Abstract
Deep venous thrombosis (DVT) is a common complication of orthopedic surgery. Genetic risk factors and high heritability carried a substantial risk of DVT. In this study, we aimed to investigate the potential association in the Han Chinese population between the polymorphisms of BDKRB2 and KNG1 and DVT after orthopedic surgery (DVTAOS). A total of 3,010 study subjects comprising 892 DVT cases and 2,118 controls were included in the study, and 39 single nucleotide polymorphisms (SNPs) in total (30 for BDKRB2 and 9 for KNG1) were chosen for genotyping. Two SNPs, rs710446 (OR = 1.27, P = 0.00016) and rs2069588 (OR = 1.29, P = 0.00056), were identified as significantly associated with DVTAOS. After adjusting for BMI, the significance of rs2069588 decreased (P = 0.0013). Haplotype analyses showed that an LD block containing rs2069588 significantly correlated with the DVTAOS risk. Moreover, bioinformatics analysis indicated that hsa-miR-758-5p and BDKRB2 formed miRNA/SNP target duplexes if the rs2069588 allele was in the T form, suggesting that rs2069588 may alter BDKRB2 expression by affecting hsa-miR-758-5p/single-nucleotide polymorphism target duplexes. Our results demonstrate additional evidence supporting that there is an important role for the KNG1 and BDKRB2 genes in the increased susceptibility of DVTAOS.
Introduction
Deep venous thrombosis (DVT) is a common disorder, in which is a blood clot occurs inside a vein; DVT has several risk factors, including acquired, inherited and mixed. Major surgery and orthopedic surgery, as acquired risk factors, carry a substantial risk of DVT. Clinically, 40% to 60% of patients acquired DVT after orthopedic surgery; 4% to 10% of those patients developed pulmonary embolism (PE), of which 5% die1. Therefore, identifying patients with a higher risk of DVT after orthopedic surgery (DVTAOS) is essential in guiding diagnosis and reducing the death rate. A growing body of literature has indicated that all strong, moderate, and weak genetic risk factors with high heritability carry a substantial risk of DVT2. Tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor-1 (PAI-1) are important biochemical constituents of the fibrinolytic system, which affects DVT3. This study found evidence that the polymorphisms in the fibrinolytic system might influence PAI-1 and t-PA3. With hundreds of mutations responsible for defective genes, deficiencies of antithrombin could lead to a high risk of DVT2.
A previous study has shown that Kng1-deficiency is associated with a decrease in thrombosis4. With the development of high-throughput DNA sequencing techniques, genome-wide association studies (GWASs) have provided supportive evidence for the polygenic nature of many complex diseases susceptibility5–11 and have identified some SNPs that contribute to the risk of DVT, such as Kininogen-1(KNG1)12. However, these results cannot explain the genetic portion of DVT. So far, the molecular mechanisms of DVT remain unknown. The KNG1 gene, which is found on human chromosome 3, also known as alpha-2-thiol proteinase inhibitor, encodes a high-molecular-weight kininogen (HMWK) and low-molecular-weight kininogen (LMWK). KNG1 is a constituent of the blood coagulation system, as is the HMWK protein. KNG1 is associated with the genetic factors of activated partial thromboplastin time (aPTT), which is a risk marker of DVT13. In addition, Hu had proven that variants of KNG1 genes potentiate the risk of thrombosis in ischemic stroke in the Chinese population14. The association between KNG1 and DVTAOS is largely unknown, so it is urgent to study this mechanism. In addition, the protected mechanism for the thrombosis of Bradykinin receptor B2 knockout (BKB2R−/−) in mice via the plasma kallikrein/kinin and renin angiotensin systems also provides new genic factor insights for DVT15. The BDKRB2 gene in humans encodes a G-protein coupled receptor for bradykinin called BKB2R; this receptor elicits many responses, including vasodilation, edema, smooth muscle spasm and pain fiber stimulation. The disfunction of BDKRB2 plays an essential role in some physiological and pathological processes, such as kidney development16, the inflammatory process in osteoarthritis17 and hepatocellular carcinoma progression18. BK, which is antithrombotic for endothelium binding to BKB2R in the intravascular system, promotes blood flow through NO, prostacyclin formation and tPA liberation19,20. Interestingly, BDKRB2 knockout mice are protected from thrombosis by increased nitric oxide and prostacyclin20. Considering the potential relationship between BDKRB2 and thrombosis, the underlying association and mechanism between BDKRB2 variants and DVT are worth investigation.
Considering the genetic heterogeneity of disease occurrence and the different etiology of DVT, the exploration of possible associations between KNG1/BDKRB2 and DVT among the Han Chinese population may shed light on the underlying mechanisms of DVT. Above all, we reasoned that alleles of KNG1 and BDKRB2 might be associated with an increased risk of DVTAOS. To test the above hypothesis, we aimed to investigate whether common variants in BDKRB2 and KNG1 have interactive effects with DVTAOS. Providing optimal thromboprophylaxis to a patient who is at DVT risk will ensure the best reductions in DVT-related morbidity and mortality from a genetic perspective via this research.
Methods
Study subjects
In the current study, we recruited 3,010 subjects undergoing knee or hip orthopedic surgery at Luoyang Orthopedic Hospital of Henan Province (Luoyang, China) from August 2012 to July 2017. Of these, 892 were diagnosed with DVTAOS and were designated as a case group; and 2,118 had no typical DVT symptoms or signs and were designated as a control group. Each subject received anticoagulants routinely within 5 hours of surgery. Two independent sonographers performed preliminary screening using lower-extremity color-Doppler ultrasound, and all subjects were assessed for DVT postoperatively within 6 days. When it was difficult to obtain a definitive diagnosis, venography was used to confirm the diagnosis. Subjects with a history of venous thromboembolism or clinical evidence of venous thromboembolism were excluded from the study. A total number of 211 study subjects were excluded for this criterion. All subjects were unrelated Han Chinese individuals. The clinical data and characteristics of all the subjects were measured or recorded and are summarized in Table 1. Both body mass index (BMI) and hyperlipidemia status showed significant difference between the DVTAOS case group and the controls. Written informed consent was obtained from all subjects. This study was performed in accordance with the ethical guidelines of the Declaration of Helsinki (version 2002) and was approved by the Medical Ethics Committee of Luoyang Orthopedic Hospital of Henan Province.
Table 1.
Characteristic information for the 3,010 study subjects.
| Case (N = 892) | Controls (N = 2,118) | Statistics | P | |
|---|---|---|---|---|
| Age | 59.3 ± 6.3 | 58.9 ± 8.1 | T = 1.2 | 0.23 |
| BMI | 25.9 ± 1.6 | 25.5 ± 1.6 | T = 6.3 | 4.93 × 10−10 |
| Gender (%) | ||||
| Male | 405 (45) | 970 (46) | ||
| Female | 487 (55) | 1148 (54) | χ2 = 0.03 | 0.87 |
| Site (%) | ||||
| hip | 519 (58) | 1224 (58) | ||
| knee | 373 (42) | 894 (42) | χ2 = 0.03 | 0.87 |
| Hypertension (%) | ||||
| Yes | 253 (28) | 585 (28) | ||
| No | 639 (72) | 1533 (72) | χ2 = 0.14 | 0.71 |
| Diabetes (%) | ||||
| Yes | 43 (5) | 95 (4) | ||
| No | 849 (95) | 2023 (96) | χ2 = 0.09 | 0.76 |
| Hyperlipidemia (%) | ||||
| Yes | 262 (30) | 523 (25) | ||
| No | 630 (70) | 1595 (75) | χ2 = 6.89 | 0.009 |
| Smoking (%) | ||||
| Yes | 156 (17) | 351 (17) | ||
| No | 736 (83) | 1767 (83) | χ2 = 0.31 | 0.58 |
| Alcohol (%) | ||||
| Yes | 128 (14) | 281 (13) | ||
| No | 764 (86) | 1837 (87) | χ2 = 0.54 | 0.46 |
SNP selection & Genotyping
Tag SNPs were searched in the study. Tag SNPs of BDKRB2 and KNG1 with minor allele frequency (MAF) >= 0.1 based on 1000 genome CHB data were selected for genotyping, and the r2 criterion used for tagging was 0.6. In total, 39 SNPs (30 for BDKRB2 and 9 for KNG1) were chosen (Supplemental Table S1). Genomic DNA was isolated from the peripheral blood using a Tiangen DNA extraction kit (Tiangen Biotech Co. Ltd, Beijing, China) according to the manufacturer’s protocol. SNP genotyping was performed using a Sequenom MassARRAY platform with iPLEX GOLD chemistry (Sequenom, San Diego, CA, USA) based on the manufacturer’s protocols. The results were processed using Sequenom Typer 4.0 software21, and the genotype data were generated from the samples. Genotyping was conducted by laboratory personnel blinded to case-control status, and the genotyping results, data entry and statistical analyses were independently reviewed by two authors. We randomly reperformed the analysis on 5% of the sample, with a concordance of 100%.
Statistical analyses
The genetic association between our selected tag SNPs and DVTAOS status were investigated in a three-step procedure. (1) We fit simple logistic models for each SNP and screened out the significant hits; (2) we examined the correlations between these significant SNPs and the clinical variables which were associated with DVTAOS status as shown in Table 1 (BMI and hyperlipidemia status) to obtain the clinical variables which could serve as potential mediators, and (3) we performed mediation analysis to examine the direct effect of targeted SNPs after adjusting those relevant clinical variables. In addition, we also constructed the linkage disequilibrium (LD) blocks for our genetic data and performed haplotype-based association analyses. Bonferroni corrections were applied for multiple comparisons. Plink22 was utilized to perform the genetic association analyses. Haploview23 was used to make LD plots. Genotypes of SNPs were coded in the additive mode in all statistical analyses.
Expression quantitative trait loci (eQTL) and bioinformatics analyses
We investigated the eQTL pattern of the significant SNPs using data extracted from the GTEx database24. The potential functional significance of the significant SNPs was examined by SIFT (for nonsynonymous SNPs)25 and the PolymiRTS Database (for SNPs located at regulatory region)26.
Results
Significant SNPs identified from simple models
Two SNPs, rs710446 (OR = 1.27, P = 0.00016) and rs2069588 (OR = 1.29, P = 0.00056), were identified as significantly associated with DVTAOS status through simple logistic models (Table 2). SNP rs710446 was a nonsynonymous change of KNG1, which altered the amino acid from IIe to Thr. SNP rs2069588 was located at the 3′ untranslated region (UTR) of the gene BDKRB2.
Table 2.
Genetic associations between single polymorphisms and DVT after orthopedic surgery.
| GENE | CHR | SNP | POS | A1 | MAF | OR | SE | L95 | U95 | STAT | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KNG1 | 3 | rs710446 | 186459927 | C | 0.27 | 1.27 | 0.06 | 1.12 | 1.43 | 3.77 | 0.00016 |
| BDKRB2 | 14 | rs2069588 | 96708667 | T | 0.17 | 1.29 | 0.07 | 1.12 | 1.48 | 3.45 | 0.00056 |
CHR: chromosome; POS:position;A1:tested allele; MAF: minor allele frequency; SE:standard error; L95: lower bound of 95% confidence interval; U95: upper bound of 95% confidence interval; STAT: statistics.
Threshold for P values used here was 0.05/39≈0.001.
Mediation analyses for SNP rs2069588
SNP rs2069588 was found to be significantly correlated with BMI (P = 0.02, Supplemental Table S2). Combined with the result that BMI was strongly associated with the disease status of DVTAOS (P = 4.93 × 10−10, Table 1), it might be a potential mediator between rs2069588 and DVTAOS. We performed a mediation analysis to examine the effect of SNP rs2069588 on DVTAOS with or without BMI included as a covariate. After adjusting for BMI, the significance of rs2069588 decreased slightly to P = 0.0013 (Table 3). The significance of BMI also decreased from 10−10 to the 10−9 level, which was obtained in a model with BMI and DVTAOS status only.
Table 3.
Mediation analysis of rs2069588 and DVT with or without BMI adjusted.
| Variables | Model1* | Model2** | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | SE | STAT | P | OR | SE | STAT | P | |
| rs2069588 | 1.29 | 0.07 | 3.45 | 5.59 × 10−4 | 1.27 | 0.07 | 3.21 | 0.0013 |
| BMI | — | — | — | — | 1.16 | 0.02 | 5.96 | 2.47 × 10−9 |
*Univariate model with genotypes of rs2069588 only.
**Multivariate model including both genotypes of rs2069588 and BMI.
Haplotype-based association analyses
A total number of 10 2-SNP LD blocks were constructed for both KNG1 and BDKRB2 (Supplemental Figure S1 and S2). One LD block, rs2069583-rs2069588 from BDKRB2, was significantly associated with DVTAOS status (P = 0.0001, Supplemental Table S3). Since rs2069588 was also identified as significant in single marker-based analyses, the significance of the LD block of rs2069583-rs2069588 could be considered a replicate for the results of the single marker-based analyses.
Significant eQTL and functional significance for rs710446 and rs2069588
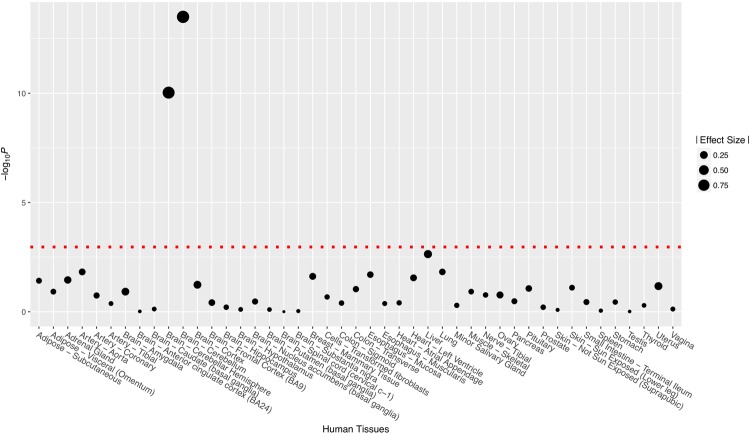
Significant eQTL signals (Fig. 1) were identified for rs2069588 on BDKRB2 from tissue of the cerebellum (NES = −0.95, P = 3.20 × 10−14) and cerebellar hemisphere (NES = −0.89, P = 9.40 × 10−11). No significant eQTL signal was identified for rs710446 on KNG1. The full results of eQTL analyses are summarized in Supplemental Table S4 and S5. SIFT predicted that rs710446 had very limited functional consequences on the protein encoded by KNG1 (rated “tolerated” for all changes), although it altered one amino acid. According to the genomic database from the University of California, Santa Cruz (http://genome.ucsc.edu/), rs2069588 acts as a 3′ untranslated region (3′ UTR) variant, which may affect microRNA binding. We used a free online tool (http://bioinfo.life.hust.edu.cn) to examine the predicted target gain or loss in microRNA binding and found that the C allele of rs2069588 causes a loss of microRNA/SNP target duplexes of hsa-miR-758-5p and BDKRB2 (Fig. 2). On the other hand, T allele of rs2069588 is a stable microRNA binding site at the 3′ UTR region of BDKRB2.
Figure 1.
eQTL pattern for rs2069588 on the gene BDKRB2 based on data extracted from the GTEx database. Threshold of the P values was 0.05/45≈0.001.
Figure 2.

The allele of rs2069588 disrupts miRNA/SNP target duplexes. Hsa-mir-758-5p and BDKRB2 produce miRNA/SNP target duplexes if the rs3025039 allele is T.
Discussion
In this study, we investigated the genetic association between DVTAOS and two loci: KNG1 and BDKRB2. Significant evidence for both loci was found to establish their association with DVTAOS. Early studies based on European populations have provided supportive evidence for the association between DVT and rs71044612. Our results on rs710446 successfully replicated these previous findings in the Han Chinese population despite focusing on DVTAOS, which is a postoperative complication of DVT. The direction of the effect and the OR in the previous study were similar to ours (OR = 1.19 to OR = 1.27). On the other hand, we identified an SNP, rs2069588, located at the 3′UTR, conferring risk of DVTAOS based on the Han Chinese population. To the best of our knowledge, our study is the first to establish the genetic association between the gene BDKRB2 and DVTAOS.
In addition to exploring the potential genetic effects in the simple model, we also noticed some potential mediators in the effects between SNPs and DVTAOS. In our study, we statistically proved that BMI might partly mediate the effect of rs2069588 to DVTAOS. From the results of our mediation analyses, we can see that when both BMI and rs2069588 were included in the logistic model, the effect size and significance of rs2069588 were reduced. Combined with the other results, which showed that BMI was significantly associated with the disease status of DVTAOS and genotypes of rs2069588, our findings indicate that part of the effect of rs2069588 on DVTAOS was mediated through BMI. In addition, BMI cannot be a confounder because the direction of effect between BMI and rs2069588 should be from rs2069588 to BMI and was unlikely to be reversed. Interestingly, it seems that our findings on the role of the mediator of BMI were not only based on statistical analyses of our data but could be supported by previous publications. BMI and obesity have long been known as risk factors for DVT27,28. On the other hand, although no evidence has been published to establish a direct connection between BDKRB2 and obesity or BMI, BDKRB2 was reported to be significantly associated with other human metabolism-related traits, including body fat modulation29 and diabetes30. In addition, interaction effects have also been identified between the SNPs of BDKRB2 and physical activity and blood pressure29. These previous findings indicated that the effect between rs2069588 and BMI might also be mediated by some other underlying factors in our study. However, the mediation effect of BMI was not identified for rs710446.
Our eQTL analyses based on the data extracted from the GTEx database revealed some significant signals for rs2069588 in BDKRB2. However, it is unclear why these eQTL signals were identified in human brain tissues. We know that eQTLs are very common in the human genome and are far more common than disease association signals. A great many SNPs have “significant” eQTL data in GTEx simply because they represent points along an eQTL association peak. Therefore, we need to be careful to interpret the results of eQTL analyses and more experimental studies are still needed in the future.
The SNP rs2069588 of BDKRB2 was predicted to be a miR-758-5p binding site and its C allele may cause a loss of the original miR-758-5p binding site. We hypothesize that its T allele as a stable miR-758-5p binding site in the 3′ UTR region of BDKRB2, and the T allele down regulates the gene expression of BDKRB2 by binding miR-758-5p compared to the C allele. It is interesting to note that the T allele of rs2069588 is the risk allele for DVTAOS and increased the risk of DVTAOS by approximately 30% in our data. Increasing evidence indicates that bradykinin plays critical roles in coagulation and fibrinolysis15,31. Previous studies revealed that by binding to the constitutive bradykinin B2 receptor in the intravascular compartment, bradykinin promotes prostacyclin and plasmin formation and tissue plasminogen activator (t-PA) liberation20, which can inhibit thrombosis. Evidence indicated that bradykinin binding to a-thrombin would substantially reduce the fibrinogen to fibrin conversion and inhibit clot formation32. Moreover, exogenous bradykinin attenuated deep venous thrombosis via reduced tissue factor (TF) expression at the mRNA and protein level in the mouse model33. Hence, the reduction of bradykinin B2 receptor expression may result in upregulation of TF factor expression, and initiate the physiological coagulation cascade, eventually leading to DVT.
A potential limitation of this study is the potential population stratification (PS) which might cause false positive results as a confounder. As ours was a candidate gene-based study, we cannot perform some statistical methods, such as principal component analysis or genomic control, to properly control PS. However, on the other hand, in order to restrict population stratification we have applied some criteria, such as recruiting samples by restricting the subjects with a stable living region34–36, during our sample collection process to reduce the genetic heterogeneity of our study subjects37,38. Another limitation is that the strategy of screening candidate SNPs was not rigorous enough to discover all potential functional SNPs, including rare variants. It is possible that these variants may contribute to DVTAOS risk in an unknown manner or in linkage disequilibrium with other undetected variants that confer a risk for DVTAOS. In addition, excluding patients with a history or clinical evidence of venous thromboembolism might cause a problem in generalization of the results of this study. Finally, as is known, the onset and development of DVTAOS can involve more genes in a complicated manner, which might be a promising direction for enrolling more related genes in future studies. In particular, future functional experiments would be valuable to understand the roles of hsa-miR-758-5p and the T allele of rs2069588 in DVTAOS. Thus, our results should be considered preliminary, and follow-up studies will be required in the future. More molecular biology experiments and model animal based studies are still needed in future to unravel the underlying biomedical mechanisms of rs710446 and its effect on DVTAOS. The aPTT assay should be performed to examine whether this SNP affects coagulation activity. In addition, further functional studies should be conducted to investigate whether this nonsynonymous SNP is more susceptible to cleavage by activated Factor XIIa or plasma kallikrein.
In sum, in this study, we gained evidence of the genetic association between DVTAOS and two loci: KNG1 and BDKRB2 in Han Chinese individuals. Further analyses have revealed the effects of BDKRB2 on DVT, which might be partly mediated through BMI or some other BMI-related underlying factors. In the future, a comprehensive study of more representative SNPs, different races, larger sample sizes, and functional experiments would be desired to confirm our findings and understand the effects of KNG1 and BDKRB2 on the risk of DVTAOS.
Electronic supplementary material
Acknowledgements
This research was totally supported by Project of Luoyang Science and Technology Development Plan (No: 1503004A) and Henan Traditional Chinese Medicine Research (No: 2014ZY02003; No: 2017ZY1005). The funding sources had no role in the design of this study, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the paper for publication.
Author Contributions
Authors Ye J. and Qing Z. conceived and designed the study. Wang Q. carried out candidate SNPs selection and statistical analyses. Cheng G., Wang X., and Chen K. conducted subject screening. Cheng G., Wang D., and Yang Y. contributed to the collection and preparation of control DNA samples. Wang Q. wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qingfeng Wang and Guoping Cheng contributed equally.
Contributor Information
Jiumin Ye, Email: anejimye@163.com.
Zhong Qing, Email: drqizhg@163.com.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34868-9.
References
- 1.Dixon J, et al. Venous thromboembolism rates in patients undergoing major hip and knee joint surgery at Waitemata District Health Board: a retrospective audit. Intern Med J. 2015;45:416–422. doi: 10.1111/imj.12702. [DOI] [PubMed] [Google Scholar]
- 2.Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. J Thromb Haemost. 2009;7:301–304. doi: 10.1111/j.1538-7836.2009.03394.x. [DOI] [PubMed] [Google Scholar]
- 3.Asselbergs FW, et al. The effects of polymorphisms in genes from the renin-angiotensin, bradykinin, and fibrinolytic systems on plasma t-PA and PAI-1 levels are dependent on environmental context. Hum Genet. 2007;122:275–281. doi: 10.1007/s00439-007-0400-9. [DOI] [PubMed] [Google Scholar]
- 4.Merkulov S, et al. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood. 2008;111(3):1274–1281. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan F, et al. Association of PDE4B polymorphisms and schizophrenia in Northwestern Han Chinese. Hum Genet. 2012;131:1047–1056. doi: 10.1007/s00439-011-1120-8. [DOI] [PubMed] [Google Scholar]
- 6.Guan F, et al. MIR137 gene and target gene CACNA1C of miR-137 contribute to schizophrenia susceptibility in Han Chinese. Schizophr Res. 2014;152:97–104. doi: 10.1016/j.schres.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Guan F, Lin H, Li L, Fu D. Genetic analysis of common variants in the HDAC2 gene with schizophrenia susceptibility in Han Chinese. Journal of human genetics. 2015;60:479–484. doi: 10.1038/jhg.2015.66. [DOI] [PubMed] [Google Scholar]
- 8.Guan F, et al. Evaluation of genetic susceptibility of common variants in CACNA1D with schizophrenia in Han Chinese. Scientific reports. 2015;5:12935. doi: 10.1038/srep12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, et al. Common variants in SLC1A2 and schizophrenia: Association and cognitive function in patients with schizophrenia and healthy individuals. Schizophr Res. 2015;169:128–134. doi: 10.1016/j.schres.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Guan F, et al. Evaluation of association of common variants in HTR1A and HTR5A with schizophrenia and executive function. Scientific reports. 2016;6:38048. doi: 10.1038/srep38048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan F, et al. Evaluation of voltage-dependent calcium channel γ gene families identified several novel potential susceptible genes to schizophrenia. Scientific reports. 2016;6:24914. doi: 10.1038/srep24914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morange PE, et al. KNG1 Ile581Thr and susceptibility to venous thrombosis. Blood. 2011;117:3692–3694. doi: 10.1182/blood-2010-11-319053. [DOI] [PubMed] [Google Scholar]
- 13.Weng LC, et al. A genetic association study of activated partial thromboplastin time in European Americans and African Americans: the ARIC Study. Human molecular genetics. 2015;24:2401–2408. doi: 10.1093/hmg/ddu732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu ZY, et al. Variants in the Atherogenic ALOX5AP, THBD, and KNG1 Genes Potentiate the Risk of Ischemic Stroke via a Genetic Main Effect and Epistatic Interactions in a Chinese Population. J Stroke Cerebrovasc. 2015;24:2060–2068. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Shariat-Madar Z, et al. Bradykinin B2 receptor knockout mice are protected from thrombosis by increased nitric oxide and prostacyclin. Blood. 2006;108(1):192–199. doi: 10.1182/blood-2006-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Dahr SS, Saifudeen Z. Interactions between BdkrB2 and p53 genes in the developing kidney. Biol Chem. 2013;394:347–351. doi: 10.1515/hsz-2012-0281. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, et al. BDKRB2 + 9/−9 bp polymorphisms influence BDKRB2 expression levels and NO production in knee osteoarthritis. Experimental biology and medicine. 2017;242:422–428. doi: 10.1177/1535370215625471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, et al. NMI promotes hepatocellular carcinoma progression via BDKRB2 and MAPK/ERK pathway. Oncotarget. 2017;8:12174–12185. doi: 10.18632/oncotarget.14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith D, Gilbert M, Owen WG. Tissue plasminogen activator release in vivo in response to vasoactive agents. Blood. 1985;66:835–839. [PubMed] [Google Scholar]
- 20.Schmaier AH. The kallikrein-kinin and the renin-angiotensin systems have a multilayered interaction. Am J Physiol-Reg I. 2003;285(1):R1–R13. doi: 10.1152/ajpcell.00554.2002. [DOI] [PubMed] [Google Scholar]
- 21.Guan F, et al. Association study of a new schizophrenia susceptibility locus of 10q24.32-33 in a Han Chinese population. Schizophr Res. 2012;138:63–68. doi: 10.1016/j.schres.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience4 (2015). [DOI] [PMC free article] [PubMed]
- 23.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 24.Lonsdale J, et al. The Genotype-Tissue Expression (GTEx) project. Nature genetics. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziebarth JD, Bhattacharya A, Chen A, Cui Y. PolymiRTS Database 2.0: linking polymorphisms in microRNA target sites with human diseases and complex traits. Nucleic Acids Res. 2012;40:D216–D221. doi: 10.1093/nar/gkr1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkin L, et al. Body Mass Index, Surgery, and Risk of Venous Thromboembolism in Middle-Aged Women A Cohort Study. Circulation. 2012;125:1897–1904. doi: 10.1161/CIRCULATIONAHA.111.063354. [DOI] [PubMed] [Google Scholar]
- 28.Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: A review. Open journal of preventive medicine. 2012;2:499–509. doi: 10.4236/ojpm.2012.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcadenti A. ADRB2, ADRB3, BDKRB2 and MTNR1B Genes Related to Body fat Modulation and Its Interaction with Physical Activity and Blood Pressure. Open Journal of Endocrine & Metabolic Diseases. 2015;5:88–97. doi: 10.4236/ojemd.2015.57012. [DOI] [Google Scholar]
- 30.Alvim RD, et al. BDKRB2 +9/−9 Polymorphism Is Associated with Higher Risk for Diabetes Mellitus in the Brazilian General Population. Exp Diabetes Res. 2012;2012:480251. doi: 10.1155/2012/480251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehringer WD, et al. Bradykinin Antagonizes the Effects of α-Thrombin[J] Inflammation. 1997;21(3):279. doi: 10.1023/A:1027345832138. [DOI] [PubMed] [Google Scholar]
- 32.Hasan AA, Amenta S, Schmaier AH. Bradykinin and its metabolite, Arg-Pro-Pro-Gly-Phe, are selective inhibitors of alpha-thrombin-induced platelet activation. Circulation. 1996;94(3):517–528. doi: 10.1161/01.CIR.94.3.517. [DOI] [PubMed] [Google Scholar]
- 33.Dong R, et al. Exogenous Bradykinin Inhibits Tissue Factor Induction and Deep Vein Thrombosis via Activating the eNOS/Phosphoinositide 3-Kinase/Akt Signaling Pathway. Cellular Physiology & Biochemistry. 2015;37(4):1592–1606. doi: 10.1159/000438526. [DOI] [PubMed] [Google Scholar]
- 34.Guan F, et al. A population-based association study of 2q32.3 and 8q21.3 loci with schizophrenia in Han Chinese. Journal of psychiatric research. 2013;47:712–717. doi: 10.1016/j.jpsychires.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, et al. 4q22.1 contributes to bone mineral density and osteoporosis susceptibility in postmenopausal women of Chinese Han population. PloS one. 2013;8:e80165. doi: 10.1371/journal.pone.0080165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan F, et al. Two-stage association study to identify the genetic susceptibility of a novel common variant of rs2075290 in ZPR1 to type 2diabetes. Scientific reports. 2016;6:29586. doi: 10.1038/srep29586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guan F, et al. Two-stage replication of previous genome-wide association studies of AS3MT-CNNM2-NT5C2 gene cluster region in a large schizophrenia case-control sample from Han Chinese population. Schizophr Res. 2016;176:125–130. doi: 10.1016/j.schres.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Jia X, et al. Two-stage additional evidence support association of common variants in the HDAC3 with the increasing risk of schizophrenia susceptibility. American journal of medical genetics. Part B, Neuropsychiatric genetics. 2016;171:1105–1111. doi: 10.1002/ajmg.b.32491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.