Abstract
Background
Cone beam CT (CBCT) imaging has been integrated into the most recent version of the Leksell Gamma Knife for the primary purpose to facilitate fractionated therapy.
Case description
This case study presents three patients where the CBCT system of the Gamma Knife Icon discovered potentially clinically significant frame shifts. In each case, patients were imaged with volumetric MR prior to stereotactic frame placement. Immediately following frame placement, diagnostic stereotactic CT imaging was acquired with a stereotactic indicator box attached to the frame. Following treatment planning and immediately before radiosurgery, a CBCT was acquired using the on-board imaging functionality of the Gamma Knife Icon, which provides a registration of the patient’s anatomy to stereotactic space independent of that provided by the stereotactic frame/fiducials. Co-registration of the CT and CBCT provides an estimate of the difference between these two estimates of stereotactic coordinates. The vector magnitudes of the differences measured at the center of stereotactic space were 0.93mm, 2.64mm and 2.18 mm for Case 1, Case 2 and Case 3 respectively.
Conclusions
Use of the CBCT functionality of the Gamma Knife Icon to verify the consistency of frame placement can prevent clinically significant targeting errors due to frame slippage or frame adapter mounting errors, and allows any required adjustments to be made without interrupting the overall treatment workflow.
Keywords: radiosurgery, cone beam CT, stereotactic frame, frame shift, Gamma Knife, quality assurance
Introduction
Gamma Knife Radiosurgery (GKRS) has traditionally involved the delivery of ablative doses (> 10 Gy) of radiation to small, well-defined intracranial volumes in a single treatment session. The mechanical design of the Gamma Knife, the use of 60Co as a radiation source and presumed absolute fixation of the patient using a stereotactic frame have been key elements which makes possible the degree of accuracy and precision required for safe delivery of such high doses of radiation[1].
Stereotactic frames serve the dual role of cranial immobilization and intracranial localization. The Leksell stereotactic “G-frame” (Elekta Instrument, AB, Stockholm) provides direct mechanical immobilization of the cranium through a rigid mechanical system. This system involves the patient’s skull, four fixation pins, four support posts, the base ring of the frame and the treatment bed [2] Recent iterations of the Gamma Knife (Perfexion™ and Icon™ models) have a mechanical frame adapter interface between the frame and the mount on the treatment bed)[3]. The dimensions of the Leksell G-frame define a targeting coordinate system, which is matched to the machine coordinates of the Gamma Knife. In earlier iterations of the Gamma Knife, stereotactic coordinates were set through the use of a mechanical trunnion system. This system required the treatment team to use scales on the stereotactic frame itself to position the patient and then “set” the proper coordinates by tightening hex-screws[1]. More recent iterations of the Gamma Knife use the treatment bed itself to position the patient at the planned coordinates, however the machine coordinates of the Gamma Knife remain directly related to the dimensions of the stereotactic frame via a calibration offset[3].
Tomographic (fan beam CT, and/or MR) or bi-plane x-ray based imaging used for Gamma Knife treatment planning are acquired pre-treatment using a modality-dependent indicator box placed over the patient’s head and attached to the base ring of the frame. The registration of the acquired images to stereotactic coordinate space assumes that this indicator box is rigidly fixed to the frame, and that the patient is immobilized during the scan.
In practice, one of the basic assumptions for GKRS is that the mechanical system of the frame is static and secure from diagnostic imaging until the end of the radiosurgical procedure. Once the patient’s frame has been applied, the entire treatment chain of a Gamma Knife procedure depends on the fixed coordinate system defined by the frame. Any violation of this assumption has the potential to result in a targeting error and a resulting decrease in treatment efficacy and potential increase in treatment toxicity[4].
The recently Gamma Knife® Icon™ includes an integrated cone beam CT (CBCT) scanner[5]. The CBCT isocenter is calibrated against the radiation isocenter of the Gamma Knife, and its known imaging geometry means that the resulting CBCT images can be used as an estimate of stereotactic coordinate space independent from the frame-defined stereotactic space. As a new quality assurance (QA) measure, CBCT scans acquired at the start of a treatment or at various points in a lengthy GKRS can be used to detect mechanical shifts in the frame system or it’s attachment to the treatment bed. If a shift is detected, the CBCT-defined coordinates can be used in place of the usual frame-defined coordinates without repeating the entire treatment-planning imaging procedure.
In this report, we present three cases that demonstrate detection of mechanical frame shift errors during GKRS in which the CBCT allowed for detection and correction immediately prior to treatment without requiring a repeat of the imaging and treatment planning workflow.
Material and Methods
Patients were investigated as part of an IRB-approved quality improvement project. Review inclusion criteria were (1) patients undergoing single session, frame based GKRS between August 2016 and August 2017, (2) patients found to have distortion in the stereotactic CT fiducials or when CT/CBCT registration differences were greater than 1 mm.
The patients in this case report were treated utilizing a standard protocol for GKRS previously described.[6,7] Prior to frame placement, a thin-sliced (~1mm thickness, volumetric acquisition, and no gap) stereotactic 3-Tesla MRI of the brain was obtained with appropriate T1 and T2 weighted sequences. On the day of GKRS, the patients were taken to an operating room and administered monitored anesthesia. The patients’ heads were prepped and draped. Local anesthetic was applied at four sites and the treating neurosurgeon affixed a Leksell G frame to each patient’s head using 4 point fixation, tightening the pins per the surgeon’s discretion and manufacturer’s recommendations[8].
Once the frame was in place, a stereotactic indicator box (Elekta Instrument, AB, Stockholm) was attached to the frame, and a thin-sliced, diagnostic quality fan beam CT of the head was obtained in the radiology department. The MRI was co-registered to this CT using the Gamma Knife treatment planning system (GammaPlan v. 11.1, Elekta Instrument AB, Stockholm) using a rigid, mutual-information based co-registration algorithm[9], with a region-of-interest (ROI) applied to exclude the stereotactic fiducial indicators imaged as part of the stereotactic CT study. A treatment plan was determined by the treating neurosurgeon, radiation oncologist, and medical physicist. The patient was then transferred to the Gamma Knife suite and placed on the Gamma Knife Icon treatment bed in treatment position with appropriate selection of Gamma angle and adjustment of the couch to avoid cervical strain in the treatment position. Immediately prior to radiosurgical delivery, a CBCT was obtained using the integrated CBCT system. The resulting images were transferred to the treatment planning system.
As previously described, the diagnostic fan beam CT (with stereotactic indicator box) and pre-treatment CBCT provide independent measures of the patient’s head position within stereotactic space. The stereotactic CT is registered to stereotactic space by identifying the fiducial marks that appear in the image due to the presence of the stereotactic indicator box. The CBCT is acquired implicitly in stereotactic space due to the CBCT calibration described above. For each case, the images were co-registered in the treatment planning system using a rigid, mutual information based co-registration algorithm[9], ROI limits were applied to the registration algorithm to exclude the stereotactic fiducial indicators on the CT scan. The resulting images were superimposed allowing direct visual comparison. Any displacement of the fixation pins relative to the skull between the two scans were identified. In addition, the treatment planning system provides an estimate of the rotational and translational differences between the stereotactic coordinate systems by mathematically decomposing the image co-registration matrix[10]:
(1).
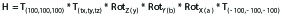
where H is the combined co-registration matrix, T(100,100,100) and T(-100,-100,-100) are the translations to and from the center of Leksell coordinate space (coordinates x=100.0mm (left-right), y=100.0mm (anterior-posterior), z=100.0mm(superior-inferior)), T(tx,ty,tz) are the translations along each orthogonal axis, and RotZ, RotY, and RotX are the rotational components around each orthogonal axis.
For each of the three cases, the rotational and translation differences was recorded from either the post-treatment report for the treatment or by retrospectively querying the treatment planning system database. The vector magnitude translational difference at center point of Leksell coordinate space was defined as:
(2).
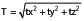
where tx, ty, and tz are the vector translations along each axis. Of note, the greater the distance from the center of rotation a given point is located, the greater the effect rotations will have on the total positional difference of that point between the coordinate system estimates. Therefore points located distant from the center point of Leksell coordinate space will have larger vector magnitude differences in the presence of rotations between the CT and CBCT images.
Results
Three patients met study inclusion criteria detailed above. This included a 66 year old male with right sided vestibular schwannoma (Case 1), a 59 year old male with a single left cerebellar metastasis (Case 2) and a 55 year old male undergoing GKRS for multiple brain metastases (Case 3). Table 1 summarizes the target and treatment planning characteristics of each case.
Table 1.
Patient and Treatment Characteristics
| Patient # | Target #/Site | Dose (Gy) | Isodose (%) | # Isocenters | Max diameter (cm) |
| 1 | 1- Right vestibular schwannoma | 12 | 50 | 4 | 1.1 |
| 2 | 1-Left cerebellar met. | 22 | 50 | 2 | 1.3 |
| 3 | 1- Left frontal met. | 22 | 50 | 3 | 1.6 |
| 3 | 2- Right frontal met. | 22 | 90 | 1 | 0.3 |
| 3 | 3- Left frontal met. | 22 | 95 | 1 | 0.4 |
| 3 | 4- Right thalamic met. | 22 | 92 | 2 | 0.3 |
| 3 | 5- Right temporal met. | 22 | 50 | 2 | 1.2 |
| 3 | 6- Left occipital met. | 22 | 90 | 1 | 0.4 |
| 3 | 7- Right parasagittal frontal met. | 22 | 93 | 1 | 0.5 |
| 3 | 8- Right parietal met. | 22 | 97 | 1 | 0.2 |
| 3 | 9- Right cerebellar met. | 22 | 97 | 1 | 1.1 |
| 3 | 10- Right parietal met. | 22 | 90 | 1 | 0.4 |
| 3 | 11- Right caudate met. | 22 | 94 | 2 | 0.3 |
| 3 | 12- Right parasagittal parietal met. | 22 | 97 | 1 | 0.3 |
| 3 | 13- Left occipital met. | 22 | 94 | 1 | 0.5 |
| met = metastasis | |||||
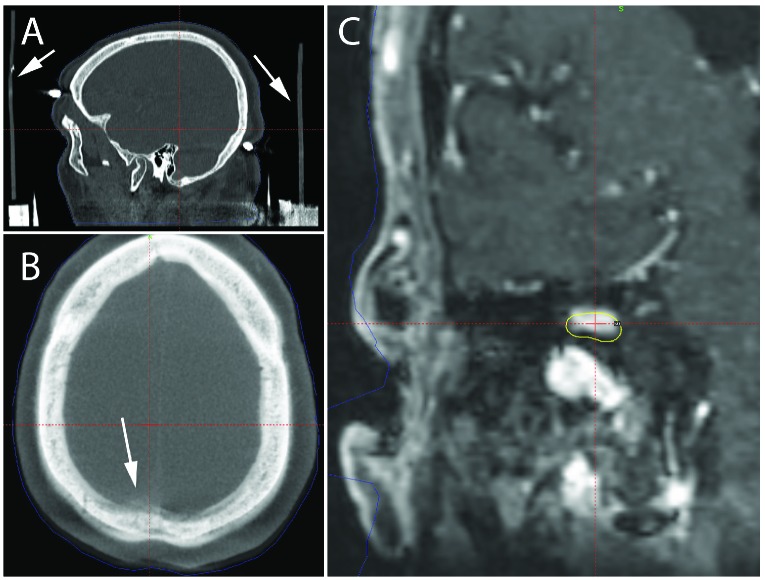
A discrepancy was detected in Case 1 when the treatment planning system reported a CT/CBCT registration difference greater in magnitude than is typically observed. Close, retrospective, inspection of the patient’s images revealed that the frame and/or frame indicator box shifted during the acquisition of the stereotactic CT scan (Figure 1A). The error in the location of the fiducial marks compared to the treatment planning system’s model of the fiducial pattern was reported by the planning system to be a mean of 0.4 mm, with a maximum error of 1.1mm.A fusion of the CT in stereotactic space as defined by the fiducial markers and the CBCT in its implicit stereotactic space shows significant mis-alignment of the two scans as shown in Figure 1B. Overlaying the treatment plan defined in the CT-based stereotactic coordinates onto the treatment planning MR co-registered to the CBCT-based stereotactic coordinates demonstrates that a significant portion of the target would not have received the intended prescription dose while permitting excess dose to normal parenchyma (Figure 1C).
Figure 1.
Treatment planning imaging for a right vestibular schwannoma. (A) The post frame placement diagnostic CT demonstrates positional shift during image acquisition, most clearly indicated by bending of the straight vertical bars of the head frame as shown by the white arrows. (B) The fusion of the Icon CBCT and diagnostic pre-procedural CT in the axial plane demonstrates misalignment, most evident in the area indicated by the white arrow. (C) The treatment plan defined by the diagnostic CT placed over the treatment planning MR co-registered to the CBCT-based stereotactic coordinates demonstrates significant portions of GTV that would have been undertreated and areas of normal parenchyma that would have received full prescription dose.
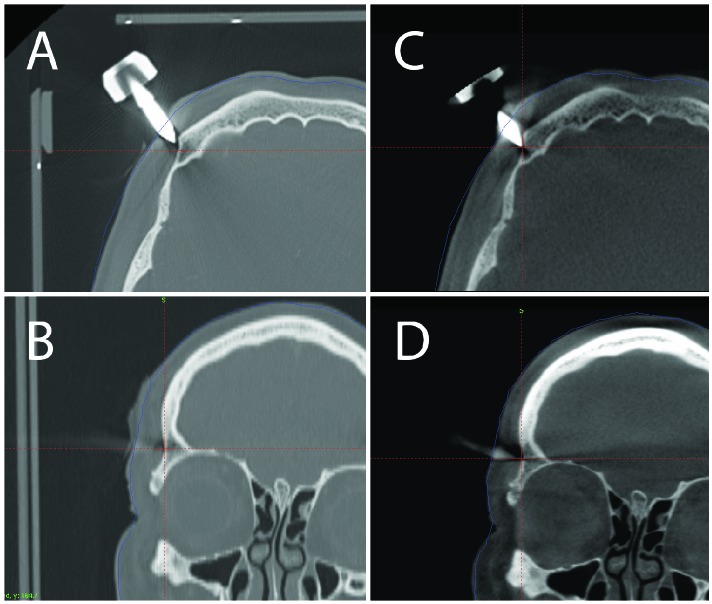
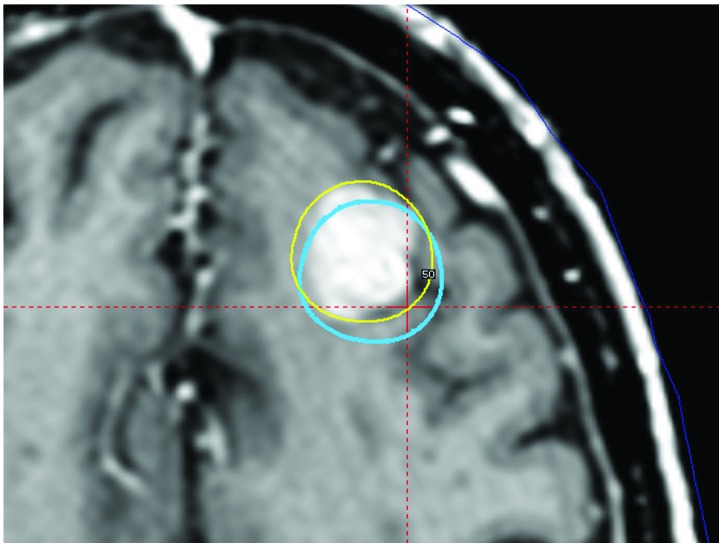
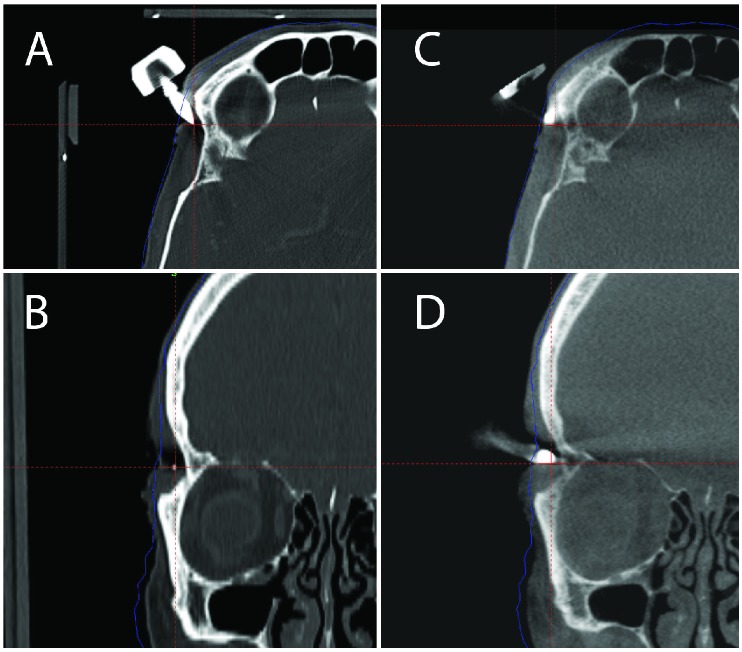
In Case 2 and Case 3, close inspection of the fixation pins on the stereotactic CT and pre-treatment CBCT scans revealed that some adjustment of the pins were required to insure proper attachment to the outer table of the cranium (Figure 2A-D, 3A-D). Reconstruction of the uncorrected treatment plan in Case 3 as compared to the intended treatment (Figure 4) demonstrates a potential targeting error due to the shift in the stereotactic coordinate system.
Figure 2.
Treatment planning for a left cerebellar metastasis. (A) Axial view of one of the frame pins on the stereotactic fan beam CT. (B) Coronal view of one of the frame pins on the stereotactic fan beam CT. (C) Axial view of the same pin on the pre-treatment CBCT. (D) Coronal view of the same pin on the pre-treatment CBCT. Comparison of the stereotactic fan beam CT with the pretreatment CBCT shows that the pin was not securely anchored with an accompanying frame shift.
Figure 4.
Treatment planning imaging for multiple brain metastases. The uncorrected plan of one of multiple brain metastasis (blue line) based off the stereotactic fan beam CT registered to the planning MRI overlaid with the corrected volume (yellow line) generated from the pretreatment CBCT registered to the planning MRI. Using the stereotactic CT for planning would have undertreated the metastasis and applied full dose to normal brain parenchyma.
The rotation and translational shifts between the CBCT and the standard stereotactic CT for the three patients are reported in Table 2. For the purpose of comparison, the vector magnitude shift introduced by the discrepancies in stereotactic coordinates was reported for the center point (coordinates: x=100.0mm; y=100.0mm; z=100.0mm of Leksell coordinate space for each patient. The resulting magnitude shifts were 0.93mm, 2.64mm, and 2.18 mm, for Case 1, Case 2, and Case 3 respectively. As the rotations are defined around the center point in stereotactic space the magnitude shift on a given isocenter in a treatment plan is proportional to the distance from this center point to the isocenter in question. For example in the third patient who had a peripheral occipital lesion, the vector magnitude shift at the lesion would have been 4.77mm.
Table 2.
Calculated Rotational and Translational Shift of the Head frame
| Patient # | Rotational Shift (degree) | Translational Shift (mm) | Overall Translation At Point (100,100,100) | ||||
| X axis | Y axis | Z axis | X axis | Y axis | Z axis | ||
| 1 | 0.3 | 0.14 | -0.06 | 0.8 | 0.02 | 0.47 | 0.93 mm |
| 2 | -0.65 | 0.36 | 0.57 | 0.91 | 1.68 | 1.68 | 2.64 mm |
| 3 | 2.32 | 1.43 | 0.24 | 0.2 | 0.07 | -2.17 | 2.18 mm |
Discussion
Stereotactic frames have been used reliably for decades in Gamma Knife radiosurgery over hundreds of thousands of cases. This study was not designed to determine the incidence of frame or frame adapter misplacement, and in fact is these are difficult to ascertain as we have not been performing CT or CBCT scans for the majority of our more than 20 years of GKRS. While we believe that the incidence of problems is likely low, the risk of adverse radiation effect from a low dose CBCT to check for frame based coordinate problems is likely even lower and seems a reasonable approach when clinically indicated.
The fundamental accuracy and precision of beam delivery using a current Gamma Knife model has been reported to be within 0.3mm[11], but this combined uncertainty depends upon rigid frame immobilization from the time the frame is affixed, throughout any stereotactic treatment planning imaging, and throughout the duration of the radiosurgical procedure itself. Each mechanical interface in the frame system is a location where there is some potential for a breakdown in the integrity of the frame system. Several prior studies have examined the uncertainty of different aspects of the procedural uncertainty of GKRS. Mack et al. looked at the whole-procedure performance using a hidden target test, performing 170 measurements over 5 years and found a mean vector displacement of 0.48±0.23mm[12]. Alternatively, Heck et al. looked specifically at a point coincident to the isocenter of the unit and found all measured data were within a radial 0.2mm sphere[13]. When the whole procedure was examined for large lesions, Ma et al. found agreement with planned dose distributions between 0.1mm and 1.6mm, which was a smaller range than predicted from quadrature summation of individual uncertainty components[11]. Massager et al. used post-GKRS enhancement on MRI in trigeminal neuralgia cases to evaluate spatial targeting accuracy and the group arrived at a median coordinate deviation on 0.91mm[14].
Figure 3.
Treatment planning imaging for multiple brain metastases. (A) Axial view of one of the frame pins on the stereotactic fan beam CT. (B) Coronal view of one of the frame pins on the stereotactic fan beam CT. (C) Axial view of the same pin on the pre-treatment CBCT. (D) Coronal view of the same pin on the pre-treatment CBCT. Comparison of the stereotactic fan beam CT with the pretreatment CBCT shows that the pin was not securely anchored with an accompanying frame shift.
In respect to the uncertainty of the stereotactic frame and frame-slippage, there is sparse published data. Using the previously described trunnion system Foote et al. investigated the incidence of coordinate slippage and found it occurred in approximately 3.5% of treated isocenters, and was associated with longer treatment time, taller patients, heavier patients, and increased number of isocenters[15]. However this mode of frame slippage is not relevant to current Gamma Knife designs, which use the treatment bed itself for positioning. In regards to potential slippage due to inadequate application of the pins MacKenzie et al. investigated the coordinate shift that occurs with the removal of a single pin and found the maximum to be 1.2mm[16]. However other studies have found GKRS using 3 fixation pins leads to negligible difference in outcome[17]. In a more clinically relevant study, setup and intrafraction motion between a Brown-Roberts-Wells stereotactic frame (Radionics, Burlington, MA) incidentally found an instance of frame slippage in one out of a total population of 102 frame-based patients enrolled in a study[18]. A failure mode and effects analysis (FMEA) of a frame-based GKRS clinical workflow created by Xu et al. assigned a poorly affixed frame a low risk priority number (RPN)[19].
Of note, neither the manufacturer (Elekta Instrument, AB), the FDA, nor the NRC provide guidelines for torque for pin tightening[8]. The tightening of pins is manual, and the tightness of the pins is at the discretion of the treating physician. After frame placement, the manufacturer recommends applying force to the base ring to check for stability but this approach has never been adequately studied for reliability or sensitivity in assessing frame changes.
In recent models (Perfexion and Icon) of the Gamma Knife, the stereotactic frame is mounted to the treatment bed via an intermediate frame adapter. In our experience the frame adapter is reliable, however the FMEA analysis by Xu et al., assigned the frame adapter the highest RPN in the study[19]. There have also been reported instances of the frame adapter not properly mounting to the frame and this ultimately resulted in a design modification of the frame adapter[20]. Mis-seating of the frame adapter would not be detected during a stereotactic MR or CT, and must be detected through disciplined visual inspection of the frame adapter. However a pre-treatment CBCT may show detectible shifts between the frame/fiducial based stereotactic coordinates determined from the stereotactic CT and the coordinates as determined by the CBCT. Further study of this indication for CBCT imaging is warranted.
Exploring the Icon system specifically, Li et al. studied the uncertainty in stereotactic coordinate definitions between a prototype CBCT attached to a Gamma Knife Perfexion and the Leksell G-frame. When looking at the intrafraction differences between the two system, they found a mean difference of 0.03 (0.05), 0.03 (0.18), and 0.03 (0.12) mm in the left-right, anterior-posterior, and superior-inferior directions (mean, (SD)). They also looked at uncertainty at time of patient setup and found maximum differences of 0.74, 0.53, and 0.68 mm along each orthogonal axis[21]. Aldahlawi et al., who found magnitude differences between the frame and CBCT coordinate definitions to be 0.21mm to 0.33mm, reinforced these results using a phantom/marker system[22]. Dutta et al. performed a study similar to that of Li et al, however on a clinical Gamma Knife Icon and found mean absolute translational differences of 0.29 (0.27), 0.24 (0.19), and 0.24(0.27) mm in the left-right, anterior-posterior, and left-right directions (mean, (SD)). Mean rotational differences were reported as 0.14(0.19), 0.16(0.21), and 0.12(0.15) degrees (mean, (SD))[23].
The small reported differences of these prior studies support both the stability of the Gamma Knife frame system, and also the sensitivity of the on-board CBCT functionality of the Gamma Knife Icon system as a QA strategy to detect abnormal differences between the two coordinate systems. As described in this case study, this QA strategy helped to mitigate a risk of mistreatment due to patient motion during a stereotactic planning CT in one case, and due to fixation pin adjustments in two other cases. Of note many Gamma Knife centers do not acquire stereotactic CT imaging after frame placement; instead they rely solely on stereotactic MR scans after frame placement and use the resulting fiducial marks to register the MR images to stereotactic coordinates. Fixation pins are generally not visible on MR scans due to induced susceptibility artifacts[24]. In this scenario it can be difficult to detect pins that have slipped or require adjustment. Significant manual handling of the frame can occur between the time of frame placement and treatment as the patient proceeds through various imaging procedures, which can include combinations of CT, MRI, and angiography depending on indication. As mentioned earlier, neither MR nor CT acquired without the patient in treatment position would detect problems with the mounting of the frame adapter on the stereotactic frame. . The integrated CBCT of the Gamma Knife Icon provides a final QA check of the frame fixation moments before the treatment commences to help mitigate these risks. The extremely low dose of the CBCT system (computed tomography dose index (CTDI) of 2.5 mGy in the low dose setting or 6.3 mGy in the high dose setting) adds negligible additional risk to the patient.
Prior to the development of the integrated CBCT system, deciding to correct a stereotactic frame placement immediately prior to treatment would have required a repeat imaging session outside the Gamma Knife suite and significant work to re-plan the treatment as any adjustment to the stereotactic frame alters the associated stereotactic coordinate system. However with the CBCT functionality of the Gamma Knife Icon the treatments highlighted in this study were simply adjusted to use the CBCT as the basis for stereotactic coordinates without significantly disrupting the normal workflow.
Conclusion
While stereotactic frames are often treated as having “perfect” fixation, in fact frames have integral mechanical uncertainties, and they are subject to rare instances of mechanical slippage. This case report presents three instances of frame shifts of a magnitude that might have resulted in clinically significant changes in delivered dose. The on-board CBCT functionality of the Gamma Knife Icon provides a new QA technique to verify the integrity of stereotactic coordinate space immediately prior to treatment, and further provides a method to continue with the procedure without interrupting it to acquire an entirely new set of imaging studies and a new treatment plan. The routine integration of CBCT into the frame-based GK workflow needs to be further studied. When clinically indicated, we believe that the on-board CBCT system of the Gamma Knife Icon has value as a QA tool unavailable on prior models of Gamma Knife that more than balances the low risk of adverse radiation effect from the low dose CBCT.
Acknowledgements
The authors would like to thank Björn Somell, Jonas Johansson, and Håkan Nordström from Elekta Instrument, AB for their assistance with the calculation of the CT to CBCT translational and rotational shifts for particular isocenter coordinates.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Authors’ disclosure of potential conflicts of interest
Dr. Schlesinger reports grant from Elekta Instrument, AB, outside the submitted work. Dr. Trifiletti reports other support from Novocure, outside the submitted work.
Drs. Dutta, Larner, Peach and Sheehan have nothing to disclose.
Author contributions
Conception and design: David Schlesinger, Jason Sheehan, M. Sean Peach, Daniel Trifiletti, Sunil Dutta, James Larner
Data collection: M. Sean Peach, David Schlesinger, Daniel Trifiletti, Sunil Dutta
Data analysis and interpretation: M. Sean Peach, David Schlesinger, Daniel Trifiletti, Sunil Dutta, Jason Sheehan
Manuscript writing: M. Sean Peach, David Schlesinger
Final approval of manuscript: David Schlesinger, Jason Sheehan, James Larner, M. Sean Peach, Sunil Dutta, Daniel Trifiletti
References
- 1. Wu A. Physics and dosimetry of the gamma knife. Neurosurg Clin N Am 1992;3(1):35-50 [PubMed] [Google Scholar]
- 2. Leksell L, Jernberg B. Stereotaxis and tomography. A technical note. Acta Neurochir (Wien) 1980;52(1-2):1-7 [DOI] [PubMed] [Google Scholar]
- 3. Lindquist C, Paddick I. The Leksell Gamma Knife Perfexion and comparisons with its predecessors. Neurosurgery 2007;61(3 Suppl):130-140; discussion 140-131 [DOI] [PubMed] [Google Scholar]
- 4. Rojas-Villabona A, Miszkiel K, Kitchen N, Jager R, Paddick I. Evaluation of the stability of the stereotactic Leksell Frame G in Gamma Knife radiosurgery. J Appl Clin Med Phys 2016;17(3):5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeverino M, Jaccard M, Patin D, Ryckx N, Marguet M, Tuleasca C, Schiappacasse L, Bourhis J, Levivier M, Bochud FO, Moeckli R. Commissioning of the Leksell Gamma Knife(R) Icon. Med Phys 2017;44(2):355-363 [DOI] [PubMed] [Google Scholar]
- 6. Sheehan JP, Yen CP, Nguyen J, Rainey JA, Dassoulas K, Schlesinger DJ. Timing and risk factors for new brain metastasis formation in patients initially treated only with Gamma Knife surgery. Clinical article. J Neurosurg 2011;114(3):763-768 [DOI] [PubMed] [Google Scholar]
- 7. Williams BJ, Xu Z, Salvetti DJ, McNeill IT, Larner J, Sheehan JP. Gamma Knife surgery for large vestibular schwannomas: A single-center retrospective case-matched comparison assessing the effect of lesion size. J Neurosurg 2013;119(2):463-471 [DOI] [PubMed] [Google Scholar]
- 8. Leksell Stereotactic System Instructions for Use, Article Number: 1007063 Rev 04 (2015-05), Elekta Instrument, AB, 2015 [Google Scholar]
- 9. Wells WM, 3rd, Viola P, Atsumi H, Nakajima S, Kikinis R. Multi-modal volume registration by maximization of mutual information. Med Image Anal 1996:1(1):35-51 [DOI] [PubMed] [Google Scholar]
- 10. Dunn F, Parberry I. 3D math primer for graphics and game development, 2nd Edition. Taylor & Francis, 2011 [Google Scholar]
- 11. Ma L, Chuang C, Descovich M, Petti P, Smith V, Verhey L. Whole-procedure clinical accuracy of gamma knife treatments of large lesions. Med Phys 2008;35(11):5110-5114 [DOI] [PubMed] [Google Scholar]
- 12. Mack A, Czempiel H, Kreiner HJ, Durr G, Wowra B. Quality assurance in stereotactic space. A system test for verifying the accuracy of aim in radiosurgery. Med Phys 2002;29(4):561-568 [DOI] [PubMed] [Google Scholar]
- 13. Heck B, Jess-Hempen A, Kreiner HJ, Schopgens H, Mack A. Accuracy and stability of positioning in radiosurgery: long-term results of the Gamma Knife system. Med Phys 2007;34(4):1487-1495 [DOI] [PubMed] [Google Scholar]
- 14. Massager N, Abeloos L, Devriendt D, Op de Beeck M, Levivier M. Clinical evaluation of targeting accuracy of gamma knife radiosurgery in trigeminal neuralgia. Int J Radiat Oncol Biol Phys 2007;69(5):1514-1520 [DOI] [PubMed] [Google Scholar]
- 15. Foote RL, Pollock BE, Link MJ, Garces YI, Kline RW. Leksell Gamma Knife coordinate setting slippage: how often, how much? J Neurosurg 2004;101(4):590-593 [DOI] [PubMed] [Google Scholar]
- 16. MacKenzie JT, Podgorsak MB, Moreland D. Validity of stereotactic frame localization during radiosurgery after one fixation pin removal. J Neurosurg 2002;97(5 Suppl):539-541 [DOI] [PubMed] [Google Scholar]
- 17. Ho JC, Luo D, Guha-Thakurta N, Ferguson SD, Ghia AJ, Yang JN, Brown PD, Voong KR. Gamma Knife Stereotactic Radiosurgery for Brain Metastases Using Only 3 Pins. Neurosurgery 2016;78(6):877-882 [DOI] [PubMed] [Google Scholar]
- 18. Ramakrishna N, Rosca F, Friesen S, Tezcanli E, Zygmanszki P, Hacker F. A clinical comparison of patient setup and intra-fraction motion using frame-based radiosurgery versus a frameless image-guided radiosurgery system for intracranial lesions. Radiother Oncol 2010;95(1):109-115 [DOI] [PubMed] [Google Scholar]
- 19. Xu AY, Bhatnagar J, Bednarz G, Flickinger J, Arai Y, Vacsulka J, Feng W, Monaco E, Niranjan A, Lunsford LD, Huq MS. Failure modes and effects analysis (FMEA) for Gamma Knife radiosurgery. Journal of Applied Clinical Medical Physics 2017;18(6):152-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Potentially incorrect mounting of frame adapter. Field Change Order 100-03-202-027, Elekta Instrument AB, 2016 [Google Scholar]
- 21. Li W, Cho YB, Ansell S, Laperriere N, Menard C, Millar BA, Zadeh G, Kongkham P, Bernstein M, Jaffray DA, Chung C. The use of cone beam computed tomography for image guided Gamma Knife stereotactic radiosurgery: Initial clinical evaluation. Int J Radiat Oncol Biol Phys 2016;96(1):214-220 [DOI] [PubMed] [Google Scholar]
- 22. AlDahlawi I, Prasad D, Podgorsak MB. Evaluation of stability of stereotactic space defined by cone-beam CT for the Leksell Gamma Knife Icon. J Appl Clin Med Phys 2017;18(3):67-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dutta SW, Kowalchuk RO, Trifiletti DM, Peach MS, Sheehan JP, Larner JM, Schlesinger D. Stereotactic shifts during frame-based image-guided stereotactic radiosurgery: Clinical measurements. International Journal of Radiation Oncology • Biology • Physics [in press] 10.1016/j.ijrobp.2018.05.042 [DOI] [PubMed] [Google Scholar]
- 24. Hargreaves BA, Worters PW, Pauly KB, Pauly JM, Koch KM, Gold GE. Metal-induced artifacts in MRI. AJR Am J Roentgenol 2011;197(3):547-555 [DOI] [PMC free article] [PubMed] [Google Scholar]