Abstract
Endothelial dysfunction and damage underlie cerebrovascular disease and ischemic stroke. We undertook corneal confocal microscopy (CCM) to quantify corneal endothelial cell and nerve morphology in 146 patients with an acute ischemic stroke and 18 age-matched healthy control participants. Corneal endothelial cell density was lower (P < 0.001) and endothelial cell area (P < 0.001) and perimeter (P < 0.001) were higher, whilst corneal nerve fibre density (P < 0.001), corneal nerve branch density (P < 0.001) and corneal nerve fibre length (P = 0.001) were lower in patients with acute ischemic stroke compared to controls. Corneal endothelial cell density, cell area and cell perimeter correlated with corneal nerve fiber density (P = 0.033, P = 0.014, P = 0.011) and length (P = 0.017, P = 0.013, P = 0.008), respectively. Multiple linear regression analysis showed a significant independent association between corneal endothelial cell density, area and perimeter with acute ischemic stroke and triglycerides. CCM is a rapid non-invasive ophthalmic imaging technique, which could be used to identify patients at risk of acute ischemic stroke.
Introduction
The major risk factors for stroke include diabetes, hypertension, smoking, dyslipidemia1–5 and metabolic syndrome6. Endothelial dysfunction is a key underlying abnormality in stroke and in those at risk of stroke, by promoting vasoconstriction and enhanced plaque vulnerability and rupture, with thrombus formation7. Endothelial dysfunction can be assessed using a variety of techniques including brachial flow-mediated dilation, cerebrovascular reactivity to L-arginine and laser Doppler8. Indeed we have previously shown impaired endothelium dependent dilatation in patients with obesity9, diabetes and hypertension10 and an association between small artery remodeling and diastolic dysfunction in obese subjects11. Patients admitted with an acute ischemic stroke have reduced forearm flow mediated dilatation and increased circulating levels of P-selectin, a marker of endothelial dysfunction12. Direct imaging of the cerebral blood vessels can identify atherosclerosis and stenosis13 and brain imaging can identify silent infarcts, cerebral microbleeds, periventricular white matter hyperintensities and perivascular spaces, which all predict a higher risk of stroke14,15. Subtle alterations in the microstructure of normal-appearing white matter also predicts stroke16. Retinal vessel dysfunction and altered structure have been related to cardiovascular disease8,17, stroke18 and recurrent stroke19.
The major function of the corneal endothelium is to regulate corneal hydration and the passage of nutrients and metabolic waste to and from stromal keratocytes20. However, it produces comparable type and amount of extracellular matrix and collagen to aortic and venous endothelium21, and exposure of corneal endothelial cells to fibrin22 or thrombin23 leads to the induction of tissue-plasminogen activator. Non-contact specular microscopy has been used to identify a reduction in corneal endothelial cell density and increased polymegathism in some studies of patients with Type 2 diabetes24 and children with Type 1 diabetes25, but not in others26.
Corneal confocal microscopy is a rapid non-invasive ophthalmic imaging technique that demonstrates corneal nerve damage in patients with diabetic and HIV neuropathy27,28, Parkinson’s disease29, multiple sclerosis30,31 and acute ischemic stroke32. We have also previously demonstrated a reduction in corneal endothelial cell density in patients with Type 1 diabetes33 and Type 2 diabetes34.
In the present study, we have utilized CCM to quantify corneal endothelial cell and nerve morphology in patients with acute ischemic stroke.
Results
Clinical and Metabolic parameters
The clinical and laboratory characteristics of the participants are given in Table 1. One hundred and forty-six patients with acute ischemic stroke, with (HbA1c ≥ 6.5%) (n = 50) and without (HbA1c ≤ 6.4%) (n = 96) type 2 diabetes mellitus (T2DM) were compared with 18 age-matched healthy control participants. The duration of diabetes in diabetic patients with ischemic stroke was 7.94 ± 7.50 years. There were no differences in age, BMI, total cholesterol, LDL and HDL between controls and stroke patients. Stroke patients had higher triglycerides (P = 0.05), HbA1c (P < 0.04), systolic blood pressure (P < 0.001) and diastolic blood pressure (P < 0.001) compared to control participants (Table 1).
Table 1.
Clinical metabolic and corneal endothelial and nerve parameters in control subjects and patients with acute ischemic stroke.
| Variables | Controls | Stroke | P value |
|---|---|---|---|
| Number of Participants | 18 | 146 | |
| Age (years) | 47.73 ± 3.10 | 48.93 ± 0.79 | 0.714 |
| Gender (M/F) | (11/7) | (141/5) | <0.001 |
| BMI (kg/m2) | 25.78 ± 0.63 | 29.40 ± 0.83 | 0.217 |
| NIHSS Score | N/A | 4.08 ± 0.33 | NA |
| Triglycerides (mmol/l) | 1.23 ± 0.24 | 1.86 ± 0.10 | 0.053 |
| Total Cholesterol (mmol/l) | 4.63 ± 0.35 | 5.05 ± 0.10 | 0.337 |
| LDL (mmol/l) | 2.96 ± 0.33 | 3.27 ± 0.09 | 0.421 |
| HDL (mmol/l) | 1.10 ± 0.07 | 0.94 ± 0.02 | 0.058 |
| BP Systolic (mmHg) | 120.40 ± 3.96 | 161.03 ± 2.47 | <0.001 |
| BP Diastolic (mmHg) | 73.60 ± 2.44 | 94.10 ± 1.41 | <0.001 |
| HbA1c (%) | 5.36 ± 0.17 | 6.83 ± 0.18 | 0.035 |
| Diabetes Duration (years) | NA | 7.94 ± 7.50 | NA |
| Mean ECD (no./mm2) | 3664.72 ± 43.88 | 3342.87 ± 27.45 | <0.001 |
| Mean ECA (µm2) | 219.81 ± 2.69 | 244.37 ± 2.05 | <0.001 |
| Mean ECP (µm) | 52.95 ± 0.35 | 55.74 ± 0.25 | <0.001 |
| Polymegathism (%) | 52.26 ± 1.31 | 52.39 ± 0.44 | 0.923 |
| Pleomorphism (%) | 33.51 ± 1.21 | 33.60 ± 0.50 | 0.953 |
| CNFD (no./mm2) | 37.54 ± 1.97 | 28.73 ± 0.65 | <0.001 |
| CNBD (no./mm2) | 73.96 ± 6.15 | 49.35 ± 2.26 | < 0.001 |
| CNFL (mm/mm2) | 21.31 ± 1.01 | 16.92 ± 0.42 | 0.001 |
BMI (Body Mass Index), NIH stroke severity (NIHSS), LDL (Low Density Lipoprotein), HDL (High Density Lipoprotein), BP (Blood Pressure), HbA1c (Glycated hemoglobin), mean ECD (Endothelial Cell Density), mean ECA (Endothelial Cell Area), mean ECP (Endothelial Cell Perimeter), CNFD (Corneal nerve fibre density), CNBD (Corneal nerve branch density), CNFL (Corneal nerve fibre length). Results are expressed as mean ± SE with significance indicated by the exact P value.
Corneal Confocal Microscopy
Corneal Endothelium
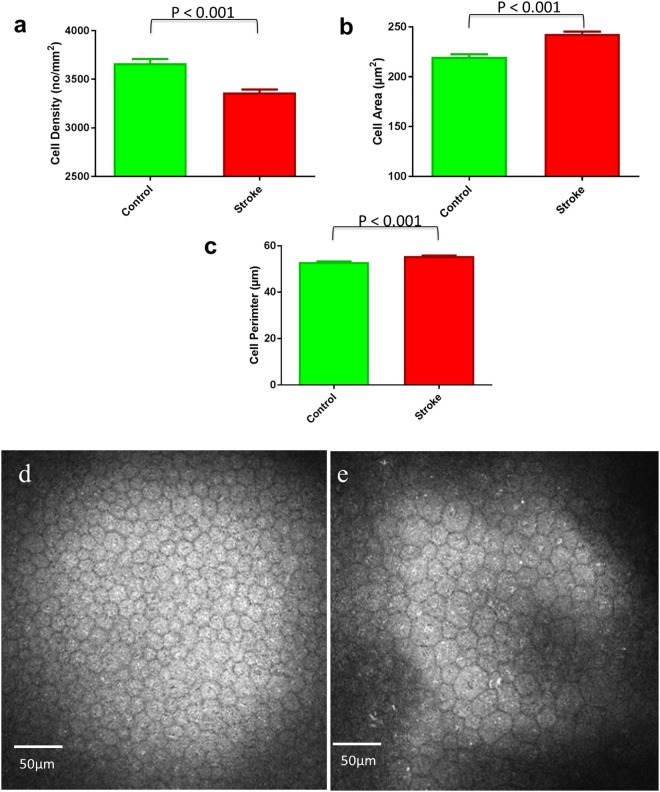
Corneal endothelial cell density was lower (P < 0.001) and endothelial cell area (P < 0.001) and perimeter (P < 0.001) were higher, but there were no significant difference in the percentage polymegathism and pleomorphism in stroke patients compared to healthy controls (Table 1; Fig. 1).
Figure 1.
Graphs showing endothelial cell density (a), endothelial cell area (b) and endothelial cell perimeter (c) expressed as Mean and SEM in participants with acute ischemic stroke and control subjects and an image of corneal endothelial cells in a control participant (d) and a patient with acute ischemic stroke (e).
There was no significant difference in corneal endothelial cell density (3363.87 ± 34.45; 3302.55 ± 45.16, P = 0.283), area (242.90 ± 2.56; 247.19 ± 3.40, P = 0.322), perimeter (55.53 ± 0.31; 56.14 ± 0.41, P = 0.247), polymegathism (52.35 ± 0.57; 52.45 ± 0.70, P = 0.920) or pleomorphism (33.61 ± 0.61; 33.59 ± 0.87, P = 0.985) in patients with and without diabetes, respectively.
Corneal Nerves
Corneal nerve fibre density (P < 0.001), corneal nerve branch density (P < 0.001) and corneal nerve fibre length (P = 0.001) were lower in patients with acute ischemic stroke compared to controls (Table 1).
Correlation between endothelial cell and nerve morphology
In all stroke patients, corneal endothelial cell density correlated with corneal nerve fiber density (r = 0.177, P = 0.033) and corneal nerve fiber length (r = 0.199, P = 0.017). Endothelial cell area and perimeter correlated with corneal nerve fiber density (r = −0.204, P = 0.014, r = −0.211, P = 0.011) and corneal nerve fiber length (r = −0.207, P = 0.013, r = −0.220, P = 0.008), respectively (Table 2). There was no significant correlation between corneal endothelial cell parameters and corneal nerve branch density or between % polymegathism and pleomorphism and corneal nerve parameters.
Table 2.
Correlation between endothelial cell and corneal nerve parameters in patients with ischemic stroke, with significant values in bold.
| Variables | CNFD | CNFL | CNBD |
|---|---|---|---|
| Endothelial Cell Density | |||
| Coefficient (r) | 0.177 | 0.199 | 0.116 |
| P | (0.033) | (0.017) | (0.166) |
| Endothelial Cell Area | |||
| Coefficient (r) | −0.204 | −0.207 | −0.128 |
| P | (0.014) | (0.013) | (0.125) |
| Endothelial Cell Perimeter | |||
| Coefficient (r) | −0.211 | −0.220 | −0.140 |
| P | (0.011) | (0.008) | (0.093) |
| Polymegathism | |||
| Coefficient (r) | −0.082 | −0.018 | −0.054 |
| P | (0.327) | (0.831) | (0.515) |
| Pleomorphism | |||
| Coefficient (r) | 0.093 | 0.068 | 0.092 |
| P | (0.263) | (0.416) | (0.271) |
ECD (Endothelial Cell Density), ECA (Endothelial Cell Area), ECP (Endothelial Cell Perimeter), CNFD (Corneal nerve fibre density), CNBD (Corneal nerve branch density), CNFL (Corneal nerve fibre length).
In stroke patients without diabetes, corneal endothelial cell density correlated with corneal nerve fiber density (r = 0.208, P = 0.042). Endothelial cell area and perimeter correlated inversely with corneal nerve fiber density (r = −0.241, P = 0.018, r = −0.236, P = 0.021) and corneal nerve fiber length (r = −0.207, P = 0.037, r = −0.216, P = 0.0035), respectively (Supplementary Table 1). There was no significant correlation between corneal endothelial cell parameters and CNBD or between % polymegathism and pleomorphism and corneal nerve parameters. In stroke patients with diabetes, there was no significant correlation between endothelial cell density, cell area or perimeter and corneal nerve parameters. Endothelial cell pleomorphism correlated with CNFD (r = 0.309, P = 0.031) and polymegathism correlated with corneal nerve fiber density (r = −0.373, P = 0.008), corneal nerve fiber length (r = −0.296, P = 0.039) and corneal nerve branch density (r = −0.334, P = 0.019) (Supplementary Table 2).
Multiple Linear Regression
There was an independent association between endothelial cell density and triglycerides (P = 0.05) (Table 3). Endothelial cell area was independently associated with higher triglycerides (P = 0.04) and acute ischemic stroke (P = 0.05) (Table 4). Endothelial cell perimeter was independently associated with higher triglycerides (P = 0.04) and acute ischemic stroke (P = 0.05) (Table 5).
Table 3.
Estimates of endothelial cell density and independent variables in multiple regression with significance.
| Parameter | Estimate | 95% CI Lower Bound | 95% CI Upper Bound | Standard Error | Significance level P Value |
|---|---|---|---|---|---|
| Dependent Variable: Endothelial Cell Density | |||||
| Constant | 3707.505 | 3127.029 | 4287.980 | 293.492 | <0.001 |
| Age | −3.873 | −10.062 | 2.315 | 3.129 | 0.218 |
| BMI | −3.159 | −8.863 | 2.545 | 2.884 | 0.275 |
| Triglycerides | −95.066 | −191.861 | 1.729 | 48.940 | 0.054 |
| Cholesterol | 144.913 | −67.325 | 357.152 | 107.309 | 0.179 |
| LDL | −110.805 | −329.658 | 108.049 | 110.654 | 0.318 |
| HDL | −269.492 | −572.551 | 33.567 | 153.228 | 0.081 |
| Systolic BP | −1.174 | −3.895 | 1.547 | 1.376 | 0.395 |
| Diastolic BP | 4.362 | −0.418 | 9.143 | 2.417 | 0.073 |
| HbA1c | 5.169 | −20.947 | 31.285 | 13.204 | 0.696 |
| Stroke | −277.299 | −595.120 | 40.523 | 160.692 | 0.087 |
Table 4.
Estimates of endothelial cell area and independent variables in multiple regression with significance.
| Parameter | Estimate | 95% CI Lower Bound | 95% CI Upper Bound | Standard Error | Significance level P Value |
|---|---|---|---|---|---|
| Dependent Variable: Endothelial Cell Area | |||||
| Constant | 217.302 | 174.154 | 260.45 | 21.816 | <0.001 |
| Age | 0.323 | −0.137 | 0.783 | 0.233 | 0.167 |
| BMI | 0.202 | −0.222 | 0.626 | 0.214 | 0.348 |
| Triglycerides | 7.564 | 0.369 | 14.759 | 3.638 | 0.039 |
| Cholesterol | −12.025 | −27.801 | 3.752 | 7.977 | 0.134 |
| LDL | 9.956 | −6.312 | 26.224 | 8.225 | 0.228 |
| HDL | 19.112 | −3.415 | 41.639 | 11.39 | 0.096 |
| Systolic BP | 0.086 | −0.117 | 0.288 | 0.102 | 0.403 |
| Diastolic BP | −0.337 | −0.692 | 0.018 | 0.18 | 0.063 |
| HbA1c | −0.679 | −2.621 | 1.262 | 0.982 | 0.49 |
| Stroke | 23.883 | 0.258 | 47.507 | 11.945 | 0.048 |
Table 5.
Estimates of endothelial cell perimeter and independent variables in multiple regression with significance.
| Parameter | Estimate | 95% CI Lower Bound | 95% CI Upper Bound | Standard Error | Significance level P Value |
|---|---|---|---|---|---|
| Dependent Variable: Endothelial Cell Perimeter | |||||
| Constant | 52.7 | 47.456 | 57.943 | 2.651 | 0.001 |
| Age | 0.035 | −0.021 | 0.091 | 0.028 | 0.218 |
| BMI | 0.025 | −0.026 | 0.077 | 0.026 | 0.330 |
| Triglycerides | 0.893 | 0.018 | 1.767 | 0.442 | 0.045 |
| Cholesterol | −1.313 | −3.23 | 0.604 | 0.969 | 0.178 |
| LDL | 1 | −0.977 | 2.977 | 0.999 | 0.319 |
| HDL | 2.271 | −0.467 | 5.008 | 1.384 | 0.103 |
| Systolic BP | 0.009 | −0.016 | 0.033 | 0.012 | 0.487 |
| Diastolic BP | −0.041 | −0.084 | 0.002 | 0.022 | 0.063 |
| HbA1c | −0.052 | −0.288 | 0.184 | 0.119 | 0.666 |
| Stroke | 2.933 | 0.062 | 5.803 | 1.451 | 0.045 |
Discussion
This is the first study to show a reduction in corneal endothelial cell density and an increase in endothelial cell size in patients with acute ischemic stroke. A study in Type 2 diabetic rats has shown impaired posterior ciliary artery relaxation and corneal nerve loss, suggesting that impaired blood flow to the trigeminal ganglion may be related to corneal nerve loss35. In the present study, we show a modest but significant correlation between the change in corneal endothelial cells and loss of corneal nerves. However, a correlation cannot imply cause and effect and common underlying abnormalities could drive both corneal endothelial cell and nerve fibre abnormalities. Indeed Olsen previously showed a higher prevalence of ischemic heart disease in patients with Fuch’s dystrophy and suggested that endothelial dystrophy and atherosclerosis may have common mechanisms36. Additionally, a number of studies of patients with corneal endothelial dystrophies have demonstrated a reduction in corneal nerve fibres37. Conversely, patients with neurotrophic keratitis and hence a primary loss of corneal nerve fibres have been shown to have endothelial cell abnormalities37,38. Furthermore, corneal nerve loss has been related to a progressive reduction in corneal endothelial cells in patients with dry eye disease39.
Diabetes, hypertension, smoking, dyslipidemia1–5,40,41, obesity and metabolic syndrome6,42 lead to endothelial dysfunction and atherosclerosis and are major risk factors for stroke. Circulating markers of endothelial dysfunction and inflammation can identify patients at risk of stroke43 and endothelial dysfunction occurs in patients with acute stroke44. Structural alterations on MRI, indicative of small vessel disease, include white matter hyperintensities, lacunes, microbleeds and perivascular spaces and are associated with an increased risk of ischemic stroke16. There is a link between abnormalities in the eye and stroke, based on observations that altered retinal vessel function, diameter and geometry are related to cardiovascular disease8,17, stroke18 and recurrent stroke19.
Loss of cells with migration and increased size of neighboring cells and a loss of their hexagonal shape, leading to increased polymegathism and pleomorphism, respectively, characterize corneal endothelial cell pathology. However, these changes are inconsistent and vary in different conditions. We show a reduction in corneal endothelial cell density and an increase in size, but no change in polymegathism or pleomorphism. A recent study in patients with Type 2 diabetes has shown a reduction in endothelial cell density and increased polymegathism, but no change in pleomorphism24. In a study of children with Type 1 diabetes, polymegathism was increased, but pleomorphism was reduced25. In subjects with HIV, endothelial cell density was preserved, but polymegathism was increased45. In the present study we also show no difference in endothelial cell morphology between patients with and without diabetes, but an association with triglycerides diastolic blood pressure and HDL. Of relevance, metabolic syndrome, characterized by raised triglycerides and blood pressure and a low HDL, is an important risk factor for stroke46. Triglycerides were also the only lipid component to confer an increased risk of stroke in the prospective EPIC-Heidelberg cohort47.
This study has several limitations including the modest number of patients with mild ischemic stroke and we did not include other types of stroke. Nevertheless, we show corneal nerve loss and an alteration in corneal endothelial cell morphology in patients with acute ischemic stroke. Larger, longitudinal studies assessing corneal endothelial cell and nerve fibre morphology in those at risk of stroke and in relation to therapies to reduce risk factors for stroke are warranted to establish the clinical utility of corneal confocal microscopy in ischemic stroke.
Methods
Subjects
This study was a prospective, non-randomized clinical study. 146 patients underwent CCM within the first week (most within three days) of admission for an acute ischemic stroke. Stroke was confirmed clinically and radiologically by a neurologist subspecialized in stroke, based on WHO criteria48. Patients underwent assessment of the NIHSS (National Institutes of Health Stroke Scale) on admission. It allows grading of the severity of stroke into minor stroke (1–4 score), moderate stroke (5–15 score), moderate to severe stroke (16–20 score) and severe stroke (21–42 score). We could not undertake CCM in participants with major weakness; therefore only patients with mild stroke were examined.
Exclusion criteria included patients with intracerebral hemorrhage, a known history of eye trauma or surgery, any corneal or anterior segment pathology including neurotrophic keratitis, trigeminal neuralgia, keratoconus, high refractive error, dry eye, contact lens wear, Fuchs corneal dystrophy, posterior corneal dystrophy and glaucoma. Age-matched healthy control participants (n = 18) were recruited and assessed from Rumailah Hospital and Hamad General Hospital in Doha, Qatar.
This study adhered to the tenets of the declaration of Helsinki and was approved by the Institutional Review Board of Weill Cornell Medicine (15–00021) and Hamad General Hospital (15304/15). Informed, written consent was obtained from all patients/guardians before participation in the study. Clinical demographic parameters, blood pressure, HbA1c, total cholesterol, HDL, LDL and triglycerides were assessed on admission.
Corneal Confocal Microscopy
All patients underwent CCM (Heidelberg Retinal Tomograph III Rostock Cornea Module, Heidelberg Engineering GmbH, Heidelberg, Germany). This device uses a 670 nm wavelength helium neon diode laser, which is a class I laser and therefore does not pose any ocular safety hazard. A 63x objective lens with a numerical aperture of 0.9 and a working distance, relative to the applanating cap (TomoCap©, Heidelberg Engineering GmbH, Heidelberg, Germany) of 0.0 to 3.0 mm is used. The size of each two-dimensional image produced is 384 μm × 384 μm with a 15° × 15° field of view and 10 μm/pixel transverse optical resolution. To perform the CCM examination, local anesthetic (0.4% benoxinate hydrochloride, Chauvin Pharmaceuticals, Chefaro, UK) was used to anaesthetize each eye and Viscotears (Carbomer 980, 0.2%, Novartis, UK) were used as the coupling agent between the cornea and the applanating cap. All patients were asked to fixate on an outer fixation light throughout the CCM scan and a CCD camera was used to correctly position the applanating cap onto the cornea. The examination took approximately 10 minutes for both eyes and was undertaken by experienced examiners (AK, GP, HA and INP), masked from the subject’s clinical status. Images of the endothelial cells and subbasal corneal nerves were captured using the “section” mode.
Image Analysis
Corneal endothelial cell morphology was undertaken in 2-3 representative central images from each eye based on the depth (endothelial cell layer), focus (sharp focused images) and position (central cornea), with a frame size of at least 25%49. The image analysis was performed blindly without the investigator being aware of whether the images were from a control subject or patient with stroke. Each image was exported to a real-time automated image analysis system (Corneal Endothelium Analysis System (CEAS))50. A central region of interest (ROI) was traced for each image to identify the optimal area for quantification, avoiding peripheral darker areas. The CEAS system consists of a cell segmentation and morphometric parameter quantification stage. The former stage can be further divided into two steps: a pre-processing step and cell contour detection step. In the pre-processing step an FFT-Band-pass filter is applied to reduce noise and enhance image quality, followed by the detection of all endothelial cells in the image using a watershed transform and a Voronoi tessellation approach. A number of clinically useful features were extracted from the segmented endothelial cell images in an automated and objective manner to accurately describe the health of the corneal endothelium and include: Mean Endothelial Cell Density (ECD) (cell/mm2), Mean Endothelial Cell Area (ECA) (µm2), Mean Endothelial Cell Perimeter (ECP) (µm), polymegathism (%) and pleomorphism (%)51 (Fig. 1). Polymegathism (coefficient of variation) was defined as the standard deviation of the cell area divided by the mean cell area. Pleomorphism was defined as the hexagonality coefficient. The mean SD of the number of cells analysed per image was 136.38+/−61.22.
6 images/subject were selected for corneal nerve image analysis52. All CCM images were analyzed using validated, purpose-written software (CCMetrics®, M. A. Dabbah, ISBE, University of Manchester, Manchester, UK)52. Corneal nerve fiber density (CNFD) (no./mm2), corneal nerve fiber branch density (CNBD) (no./mm2) and corneal nerve fiber length (CNFL) (mm/mm2) were manually quantified.
Statistical analysis
All statistical analysis was carried out using IBM SPSS Statistics software Version 24. Normality of the distribution of data was examined using the Kolmogorov-Smirnov test, and by visual inspection of the histogram and a normal Q-Q plot. Data is expressed as the mean ± standard error (Table 1). Statistical justification for the number of participants was based on a power analysis using the freeware program G*Power version 3.0.10 for α (type 1 error) of 0.05 and power (1 − type 2 error) of 0.80 using corneal nerve fibre density mean (37.12 vs 29.18) and standard deviation (8.35 and 7.16) comparing healthy controls to patients with stroke32.
The statistical distribution of healthy controls and patients with acute ischemic stroke and between stroke patients with and without diabetes was compared using the unpaired t test (2-tailed) (normally distributed variables) and Mann-Whitney test (non-normally distributed variables). Bonferroni correction was applied to control for multiple testing where P = 0.006, based on eight independent observations.
To investigate the association between risk factors for stroke and corneal endothelial cell parameters, Pearson correlation was performed and multiple linear regression was conducted to assess the association between endothelial cell abnormalities and co-variates. Significance level was set at P = 0.05. Prism 6 (version 6.0 g, Graphpad software Inc., CA, USA) was used to plot the graphs.
Electronic supplementary material
Acknowledgements
We thank Dr. Pooja and Dr. Rumaysa for their help with recruitment of the stroke patients and Paula Burke, Mark Santos, Sujatha Joseph and Deborah Morgan for their contribution in the data collection. We also thank Soha Dargham for her help in the statistical analysis. Supported by Qatar National Research Fund Grant BMRP20038654.
Author Contributions
A.K. designed the study, performed the C.C.M. assessment, analyzed and interpreted the data and wrote the manuscript. G.P., H.A.M., I.P. performed the C.C.M. assessment. S.K., N.A., F.W. performed clinical examination and recruited the participants. R.Q., S.A.F. performed image analysis and wrote the manuscript. B.B., F.S. recruited the patients and collated the data. A.S. reviewed and revised the manuscript. R.A.M. designed and oversaw the study, secured the I.R.B. approval, wrote and revised the manuscript. All authors read and approved the final manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35298-3.
References
- 1.Putaala J, et al. Diabetes mellitus and ischemic stroke in the young: clinical features and long-term prognosis. Neurology. 2011;76:1831–1837. doi: 10.1212/WNL.0b013e31821cccc2. [DOI] [PubMed] [Google Scholar]
- 2.Baird TA, et al. The influence of diabetes mellitus and hyperglycaemia on stroke incidence and outcome. J Clin Neurosci. 2002;9:618–626. doi: 10.1054/jocn.2002.1081. [DOI] [PubMed] [Google Scholar]
- 3.Jia Q, et al. Diabetes and poor outcomes within 6 months after acute ischemic stroke: the China national stroke registry. Stroke. 2011;42:2758–2762. doi: 10.1161/STROKEAHA.111.621649. [DOI] [PubMed] [Google Scholar]
- 4.Kiyohara Y, Ueda K, Fujishima M. Smoking and cardiovascular disease in the general population in Japan. J Hypertens Suppl. 1990;8:S9–15. doi: 10.1097/00004872-199006002-00003. [DOI] [PubMed] [Google Scholar]
- 5.Shuaib A. Alteration of blood pressure regulation and cerebrovascular disorders in the elderly. Cerebrovasc Brain Metab Rev. 1992;4:329–345. [PubMed] [Google Scholar]
- 6.Heymann EP, Goldsmith D. Best approaches in the battle against Globesity? Learning lessons from our experience tackling HIV-AIDS and tobacco smoking. JRSM Short Reports. 2012;3:1–9. doi: 10.1258/shorts.2012.011159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajendran P, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flammer AJ, et al. The assessment of endothelial function. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aghamohammadzadeh R, et al. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol. 2013;62:128–135. doi: 10.1016/j.jacc.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik RA, et al. Effects of angiotensin type-1 receptor antagonism on small artery function in patients with Type 2 diabetes mellitus. Hypertension. 2005;45:264–269. doi: 10.1161/01.HYP.0000153305.50128.a1. [DOI] [PubMed] [Google Scholar]
- 11.Khavandi K, et al. Abnormal remodeling of subcutaneous small arteries is associated with early diastolic impairment in metabolic syndrome. J Am Heart Assoc. 2017;6:1–9. doi: 10.1161/JAHA.116.004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blum A, et al. Endothelial dysfunction and procoagulant activity in acute ischemic stroke. J Vasc Interv Neurol. 2012;5:33–39. [PMC free article] [PubMed] [Google Scholar]
- 13.Imam YZ, D’Souza A, Malik RA, Shuaib A. Secondary stroke prevention: improving diagnosis and management with newer technologies. Transl Stroke Res. 2016;7:458–477. doi: 10.1007/s12975-016-0494-2. [DOI] [PubMed] [Google Scholar]
- 14.Debette S, Markus H. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:1–9. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buyck J-F, et al. Cerebral white matter lesions are associated with the risk of stroke but not with other vascular events. Stroke. 2009;40:2327–2331. doi: 10.1161/STROKEAHA.109.548222. [DOI] [PubMed] [Google Scholar]
- 16.de Groot M, et al. Changes in normal-appearing white matter precede development of white matter lesions. Stroke. 2013;44:1037–1042. doi: 10.1161/STROKEAHA.112.680223. [DOI] [PubMed] [Google Scholar]
- 17.Nägele MP, et al. Retinal microvascular dysfunction in heart failure. Eur Heart J. 2017;39:47–56. doi: 10.1093/eurheartj/ehx565. [DOI] [PubMed] [Google Scholar]
- 18.Wu H-Q, et al. The association between retinal vasculature changes and stroke: a literature review and meta-analysis. Int J Ophthalmol. 2017;10:109–114. doi: 10.18240/ijo.2017.01.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuo Y, et al. Prediction factors of recurrent stroke among chinese adults using retinal vasculature characteristics. J Stroke Cerebrovasc Dis. 2017;26:679–685. doi: 10.1016/j.jstrokecerebrovasdis.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 20.He Z, et al. 3D map of the human corneal endothelial cell. Sci Rep. 2016;6:29047. doi: 10.1038/srep29047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sage H, Pritzl P, Bornstein P. Secretory phenotypes of endothelial cells in culture: comparison of aortic, venous, capillary, and corneal endothelium. Arterioscler Thromb Vasc Biol. 1981;1:427–442. doi: 10.1161/01.atv.1.6.427. [DOI] [PubMed] [Google Scholar]
- 22.Ramsby M, Kreutzer D. Fibrin induction of tissue plasminogen activator expression in corneal endothelial cells in vitro. Invest Ophthalmol Sci. 1993;34:3207–3219. [PubMed] [Google Scholar]
- 23.Fukushima M, Nakashima Y, Sueishi K. Thrombin enhances release of tissue plasminogen activator from bovine corneal endothelial cells. Invest Ophthalmol Sci. 1989;30:1576–1583. [PubMed] [Google Scholar]
- 24.El-Agamy A, Alsubaie S. Corneal endothelium and central corneal thickness changes in Type 2 diabetes mellitus. Clin Ophthalmol. 2017;11:481–486. doi: 10.2147/OPTH.S126217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anbar M, Ammar H, Mahmoud RA. Corneal endothelial morphology in children with type 1 diabetes. J Diabetes Res. 2016;2016:1–8. doi: 10.1155/2016/7319047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leelawongtawun W, Suphachearaphan W, Kampitak K, Leelawongtawun R. A comparative study of corneal endothelial structure between diabetes and non-diabetes. J Med Assoc Thai. 2015;98:484–488. [PubMed] [Google Scholar]
- 27.Alam U, et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PloS One. 2017;12:e0180175. doi: 10.1371/journal.pone.0180175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemp HI, et al. Use of corneal confocal microscopy to evaluate small nerve fibers in patients with human immunodeficiency virus. JAMA Ophthalmol. 2017;137:795–800. doi: 10.1001/jamaophthalmol.2017.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kass-Iliyya L, et al. Small fiber neuropathy in Parkinson’s disease: a clinical, pathological and corneal confocal microscopy study. Parkinsonism Relat Disord. 2015;21:1454–1460. doi: 10.1016/j.parkreldis.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petropoulos IN, et al. Corneal confocal microscopy: an imaging endpoint for axonal degeneration in multiple sclerosis. Invest Ophthalmol Sci. 2017;58:3677–3681. doi: 10.1167/iovs.17-22050. [DOI] [PubMed] [Google Scholar]
- 31.Bitirgen G, Akpinar Z, Malik RA, Ozkagnici A. Use of corneal confocal microscopy to detect corneal nerve Loss and increased dendritic cells in patients with multiple sclerosis. JAMA Ophthalmol. 2017;135:777–782. doi: 10.1001/jamaophthalmol.2017.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan A, et al. Corneal confocal microscopy detects corneal nerve damage in patients admitted with acute ischemic stroke. Stroke. 2017;48:3012–3018. doi: 10.1161/STROKEAHA.117.018289. [DOI] [PubMed] [Google Scholar]
- 33.Szalai E, et al. Early corneal cellular and nerve fiber pathology in young patients with Type 1 diabetes mellitus identified using corneal confocal microscopy. Invest Ophthalmol Sci. 2016;57:853–858. doi: 10.1167/iovs.15-18735. [DOI] [PubMed] [Google Scholar]
- 34.Bitirgen G, Ozkagnici A, Malik R, Kerimoglu H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with Type 2 diabetes mellitus. Diabet Med. 2014;31:431–438. doi: 10.1111/dme.12324. [DOI] [PubMed] [Google Scholar]
- 35.Davidson EP, Coppey LJ, Holmes A, Yorek MA. Changes in corneal innervation and sensitivity and acetylcholine-mediated vascular relaxation of the posterior ciliary artery in a Type 2 diabetic rat. Invest Ophthalmol Sci. 2012;53:1182–1187. doi: 10.1167/iovs.11-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen T. Is there an association between Fuchs’ endothelial dystrophy and cardiovascular disease? Graefes Arch Clin Exp Ophthalmol. 1984;221:239–240. doi: 10.1007/BF02134146. [DOI] [PubMed] [Google Scholar]
- 37.Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf. 2017;15:15–47. doi: 10.1016/j.jtos.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller RT, et al. In vivo confocal microscopy demonstrates bilateral loss of endothelial cells in unilateral herpes simplex keratitis. Invest Ophthalmol Sci. 2015;56:4899–4906. doi: 10.1167/iovs.15-16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kheirkhah A, Satitpitakul V, Hamrah P, Dana R. Patients with dry eye disease and low subbasal nerve density are at high risk for an accelerated corneal endothelial cell loss. Cornea. 2017;36:196–201. doi: 10.1097/ICO.0000000000001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonita R, Scragg R, Stewart A, Jackson R, Beaglehole R. Cigarette smoking and risk of premature stroke in men and women. Br Med J (Clin ResEd) 1986;293:6–8. doi: 10.1136/bmj.293.6538.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amarenco P, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 42.Letra, L. & Sena, C. Cerebrovascular disease: consequences of obesity-induced endothelial dysfunction in Obesity and Brain Function (eds Letra, L. & Seiça, R.) 163–189 (Springer International Publishing, 2017). [DOI] [PubMed]
- 43.Chung J-W, et al. Distinct roles of endothelial dysfunction and inflammation in intracranial atherosclerotic stroke. Eur J Neurol. 2017;77:211–219. doi: 10.1159/000460816. [DOI] [PubMed] [Google Scholar]
- 44.Omisore AD, Ayoola OO, Ibitoye BO, Fawale MB, Adetiloye VA. Sonographic evaluation of endothelial function in brachial arteries of adult stroke patients. J Ultrasound Med. 2017;36:345–351. doi: 10.7863/ultra.16.03100. [DOI] [PubMed] [Google Scholar]
- 45.Pathai S, et al. Corneal endothelial cells provide evidence of accelerated cellular senescence associated with HIV infection: a case-control study. PLoS One. 2013;8:e57422. doi: 10.1371/journal.pone.0057422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, et al. Is metabolic syndrome associated with the risk of recurrent stroke: a meta-analysis of cohort studies. J Stroke Cerebrovasc Dis. 2017;26:2700–2705. doi: 10.1016/j.jstrokecerebrovasdis.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Katzke VA, Sookthai D, Johnson T, Kühn T, Kaaks R. Blood lipids and lipoproteins in relation to incidence and mortality risks for CVD and cancer in the prospective EPIC–Heidelberg cohort. BMC Medicine. 2017;15:1–8. doi: 10.1186/s12916-017-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sacco RL, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kheirkhah A, Saboo US, Marmalidou A, Dana R. Overestimation of corneal endothelial cell density in smaller frame sizes in in vivo confocal microscopy. Cornea. 2016;35:363–369. doi: 10.1097/ICO.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 50.Al-Fahdawi S, et al. A fully automated cell segmentation and morphometric parameter system for quantifying corneal endothelial cell morphology. Comput Methods Programs Biomed. 2018;160:11–23. doi: 10.1016/j.cmpb.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Sharif MS, et al. An efficient intelligent analysis system for confocal corneal endothelium images. Comput Methods Programs Biomed. 2015;122:421–436. doi: 10.1016/j.cmpb.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Petropoulos IN, et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Invest Ophthalmol Sci. 2014;55:2071–2078. doi: 10.1167/iovs.13-13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.