Abstract
The brain–gut–microbiota axis is increasingly viewed as a novel paradigm in neuroscience with the capacity to generate innovative therapies for patients with psychiatric illnesses. Psychobiotics, defined as live bacteria, which when ingested in adequate amounts, confer mental health benefits, are increasingly of interest, as preclinical trials continue to show promising results. Particularly in stress‐related, anxiety and depressive disorders, there is potential for psychobiotics to deliver new therapies. The question of which microbes may prove to be the most promising psychobiotic in delivering such therapies at a clinical level is of great importance. Here we look at the characteristics of psychobiotics, in an attempt to present an outline from which the identification of potential new psychobiotics may be possible.
Linked Articles
This article is part of a themed section on When Pharmacology Meets the Microbiome: New Targets for Therapeutics? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.24/issuetoc
Abbreviations
- B‐GOS
Bimuno galacto‐oligosaccharides
- CRP
C‐reactive protein
- FMT
faecal microbiota transplant
- FOS
fructo‐oligosaccharides
- GOS
galacto‐oligosaccharides
- HMOs
human milk oligosaccharides
- HPA
hypothalamic–pituitary–adrenal axis
- IBD
inflammatory bowel disease
- MAMPs
microbe‐associated molecular patterns
- SCFAs
short‐chain fatty acids
- TLR
toll‐like receptor
Gut–brain–microbiota axis
The brain–gut–microbiota axis consists of several key components, the CNS, the neuroendocrine system, the neuroimmune system and most importantly the gut microbiota. The gut microbiome is composed of all microorganisms and their genomes, in the intestinal space. Bacterial concentrations in the gut range from 101–103 g‐1 in the upper intestines to 1011–1012 g‐1 in the colon (O'Hara and Shanahan, 2006). The complex interaction between these components operates in a bidirectional communication network between the brain and the gut. Evidence continues to demonstrate that the gut microbiota has a significant impact on brain function (Stilling et al., 2014). Many studies have now demonstrated that the gut–brain–microbiota axis contributes to the regulation of brain physiology and ultimately behaviour. Various signalling pathways have been suggested, all of which demonstrate the link between brain modulation, behaviour and the gut microbiome (Cryan and Dinan, 2015).
The term probiotic was first introduced by Metchnikoff in 1908. Probiotics today are defined as live organisms that when ingested in adequate amounts, exert a health benefit (Dinan and Quigley, 2011). However, there is an ongoing need to revise and refine this definition as more research is conducted in this area. For example, it has been demonstrated that even dead probiotic microorganisms can induce immune reactions when ingested in adequate amounts (Dinan et al., 2013). Specific probiotics appear to have benefits in particular disease states and disorders.
Prebiotics are indigestible food material, for example, oligosaccharides, with selective fermentation in the colon, which affects the microbiome by inducing growth, activity or both, of one or several bacteria in the microbiome (Roberfroid et al., 2010). Prebiotics are of interest as several studies have confirmed certain prebiotics can alter the gut microbiota to reduce low‐grade inflammation (Bindels et al., 2015). They can also inhibit the growth of pathogenic bacteria. Synbiotics are described as ‘mixtures of probiotics and prebiotics that beneficially affect the host by improving the survival and implantation of live microbial dietary supplements in the gastrointestinal tract, by selectively stimulating the growth and/or by activating the metabolism of one or a limited number of health‐promoting bacteria, thus improving host welfare’ (Roberfroid et al., 2010). Probiotic preparations, containing multiple strains of probiotics, have also shown promise in disorders such as ulcerative colitis (Wasilewski et al., 2015). These ‘polybiotic’ preparations may prove of significant interest in the future development of psychobiotics.
Since the term psychobiotics was first introduced in 2012 and defined as ‘a live organism that, when ingested in adequate amounts, produces a health benefit in patients suffering from psychiatric illness’ (Dinan et al., 2013), the search for new and novel therapeutics in this area continues (Figure 1). The term psychobiotics has also been expanded to include prebiotics (Sarkar et al., 2016) and may feasibly confer benefits in both the patient population and the at risk population. In preclinical studies demonstrating the effects of the gut microbiome on neuroimmune, neural and hormonal pathways, the suggestion is that the gut microbiota can be modulated to treat neuropsychiatric disorders, or at the very least offer adjunctive treatment options for these disorders (Cryan and Dinan, 2015; Kelly et al., 2016b). Although much has been made of stress‐related psychiatric disorders, such as depression and anxiety, as the exploration of the complex and not fully discovered relationship between the brain–gut–microbiota axis continues, there is enormous scope for psychobiotics as therapeutics in patients suffering from a wide variety of psychiatric illnesses.
Figure 1.
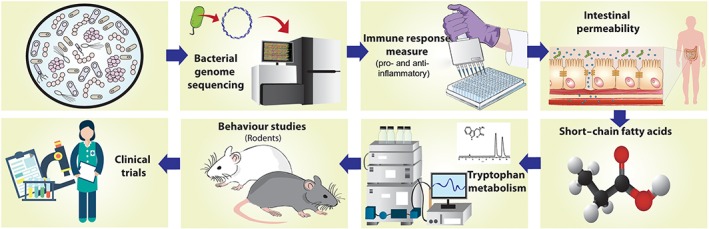
The search for psychobiotics. This illustrates the potential pathway for psychobiotic identification. The first step in the process is genome sequencing and comparison with strains known to have psychobiotic potential. Determining the potential to generate short‐chain fatty acids (SCFAs) and tryptophan is a fundamental step in the process, as is the ability to demonstrate anti‐inflammatory activity and have an effect on the intestinal barrier function. Behavioural studies in animals precede any human intervention studies.
In the search for psychobiotics, previous studies have focussed on strains of bacteria secreting http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1067 tryptophan and short‐chain fatty acids (SCFAs) or impacting the hypothalamic–pituitary–adrenal axis (HPA) and the inflammatory response pathway (Jokela et al., 2014). All of these parameters have shown anomalies in stress and especially in depression and anxiety disorders.
How do we identify a beneficial bacterium for neuropsychiatry treatment? How do we set out to discover potential new psychobiotics? In this review we discuss the attributes of known psychobiotics and the features, which provide benefits for hosts. We discuss how these benefits are known to affect the brain–gut–microbiota axis. If new putative psychobiotics are identified, how can we know which might work and allow us to focus only on trials most likely to succeed? Here we propose a strategy for the identification of novel psychobiotics.
Brain–gut–microbiota axis abnormalities in depression and related disorders
Depression is an intricate mood disorder affecting 121 million people worldwide. The WHO predicts an increase in depressive disorders second only to cardiovascular disease by 2020 in terms of total disease burden worldwide. Depression is often reoccurring, frequently has comorbidity with anxiety and is associated with significant disability worldwide (Kelly et al., 2016b). Depression results in a dysregulation of neuroendocrine, neuroimmune, neurotransmitter and endocrine functions (Jokela et al., 2014). A feature of depression, which is repeatedly demonstrated, is the low‐grade inflammatory response observed in this patient population (Kelly et al., 2016a). Specific pro‐inflammatory biomarkers have been identified as being of significance in depression including raised http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5073 and C‐reactive protein (CRP) (O'Brien et al., 2004). Such is the impact of certain pro‐inflammatory cytokines such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4955 that an injection of this cytokine is known to induce a depressive episode (McNutt et al., 2012). Stress can influence the development of the intestinal barrier and has been linked with increased permeability of the gut (Moussaoui et al., 2014). Kelly et al. (2016a) have recently shown a significant reduction in microbiota richness and diversity in those suffering with depression. Furthermore, transferring the altered gut microbiota to germ‐free rats induced a depressed phenotype in these rats. This study suggests that the gut microbiota may play a causal role in the pathophysiology of depression (Figure 2).
Figure 2.
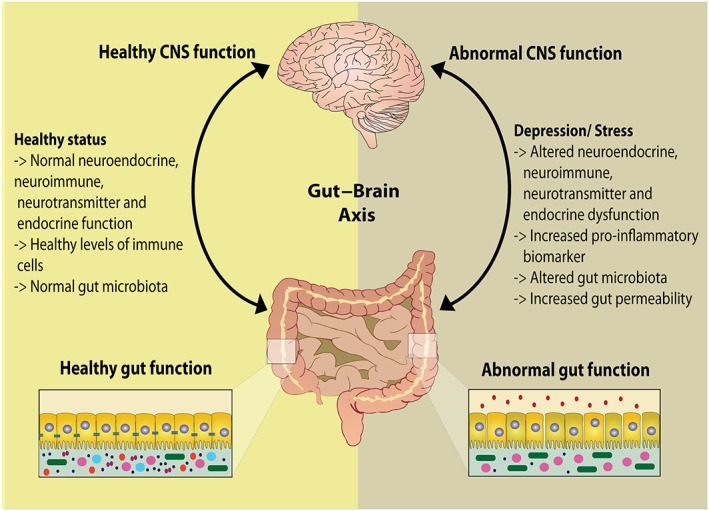
The gut–brain axis. Comparison between a healthy gut–brain system and an abnormal system. In negative affective states such as stress, anxiety or depression, the expression of pro‐inflammatory markers increases, gut microbiota and gut permeability are altered and the gut–brain axis is in a state of dysbiosis.
Genomic identification of psychobiotics
The most probable method with which to quickly and efficiently identify potential new psychobiotics is to fully characterize and analyse individual bacteria currently known to have beneficial effects. Individual bacterial strains with various probiotic properties should be assessed to unveil the genetic description of those with beneficial phenotypes. This has begun in earnest with several recent studies describing whole genomes of various probiotics (Arnold et al., 2017). Mapping of the entire gut microbiome has revealed most genes to be bacterial in origin (Qin et al., 2010). Metagenomic sequencing approaches to evaluate the microbiome are useful; however, these should be enhanced with proteomic and metabolomic analyses to determine which microbial genes and proteins are expressed in various conditions (Tremaroli and Bäckhed, 2012). Such inter‐omic analyses allows for the investigation and characterization of the gut microbiota ecosystem. Work to date has unveiled various genetic traits, including horizontal gene transfer in some strains of Lactobacillus (Douillard et al., 2013). Bile resistance and anti‐microbial activity have all been identified in studies in the microbiota (Siezen and van Hylckama Vlieg, 2011). Further characterization will enable a genetic signature to be built in order to identify the most appropriate psychobiotics for those suffering with psychiatric conditions. Such an approach should hasten discovery and help eliminate unsuitable microbes.
Studies have highlighted the relationship between the gut microbiome and the mucosal proteome (Presley et al., 2012), indicating that the bacterial composition of the microbiome may alter the mucosal proteome. McHardy et al. (2013) demonstrated the importance of considering the proteome, metabolome and the genome concurrently. The inter‐omic play between the microbiome and the mucosa is of upmost importance and should not be discarded when considering the complexities of the gut–brain axis and the potential for probiotics to alter the axis in various ways. Noecker et al. (2016) presented a framework to take into account all ‘omic’ interactions, in an attempt to evaluate the microbiome at a multi‐omic, multisystem level, rather than evaluating all aspects individually. This framework may permit a better understanding of all factors affecting the microbiome and the complex multidimensional interactions at play. It is likely that the genomics of both the host, the composites of the microbiome and the proteomic and metabolomic composition will also have a significant role in furthering our understanding of disease states.
Anti‐inflammatory action and immune response and its role in psychobiotic identification
The microbiome interacts with the gut epithelial surface to trigger immune responses, and in early life, plays a key role in shaping the neuroimmune evolution (O'Hara and Shanahan, 2006). In particular, toll‐like receptors (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=316), which recognize various structural components of bacteria, can trigger pro‐inflammatory responses (McCusker and Kelley, 2013). An altered gut microbiome in early development can predispose to such varied conditions as inflammatory bowel disease (IBD), increased stress reactivity and predisposition to anxiety and depression (Dinan et al., 2013). Most probiotics tested have an anti‐inflammatory action, which may be beneficial to a multiplicity of states, including such diverse diseases as IBD, post‐operative states and psychiatric disorders such as anxiety and depression. Dai et al. (2013) demonstrated that certain probiotics induce an http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4975‐mediated anti‐inflammatory response and down‐regulate the expression of a variety of inflammatory drivers including TNF‐α and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998. IL‐6 together with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4985, IL‐1 and TNF‐α are hallmarks of a depressive disorder and are well characterized and readily identifiable in this affective state.
Probiotic activity has been shown to trigger immune responses stimulating the anti‐inflammatory pathways based on bacteria genera and microbe‐associated molecular patterns (MAMPs) (Mackey and McFall, 2006). MAMPs are thought to trigger such anti‐inflammatory cytokines as IL‐10 by stimulating pattern‐recognition receptors (Chu and Mazmanian, 2013). In particular, some beneficial bacteria such as Bifidobacteria appear to prevent TLRs being activated and initiating a pro‐inflammatory response (Zhou et al., 2015). Prebiotics and probiotics have been shown to interact with TLRs initiating both pro‐inflammatory and anti‐inflammatory responses (Takeda and Akira, 2005). There is mounting evidence that prebiotics prevent MAMPs triggering immune responses. This mechanism is most likely occurring through a direct interaction on the gut epithelium with oligosaccharides (Sarkar et al., 2016). Furthermore, the mere presence of probiotics can have an anti‐inflammatory effect. Most are Gram positive and lack http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5019, reducing pro‐inflammatory responses as they colonize the gut (Gayathri and Rashmi, 2017). Neonatal studies have shown that the prebiotic human milk oligosaccharides (HMOs) are particularly adept at inhibiting pro‐inflammatory states (Wickramasinghe et al., 2015). It seems reasonable to assume that bacteria, which induce an anti‐inflammatory response, are likely to show psychobiotic activity.
The microbiota and intestinal barrier permeability
The gut barrier has a pivotal role in defending the body through its epithelium, by maintaining pathogens and toxins while regulating the absorption of nutrients. The microbiota composition has an intricate relationship with the permeability of the epithelial membrane, with the microbiota and its metabolites having a role in altering the permeability of the membrane (Jakobsson et al., 2015). Increased permeability of the intestinal epithelium has been associated with a pro‐inflammatory state (Kelly et al., 2016b). In response to an acute stress, the colonic paracellular permeability has been shown to increase and can be linked to both inflammatory responses and translocation of the gut microbiota (Moussaoui et al., 2014). Rat pups subjected to early life stress in the form of maternal separation demonstrated altered gut microbiota, increased plasma corticosterone and an overall increase in systemic immune responses (O'Mahony et al., 2009). This may increase susceptibility to pro‐inflammatory diseases such as depression and anxiety in the future. Increased permeability of the intestinal barrier allows certain Gram‐negative bacteria components to translocate from the gut and trigger pro‐inflammatory pathways (Lucas and Maes, 2013). This is the suspected mechanism of action for the inflammatory state observed in depression. Higher immunoglobulin (IgA and IgM)‐mediated immune responses to these Gram‐negative bacteria are also observed in depression (Maes et al., 2013).
Lactobacillus helveticus R0052 has demonstrated its ability to reduce intestinal permeability as a result of a barrier effect (Messaoudi et al., 2011). Kelly et al. (2016a) have shown reduced gut microbiota diversity at the phyla level in rats administered a faecal microbiota transplant (FMT) from depressed patients. This shows the significant alteration in microbiota in neuropsychiatric stress‐related disorders. The study also showed increased CRP in plasma of FMT rats revealing a pro‐inflammatory response at the systemic level related to the altered microbiota. It can be reasonably surmised that the plasma response was secondary to an altered epithelial permeability as a result of dysbiosis following FMT from depressed subjects. If intestinal permeability is compromised, the ensuing immune response could trigger an array of neuropsychiatric responses. Recent studies have highlighted the role of prebiotics in enhancing the microbiota present in the gut. Particular interest is arising in fructo‐oligosaccharides (FOS) and galacto‐oligosaccharides (GOS). A recent study by Holscher et al. (2017) has demonstrated that administration of HMOs enhances gut epithelial differentiation and maturation. This study strengthens the theory that oligosaccharides directly affect the epithelium, and by preventing a ‘leaky gut’, minimize pro‐inflammatory responses. There is potential to use psychobiotics to target the gut epithelium and to reduce permeability in an effort to maintain integrity and reduce immune responses.
Hypothalamic–pituitary–adrenal axis and the stress response
The HPA circuit has long been recognized as an important regulatory loop between the brain and gut. Hyper‐activation of the HPA has been identified in various psychological and affective states, including depression, anxiety and stress (Flandreau et al., 2012). However, the question of whether this affective state triggers overactivation of the HPA or whether hyperactivity of the HPA results in the affective state has been the subject of some debate. The primary hypothalamic regulatory peptide is http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=912; in response to psychological stress, it is released by neurotransmittors such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=505 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5 (Dinan and Cryan, 2012). Studies evaluating the altered HPA responses in patients with both major depression and irritable bowel disease indicate this may be induced by increased gut permeability (Dinan and Cryan, 2012). The use of germ‐free animals has exposed interesting findings in the role of the microbiota and the development of the HPA. In the absence of microbiota in the gut, TLRs have reduced expression in gut epithelia, and thus, they are not present to initiate the immune cascade, which results in activation of the HPA (Gosselin and Rivest, 2008). The stress response in germ‐free animals results in exaggerated release of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2869, a response fully reversed by monoassociation with Bifidobacterium infantis (Kelly et al., 2016b). The role of the microbiota in the development of appropriate stress responses and the development of the HPA is demonstrated in this study. A microbiota demonstrating abilities to reduce a stress response is most clearly showing psychobiotic potential. An ongoing question is how best to measure a stress response? Many endocrine studies utilize salivary or plasma cortisol sampling, and other studies measure various cytokine markers. A consensus has yet to be reached.
Short‐chain fatty acids and psychobiotic potential
SCFAs are the metabolites of indigestible macronutrients derived from for example plant polysaccharides. The administration of prebiotics increases SCFA production strengthening this concept (Psichas et al., 2015). The enzymes that digest these polysaccharides are supplied in the human gut by the microbiome (Qin et al., 2010). SCFAs have increasingly been investigated for potential psychobiotic therapy. The SCFAs include http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1059, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1058 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1062. Butyrate, in particular, has been demonstrated to have antidepressant properties in a number of studies. It has the ability to cross the blood–brain barrier, has been shown to have numerous neuroprotective abilities and has been demonstrated to have cognitive and antidepressant properties (Han et al., 2014). Further support as to the antidepressant effects of butyrate were demonstrated by Moretti et al. (2011) who showed systemic butyrate injections reduced stress behaviours and had an antidepressant effect in rats. This action is in keeping with butyrate as an epigenetic modulator via its regulation of gene expression through inhibition of histone deacetylase (Stilling et al., 2014). It has been noted that SCFAs directly affect the mucosal immune system, can alter the HPA axis, and through these mechanisms appear to alter the central neurotransmission (Perry et al., 2016). Butyrate's ability to act as a neuroprotective agent together with its effects on memory and cognition is of particular interest given that loss of cognitive abilities is a long recognized and undertreated feature of recurrent and severe depressive disorders. As our understanding of SCFAs expands, their potential use as therapeutics becomes more tangible, and we foresee a role for SCFAs in the evolution of psychobiotics.
Tryptophan metabolism
Tryptophan is an essential amino acid that must be ingested in the diet. The brain has limited tryptophan storage capacity and consequently a constant supply is required. Certainly reduced peripheral levels of tryptophan are associated with a depressive phenotype (Ogawa et al., 2014). The brain–gut–microbiota axis and tryptophan metabolism are inherently linked, with tryptophan levels and availability influenced directly by the gut microbiota (Desbonnet et al., 2008). Bacteria such as Bifidobacteria have been shown to increase peripheral tryptophan levels (Desbonnet et al., 2008) and certainly have psychobiotic potential. The majority of 5‐HT is synthesized in the gut by enterochromaffin cells from tryptophan and bacteria are thought to play a role in this metabolism (Clarke et al., 2013). Specific microbiota metabolites have been shown to increase colonic and plasma 5‐HT levels (Kelly et al., 2016a), and in germ‐free mice, levels of 5‐HT increase almost threefold when colonized by gut microbes (Clarke et al., 2013). Bifidobacteria, with the ability to raise peripheral tryptophan, are a true target for psychobiotic development.
Diet and mental illness
Diet in a healthy adult is the main determinant of gut microbiota composition. The association between diet and psychiatric disorders, particularly depression and anxiety, has long been suggested, and gut microbes may be the important linking factor. An association between the Mediterranean diet and a reduction in depression is well established. The Mediterranean diet is defined as being high in plant foods (fruit, vegetables, breads, cereals, potatoes, beans, nuts and seeds), fresh fruit, olive oil, moderate amounts of dairy products (cheese and yogurt), and fish and poultry, zero to four eggs consumed weekly, red meat consumed in low amounts and wine consumed in low‐to‐moderate amounts, traditionally with meals. Even moderate adherence to the Mediterranean diet results in reduced risk of depression (Psaltopoulou et al., 2013). Other studies have demonstrated that a more ‘Western diet’, defined as a diet high in processed or fried foods, refined grains, sugary products and beer, are associated with higher reported levels of anxiety and depression in women (Jacka et al., 2010). Analysis of the microbiota in those on a Mediterranean diet may help identify bacterial strains with psychobiotic activity.
What behavioural studies in rodents help to identify psychobiotics?
A number of well‐validated rodent behavioural tests are used to assess the effects of altering gut microbiota on mental states. The rodent response in these tests allows for inference of effects from specific bacteria. In approach orientation, time spent exploring new environments is a key behavioural feature, as it is crucial for the rodent to do so in order to adapt to novel and changing environments. Stress and anxiety are shown to significantly reduce exploration behaviours. The forced swim test in rodents is based on progressive immobility displayed by the rodent when forced to swim in a container of water. Immobility in the forced swim test has long been associated with increased anxiety and depressive behaviours in rodents. The test has frequently been used to identify the antidepressant potential of drugs and now psychobiotics.
Germ‐free mice demonstrate reduced anxiety by showing more exploratory behaviours in approach orientation tests (Diaz Heijtz et al., 2011). However, once germ‐free mice are exposed to stress, they exhibit elevated and exaggerated glucocorticoid responses and reduced exploratory behaviours (Desbonnet et al., 2015). The use of faecal transfer of the microbiota of interest to germ‐free rodents has proved to be beneficial in examining how the gut microbiota may alter a rodent's behavioural phenotype. Bercik et al. (2011) have shown that transferring the microbiota from stress‐sensitive mice strains to non‐anxious mice strains can alter phenotype and reduce exploratory behaviours inferring increased anxiety in the recipient mice.
The testing of potential psychobiotics using rodent behavioural tests is an important step in psychobiotic identification.
Prebiotics, polybiotics and synbiotics as psychobiotics
Prebiotics have been defined as ‘a non‐digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon’ (Roberfroid et al., 2010). Studies have shown prebiotics may have an ability to survive for extended periods in the gut, and one study has shown their potential to promote long‐standing benefits up to a year after administration (Oliveros et al., 2016). This makes prebiotics increasingly attractive as vehicles for modulating the brain–gut–microbiota axis.
The probiotic ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ has a transient modulating effect on the gut microbiota. Multiple studies show improved functionality, which is quickly lost on withdrawal of the probiotic. For example, significantly, there is reduced anxiety in mice within 1 week of probiotic treatment, with no effect observed at 3 weeks post‐treatment (Matthews and Jenks, 2013). Patients with IBD report an emergence of symptoms within days of discontinuation of probiotics.
A modified GOS (Bimuno GOS or B‐GOS) and FOS has been shown to have neuroprotective properties in animal models, with administration of B‐GOS showing clear evidence of increasing the availability of brain‐derived neurotrophic factor (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4872) in the hippocampal region (Savignac et al., 2015). There is also evidence of increased cognitive abilities, particularly in the area of memory, following increased BDNF levels (Williams et al., 2016). Other studies involving B‐GOS and FOS have established that the administration of B‐GOS significantly lowers the salivary cortisol response (Schmidt et al., 2015). B‐GOS, when fed to mice, reduces anxiety and expected cytokine stress responses. The results of this study indicate that B‐GOS exerts its anxiolytic effect through modulation of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=6 receptors (Savignac et al., 2015). Human studies have also demonstrated benefits from B‐GOS in emotional processing (Schmidt et al., 2015). All of which further support the potential role of oligosaccharides and, in particular, B‐GOS as a potential psychobiotics, whether alone or as adjunctive therapy in affective and anxiety disorders.
Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 demonstrate anti‐inflammatory properties on intestinal epithelial cells (Messaoudi et al., 2011). Also, administration of poly‐psychobiotics L. helveticus R0052 and B. longum R0175 concurrently has shown good evidence of reducing depressive behaviours in mice post‐myocardial infarction (Arseneault‐Breard et al., 2012), while showing the ability to reduce anxiety levels in healthy volunteers and decrease 24 h urinary cortisol output. The recurrent difficulties with these studies is that probiotics, whether administered as a multiple strain or single strain, are transient and do not colonize the gut permanently.
Increasingly of interest is synbiotics, a prebiotic co‐administered with a probiotic. Some studies have demonstrated good use of synbiotics in IBD (Wasilewski et al., 2015); they have been shown to induce significant reductions in TNF‐α, which were maintained for up to 6 months post‐treatment.
VSL #3 is a probiotic preparation or formula composed of eight live freeze‐dried bacterial species that are normal components of the human gastrointestinal microflora, including four strains of lactobacilli (Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus and Lactobacillus delbrueckii ssp. bulgaricus), three strains of Bifidobacteria (B. longum, Bifidobacterium breve and B. infantis) and Streptococcus salivarius ssp. thermophilus. VSL #3 has demonstrated good results for patients with conditions such as ulcerative colitis and irritable bowel syndrome (Wasilewski et al., 2015). This probiotic preparation could be considered a polybiotic, and there is the potential to co‐administer a probiotic formula in the search for psychobiotic therapies. The potential for synbiotics, the amalgamation of probiotics and prebiotics, is increasingly of interest in the search for psychobiotics. So far, no major studies of synbiotics in humans with mental health disorders have been reported.
There may also be a need to administer multi‐strain probiotics or cocktails of probiotics and prebiotics in order to confer less transient and more long‐term benefits. There is as yet no consensus on whether psychobiotic potential is achieved best with a single‐strain probiotic, a multi‐strain polybiotic or synbiotics, a prebiotic co‐administered with a probiotic.
Discussion
Determining the preclinical markers that best identify psychobiotics that will benefit patients with depression and related disorders is a major challenge. Only by adequate preclinical evaluation coupled with good clinical studies will effective algorithms emerge for identifying likely psychobiotics. Not all prebiotic, probiotic or synbiotics will have psychobiotic potential, and not all that show preclinical promise will be suitable for further development.
In investigating and developing psychobiotics, it is important to focus on those strains that have shown effects on behaviour and gut permeability, are neuroactive and reduce pro‐inflammatory and stress responses in preclinical studies. Even this strategy will have difficulties however. For example, Lactobacillus rhamnosus strain JB‐1 demonstrated an ability to reduce stress‐related behaviour and http://www.sciencedirect.com/topics/page/Corticosterone release and alter central expression of http://www.sciencedirect.com/topics/page/GABA_receptor in an anxious mouse strain (Bravo et al., 2011). L. rhamnosus was therefore predicted to have good potential as a psychobiotic. However, these promising preclinical findings did not translate in healthy male volunteers. There was no difference between L. rhamnosus and placebo in an 8 week trial in healthy volunteers with a crossover design (Kelly et al., 2017). This highlights the need for preclinical trials to be moved quickly forward to interventional studies in populations with anxiety, depression and stress‐related disorders.
Other more promising progress has been made. A double‐blind, placebo‐controlled, randomized study was conducted on volunteers with symptoms of stress. Subjects received a probiotic (Probio‐Stick; Lallemand SAS, Saint‐Simon, France) containing L. helveticus R0052 and B. longum R00175 (3 × 109 colony‐forming units per sachet stick) or an identical placebo without probiotics for a 3 week period. Though this study showed reductions in stress‐related abdominal discomfort, it did not have any effect on other symptoms of stress, for example, sleep. A further follow‐up double‐blind randomized control study by of the same preparation delivered for 4 weeks showed significantly reduced stress levels in volunteers as recorded using various stress measurement tools (Hopkins Symptom Checklist, the Hospital Anxiety and Depression Scale, the Perceived Stress Scale, the Coping Checklist and a 24 h urinary free cortisol collection. In 2016, on foot of both studies, Lallemands's Probio'Stick® (L. helveticus R0052 and B. longum Rossell®‐175 in a stick presentation) has been accepted by the Canadian health authorities for benefits in the area of stress, anxiety and mood.
Personalized psychobiotics may have a role in the future. Many researchers have now recognized that though identical bacterial species colonize the human gut, varying genetics, life experiences and exposures alter the composition of the gut microbiota. This may affect the clinical presentation of the individual in various affective or stress‐related disorders. This will alter the best ‘prescribed’ probiotic required to address the individual's anxiety or depression. Presently, however, the priority lies in identifying generic psychobiotics beneficial to many patients, prior to individualization becoming a reality.
As research continues, the more fundamental the gut microbiota appears to be in affecting the entire system, at almost all conceivable levels. In order to confer the most benefit from potential psychobiotics and develop future therapeutics, continuing to evaluate the manner in which they are altering the gut microbiota is of upmost importance. The ability of potential psychobiotics to act on the areas outlined here will allow researchers to target trials on probiotics, probiotic formulations, prebiotics or synbiotics showing preclinical promise and avoid wasting valuable cost and time on unlikely candidates. A targeted and predictive approach may, hopefully, allow the development of psychobiotics in a timely fashion.
Nomenclature of targets and ligands
Key protein targets in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2015), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Author contributions
A.B., K.S., J.C. and T.D. have all contributed substantially to the concept and design of this review article. All have substantially contributed with drafting and revising the article. All have been involved in the final draft approval and the final version submitted herewith.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors are supported in part by Science Foundation Ireland in the form of a centre grant (Alimentary Pharmabiotic Centre grant number SFI/12/RC/2273) and received funding from the European Community's Seventh Framework Programme Grant MyNewGut under grant agreement no. FP7/2007‐2013. The centre has conducted studies in collaboration with several companies including GSK, Pfizer, Cremo, Suntory, Wyeth and Mead Johnson.
Bambury, A. , Sandhu, K. , Cryan, J. F. , and Dinan, T. G. (2018) Finding the needle in the haystack: systematic identification of psychobiotics. British Journal of Pharmacology, 175: 4430–4438. 10.1111/bph.14127.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JW, Monteagudo‐Mera A, Altermann E, Cadenas MB, Thompson AL, Azcarate‐Peril MA (2017). Genome sequences of potential probiotic Lactobacillus rhamnosus isolates from human infants. Genome Announc 5: e00107–e00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault‐Breard J, Rondeau I, Gilbert K, Girard SA, Tompkins TA, Godbout R et al (2012). Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post‐myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br J Nutr 107: 1793–1799. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J et al (2011). The intestinal microbiota affect central levels of brain‐derived neurotropic factor and behavior in mice. Gastroenterology 141: 599–609.e593. [DOI] [PubMed] [Google Scholar]
- Bindels LB, Delzenne NM, Cani PD, Walter J (2015). Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12: 303–310. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG et al (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108: 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Mazmanian SK (2013). Innate immune recognition of the microbiota promotes host‐microbial symbiosis. Nat Immunol 14: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F et al (2013). The microbiome–gut–brain axis during early life regulates the hippocampal serotonergic system in a sex‐dependent manner. Mol Psychiatry 18: 666–673. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG (2015). Gut microbiota: microbiota and neuroimmune signalling – Metchnikoff to microglia. Nat Rev Gastroenterol Hepatol 12: 494–496. [DOI] [PubMed] [Google Scholar]
- Dai C, Zheng C‐Q, Meng F‐j, Zhou Z, Sang L‐x, Jiang M (2013). VSL# 3 probiotics exerts the anti‐inflammatory activity via PI3k/Akt and NF‐κB pathway in rat model of DSS‐induced colitis. Mol Cell Biochem 374: 1–11. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney RD et al (2015). Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun 48: 165–173. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG (2008). The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 43: 164–174. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A et al (2011). Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 108: 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF (2012). Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37: 1369–1378. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Quigley EM (2011). Probiotics in the treatment of depression: science or science fiction? Aust N Z J Psychiatr 45: 1023–1025. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Stanton C, Cryan JF (2013). Psychobiotics: a novel class of psychotropic. Biol Psychiatry 74: 720–726. [DOI] [PubMed] [Google Scholar]
- Douillard FP, Ribbera A, Kant R, Pietilä TE, Järvinen HM, Messing M et al (2013). Comparative genomic and functional analysis of 100 Lactobacillus rhamnosus strains and their comparison with strain GG. PLoS Genet 9: e1003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB (2012). Chronic overexpression of corticotropin‐releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene‐expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology 37: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayathri D, Rashmi B (2017). Mechanism of development of depression and probiotics as adjuvant therapy for its prevention and management. Ment Health Prev 5: 40–51. [Google Scholar]
- Gosselin D, Rivest S (2008). MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol Psychiatry 13: 480–497. [DOI] [PubMed] [Google Scholar]
- Han A, Sung Y‐B, Chung S‐Y, Kwon M‐S (2014). Possible additional antidepressant‐like mechanism of sodium butyrate: targeting the hippocampus. Neuropharmacology 81: 292–302. [DOI] [PubMed] [Google Scholar]
- Holscher HD, Bode L, Tappenden KA (2017). Human milk oligosaccharides influence intestinal epithelial cell maturation in vitro . J Pediatr Gastroenterol Nutr 64: 296–301. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O'Reilly SL et al (2010). Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry 167: 305–311. [DOI] [PubMed] [Google Scholar]
- Jakobsson HE, Rodriguez‐Pineiro AM, Schutte A, Ermund A, Boysen P, Bemark M et al (2015). The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 16: 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M, Hamer M, Singh‐Manoux A, Batty GD, Kivimaki M (2014). Association of metabolically healthy obesity with depressive symptoms: pooled analysis of eight studies. Mol Psychiatry 19: 910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N et al (2017). Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB‐1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun 61: 50–59. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, O'Brien C, Patterson E, El Aidy S, Deane J et al (2016a). Transferring the blues: depression‐associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82: 109–118. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Clarke G, Cryan JF, Dinan TG (2016b). Brain–gut–microbiota axis: challenges for translation in psychiatry. Ann Epidemiol 26: 366–372. [DOI] [PubMed] [Google Scholar]
- Lucas K, Maes M (2013). Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol 48: 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D, McFall AJ (2006). MAMPs and MIMPs: proposed classifications for inducers of innate immunity. Mol Microbiol 61: 1365–1371. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, Berk M, Geffard M, Bosmans E (2013). In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS‐damaged neoepitopes. Acta Psychiatr Scand 127: 344–354. [DOI] [PubMed] [Google Scholar]
- Matthews DM, Jenks SM (2013). Ingestion of Mycobacterium vaccae decreases anxiety‐related behavior and improves learning in mice. Behav Processes 96: 27–35. [DOI] [PubMed] [Google Scholar]
- McCusker RH, Kelley KW (2013). Immune‐neural connections: how the immune system's response to infectious agents influences behavior. J Exp Biol 216: 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHardy IH, Goudarzi M, Tong M, Ruegger PM, Schwager E, Weger JR et al (2013). Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter‐relationships. Microbiome 1: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt MD, Liu S, Manatunga A, Royster EB, Raison CL, Woolwine BJ et al (2012). Neurobehavioral effects of interferon‐α in patients with hepatitis‐C: symptom dimensions and responsiveness to paroxetine. Neuropsychopharmacology 37: 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A et al (2011). Assessment of psychotropic‐like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 105: 755–764. [DOI] [PubMed] [Google Scholar]
- Moretti M, Valvassori SS, Varela RB, Ferreira CL, Rochi N, Benedet J et al (2011). Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav Pharmacol 22: 766–772. [DOI] [PubMed] [Google Scholar]
- Moussaoui N, Braniste V, Ait‐Belgnaoui A, Gabanou M, Sekkal S, Olier M et al (2014). Changes in intestinal glucocorticoid sensitivity in early life shape the risk of epithelial barrier defect in maternal‐deprived rats. PLoS One 9: e88382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noecker C, Eng A, Srinivasan S, Theriot CM, Young VB, Jansson JK et al (2016). Metabolic model‐based integration of microbiome taxonomic and metabolomic profiles elucidates mechanistic links between ecological and metabolic variation. mSystems 1: e00013–e00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien SM, Scott LV, Dinan TG (2004). Cytokines: abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol Clin Exp 19: 397–403. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Fujii T, Koga N, Hori H, Teraishi T, Hattori K et al (2014). Plasma l‐tryptophan concentration in major depressive disorder: new data and meta‐analysis. J Clin Psychiatry 75: e906–e915. [DOI] [PubMed] [Google Scholar]
- O'Hara AM, Shanahan F (2006). The gut flora as a forgotten organ. EMBO Rep 7: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveros E, Ramirez M, Vazquez E, Barranco A, Gruart A, Delgado‐Garcia JM et al (2016). Oral supplementation of 2′‐fucosyllactose during lactation improves memory and learning in rats. J Nutr Biochem 31: 20–27. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho A‐M, Quigley EM et al (2009). Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 65: 263–267. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL et al (2016). Acetate mediates a microbiome–brain–β‐cell axis to promote metabolic syndrome. Nature 534: 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley LL, Ye J, Li X, LeBlanc J, Zhang Z, Ruegger PM et al (2012). Host–microbe relationships in inflammatory bowel disease detected by bacterial and metaproteomic analysis of the mucosal–luminal interface. Inflamm Bowel Dis 18: 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N (2013). Mediterranean diet, stroke, cognitive impairment, and depression: a meta‐analysis. Ann Neurol 74: 580–591. [DOI] [PubMed] [Google Scholar]
- Psichas A, Sleeth M, Murphy K, Brooks L, Bewick G, Hanyaloglu A et al (2015). The short chain fatty acid propionate stimulates GLP‐1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 39: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I et al (2010). Prebiotic effects: metabolic and health benefits. Br J Nutr 104: S1–63. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ (2016). Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci 39: 763–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF (2015). Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res 287: 59–72. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PW (2015). Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl) 232: 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen RJ, van Hylckama Vlieg JE (2011). Genomic diversity and versatility of Lactobacillus plantarum, a natural metabolic engineer. Microb Cell Fact 10: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2015). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling RM, Dinan TG, Cryan JF (2014). Microbial genes, brain & behaviour – epigenetic regulation of the gut–brain axis. Genes Brain Behav 13: 69–86. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S (2005). Toll‐like receptors in innate immunity. Int Immunol 17: 1–14. [DOI] [PubMed] [Google Scholar]
- Tremaroli V, Bäckhed F (2012). Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249. [DOI] [PubMed] [Google Scholar]
- Wasilewski A, Zielinska M, Storr M, Fichna J (2015). Beneficial effects of probiotics, prebiotics, synbiotics, and psychobiotics in inflammatory bowel disease. Inflamm Bowel Dis 21: 1674–1682. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe S, Pacheco AR, Lemay DG, Mills DA (2015). Bifidobacteria grown on human milk oligosaccharides downregulate the expression of inflammation‐related genes in Caco‐2 cells. BMC Microbiol 15: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Chen L, Savignac HM, Tzortzis G, Anthony DC, Burnet PW (2016). Neonatal prebiotic (BGOS) supplementation increases the levels of synaptophysin, GluN2A‐subunits and BDNF proteins in the adult rat hippocampus. Synapse 70: 121–124. [DOI] [PubMed] [Google Scholar]
- Zhou W, Lv H, Li M, Su H, Huang L, Li J et al (2015). Protective effects of Bifidobacteria on intestines in newborn rats with necrotizing enterocolitis and its regulation on TLR2 and TLR4. Genet Mol Res 14: 11505–11514. [DOI] [PubMed] [Google Scholar]


