Abstract
The commensal gut microbiota is an environmental factor that has been implicated in the development of cardiovascular disease. The development of atherosclerotic lesions is largely influenced not only by the microbial‐associated molecular patterns of the gut microbiota but also by the meta‐organismal trimethylamine N‐oxide pathway. Recent studies have described a role for the gut microbiota in platelet activation and arterial thrombosis. This review summarizes the results from gnotobiotic mouse models and clinical data that linked microbiota‐induced pattern recognition receptor signalling with atherogenesis. Based on recent insights, we here provide an overview of how the gut microbiota could affect endothelial cell function and platelet activation, to promote arterial thrombosis.
Linked Articles
This article is part of a themed section on When Pharmacology Meets the Microbiome: New Targets for Therapeutics? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.24/issuetoc
Abbreviations
- ApoE
apolipoprotein E
- CVD
cardiovascular disease
- DMB
3,3‐dimethyl‐1‐butanol
- MAMPs
microbial‐associated molecular patterns
- NLR
NOD‐like receptor
- Pam3CSK4
N‐Palmitoyl‐S‐[2,3‐bis(palmitoyloxy)‐(2RS)‐propyl]‐[R]‐cysteinyl‐[S]‐seryl‐[S]‐lysyl‐[S]‐lysyl‐[S]‐lysyl‐[S]‐lysine
- PG
peptidoglycan
- PRR
pattern recognition receptor
- TLR
toll‐like receptor
- TMA
trimethylamine
- TMAO
trimethylamine N‐oxide
- VLDL
very low density lipoprotein
- VWF
von Willebrand factor
Introduction
The intestinal microbiota is the sum of trillions of microorganisms that reside in the gastrointestinal tract (Bäckhed et al., 2015; Xiao et al., 2015). The microbiome is largely influenced by host genetics, body site, diet, antibiotics and lifestyle factors (Gilbert et al., 2018). This complex microbial ecosystem regulates, among others, the vascularization and architecture of the small intestine (Reinhardt et al., 2012; Khandagale and Reinhardt, 2018), the maintenance of the structural integrity of the gut mucosal barrier (Cani et al., 2008; Muccioli et al., 2010), inflammatory tone (Bain et al., 2014; Balmer et al., 2014a; Zhang et al., 2015a) and host energy metabolism (Bäckhed et al., 2007; Heiss and Olofsson, 2017).
Nutrition and related changes in the gut microbiota influence the intestinal barrier function, increasing gut permeability (Cani et al., 2008; Thaiss et al., 2018). Bacterial products, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5044s and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5019 constantly leak into the portal circulation (Clarke et al., 2010; Balmer et al., 2014a,b), promoting the development of metabolic inflammation (Cani et al., 2008; Caesar et al., 2012). The remote signalling of microbiota‐derived metabolites and microbial‐associated molecular patterns (MAMPs) can promote disease progression, for instance, of liver fibrosis (Lelouvier et al., 2016), non‐alcoholic fatty liver disease (Bashiardes et al., 2016; Janssen et al., 2017) and hepatocellular carcinoma (Roderburg and Luedde, 2014; Li et al., 2016a).
Interestingly, the microbiota is not only associated with liver pathologies. During the past decade, a number of clinical and animal studies have provided a substantial amount of association‐based evidence, linking the commensal microbiota with the development of cardiovascular disease (CVD) (Ott et al., 2006; Koren et al., 2011; Fåk et al., 2015; Emoto et al., 2017) and cerebrovascular diseases (Koren et al., 2011; Karlsson et al., 2012; Fåk et al., 2015; Benakis et al., 2016), which include arterial thrombosis (Table 1). However, the underlying mechanisms that are triggered by signalling‐active molecules derived from gut microbial communities, contributing to the progression of cardiovascular disease and promoting the development of arterial thrombosis, are largely unresolved (Komaroff, 2018).
Table 1.
Gut microbes associated with atherosclerosis
| Link to atherosclerosis | Bacterial species | Habitat | Reference |
|---|---|---|---|
| Bacteria found in the blood that were associated with cardiovascular disease risk | Helicobacter pylori | GI tract | Patel et al. (1995) |
| Prevotella nigrescens a | Oral flora | Yakob et al. (2011) | |
| Porphyromonas gingivalis a | Oral flora, upper GI tract, respiratory tract and colon | Yakob et al. (2011) and Haraszthy et al. (2000) | |
| Microbiota isolated from carotid plaque | Bacteroides forsythus | Oral cavity | Haraszthy et al. (2000) |
| Bacteria found in atherosclerotic plaque | Staphylococcus sp.a (epidermidis, aureus, haemolyticus and hominis) | Skin and respiratory tract | Ott et al. (2006) and Koren et al. (2011) |
| Proteus vulgaris | Intestinal tract | Ott et al. (2006) | |
| Klebsiella pneumonia | Oral flora, skin and intestine | ||
| Enterobacteriaceae bacterium | Gut flora | ||
| Enterobacter dissolvens | Gut flora | ||
| Pantoea agglomerans | Gut flora | ||
| Citrobacter freundii | GI tract | ||
| Enterobacter cloacae | Gut flora | ||
| Streptococcus a | Skin | ||
| Nocardia sp. | Oral cavity | ||
| Propionibacterineae a | Skin | Koren et al. (2011) | |
| Granulicatella a | GI tract | ||
| Streptococcus a | Gut flora | ||
| Subdoligranulum a | Gut flora | ||
| Ruminococcus a | Gut flora | ||
| Veillonella a | Oral cavity and gut flora | ||
| Chryseomonas | Oral cavity | ||
| Lachnospiraceae a | Gut flora | ||
| Microbiota from faecal samples associated with atherosclerosis | Collinsela | Gut flora | Karlsson et al. (2012) |
| Ruminococcus a | Gut flora | Jie et al. (2017) and Emoto et al. (2017) | |
| Clostridium a | Gut flora | ||
| Escherichia | Gut flora | ||
| Subdoligranulum | Gut flora | ||
| Streptococcus a | Gut flora | ||
| Coprobacillus | Gut flora | ||
| Enterococcus | Gut flora | ||
| Bifidobacterium | GI tract | Emoto et al. (2017) | |
| Prevotella | Oral flora | ||
| Lactobacillales | Disperse | ||
| Bacteroides | GI tract | ||
| Lachnospiraceae a | Gut flora | Koren et al. (2011) | |
| Veillonella a | Oral cavity and gut flora | ||
| Enterobacter cloacae | Gut flora | ||
| Subdoligranulum a | Gut flora | ||
| Microbiota, isolated from oral cavity, associated with atherosclerosis | Streptococcus a | Gut flora | Koren et al. (2011) |
| Propionibacterineae a | Skin | ||
| Rothia | Oral cavity | ||
| Corynebacterium | Gut flora | ||
| Staphylococcus a | Skin and respiratory tract | ||
| Veillonella a | Oral cavity and gut flora | Koren et al. (2011) and Fåk et al. (2015) | |
| Anaeroglobus | Oral flora | Fåk et al. (2015) | |
| Odoribacter | Oral flora | ||
| Porphyromonas a | Oral flora, upper GI tract, respiratory tract and colon | ||
| Prevotella a | Oral flora | ||
| Coprobacillus | Gut flora |
GI, gastrointestinal.
Bacteria found in different samples.
There is increasing evidence for the contributory role of the gut microbiota in the development of CVD and in arterial thrombosis. Initial mechanistic studies have revealed that http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5521 (TMA), a choline‐derived metabolite produced by the TMA‐lyases (cutC) and the carnitine oxygenase (cntA) of gut microbes that is absorbed into the portal circulation and converted to https://pubchem.ncbi.nlm.nih.gov/compound/1145 (TMAO) by flavin monooxygenases in the liver, is associated with CVD. TMAO is associated with increased atherogenesis in mice and humans (Wang et al., 2011; Koeth et al., 2013). Furthermore, this metabolite was demonstrated to facilitate platelet activation, thus promoting arterial thrombus formation (Zhu et al., 2016).
In addition, innate immune pathways contribute to the development of atherosclerosis (Björkbacka et al., 2004) and foster arterial thrombosis (Ren et al., 2014). A role of gut microbiota‐induced http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1752 (TLR2) signalling in promoting arterial thrombus growth has recently been demonstrated with germ‐free mouse models (Jäckel et al., 2017) (Figure 1). Furthermore, clinical studies have provided strong evidence for the involvement of innate immune pathways in CVD, showing that the blockade of IL‐1β signalling reduced cardiovascular mortality (Ridker et al., 2017). Hence, it will be intriguing to reveal the molecular mechanisms that link the gut microbiota to arterial thrombosis.
Figure 1.
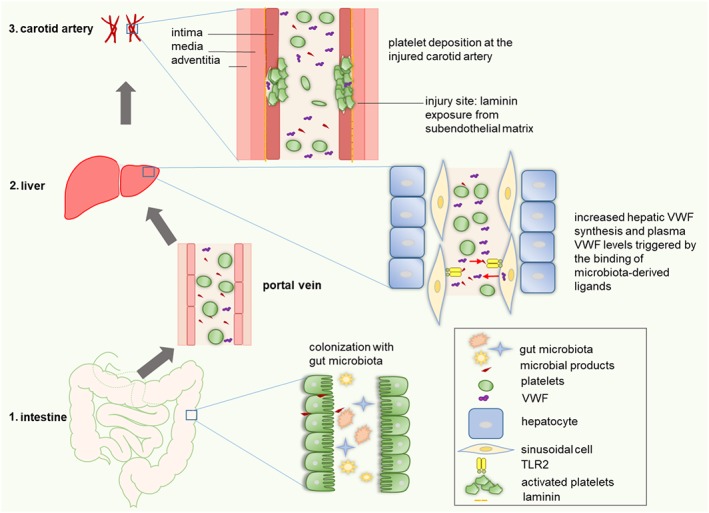
Remote signalling by the microbiota stimulates VWF synthesis in the hepatic endothelium of mice, promoting thrombus growth in the ligation‐injured carotid artery. (1) Microbial products from the gut lumen translocate from the gut lumen to the liver, where they (2) stimulate TLR2, leading to increased hepatic VWF synthesis and release into the circulation. (3) Elevated VWF plasma levels promote platelet deposition and thrombus growth in the injured carotid artery.
In this review, we provide an overview of the role of the commensal microbiota as a modulating factor of atherogenesis and its involvement in augmenting prothrombotic platelet function. We specifically discuss the functional role of the intestinal microbiota as a novel risk factor in arterial thrombosis.
Microbial metabolites and microbe‐associated molecular patterns from the gut microbiota float in the circulation
Under physiological conditions, the barrier function of the epithelial lining (Marchiando et al., 2010) and the gut vascular barrier (Spadoni et al., 2015) restrict colonizing gut microbes to the intestinal lumen. However, small amounts of bacteria are constantly taken up by dendritic cells and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=74 mononuclear phagocytes, reaching the mesenteric lymph nodes (Huang et al., 2000; Diehl et al., 2013) to preserve peripheral tolerance (Probst et al., 2014). In contrast to live bacteria, bacterial breakdown products and bacterial metabolites leak into the portal circulation, thus reaching remote body sites (Balmer et al., 2014b) and could be detected in the plasma of mice and humans (Amar et al., 2008; Clarke et al., 2010). Factors that affect gut microbial ecology (e.g. nutrition, antibiotics and oxidative stress) can perturb the intestinal barrier function. High‐fat diet increases the leakage of gut microbial products, such as LPS, into the circulation (Cani et al., 2008). This 2.5‐fold rise in plasma LPS was termed metabolic endotoxemia and results in low‐grade inflammation. MAMPs (e.g. LPS, peptidoglycans, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5024, lipoteichoic acid and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4931) are recognized by http://www.guidetopharmacology.org/GRAC/ReceptorFamiliesForward?type=CATALYTICRECEPTOR&familyId=302 (PRRs) such as TLRs, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=317 (NLRs) and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=940 receptors. Interestingly, antibiotic treatment abolished the effect of high‐fat diet, indicating the involvement of the gut microbiota in increasing gut permeability, which was associated with a reduced expression of epithelial tight junction proteins (Cani et al., 2008). Under conditions of acute intestinal inflammation, the intestinal barrier function is severely perturbed. Then, the liver acts as a firewall to clear blood‐borne gut bacteria from the mesenteric and systemic vasculature and to prevent systemic spreading (Balmer et al., 2014b).
In addition to innate immune receptor agonists, gut microbial metabolites, such as TMA, are also translocated from the gut into the circulation (al‐Waiz et al., 1992). A detailed understanding of the link between diet, microbiota profile and intestinal barrier is crucial, as both PRR signalling and the meta‐organismal TMAO pathway promote CVD (Björkbacka et al., 2004; Wang et al., 2011; Koeth et al., 2013) and arterial thrombosis (Ren et al., 2014; Zhu et al., 2016).
Microbial‐associated molecular patterns derived from the gut microbiota affect the development of atherosclerotic lesions
Rupture (Badimon and Vilahur, 2014) and erosion (Quillard et al., 2017) of atherosclerotic plaques are considered the primary cause of arterial thrombosis. Pattern recognition receptors (PRRs), such as http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1754, are functionally expressed by myeloid cells (Xu et al., 2001), platelets (D'Atri and Schattner, 2017) and the vascular endothelium (Dunzendorfer et al., 2004). In atherosclerotic plaques from patients undergoing endartererctomy and in biopsies of the internal mammary artery from patients undergoing bypass surgery, the expression of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1751, TLR2 and TLR4 was increased and localized to endothelial cells and macrophages (Edfeldt et al., 2002). Epidemiological studies on carotid artery atherosclerosis (Kiechl et al., 2002) and various animal models (Björkbacka et al., 2004) established that innate immune receptor signalling is an important determinant of atherogenesis. Furthermore, in hypertensive endarterectomy patients, blood LPS levels were significantly elevated, and macrophages in atherosclerotic plaque specimens and carotid arteries stained positive for LPS (Carnevale et al., 2018). Demonstrating the functional involvement of LPS, the intravenously administered TLR4 agonist LPS from Escherichia coli accelerated the formation of atherosclerotic lesions, in a hypercholesterolemic rabbit model, as evaluated by increased lesion size, lesion thickness and lesion volume (Lehr et al., 2001). Collectively, these studies demonstrate a role for MAMPs in the development of atherosclerosis.
Strong evidence for the role of TLR signalling in atherogenesis comes from genetic mouse studies. In TLR4−/− × ApoE−/− atherosclerotic mice that were fed for 6 weeks with 0.15% cholesterol‐rich diet, the aortic plaque area and macrophage infiltration of the aortic sinus plaques were reduced (Michelsen et al., 2004). Importantly, the effect of MAMPs on atherogenesis is not restricted to LPS and TLR4 signalling of myeloid cells (Coenen et al., 2009), as TLR2‐deficiency led to a reduction in atherosclerotic lesion size in the LDL receptor (LDLR)‐deficient and the apolipoprotein E (ApoE)‐deficient atherosclerosis model (Mullick et al., 2005; Liu et al., 2008). In contrast to the atherogenic role of TLR4 under low‐fat diet conditions, in the LDLR‐deficient atherosclerosis mouse model (Coenen et al., 2009), bone marrow transplantation experiments showed that the lack of TLR2 in myeloid cells did not reduce the development of atherosclerotic lesions in hypercholesterolemic LDLR‐deficient mice, indicating that TLR2 signalling in the vascular endothelium promotes the development of atherosclerotic lesions (Mullick et al., 2005, 2008). The role of endothelial TLR2 in atherogenesis was further corroborated, as stimulation of TLR2/1‐mediated signalling with the synthetic ligand http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8558 increased atherosclerotic burden in hypercholesterolemic LDLR‐deficient mice, but not in mice with total TLR2‐deficiency or with TLR2‐deficiency in myeloid cells (Mullick et al., 2005). Taken together, there is strong evidence for the contribution of TLR signalling of macrophages and endothelial cells in atherogenesis.
The commensal gut microbiota is a tonic‐activating factor of TLRs and other PRRs not only in the intestine (Hörmann et al., 2014; Nigro et al., 2014) but also in remote organs, such as the bone marrow (Khosravi et al., 2014; Balmer et al., 2014a). In a clinical study, the functional characterization of the gut metagenomes of patients with symptomatic atherosclerosis showed an enrichment in genes encoding for the peptidoglycan biosynthesis pathway (Karlsson et al., 2012). Therefore, MAMPs derived from the gut microbiota have to be considered as drivers of atherosclerotic lesion formation.
Causal evidence for the role of the gut microbiota in atherosclerosis comes from antibiotic‐treated and germ‐free mouse atherosclerosis models (Table 2). When the commensal gut microbiota of ApoE‐deficient mice was extensively reduced by treatment with a cocktail of the antibiotics vancomycin, neomycin, metronidazole and ampicillin, no difference in atherosclerotic lesion size was found on a standard chow diet, whereas the antibiotic cocktail reduced the proatherogenic effect of a 1% choline‐enriched diet (Wang et al., 2011). Similarly, when atherosclerosis‐prone ApoE‐deficient mice on an atherogenic diet were treated with the broad‐spectrum antibiotic ampicillin, the reduction of the gut microbiota had beneficial effects on cardiovascular risk factors, such as improved glucose tolerance, reduced plasma LDL and very LDL (VLDL) levels and decreased atherosclerotic lesion size (Rune et al., 2016). In contrast, germ‐free ApoE‐deficient mice that were kept for 20 weeks on a chow diet were reported to have increased total plasma cholesterol levels, increased LDL and VLDL levels, but decreased triglyceride levels (Kasahara et al., 2017). In this study, germ‐free ApoE‐deficient mice on a chow diet showed reduced lesion size and a reduced quantity of macrophages in the aortic sinus plaques (Kasahara et al., 2017). However, a previous study has reported that germ‐free ApoE‐deficient mice that were fed a diet that lacked cholesterol had a significantly reduced vessel lumen and an increased volume of the atherosclerotic plaque in the thoracic aorta, compared with conventionally raised ApoE‐deficient mice on the same diet (Stepankova et al., 2010). When 8 week old germ‐free ApoE‐deficient mice and conventionally raised ApoE‐deficient mice were kept on a diet containing 2% cholesterol for 3–4 months, no significant difference was found in the area fraction of the free vessel lumen or in the volume of the atherosclerotic plaque. In agreement with the study of Kasahara et al., serum cholesterol levels were also increased in germ‐free ApoE‐deficient mice in this study (Stepankova et al., 2010). In a recent study with germ‐free ApoE‐deficient mice, it was confirmed that 12 weeks of Western diet feeding did not result in changed atherosclerotic lesion size in the aortic root, excluding a proatherogenic effect of dietary choline supplementation (Lindskog Jonsson et al., 2018). Interestingly, the colonization of conventionally raised ApoE‐deficient mice that were on an atherogenic Western diet for 8 weeks with Akkermansia muciniphila, an abundant colonizer of the Verrucomicrobia phylum that counteracts metabolic endotoxemia and is decreased in obese leptin‐deficient and in diet‐induced obese mice (Everard et al., 2013), resulted in reduced atherosclerotic lesion size (Li et al., 2016b). The authors of this study suggested that the association of hyperlipidaemic ApoE‐deficient mice with Akkermansia muciniphila ameliorated vascular inflammation in the aorta. Furthermore, in this study, gavage of ApoE‐deficient mice on a Western diet with Akkermansia muciniphila reduced intestinal permeability, a critical determinant of metabolic endotoxemia (Cani et al., 2008). However, due to the low numbers of mice used in these studies, the different types of diets used, the partly controversial results and the severity of the ApoE atherosclerosis model (Table 2), additional germ‐free mouse studies, addressing also early atherosclerosis, are indispensable to clarify the role of gut commensals in atherogenesis.
Table 2.
The outcome of different diets in antibiotic‐treated and germ‐free ApoE mouse models
| Diet | Microbiota‐dependent influence on atherogenesis | Reference |
|---|---|---|
| ApoE−/− mice were raised on chow diet until 4 weeks of age and then transferred on a control diet (0.08% choline) or a normal chow with high choline content (1% choline) for 16 weeks. | When C57BL/6J ApoE−/− mice were treated with a cocktail of vancomycin (0.5 g·L−1), neomycin sulfate (1 g·L−1), metronidazole (1 g·L−1) and ampicillin (1 g·L−1) at weaning via the drinking water, the choline‐diet dependent increase in foam cell formation and macrophage total cholesterol content were suppressed. Also, the choline‐diet induced increase in atherosclerotic lesion size and macrophage content in the aorta was reduced by the antibiotics. | Wang et al. (2011) |
| ApoE−/− mice were fed gluten‐free Western diet (21% fat, 50% carbohydrate, 20% protein, 2% choline bitartrate and 1.5% cholesterol) from weaning until 16 weeks of age. | When B6.129P2‐ApoEtm1Unc N11 mice received tap water with ampicillin (1 g·L−1), the treated mice gained more body weight, transiently improved glucose tolerance, and lowered total plasma cholesterol, LDL and VLDL. The antibiotic‐treated group on Western diet was protected from the development of atherosclerotic lesions (en face plaque assessment). | Rune et al. (2016) |
| ApoE−/− mice were fed a chow diet composed of 20% calories from fat, 50% calories from carbohydrates and 30% calories from protein. | Germ‐free C57BL/6 ApoE−/− mice showed increased plasma cholesterol and LDL cholesterol levels and a decrease in triglyceride levels compared to conventionally raised controls. Also, liver total cholesterol was increased in the germ‐free state. In contrast to conventionally raised ApoE−/− mice, the germ‐free ApoE−/− mice had conjugated bile acids. Germ‐free ApoE−/− mice were resistant to the development of atherosclerosis, as shown by reduced plaque area in the aortic root and reduced macrophage‐positive area per plaque area. | Kasahara et al. (2017) |
| ApoE−/− mice were fed a standard diet, containing 0% cholesterol and 3% fat. These mice were compared to a hypercholesteric diet containing 2% cholesterol, 5% tallow fat and 3% fish fat fed at the age of 8 weeks for 3–4 months. | Germ‐free B6.129P2‐ApoEtm1Unc/J mice on the standard diet had significantly reduced vessel lumen and an increased atherosclerotic plaque volume in the thoragic aorta. This difference was abolished when mice were kept on the hypercholesteric diet. This study also reported increased plasma cholesterol levels in the germ‐free ApoE−/− mice. | Stepankova et al. (2010) |
| 8‐week‐old conventionally raised ApoE−/− mice on a normal chow or a Western diet for 8 weeks. Mice were gavaged daily for a period of 8 weeks with 5 × 109 cfu Akkermansia muciniphila. | Akkermansia muciniphila was reduced in the faeces of the Western diet‐fed ApoE−/− mice. Treatment with live Akkermansia muciniphila substantially reduced the lesion area and size in ApoE−/− mice fed a Western diet but did not alter lipid metabolism in this model. Akkermansia muciniphila ameliorated aortic and systemic inflammation in Western diet‐fed ApoE−/− mice, as shown by MOMA, CCL2 and ICAM‐1 staining in the aortic arch. This was associated with decreased permeability and reduced serum LPS. | Li et al. (2016b) |
Pattern recognition receptor signalling induced by the gut microbiota and microbiota‐dependent choline metabolism promote arterial thrombus growth
PRR signalling of platelets (Zhang et al., 2015b; Biswas et al., 2017) and endothelial cells (Ren et al., 2014; Jäckel et al., 2017) promotes arterial thrombosis in the mouse ferric chloride and the ligation injury model of the carotid artery. Arterial thrombus formation is supported by http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1763, TLR2 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1759 in platelets (Panigrahi et al., 2013; Zhang et al., 2015b; Biswas et al., 2017) as well as TLR2 and TLR4 in endothelial cells (Ren et al., 2014; Jäckel et al., 2017). As the gut microbiota is a source of physiologically active PRR agonists in the plasma (Clarke et al., 2010; Balmer et al., 2014a), it is crucial to explore the role of this microbial ecosystem in arterial thrombosis.
There is emerging evidence from germ‐free mouse models that colonization with a gut microbiota promotes thrombus formation in the carotid artery, as demonstrated in the ligation injury model on normal chow diet (Jäckel et al., 2017) and in the ferric chloride injury model by feeding a choline‐rich diet (Zhu et al., 2016). As endothelial cells in the liver encounter blood from the intestine via the portal vein, it is interesting to note that hepatic http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1759 (VWF) expression, an integral component of the acute phase response, is increased by the presence of a gut microbiota (Jäckel et al., 2017). Consistent with these results, plasma levels of VWF and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2607 levels were decreased in germ‐free mice compared with those in their conventionally raised counterparts. Similar to germ‐free mice, hepatic endothelial VWF expression and VWF plasma levels were reduced in TLR2‐deficient mice compared with wild type control mice, but this difference was abolished when comparing germ‐free TLR2‐deficient mice with germ‐free wild type littermate controls (Jäckel et al., 2017). Demonstrating the involvement of the gut microbiota, recolonization of germ‐free TLR2‐deficient mice and their wild type littermate controls re‐established the difference in hepatic VWF expression. In this study, we could show that MAMPs taken up via the enteric route are clearly important for the function of the liver endothelium, as hepatic VWF expression could also be up‐regulated by administration of the TLR2/6 agonist lipoteichoic acid to germ‐free mice via the drinking water. In the carotid artery ligation model, platelet deposition to the injury site was dependent on microbiota‐triggered TLR2 signalling and the TLR2‐dependent increase in VWF plasma levels. Importantly, platelet depletion experiments virtually excluded a role of platelet TLR2 in platelet deposition to the arterial ligation injury site. In static adhesion experiments on laminin coatings, the http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6588 acid‐motif in the C4 domain of VWF, which primarily interacts with the platelet http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=760 http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2579&familyId=760&familyType=CATALYTICRECEPTOR, was identified to mediate this platelet deposition defect observed in TLR2‐deficient mice. Taken together, these data from gnotobiotic mouse models support a functional role of microbiota‐stimulated TLR2 signalling in arterial thrombosis (Figure 1).
In addition to the pattern recognition of endothelial cells, the TMAO meta‐organismal pathway has been uncovered as a microbiota‐dependent factor that is predictive for thrombotic event risk and augments thrombus formation in the carotid artery of laboratory mice (Zhu et al., 2016). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4551 is catabolized by the intestinal microbiota, leading to the formation of TMA by bacterial TMA‐lyase enzymes. Meanwhile, a number of TMA‐producing bacterial strains have been identified and characterized (Table 3). TMA is in turn metabolized to TMAO in the liver by the hepatic flavin monooxygenase enzyme family (Wang et al., 2011; Koeth et al., 2013). TMAO was proposed to promote microbiota‐dependent atherosclerosis, but there are experimental and clinical studies that could not confirm this link (Meyer et al., 2016; Lindskog Jonsson et al., 2018). Although, in mice, the choline‐derived metabolite TMAO induced vascular inflammation in the aorta and evoked phosphorylation of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1499/http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514/NF‐κB pathway in human aortic endothelial cells and vascular smooth muscle cells, the prothrombotic phenotype caused by TMAO was due to platelet hyperreactivity (Zhu et al., 2016, 2017). Intraperitoneal injections of TMAO, yielding plasma TMAO concentrations of approximately 100 μM, 0.12% v/v TMAO in drinking water, or dietary 1% choline supplementation of the diet, reduced the time to occlusion in the ferric chloride injury model of the carotid artery. In this study, the effects of dietary TMAO and choline were further confirmed in a photochemical mouse thrombosis model (Zhu et al., 2016). The prothrombotic phenotype of dietary choline supplementation in the ferric chloride carotid artery model was absent in germ‐free mice, but importantly, it could be re‐established in conventionalized mice. Furthermore, Zhu and co‐workers demonstrated that the TMAO‐dependent augmentation in platelet aggregation and ferric chloride‐induced carotid artery thrombosis could be transplanted into germ‐free recipient mice depending whether the transplanted cecal microbiota was derived from a high TMA‐producing donor mouse strain (C57BL/6J) or from a low TMA‐producing donor mouse strain (NZW/LacJ). Independent of the agonist used, TMAO increased the sensitivity of the aggregation response of human platelets in PRP and washed platelets. Moreover, TMAO increased platelet deposition to a collagen‐coated surface in whole blood under flow conditions. Mechanistically, TMAO treatment of platelets resulted in an enhanced stimulus‐dependent release of Ca2+ from intracellular stores, which in washed human platelets correlated with the augmentation of the second messenger http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4222. Of note, the effect of the choline diet, increasing platelet aggregation, was mediated by a factor contained in platelet‐poor plasma. An interventional clinical study has recently demonstrated that a choline‐rich diet for 2 months augments the extent of ADP‐induced platelet aggregation in vegetarians and omnivores (Zhu et al., 2017). Interestingly, in this study, administration of aspirin prior to the choline‐supplementation attenuated the plasma TMAO‐dependent increase in ADP‐induced platelet aggregation (Zhu et al., 2017). To date, the exact prothrombotic action of microbiota‐derived TMAO is still not completely resolved, in so far as no ‘chemical sensor’ for TMAO has been identified and characterized in platelets.
Table 3.
Overview on identified TMA‐producing bacteria
| Bacterial species | Study description | Reference |
|---|---|---|
| Shigella alkalescens | The choline reaction was suggested to separate S. alkalescens from S. paradysenteriae. | Wood and Keeping (1944) |
| Vibrio cholinicus | Vibrio cholinicus N sp., a choline‐fermenting organism, was isolated from black mud from a small stagnant creek. | Hayward and Stadtman (1959) and Hayward and Stadtman (1960) |
| Clostridium sp. | Identification of 26 betaine, choline, creatine and ethanoamine degrading Clostridium species. | Möller et al. (1986) |
| Proteus mirabilis | The cleavage of choline to trimethylamine and acetaldehyde by extracts of Proteus mirabilis was described. | Sandhu and Chase Jr (1986) and Jameson et al. (2016) |
| Streptococcus sanguis I | Mixed bacterial flora was cultured from dental plaque and saliva and the only TMA‐forming bacterium was identified as Streptococcus sanguis I. | Chao and Zeisel (1990) |
| Desulfovibrio desulfuricans G20 | By position‐specific iterative (PSI)‐blast, genes encoding homologues of EutG, EutE and microcompartment protein EutM from Salmonella enterica were found in the genome of Desulfovibrio desulfuricans (ATTC 27774) and the choline utilization (cut) gene cluster was demonstrated to be responsible for choline metabolism and TMA production. | Craciun and Balskus (2012) |
| Klebsiella pneumoniae | The structure and function of the cutC choline lyase from the human microbiota bacterium Klebsiella pneumoniae was characterized. | Kalnins et al. (2015) |
|
cutC: Clostridium XIVa strains and Eubacterium sp. strain AB3007 cntA: Gammaproteobacteria, in particular Escherichia/Shigella |
Databases for genes of the main TMA‐synthesis pathways (cutC, cntA) were established and gene‐targeted assays were designed for quantitative PCR coupled to sequencing of PCR products. The TMA‐producing communities in the faecal samples of 50 individuals were characterized. | Rath et al. (2017) |
The gut microbiota as a novel therapeutic target in CVD and arterial thrombosis
As plasma levels of TMAO correlated with CVD progression and with the incidence of arterial thrombosis (Wang et al., 2011; Zhu et al., 2016, 2017), and as the bacterial strains that exert TMA‐lyase enzyme activity and the wide distribution of the choline utilization (cut) gene cluster in the human intestinal tract are increasingly recognized (Craciun and Balskus, 2012; Koeth et al., 2013; Martínez‐del Campo et al., 2015; Rath et al., 2017) (Table 3), microbiome analyses of faecal samples from patients with diagnosed early atherosclerosis could become an interesting diagnostic option in the future. This may especially be useful to predict disease progression of CVD patients and to decide on nutritional or probiotic interventions. However, it is essential that future clinical studies clarify the conditions under which the TMAO metabolite is associated with increased CVD and arterial thrombosis risk, particularly as others have not found this association (Meyer et al., 2016).
Targeting the enzymes of gut microbes with the aim of reducing the synthesis of microbial metabolites that promote cardiovascular disease and arterial thrombosis in the human host or to enhance beneficial microbial synthesis pathways is currently developing as a promising therapeutic option (Brown and Hazen, 2017). The specific non‐lethal inhibition of gut microbial TMA‐lyase enzymes could be of therapeutic value to reduce the progression of atherosclerotic lesion development, and TMA‐lyases could be a useful target for anti‐thrombotic prophylaxis (Wang et al., 2015). Indeed, in mice fed a high choline or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4780 diet, the inhibition of TMA production by https://www.pubchem.ncbi.nlm.nih.gov/compound/12233 (DMB), a non‐lethal inhibitor of the TMA‐lyases of gut microbes, lowered plasma TMAO levels. In the ApoE‐deficient mouse atherosclerosis model, fed a chemically defined chow diet containing 1% choline, the administration of 1% (v/v) DMB in the drinking water reduced foam cell formation in the atherosclerotic plaques and reduced atherosclerotic lesion size in the aortic root (Wang et al., 2015). Because of the risk of toxic side effects, further studies need to address whether pharmacological targeting of gut bacterial TMA‐lyases can be considered a possible and safe approach.
LPS‐induced activation of TLR4 has been demonstrated in murine and human platelets (see Vallance et al., 2017), and in addition to TLR2, TLR4 expressed by the vascular endothelium promoted arterial thrombosis in mice (Ren et al., 2014). However, it appears challenging if not impossible to target pharmacologically, the microbiota‐derived microbial patterns or the innate immune receptors that drive atherogenesis and arterial thrombosis, as their balanced function is vital for host defence and tissue homeostasis (Rakoff‐Nahoum et al., 2004). Nevertheless, the CANTOS trial, including patients with a history of myocardial infarction and elevated https://www.uniprot.org/uniprot/P02741, has shown that therapeutic inhibition of the inflammatory pathways downstream of pattern recognition, that is, the blockade of IL‐1β with canacinumab, reduced http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 plasma levels and lowered the incidence of recurrent cardiovascular events (Ridker et al., 2017).
In summary, distinct metabolic pathways of the human gut microbiota are currently being identified as promising druggable targets to combat the progression of cardiometabolic diseases.
Concluding remarks
Meanwhile, there is compelling evidence from metagenomics analyses of patient stool samples, clinical studies with patient specimens and gnotobiotic mouse models that implicate the commensal gut microbiota in the development of cardiovascular and cardiometabolic disease. Only recently, the gut microbiota has been identified as a factor affecting arterial thrombosis, but clearly, further investigations with gnotobiotic mouse models are needed to pinpoint the microbiota‐dependent mechanisms that can modulate arterial thrombus growth. In the future, well‐designed prospective clinical studies are required to analyse the translational significance of the identified microbiota‐regulated factors. This should lead to interventional studies with selective inhibitors that target the meta‐organismal pathways promoting CVD and arterial thrombosis.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c).
Author contributions
K.K. and C.R. wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
C.R. received funding by the German Federal Ministry of Education and Research (01EO1503), Deutsche Forschungsgemeinschaft (DFG) individual grants to C.R. (RE 3450/3‐1, RE 3450/5‐1 and RE 3450/5‐2) and a project grant from the Boehringer Ingelheim Foundation.
Kiouptsi, K. , and Reinhardt, C. (2018) Contribution of the commensal microbiota to atherosclerosis and arterial thrombosis. British Journal of Pharmacology, 175: 4439–4449. 10.1111/bph.14483.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al‐Waiz M, Mikov M, Mitchell SC, Smith RL (1992). The exogenous origin of trimethylamine in the mouse. Metabolism 41: 135–136. [DOI] [PubMed] [Google Scholar]
- Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC et al (2008). Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 87: 1219–1223. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI (2007). Mechanisms underlying the resistance to diet‐induced obesity in germ‐free mice. Proc Natl Acad Sci U S A 104: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva‐Datchary P et al (2015). Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17: 690–703. [DOI] [PubMed] [Google Scholar]
- Badimon L, Vilahur G (2014). Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med 276: 618–632. [DOI] [PubMed] [Google Scholar]
- Bain CC, Bravo‐Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S et al (2014). Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15: 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer ML, Schürch CM, Saito Y, Geuking MB, Li H, Cuenca M et al (2014a). Microbiota‐derived compounds drive steady‐state granulopoiesis via MyD88/TICAM signaling. J Immunol 193: 5273–5283. [DOI] [PubMed] [Google Scholar]
- Balmer ML, Slack E, de Gottardi A, Lawson MA, Hapfelmeier S et al (2014b). The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med 6: 237ra66. [DOI] [PubMed] [Google Scholar]
- Bashiardes S, Shapiro H, Rozin S, Shibolet O, Elinav E (2016). Non‐alcoholic fatty liver and the gut microbiota. Mol Metab 5: 782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M et al (2016). Commensal microbiota affects ischemic stroke outcome by regulating γδ T cells. Nat Med 22: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Zimman A, Gao D, Byzova TV, Podrez EA (2017). TLR2 plays a key role in platelet hyperreactivity and accelerated thrombosis associated with hyperlipidemia. Circ Res 121: 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA et al (2004). Reduced atherosclerosis in MyD88‐null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med 10: 416–421. [DOI] [PubMed] [Google Scholar]
- Brown JM, Hazen SL (2017). Targeting of microbe‐derived metabolites to improve human health: the next frontier for drug discovery. J Biol Chem 292: 8560–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar R, Reigstad CS, Bäckhed HK, Reinhardt C, Ketonen M, Lundén GÖ et al (2012). Gut‐derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 61: 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM et al (2008). Changes in gut microbiota control metabolic endotoxemia‐induced inflammation in high‐fat diet‐induced obesity and diabetes in mice. Diabetes 57: 1470–1481. [DOI] [PubMed] [Google Scholar]
- Carnevale R, Nocella C, Petrozza V, Cammisotto V, Pacini L, Sorrentino V et al (2018). Localization of lipopolysaccharide from Escherichia coli into human atherosclerotic plaque. Sci Rep 8: 3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CK, Zeisel SH (1990). Formation of trimethylamine from dietary choline by Streptococcus sanguis I, which colonizes the mouth. J Nutr Biochem 1: 89–97. [DOI] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN (2010). Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen KR, Gruen ML, Lee‐Young RS, Puglisi MJ, Wasserman DH, Hasty AH (2009). Impact of macrophage toll‐like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia 52: 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciun S, Balskus EP (2012). Microbial conversion of choline to trimethylamine requires a glycil radical enzyme. Proc Natl Acad Sci U S A 109: 21307–21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Atri LP, Schattner M (2017). Platelet toll‐like receptors in thromboinflammation. Front Biosci (Landmark Ed) 22: 1867–1883. [DOI] [PubMed] [Google Scholar]
- Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A et al (2013). Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 494: 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunzendorfer S, Lee HK, Soldau K, Tobias PS (2004). Toll‐like receptor 4 functions intracellularly in human coronary artery endothelial cells: roles of LBP and sCD14 in mediating LPS responses. FASEB J 18: 1117–1119. [DOI] [PubMed] [Google Scholar]
- Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ (2002). Expression of toll‐like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105: 1158–1161. [PubMed] [Google Scholar]
- Emoto T, Yamashita T, Kobayashi T, Sasaki N, Hirota Y, Hayashi T et al (2017). Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels 32: 39–46. [DOI] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB et al (2013). Cross‐talk between Akkermansia muciniphila and intestinal epithelium controls diet‐induced obesity. Proc Natl Acad Sci U S A 110: 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåk F, Tremaroli V, Bergström G, Bäckhed F (2015). Oral microbiota in patients with atherosclerosis. Atherosclerosis 243: 573–578. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R (2018). Current understanding of the human microbiome. Nat Med 24: 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ (2000). Identification of periodontal pathogens in atheromatous plaques. J Periodontol 71: 1554–1560. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS guide to pharmacology in 2018: updates and expansion to encompass the new guide to immunopharmacology. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward HR, Stadtman TC (1959). Anaerobic degradation of choline. I. Fermentation of choline by an anaerobic, cytochrome‐producing bacterium, Vibrio cholinicus n. sp. J Bacteriol 78: 558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward HR, Stadtman TC (1960). Anaerobic degradation of choline. II. Preparation and properties of cell‐free extracts of Vibrio chlinicus. J Biol Chem 235: 538–543. [PubMed] [Google Scholar]
- Heiss CN, Olofsson LE (2017). Gut microbiota‐dependent modulation of energy metabolism. J Innate Immun 10: 163–171. 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörmann N, Brandão I, Jäckel S, Ens N, Lillich M, Walter U et al (2014). Gut microbial colonization orchestrates TLR2 expression, signaling and epithelial proliferation in the small intestinal mucosa. PLoS One 9: e113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD et al (2000). A discrete subpopulation of dendritic cells transports apaptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med 191: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäckel S, Kiouptsi K, Lillich M, Hendrikx T, Khandagale A, Kollar B et al (2017). Gut microbiota regulate hepatic von Willebrand factor synthesis and arterial thrombus formation via toll‐like receptor‐2. Blood 130: 542–553. [DOI] [PubMed] [Google Scholar]
- Jameson E, Fu T, Brown IR, Paszkiewicz K, Purdy KJ, Frank S et al (2016). Anaerobic choline metabolism in microcompartments promotes growth and swarming of Proteus mirabilis. Environ Microbiol 18: 2886–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen AWF, Houben T, Katiraei S, Dijk W, Boutens L, van der Bolt N et al (2017). Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: a potential role for bile acids. J Lipid Res 58: 1399–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S et al (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 8: 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins G, Kuka J, Grinberga S, Makrecka‐Kuka M, Liepinsh E, Dambrova M et al (2015). Structure and function of CutC choline lyase from human microbiota bacterium Klebsiella pneumoniae. J Biol Chem 290: 21732–21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D et al (2012). Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3: 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K, Tanoue T, Yamashita T, Yodoi K, Matsumoto T, Emoto T et al (2017). Commensal bacteria at the crossroad between cholesterol homeostasis and chronic inflammation in atherosclerosis. J Lipid Res 58: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandagale A, Reinhardt C (2018). Gut microbiota – architects of small intestinal capillaries. Front Biosci (Landmark Ed) 23: 752–766. [DOI] [PubMed] [Google Scholar]
- Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Goodridge HS et al (2014). Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15: 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E et al (2002). Toll‐like receptor 4 polymorphisms and atherogenesis. N Engl J Med 347: 185–192. [DOI] [PubMed] [Google Scholar]
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT et al (2013). Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaroff AL (2018). The microbiome and risk for atherosclerosis. JAMA 19: 2381–2382. [DOI] [PubMed] [Google Scholar]
- Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V et al (2011). Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A 108 (Suppl. 1): 4592–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr HA, Sagban TA, Ihling C, Zähringer U, Hungerer KD, Blumrich M et al (2001). Immunopathogenesis of atherosclerosis: endotoxin accelerates atherosclerosis in rabbits on hypercholesterolemic diet. Circulation 104: 914–920. [DOI] [PubMed] [Google Scholar]
- Lelouvier B, Servant F, Païssé S, Bruner AC, Benyahya S, Serino M et al (2016). Changes in blood microbiota profiles associated with liver fibrosis in obese patients: a pilot analysis. Hepatology 64: 2015–2027. [DOI] [PubMed] [Google Scholar]
- Li J, Sung CY, Lee N, Ni Y, Pihlajamäki J, Panagiotou G et al (2016a). Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A 113: E1306–E1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lin S, Vanhoutte PM, Woo CW, Xu A (2016b). Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia‐induced inflammation in Apoe−/− mice. Circulation 133: 2434–2446. [DOI] [PubMed] [Google Scholar]
- Lindskog Jonsson A, Caesar R, Akrami R, Reinhardt C, Fåk Hållenius F, Borén J et al (2018). Impact of gut microbiota and diet on the development of atherosclerosis in Apoe−/− mice. Arterioscler Thromb Vasc Biol. 10.1161/ATVBAHA118.311233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC 3rd et al (2008). Toll‐like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis 196: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando AM, Graham WV, Turner JR (2010). Epithelial barriers in homeostasis and disease. Annu Rev Pathol 5: 119–144. [DOI] [PubMed] [Google Scholar]
- Martínez‐del Campo A, Bodea S, Hamer HA, Marks JA, Haiser HJ, Turnbaugh PJ et al (2015). Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut microbiota. MBio 6: e00042–e00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KA, Benton TZ, Bennett BJ, Jacobs DR Jr, Loyd‐Jones DM, Gross MD et al (2016). Microbiota‐dependent metabolite trimethylamine N‐oxide and coronary artery calcium in the coronary artery risk development in young adults study (CARDIA). J Am Heart Assoc 5: e003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM et al (2004). Lack of toll‐like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA 101: 10679–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller B, Hippe H, Gottschalk G (1986). Degradation of various amine compounds by mesophilic clostridia. Arch Microbiol 45: 85–90. [DOI] [PubMed] [Google Scholar]
- Muccioli GG, Naslain D, Bäckhed F, Reigstad C, Lambert DM, Delzenne NM et al (2010). The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 6: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick AE, Tobias PS, Curtiss LK (2005). Modulation of atherosclerosis in mice by toll‐like receptor 2. J Clin Invest 115: 3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick AE, Soldau K, Kiosses WB, Bell TA 3rd, Tobias PS, Curtiss LK (2008). Increased endothelial expression of toll‐like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med 205: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro G, Rossi R, Commere PH, Jay P, Sansonetti PJ (2014). The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe 15: 792–798. [DOI] [PubMed] [Google Scholar]
- Ott SJ, El Mokhtari NE, Musfeldt M, Hellmig S, Freitag S, Rehman A et al (2006). Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation 113: 929–937. [DOI] [PubMed] [Google Scholar]
- Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG et al (2013). Engagement of platelet toll‐like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ Res 112: 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Mendall MA, Carrington D, Strachan DP, Leatham E, Molineaux N et al (1995). Association of Helicobacter pylori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factors. BMJ 311: 711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst HC, Muth S, Schild H (2014). Regulation of the tolerogenic function of steady‐state DCs. Eur J Immunol 44: 927–933. [DOI] [PubMed] [Google Scholar]
- Quillard T, Franck G, Mawson T, Folco E, Libby P (2017). Mechanisms of erosion of atherosclerotic plaques. Curr Opin Lipidol 28: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff‐Nahoum S, Paglino J, Eslami‐Varzaneh F, Edberg S, Medzhitov R (2004). Recognition of commensal microflora by toll‐like receptors is required for intestinal homeostasis. Cell 118: 229–241. [DOI] [PubMed] [Google Scholar]
- Rath S, Heidrich B, Pieper DH, Vital M (2017). Uncovering the trimethylamine‐producing bacteria of the human gut microbiota. Microbiome 5: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt C, Bergentall M, Greiner TU, Schaffner F, Östergren‐Lundén G, Petersen LC et al (2012). Tissue factor and PAR1 promote microbiota‐induced intestinal vascular remodelling. Nature 483: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Li R, Luo M, Chen N, Deng X, Yan K et al (2014). Endothelial cells but not platelets are the major source of toll‐like receptor 4 in the arterial thrombosis and tissue factor expression in mice. Am J Physiol Regul Integr Comp Physiol 307: R901–R907. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C et al (2017). Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- Roderburg C, Luedde T (2014). The role of the gut microbiome in the development and progression of liver cirrhosis and hepatocellular carcinoma. Gut Microbes 5: 441–445. [DOI] [PubMed] [Google Scholar]
- Rune I, Rolin B, Larsen C, Nielsen DS, Kanter JE, Bornfeldt KE et al (2016). Modulating the gut microbiota improves glucose tolerance, lipoprotein profile and atherosclerotic plaque development in ApoE‐deficient mice. PLoS One 11: e0146439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu SS, Chase T Jr (1986). Aerobic degradation of choline by Proteus mirabilis: enzyme requirements and pathway. Can J Microbiol 32: 743–750. [DOI] [PubMed] [Google Scholar]
- Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A et al (2015). A gut‐vascular barrier controls the systemic dissemination of bacteria. Science 350: 830–834. [DOI] [PubMed] [Google Scholar]
- Stepankova R, Tonar Z, Bartova J, Nedorost L, Rossman P, Poledne R et al (2010). Absence of microbiota (germ‐free conditions) accelerates the atherosclerosis in ApoE‐deficient mice fed standard low cholesterol diet. J Atheroscler Thromb 17: 796–804. [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E et al (2018). Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359: 1376–1383. [DOI] [PubMed] [Google Scholar]
- Vallance TM, Zeuner M‐T, Williams HF, Widera D, Vaiyapuri S (2017). Toll‐like receptor 4 signalling and its impact on platelet function, thrombosis, and hemostasis. Mediators Inflamm 2017: 9605894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B et al (2011). Gut flore metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E et al (2015). Non‐lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163: 1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AJ, Keeping FE (1944). The formation of trimethylamine from choline as a characteristic of Shigella alkalescens. J Bacteriol 47: 309–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Feng Q, Liang S, Sonne SB, Xia Z, Qiu X et al (2015). A catalog of the mouse gut metagenome. Nat Biotech 33: 1103–1108. [DOI] [PubMed] [Google Scholar]
- Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC et al (2001). Toll‐like receptor‐4 is expressed by macrophages in murine and human lipid‐rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104: 3103–3108. [DOI] [PubMed] [Google Scholar]
- Yakob M, Söder B, Meurman JH, Jogestrand T, Nowak J, Söder PÖ (2011). Prevotella nigrescens and Porphyromonas gingivalis are associated with signs of carotid atherosclerosis in subjects with and without periodontitis. J Perodontal Res 46: 749–755. [DOI] [PubMed] [Google Scholar]
- Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ et al (2015a). Neutrophil ageing is regulated by the microbiome. Nature 525: 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang S, Hu L, Zhai L, Xue R, Ye J et al (2015b). Nucleotide‐binding oligomerization domain 2 receptor is expressed in platelets and enhances platelet activation and thrombosis. Circulation 131: 1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z et al (2016). Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Wang Z, Tang WHW, Hazen SL (2017). Gut microbe‐generated trimethylamine N‐oxide from dietary choline is prothrombotic in subjects. Circulation 135: 1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]


