organ volumes receiving at least 25 or 20 Gy, respectively.
Figure 1.
a) Axial image from a patient with ascites treated with SBRT as a bridge to transplant for a HCC in segment 4A, 40 Gy in 5 fractions. The pink circle delineated by black arrows represents the planning target volume (PTV) with surrounding isodose lines. Functional treatment planning with 3D-CT/SPECT was utilized. The blue color wash which is outlined in a black line demonstrates the SPECT volumes that represent the functionally active hepatic parenchyma. The whole liver is delineated by the red color wash. The radiation plan was constructed with the goal of best avoidance of the SPECT volume. b) DVH histogram showing the SPECT_NLV-PTV (blue line) and the Liver-PTV volume (brown line). For treatment, the liver dose constraints are imposed exclusively on residual function liver volumes defined on the 3D-CT/SPECT, which represents the more restrictive volume.
Toxicity was defined by the Common Terminology Criteria for Adverse Events CTCAE (v4.03). (21) Progressive cirrhosis was defined as either progression of CP score ≥2 or a change in MELD-Na score leading to increased 3-month waiting list mortality. (22, 23) Radiation induced liver disease (RILD) was defined as either nonmalignant ascites with elevation of alkaline phosphatase more than two times the upper limits of normal, without increase in bilirubin and transaminase levels (classic RILD) or transaminase levels more than 5 times the upper limits of normal or pretreatment level (non-classic RILD). (24, 25) Patients were followed by the multidisciplinary team, including the transplant team, with imaging and lab work obtained per OPTN policy. (26) The patient was assessed at 1 month and then at least at every three-month intervals until liver transplantation in the radiation oncology department. No patients were lost to follow-up. The operative notes and the first postsurgical follow-up were reviewed in detail. Radiographic Tumor response was determined by MRI or CT imaging of the liver using the Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST (mRECIST). (27, 28) Radiographic local control was defined as no progression of disease within the planning target volume. Survival, control, and progressive cirrhosis were evaluated via Kaplan Meier analysis. Calculations were performed using SPSS 20.0.
Results
The median age was 60 years (range 48-69 years), with all patients having an Eastern Cooperative Oncology Group performance status of ≤2. Three patients required SBRT for tumor downsizing to be listed within the Milan Criteria for transplant. At the time of SBRT, the median CP score was 9 (range 8-11) and MELD-Na 14 (range 9-24) adjusted for the serum sodium concentration. Seven patients were CP B score ≥8 and 5 patients were CP C. The cirrhosis was secondary to hepatitis C in the majority of the patients (66%), with nonalcoholic fatty liver disease, iron overload and alcohol representing the other causes. There was no other liver-directed therapy besides two patients who had undergone a prior transarterial chemoembolization (TACE). TACE was used one month prior to SBRT for radiosensitization in one patient with two lesions, with an inferior right hepatic lesion precluding dose escalation (delivered 30 Gy in 5 fractions) given adjacency to the bowel. The other patient did not have radiographic response to TACE.
Five patients had two HCC lesions treated, resulting in a total of 17 HCC lesions treated. The median tumor max dimension was 2.3 cm (range 1.3-5.2 cm). The median pre-treatment AFP was 13.5 (range 2.4-312). The median dose delivered was 40 Gy in 5 fractions (range 30-50 Gy in 4-6 fractions), and the median Biologically Effective Dose (BED) Gy3 was 146. The median gross tumor volume size was 14.5 cc and median planning target volume size was 37.5 cc (range 9-164 cc).
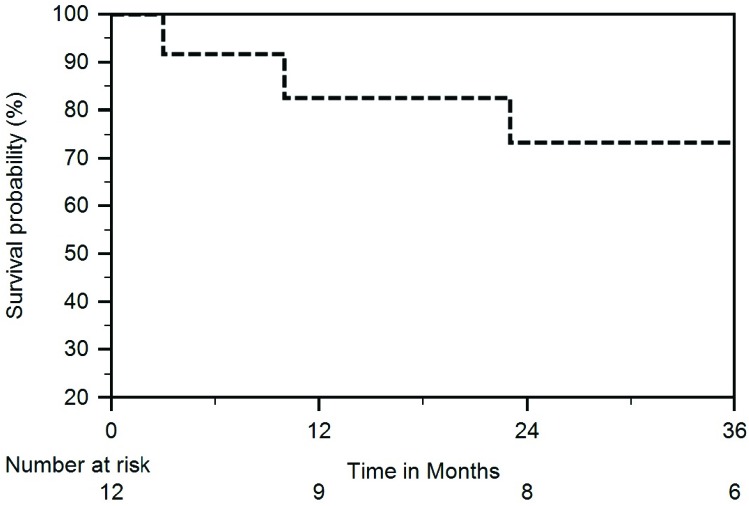
The median time between completion of SBRT and liver transplant was 5 months (range 2-10 months). Four patient’s operative notes reflected adhesions or radiation changes, one secondary to previous spontaneous bacterial peritonitis, two from spontaneous bacterial peritonitis and radiation, and one from radiation alone. In regards, to the patient with radiation changes alone the operative note identified the areas of radiation in the margin of segment 4 and the right lobe of the liver, with some retraction and fibrosis noted on the surface of the liver. There were no complications experienced during surgery or in the postoperative period for this patient. The median follow-up and survival was 40 months (range 3-70 months) and 46 months (range 3-70 months), respectively. Out of the 12 patients treated with the intent to proceed with OLT, 11 had successful OLT with one dying prior to receiving OLT. The one patient succumbed to renal/hepatic failure before obtaining a liver transplant or restaging imaging at 3 months after SBRT to two lesions (planning target volumes of 72 cc and 40 cc). At the time of SBRT, the patient was 69 years old with CP-C10, MELD-Na 18, grade 2 encephalopathy and ascites. The patient did not have liver enzyme elevations to define RILD. The Kaplan-Meier actuarial overall survival estimates at 1 through 4-years after completion of SBRT were 91%, 91%, 68%, and 57% (Figure 2). After transplant, 4 of the 11 patients have died. The cause of death included recurrent hepatitis C and liver failure, chronic kidney disease resulting in kidney failure, pneumonia causing septic shock, and metastatic disease from HCC. One patient developed metastatic disease a year ago from a neuroendocrine tumor to the lungs and retroperitoneum and remains alive 3 years and 9 months after SBRT.
Figure 2.
Overall survival for the entire group
Restaging imaging after SBRT was obtained in 11 out of the 12 patients, with 100% radiographic local control. One patient progressed distantly at 38 months from SBRT, with no patients recurring in the liver. The median decrease in size of the HCC lesion was 60%, with all lesions having a treatment response within the first 3-6 months on restaging CT or MRI. Only one patient did not have evidence of radiographic response, however the lesion lost arterial phase enhancement, which was deemed to represent a treatment effect and remained within Milan criteria for transplant. Radiographic response (complete response and partial response) as evaluated by the mRECIST criteria was 80%. Five lesions had complete radiographic response to SBRT by mRECIST criteria. Out of 5 patients who had elevated pretreatment AFP, 4 normalized by 6 months. Five patients had a pathologic complete response (pCR) on explanted liver (46%), with two additional patients having extensive or grossly necrotic pathologic findings within treated HCC. Neither of the two patients treated with TACE had a pCR. One patient had residual HCC on explant pathology that was similar in size to the pre-SBRT imaging, though radiographically he did have a 0.7 cm decrease in size of the HCC.
Grade ≤2 fatigue was the most prevalent acute adverse event, occurring in 50% of the patients. There were two Grade 1 gastrointestinal toxicities. No patients were formally diagnosed with radiation induced liver disease (RILD), with 4 patients developing grade ≤2 transient elevation of serum transaminases, alkaline phosphatase, or bilirubin. The median time to progression of Child-Pugh score ≥2 was 9.7 months while median time to MELD-Na progression was not met before the liver transplant.
Discussion
To our knowledge this is one of the largest series of patients evaluating the clinical outcomes and toxicity of using SBRT as a bridge to transplant or downsizing to OLT in patients with CP scores ≥8 hepatic cirrhosis. In the setting of severe cirrhosis, local control is difficult to achieve without compromising poor liver function. In our series, residual hepatic function was preserved without affecting the patients’ 3-month mortality on the liver transplant waitlist as the median time to MELD-Na progression was not met before liver transplant. We believe this finding is not only attributable to the precise tumor targeting with SBRT technique but also to the functional liver planning with SPECT/CT, allowing for conformal avoidance of functionally active, well-perfused hepatic parenchyma. The overall treatment toxicity in our study was below Grade 3, with no evidence of radiation induced liver disease, and predominately consisting of Grade ≤2 fatigue. This toxicity profile is similar to our recently published data on 15 transplant-ineligible patients with CP score ≥8 cirrhosis that were treated with SBRT and functional treatment planning, with no Grade ≤3 toxicity or radiation induced liver disease. (12, 17, 29, 30) In addition, in a retrospective review by Mohamed et. al., the most common side effect in the 24 patients undergoing SBRT for bridge to transplant was Grade 1-2 fatigue, with no grade 3 or 4 toxicity seen. (31)
In regard to the effectiveness of treatment, our results compare favorably to the limited published series on patients with CP score ≥8 cirrhosis treated with SBRT as a bridge to transplant. In an abstract, Culleton et. al. reported on outcomes in patients with CP B or C cirrhosis treated with SBRT, although the majority having CP B score 7 liver function and the median dose was 33 Gy in 6 fractions. Ten patients were treated as a bridge to transplant. The median survival of patients treated as a bridge to transplant was 30.7 months in their series, compared to 46 months in our series of patients. (15)
The excellent local control, radiographic downsizing, and response seen on explant liver pathology are promising, and mirror other results from bridge to transplant SBRT studies. The 100% radiographic local control is similar to other studies published, including the Indiana University Simon Cancer Center series which included 21 CP A and B patients who proceeded to OLT, and had no local failures prior to OLT. (14, 32) In regard to radiographic downsizing, the Mannina series reported pre-OLT radiographic response rates (CR + PR) ranging from 52% (RECIST) to 86% (mRECIST), which is consistent with the 70% (RECIST) and 80% (mRECIST) seen in our series of patients treated with a similar median dose fractionation. (33) In a recently published review article by Murray and Dawson the complete pathologic responses have been reported in 14%-27% of lesions. (7) Our experience of a complete pathologic response is higher, with 46% of patients having complete pathologic response, which may be due to limited sample size.
It is important to highlight that liver SBRT in patients with CP score ≥8 cirrhosis is still controversial. In addition, in our recently published paper on transplant-ineligible HCC patients with CP score ≥ 8 cirrhosis prognosis after liver SBRT was poor and broadly similar prognosis would probably be expected with optimal supportive care. (30) Thus, it is important to work in a multidisciplinary fashion with severely cirrhotic patients eligible for liver SBRT being upfront candidates for OLT. We excluded three patients who were treated as a bridge to transplant however were delisted secondary to relapse on prohibitive substance use. We recommended continued counseling of the patient with each physician interaction to reinforce the need for transplant and the lifestyle changes to remain eligible.
This report has limitations, including retrospective design and its inherent biases, and a limited sample size. However, this report has value due to scarcity of previously published data on the subject, uniformity of our treatment planning procedures, and completeness of follow-up data.
Our single institutional review suggests that liver SBRT with functional treatment planning can be used safely as a bridge to OLT or for tumor downsizing in select patients with CP score ≥8 cirrhosis, who may otherwise progress prior to undergoing transplant. SBRT demonstrated excellent local control and radiographic response without grade 3 or higher acute toxicity, resulting in successful transplants and an opportunity for long-term survival.
Acknowledgments
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Conception and design: Alexander Kirichenko, Steven Gresswell, Ngoc Thai, Tadahiro Uemura, Lorenzo Machado
Data collection: Steven Gresswell
Data analysis and interpretation: Steven Gresswell, Shaakir Hasan
Manuscript writing: Steven Gresswell, Rachel Tobillo, Shaakir Hasan
Final approval of manuscript: Steven Gresswell, Rachel Tobillo, Shaakir Hasan, Alexander Kirichenko, Ngoc Thai, Tadahiro Uemura, Lorenzo Machado
References
- 1. Mittal S. Epidemiology of HCC: Consider the population. 2013;47(0):S2-6. doi: 10.1097/MCG.0b013e3182872f29. PubMed PMID: 23632345; PubMed Central PMCID: PMCPMC3683119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Harper AM, et al. OPTN/SRTR 2016 Annual Data Report: Liver. American Journal Of Transplantation. 2018;18 Suppl 1:172-253. Epub 2018/01/03. doi: 10.1111/ajt.14559. PubMed PMID: 29292603. [DOI] [PubMed] [Google Scholar]
- 3. Rahimi RS, Trotter JF. Liver transplantation for hepatocellular carcinoma: outcomes and treatment options for recurrence. Ann Gastroenterol. 28 Greece2015. p. 323-30. [PMC free article] [PubMed] [Google Scholar]
- 4. Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transplantation. 2011;17 Suppl 2:S44-57. Epub 2011/06/23. doi: 10.1002/lt.22365. PubMed PMID: 21695773. [DOI] [PubMed] [Google Scholar]
- 5. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of hepatocellular carcinomas in patients with cirrhosis. New England Journal of Medicine. 1996;334(11):693-700. doi: 10.1056/nejm199603143341104. PubMed PMID: 8594428. [DOI] [PubMed] [Google Scholar]
- 6. Ghaoui R, Garb J, Gordon F, Pomfret E. Impact of geography on organ allocation: Beyond the distance to the transplantation center. World Journal of Hepatology. 2015;7(13):1782-7. doi: 10.4254/wjh.v7.i13.1782. PubMed PMID: 26167251; PubMed Central PMCID: PMCPMC4491907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murray LJ, Dawson LA. Advances in stereotactic body radiation therapy for hepatocellular carcinoma. Seminars in Radiation Oncology. 27(3):247-55. doi: 10.1016/j.semradonc.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 8. Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, V Wu, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transplantation. 2002;8(10):873-83. Epub 2002/10/03. doi: 10.1053/jlts.2002.34923. PubMed PMID: 12360427. [DOI] [PubMed] [Google Scholar]
- 9. She WH, Cheung TT. Bridging and downstaging therapy in patients suffering from hepatocellular carcinoma waiting on the list of liver transplantation. Transl Gastroenterol Hepatol. 12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358-80. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 11. (NCCN) NCCN Hepatobiliary Cancers [January 10, 2018]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. [Google Scholar]
- 12. Hasan S, Kudithipudi V, Thai NV, Kirichenko AV. Stereotactic body radiation therapy (SBRT) with functional treatment planning for hepatocellular carcinoma (HCC) in patients with early-stage cirrhosis. International Journal of Radiation Oncology • Biology • Physics. 96(2):E182. doi: 10.1016/j.ijrobp.2016.06.1047. [DOI] [Google Scholar]
- 13. Moore A, Cohen-Naftaly M, Tobar A, Kundel Y, Benjaminov O, Braun M, et al. Stereotactic body radiation therapy (SBRT) for definitive treatment and as a bridge to liver transplantation in early stage inoperable Hepatocellular carcinoma. Radiat Oncol. 12 London 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transplantation. 2012;18(8):949-54. Epub 2012/04/03. doi: 10.1002/lt.23439. PubMed PMID: 22467602. [DOI] [PubMed] [Google Scholar]
- 15. Culleton S, Jiang H, Kim J-HJ, Brierley JD, Brade AM, Ringash J, et al. Outcomes following stereotactic body radiotherapy for patients with Child-Pugh B/C hepatocellular carcinoma. Journal of Clinical Oncology. 2013;31(4_suppl):167-. doi: 10.1200/jco.2013.31.4_suppl.167. [DOI] [PubMed] [Google Scholar]
- 16. Facciuto ME, Singh MK, Rochon C, Sharma J, Gimenez C, Katta U, et al. Stereotactic body radiation therapy in hepatocellular carcinoma and cirrhosis: evaluation of radiological and pathological response. Journal Of Surgical Oncology. 2012;105(7):692-8. Epub 2011/10/01. doi: 10.1002/jso.22104. PubMed PMID: 21960321. [DOI] [PubMed] [Google Scholar]
- 17. Kirichenko A, Gayou O, Parda D, Kudithipudi V, Tom K, Khan A, et al. Stereotactic body radiotherapy (SBRT) with or without surgery for primary and metastatic liver tumors. HPB (Oxford). 2016;18(1):88-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gayou O, Day E, Mohammadi S, Kirichenko A. A method for registration of single photon emission computed tomography (SPECT) and computed tomography (CT) images for liver stereotactic radiotherapy (SRT). Medical Physics. 2012;39(12):7398-401. Epub 2012/12/13. doi: 10.1118/1.4766877. PubMed PMID: 23231289. [DOI] [PubMed] [Google Scholar]
- 19. Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology (Baltimore, Md). 1995;21(5):1317-21. Epub 1995/05/01. PubMed PMID: 7737637. [PubMed] [Google Scholar]
- 20. Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: A consensus panel report from the radioembolization brachytherapy oncology consortium. International Journal of Radiation Oncology, Biology, Physics. 2007;68(1):13-23. Epub 2007/04/24. doi: 10.1016/j.ijrobp.2006.11.060. PubMed PMID: 17448867. [DOI] [PubMed] [Google Scholar]
- 21. Services USDoHaH Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2010. [Google Scholar]
- 22. Culleton S, Jiang H, Haddad CR, Kim J, Brierley J, Brade A, et al. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B C or hepatocellular carcinoma. Radiotherapy and Oncology. 2014;111(3):412-7. [DOI] [PubMed] [Google Scholar]
- 23. Singal AK, Kamath PS. Model for end-stage liver disease. J Clin Exp Hepatol. 32013. p. 50-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang T, Zhao Y-T, Wang Z, Li C-R, Jin J, Jia AY, et al. Efficacy and safety of intensity-modulated radiotherapy following transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Medicine. 2016;95(21):e3789. doi: 10.1097/md.0000000000003789. PubMed PMID: 00005792-201605240-00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim J, Jung Y. Radiation-induced liver disease: Current understanding and future perspectives. Exp Mol Med. 2017;49(7):e359. Epub 2017/07/22. doi: 10.1038/emm.2017.85. PubMed PMID: 28729640; PubMed Central PMCID: PMCPMC5565955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. OPTN Policy 9.3 Allocation of livers. Available from: https://optn.transplant.hrsa.gov/governance/policies/. [Google Scholar]
- 27. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer (Oxford, England : 1990). 2009;45(2):228-47. Epub 2008/12/23. doi: 10.1016/j.ejca.2008.10.026. PubMed PMID: 19097774. [DOI] [PubMed] [Google Scholar]
- 28. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Seminars In Liver Disease. 2010;30(1):52-60. Epub 2010/02/23. doi: 10.1055/s-0030-1247132. PubMed PMID: 20175033. [DOI] [PubMed] [Google Scholar]
- 29. Kudithipudi V, Day E, Thai N, Kirichenko A. Liver stereotactic radiotherapy (SRT) with functional treatment planning for patients with intermediate stage hepatocellular carcinoma (HCC). Journal of Radiation Oncology; 2017. p. 371-7. [Google Scholar]
- 30. Valakh V, Williams G. Stereotactic body radiotherapy for hepatocellular carcinoma in the setting of poor liver function: A case series. Journal of Clinical Oncology. 2017;35(4_suppl):493-. doi: 10.1200/JCO.2017.35.4_suppl.493. [DOI] [Google Scholar]
- 31. Mohamed M, Katz AW, Tejani MA, Sharma AK, Kashyap R, Noel MS, et al. Comparison of outcomes between SBRT, yttrium-90 radioembolization, transarterial chemoembolization, and radiofrequency ablation as bridge to transplant for hepatocellular carcinoma. Advances in Radiation Oncology. 2016;1(1):35-42. Epub 2015/12/29. doi: 10.1016/j.adro.2015.12.003. PubMed PMID: 28799575; PubMed Central PMCID: PMCPMC5506745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. International Journal of Radiation Oncology, Biology, Physics. 2011;81(4):e447-53. Epub 2011/06/08. doi: 10.1016/j.ijrobp.2011.04.011. PubMed PMID: 21645977. [DOI] [PubMed] [Google Scholar]
- 33. Mannina EM, Cardenes HR, Lasley FD, Goodman B, Zook J, Althouse S, et al. Role of stereotactic body radiation therapy before orthotopic liver transplantation: Retrospective evaluation of pathologic response and outcomes. International Journal of Radiation Oncology, Biology, Physics. 2017;97(5):931-8. Epub 2017/03/24. doi: 10.1016/j.ijrobp.2016.12.036. PubMed PMID: 28333015. [DOI] [PubMed] [Google Scholar]