Abstract
Aim
Metoprolol (a CYP2D6 substrate) is often co‐prescribed with paroxetine/fluoxetine (a CYP2D6 inhibitor) because the clinical relevance of this drug–drug interaction (DDI) is still unclear. This review aimed to systematically evaluate the available evidence and quantify the clinical impact of the DDI.
Method
Pubmed, Web of Science, Cochrane Library and Embase were searched for studies reporting on the effect of the DDI among adults published until April 2018. Data on pharmacokinetics, pharmacodynamics and clinical outcomes from experimental, observational and case report studies were retrieved. The protocol of this study was registered in PROSPERO (CRD42018093087).
Results
We found nine eligible articles that consisted of four experimental and two observational studies as well as three case reports. Experimental studies reported that paroxetine increased the AUC of metoprolol three to five times, and significantly decreased systolic blood pressure and heart rate of patients. Case reports concerned bradycardia and atrioventricular block due to the DDI. Results from observational studies were conflicting. A cohort study indicated that the DDI was significantly associated with the incidence of early discontinuation of metoprolol as an indicator of the emergence of metoprolol‐related side effects. In a case–control study, the DDI was not significantly associated with bradycardia.
Conclusion
Despite the contradictory conclusions from the current literature, the majority of studies suggest that the DDI can lead to adverse clinical consequences. Since alternative antidepressants and beta‐blockers with comparable efficacy are available, such DDIs can be avoided. Nonetheless, if prescribing the combination is unavoidable, a dose adjustment or close monitoring of the metoprolol‐related side effects is necessary.
Keywords: CYP2D6, drug–drug interaction, metoprolol, paroxetine/fluoxetine
What is Already Known about this Subject
Metoprolol and paroxetine/fluoxetine combination may trigger a CYP2D6 mediated drug–drug interaction (DDI).
Metoprolol and paroxetine/fluoxetine co‐prescription is still observed in clinical practice because the clinical relevance of this DDI is unclear.
What this Study Adds
Despite the conflicting conclusions from the current published literature, the majority of studies suggest that the DDI can cause adverse clinical consequences.
It is prudent to avoid the concurrent use of metoprolol and paroxetine/fluoxetine because alternative and safe antidepressants and beta‐blockers are available. However, if the prescription of the combination is unavoidable, a dose adjustment or close monitoring of metoprolol‐related side effects is necessary.
Introduction
Cardiovascular diseases and depression are still among the most prevalent diseases in the world and they often coincide in patients 1, 2, 3, 4. This situation leads to the co‐prescription of drugs for treating these chronic illnesses. The selective http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=28‐blocker metoprolol is one of the preferred beta‐blockers in general practice guidelines and widely prescribed for patients with cardiac diseases in the Netherlands, New Zealand and US 5, 6, 7, 8, 9, 10. Meanwhile, because of the favourable safety profile, selective http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5 reuptake inhibitors (SSRIs) such as paroxetine and fluoxetine are commonly used to treat depression in this patient population 11, 12. There have been several studies reporting that metoprolol and paroxetine/fluoxetine are commonly co‐prescribed in clinical practice 8, 13. Bahar et al. reported that among all co‐prescriptions of beta‐blockers with paroxetine/fluoxetine during 1994–2014 for elderly patients in the community pharmacies in the Netherlands, 52% of them was metoprolol‐paroxetine/fluoxetine combination. The numbers of other beta‐blockers combined with paroxetine/fluoxetine were 17%, 12% and 19% for atenolol, bisoprolol and any other beta‐blockers respectively 8.
Metoprolol is mainly metabolised by oxidation in the liver 14. However, since the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1329&familyId=262&familyType=ENZYME enzyme is most involved in metoprolol metabolism, strong inhibitors of CYP2D6, such as paroxetine and fluoxetine, may trigger a drug–drug interaction (DDI) which can potentially produce metoprolol‐related toxicities due to an impaired metoprolol clearance 15, 16, 17, 18, 19. Therefore, in a drug database for a computerised DDI surveillance system [such as G‐standaard from the ‘Royal Dutch Association for the Advancement of Pharmacy’ (KNMP)], this DDI is flagged to warn health care providers about the potential risks. However, another drug database for drug–drug interaction alerting system (such as Pharmabase from the Health Base Foundation) decided not to give a signal because of the uncertainties surrounding the DDI 8. The decision whether to provide a safety alert or not for the combination has been reported to influence the number of metoprolol and paroxetine/fluoxetine co‐prescriptions 8. Consequently, the potential metoprolol‐related side effects due to the DDI are not always prevented by the presence of DDI alerts. Hence, it is important to determine the clinical relevance of the DDI.
In the current study, we therefore aimed to evaluate all published studies regarding the clinical impact of the concurrent use of metoprolol and paroxetine/fluoxetine and to quantify potential risks. In addition, we aimed to formulate recommendations on how to manage this DDI in the clinic.
Methods
This study was reported based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐analyses) guideline 20. The study protocol was registered in PROSPERO under number CRD42018093087 (http://www.crd.york.ac.uk). This systematic review only included experimental or observational studies and case reports (without language restrictions) conducted among adults. In addition, articles were included if they (1) concerned a metoprolol and paroxetine/fluoxetine combination and (2) reported the outcomes of the interaction. We excluded conference abstracts, reviews/editorials/letters as well as in vitro and animal studies.
Search strategy
Original studies about the metoprolol and paroxetine/fluoxetine interaction were systematically searched. Pubmed, Web of Science, Cochrane library (limited to clinical trials) and Embase databases were used to identify articles published before April 1, 2018. Search terms included metoprolol, paroxetine, fluoxetine, pharmacokinetics (PK) or pharmacodynamics (PD) parameters, or other relevant outcomes (such as dose adjustment or early discontinuation of metoprolol after the start of the combination). The search queries are available in the Supporting Information Data S1. Records from all databases were exported to a web‐based reference manager, RefWorks, and duplicate records were removed.
Record selection
Titles and abstracts from the unique records were screened by two reviewers independently (MAB and JK) to identify eligible articles. In case of disagreement, discussion to achieve consensus was initiated, and if necessary, a third reviewer was involved (BW). After the initial title and abstract screening, full texts from the eligible records were evaluated also independently by MAB and JK to come to a final selection. The level of inter‐rater agreement was calculated by using a percentage of agreement and reliability Cohen's kappa (ĸ) statistic.
Data extraction
Selected articles were used to extract data on study design, study population, metoprolol and paroxetine/fluoxetine dose, co‐medication, PK/PD data or other relevant outcomes such as odd ratio (OR) or relative risk (RR), and metabolic profiles of participants. Studies were categorised based on their study design: (1) experimental studies, (2) observational studies or (3) case reports. If the study was performed in a way that researchers intentionally gave the combination of metoprolol‐paroxetine/fluoxetine to healthy volunteers/patients in order to observe its outcomes, the study was regarded as an experimental study. However, if researchers only observed the effects of the interaction from the patients with/without the combination without doing any intervention or modification, the study was included as an observational study. Lastly, if the article was a detailed scientific report regarding a single observation about the clinical outcome of the interaction, it was included as a case report.
Quality assessment of included articles
To assess the quality of the studies included in this systematic review, we used the Joanna Briggs Institute critical appraisal tools for assessing the methodological quality of case reports, randomized clinical trials, quasi‐experimental studies, and observational studies (case control and cohort studies) 21. Additionally, we used the National Heart, Lung, and Blood Institute quality assessment tool for assessing the quality of a before‐after study with no control group 22. The complete list of questions from each tools can be found in the Supporting Information Data S2.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 23, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 24, 25.
Results
Database searches and record selection
The systematic literature search in Pubmed, Web of Science, Cochrane library, and Embase resulted in 354, 249, 81 and 894 records respectively. After removal of duplicates (n = 262), 1316 unique records were selected for title and abstract screening. Title and abstract screening resulted in 44 eligible records of which the full texts were evaluated. Full text evaluation led to the exclusion of 35 publications because no metoprolol and paroxetine/fluoxetine combinations were reported (n = 27), no clinical outcomes were reported (n = 5), and they were not original studies (n = 3) (Figure 1). A full overview of the included studies and characteristics is shown in Table 1. The percentage of agreement between reviewers for titles and abstracts screening was 98% with kappa value 0.68 (good) 26. Meanwhile, the percentage of agreement for full text screening was 97% with kappa value 0.93 (very good) 26.
Figure 1.
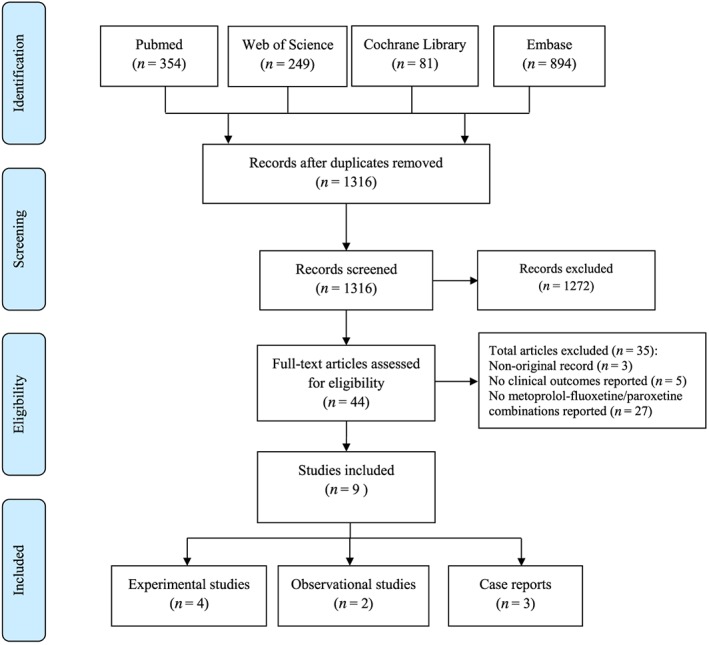
Flow diagram literature search and screening process
Table 1.
Study characteristics
| Reference | Study design | Size (n) | Population type | Co‐medication | Metoprolol Dose | SSRI | Dose |
|---|---|---|---|---|---|---|---|
| O. Onalan et al. 33 | Case report | 1 | Patient (63 years old) | Alprazolam | 50 mg daily | Paroxetine | 20 mg day−1 |
| T. Walley et al. 19 | Case report | 1 | Patient (54 years old) | n/a | 100 mg daily | Fluoxetine | 20 mg day−1 |
| F. König et al. 18 | Case report | 1 | Patient (62 years old) | Lithium | 50 mg twice daily | Paroxetine | 20 mg day−1 |
| S. Stout et al. 27 | Prospective, open‐label, randomized, crossover clinical trial | 10 | Healthy volunteers (18–45 years old) | No | 50 mg single dose (IR), 100 mg single dose (ER) | Paroxetine | 10 mg day−1 b |
| R. Parker et al. 28 | Prospective, open‐label, randomized, crossover clinical trial | 15 | Healthy volunteers (≥21 years old) | No | 100 mg single dose (ER), 200 mg single dose (ER), 200 mg divided in 2 administrations (IR) | Paroxetine | 20 mg day−1 |
| A. Hemeryck et al. 29 | Open trial, pre‐test/post‐test designs (without control group) | 8 | Healthy volunteers (20–29 years old) | No | 100 mg single dose (IR) | Paroxetine | 20 mg day−1 |
| K. Goryachkina et al. 30 | Open‐label, non‐randomized, pre‐test/post‐test designs (with control group) | 17 | AMI patients, with (study group) or without depression (control group) (47–80 years old). | aspirin (13 patients), enalapril (5), spironolactone (1), perindopril (8), quinapril (1), mononitrate (5), trimetazidine (1), simvastatin (1), indapamide (1), clopidogrel (5), iron preparations (2), omeprazole (2), nifedipine (slow release) (3), warfarin (1), amlodipine (1), hydrochlorothiazide (1), ketorolac (1), molsidomine (1) and rosuvastatin (1). | Mean 75 ± 39 mg/day (IR or ER) | Paroxetine | 20 mg day−1 |
| P.A. Kurdyak et al. 31 | Nested case–control | Cases: 99; Control: 394 | Patient (≥66 years old) | Yesa | n/a | Paroxetine or Fluoxetine | n/a |
| M.A. Bahar et al. 32 | Cohort study | Metoprolol‐paroxetine/fluoxetine group: 528; Metoprolol‐citalopram group: 673, and Metoprolol‐mirtazapine group: 625. | Patient (≥60 years old) | Noc | n/a | Paroxetine or Fluoxetine | n/a |
AMI, Actue Miocardial Infarctionl; ER, Extended Release; IR, Immediate Release
No detailed information about co‐medication available; the number of other CYP2D6 inhibitors and chronotropic drugs are adjusted in the multivariate analysis.
After reaching steady state concentrations
patients with other CYP2D6 inhibitors, any other antidepressants beside the studied drugs or using chronotropic drugs were excluded.
Characteristics of included studies
Experimental studies
We found four experimental studies on the relevant combinations. Two of these studies were performed in healthy volunteers and used a prospective open‐label, randomized, crossover study design 27, 28. One study was also conducted in healthy participants but used an open trial with pre‐ and post‐design (without a control group) 29. The fourth study was a nonrandomized intervention study (pre‐and post‐design with a reference group) performed in patients with acute myocardial infarction 30. The distribution of characteristics of each study can be viewed in Table 1.
Observational studies
Two observational studies were included. The first one was a nested case–control study performed by Kurdyak et al. using a study population of Ontario residents with a minimum age of 66 years who were using metoprolol (332 254 patients) 31. The cases were metoprolol users who were hospitalized due to bradycardia and newly treated either with a CYP2D6 inhibitor (fluoxetine/paroxetine) or with a non‐potent CYP2D6 inhibitor (fluvoxamine/citalopram/venlafaxine/sertraline) (99 patients). Meanwhile, the control group consisted of metoprolol users without hospitalization and who were newly treated with the studied drugs (394 patients). The second study is a retrospective cohort study by Bahar et al. using a prescription database (IADB.nl) among Dutch elderly (≥60 years) patients with metoprolol prescription (64 578 patients) who were co‐prescribed with paroxetine/fluoxetine (528 patients), with citalopram (673 patients), and with mirtazapine (625 patients) 32. No PK data were presented in these studies.
Case reports
Three case reports were included in this systematic review 18, 19, 33. The first case report concerned a 63‐year‐old woman who was known with depression and hypertension. The patient received paroxetine andalprazolam for one year after which metoprolol was added to the treatment regimen 33. A second case report concerned a 54‐year‐old depressed man with angina. Initially only metoprolol was prescribed. Fluoxetine was added one month later to treat his depression 19. The last case study reported a case of a 62‐year‐old female patient diagnosed with hypertrophic cardiomyopathy, bipolar disorder and depression, who received both metoprolol and paroxetine 18. None of the case reports presented PK data.
Pharmacokinetic data
Experimental studies
All experimental studies showed significant increases in metoprolol exposure, as measured in the area under the concentration curve (AUC) 27, 28, 29, 30 (Table 2). Hemeryck et al. reported significant increases of metoprolol PK parameters when combined with paroxetine. During the combination treatment, the mean [S] and [R]‐metoprolol AUCs were five and seven times higher, respectively, than with treatment with metoprolol alone 29. Stout et al. reported approximately three‐fold increase in the [S]‐metoprolol AUC, regardless of the metoprolol dosage forms 27. Furthermore, a study by Parker et al. showed comparable results that paroxetine increased the [S]‐metoprolol AUC about three‐fold, regardless of the metoprolol formulations and doses 28. The last two studies showed that the [S]/[R] AUC ratios of metoprolol were significantly decreased after paroxetine intake in the range of 27 to 30%. These results indicate that paroxetine inhibition was greater in [R] than [S] metoprolol causing a loss of stereoselective metoprolol metabolism. Finally, Goryachkina et al. reported a four‐fold increase of the total metoprolol AUC when it was combined with paroxetine 30. Moreover, metoprolol metabolite concentration was significantly decreased by 77% 30.
Table 2.
Overview of the clinical outcomes per study
| Reference | Clinical outcomes interaction | CYP2D6 profile | ||
|---|---|---|---|---|
| Pharmacokinetics | Pharmacodynamics | Other outcomes | ||
| O. Onalan et al. 33 | n/a | Complete AV‐Block | n/a | n/a |
| T. Walley et al. 19 | n/a | Bradycardia, lethargy | n/a | n/a |
| F. König et al. 18 | n/a | Bradycardia, lethargy | n/a | n/a |
| S. Stout et al. 27 |
↑mean AUC [S]‐metoprolol IR (270%, P < 0.001), ↑mean AUC [R]‐metoprolol IR (419%, P < 0.001), ↑mean AUC [S]‐metoprolol ER (246%, P < 0.001), ↑mean AUC [R]‐metoprolol ER (334%, P < 0.001),↓[S]/[R]‐ratio (29% and 30% for IR and ER resp., P < 0.001) |
↓mean systolic blood pressure (7.3%, P < 0.001) no changes in HR or P‐R interval observed between baseline and any of the study phases | n/a | n/a |
| R. Parker et al. 28 |
↑mean AUC [S]‐metoprolol IR (209%, P < 0.05) ↑mean AUC [R]‐metoprolol IR (288%, P < 0.05) ↑mean AUC [S]‐metoprolol 100 mg ER (220%, P < 0.05) ↑mean AUC [R]‐metoprolol 100 mg ER(220%, P < 0.05) ↑mean AUC [S]‐metoprolol 200 mg ER (210%, P < 0.05) ↑mean AUC [R]‐metoprolol 200 mg ER (297%, P < 0.05) ↓[S]/[R]‐ratio metoprolol IR (27%, P < 0.05) ↓[S]/[R]‐ratio metoprolol 100 mg ER (27%, P < 0.05) ↓[S]/[R]‐ratio metoprolol 200 mg ER (27%, P < 0.05) |
↓AUEC exercise HR IR formulation (12%, P < 0.05), ↓AUEC exercise HR 100 mg ER (8.6%, P < 0.05), ↓AUEC exercise HR 200 mg ER (9.5%, P < 0.05), ↓AUEC exercise systolic blood pressure IR formulation (7.5%, P < 0.05), ↓AUEC exercise systolic blood pressure 100 mg ER (9.2%, P < 0.05), ↓AUEC exercise systolic blood pressure 200 mg ER (11.1%, P < 0.05) |
n/a | CYP2D6*1/*1 (n = 3), CYP2D6*1/*2 (n = 4), Other [at least 1 active CYP2D6 allele] (n = 8) |
| A. Hemeryck et al. 29 | ↑mean AUC [S]‐metoprolol (408%, P < 0.001), ↑mean AUC [R]‐metoprolol (693%, P < 0.001) |
↑AUEC reduction in exercise HR (46%, P < 0.01)b
↑AUEC reduction in exercise systolic blood pressure (97%, P < 0.05)b |
n/a | Extensive metabolizers |
| K. Goryachkina et al. 30 | ↑mean AUC metoprolol (321%, P < 0.0001), ↓mean AUC α‐hydroxy‐metoprolol (77%, P < 0.0001), | ↓AUEC resting HR (13%, P = 0.0007) severe postural hypotension (n = 1)abradycardia [<45 BPM] (n = 1)a | n/a | CYP2D6*1/*1 (n = 9), CYP2D6*1/*3 (n = 3), CYP2D6*1/*4 (n = 5) |
| P.A. Kurdyak et al. 31 | n/a | Compared to fluvoxamine,citalopram, and venlafaxine, ‐metoprolol: Bradycardia (OR = 1.01, 95% CI 0.53–1.94) | n/a | |
| M.A. Bahar et al. 32 | n/a | n/a |
Compared to citalopram‐metoprolol: Early discontinuation of metoprolol (OR = 1.07, 95% CI:0.77–1.48); dose adjustment of metoprolol (OR = 1.00, 95% CI 0.65–1.54) Compared to mirtazepine‐metoprolol: Early discontinuation of metoprolol (OR = 1.43, 95% CI 1.01–2.02); dose adjustment of metoprolol (OR = 1.00, 95% CI 0.65–1.54) |
n/a |
AUC, Area under the Concentration Curve; AUEC, Area under the Effect Curve; BPM, Beats per minute; ER, Extended Release; HR, Heart Rate; IR, Immediate Release; MR, Metabolic Ratio; OR, Odds Ratio.
Patient carrying one non‐functional CYP2D6 allele.
% change from the baseline (before metoprolol intake) in 4‐min exercise tests.
Pharmacodynamic data
Experimental studies
The evaluation of the PD outcomes of the experimental studies showed comparable results. Hemeryck et al. found an increase in β1‐blocking effects of metoprolol after paroxetine administration. The β1‐blocking effect of metoprolol at different time points (before and after paroxetine treatment) was defined as the percentage of change in the heart rate and systolic blood pressure compared to the baseline (before metoprolol administration) in a 4‐min exercise test. Concomitant paroxetine administration enhanced the reduction of exercise‐induced heart rate and systolic blood pressure by 46% and 97%, respectively 29. Similar effects on the β1‐blocking capacities of metoprolol were found by Parker et al. Yet, the study reported the area under the effect time curve (AUEC) for both the exercise‐induced heart rate and systolic blood pressure responses from 0–24 h after each metoprolol doses/formulations administrations to determine the total β1‐blocking effect before and after paroxetine co‐administration 28. Paroxetine significantly reduced the exercise heart rate AUEC in patients treated with immediate‐release metoprolol (metoprolol IR), extended‐release metoprolol (metoprolol ER) 100 mg, and metoprolol ER 200 mg by 12%, 8.6% and 9.5%, respectively. In addition, paroxetine significantly decreased the exercise systolic pressure AUEC in patients treated with metoprolol IR, metoprolol ER 100 mg, and metoprolol ER 200 mg by 7.5%, 9.2%, and 11%, respectively. No significant differences were found between patients treated with the different formulations in systolic blood pressure or heart rate AUEC 28.
Stout et al. reported only limited PD outcomes. A comparison of the resting systolic blood pressure between metoprolol only and metoprolol/paroxetine phases showed that paroxetine was able to significantly alleviate the mean resting systolic blood pressure by approximately 7.3%. No differences were found in the pharmacodynamic outcomes from the different metoprolol formulations 27.
Goryachkina et al. reported a significant decrease in resting heart rates in the study group after the addition of paroxetine, with the heart rate AUEC decreasing by 13%. Moreover, this decrease was not observed in the metoprolol only group. They reported that there were two patients needing a dose adjustment of metoprolol because they developed severe postural hypotension and bradycardia. Both patients had one inactive allele of CYP2D6. No physical exertion tests were performed in this study because such tests were not part of the standard clinical management of acute myocardial infarction (AMI) patients 30.
Case–control study
Within the metoprolol receiving cohort, Kurdyak et al. observed 8232 cases that were hospitalised due to bradycardia. Of the 8232 hospitalised cases, 99 patients were found to be newly treated with an SSRI within the 30 days prior to hospitalisation. Paroxetine or fluoxetine was prescribed in 23 of these cases (23.2%). No evidence for an increased risk for bradycardia was found in this study (OR 1.01; 95% CI 0.53–1.94, P = 0.98) 31.
Case reports
All case reports presented patients with cardiac adverse events after concomitant use of metoprolol and paroxetine/fluoxetine. Two studies reported the emergence of bradycardia (36 bpm and 41 ppm) 18, 19, 33. Moreover, one case presented a complete atrioventricular block that was attributed to the DDI 33. In the report by Walley et al., the heart rate returned to normal after discontinuation of fluoxetine. Moreover, no bradycardia was observed after fluoxetine rechallenge without concomitant metoprolol use 19. In the case presented by König et al., bradycardia persisted even after a reduction of the metoprolol dose (50 mg/day to 25 mg day−1). It was therefore decided to discontinue metoprolol, which resulted in a normalisation of the heart rate 18.
Onalan et al. described a patient that presented with a complete AV block while being treated with both metoprolol and paroxetine. The patient had been transferred to their clinic because she was being considered for a permanent pacemaker. Because a possible DDI was suspected, paroxetine and metoprolol were discontinued. Five days after paroxetine and metoprolol discontinuation, the AV block was completely recovered. Rechallenge with unchanged doses of metoprolol or paroxetine only did not induce bradycardia 33.
Other relevant outcomes
A cohort study by Bahar et al. used two proxy outcomes, early discontinuation and dose adjustment of metoprolol, as indicators of the appearance of metoprolol‐related side effects after the start of paroxetine/fluoxetine in metoprolol prescription. The study had no information on pharmacokinetics and pharmacodynamics parameters of metoprolol. Compared to the metoprolol‐citalopram combination, metoprolol‐paroxetine/fluoxetine was not significantly correlated with the early discontinuation (OR = 1.07, 95% CI:0.77–1.48) and dose adjustment of metoprolol (OR = 0.87, 95% CI:0.57–1.33). However, because of the reported weak inhibitory capacity of citalopram on the metabolic activity of CYP2D6, the authors suggested a second comparator, mirtazapine‐metoprolol 16, 34, 35. The comparison of the interacting combination with the second control showed that metoprolol‐paroxetine had a significant correlation with the early discontinuation of metoprolol (OR = 1.43, 95% CI:1.01–2.02) but not the dose adjustment of metoprolol (OR = 1.00, 95% CI:0.65–1.54) 32.
Co‐medication
Co‐medication might be a source of additional drug–drug interactions with both metoprolol and paroxetine/fluoxetine. As far as reported, the influence of co‐medication in the publications included in this review was limited. In the two cases reports, Onalan et al. and König et al. reported that beside the combination of metoprolol and paroxetine, patients were co‐medicated with alprazolam and lithium, respectively. Both drugs have no PK/PD interaction with either metoprolol or paroxetine 36, 37. In a study reported by Goryachkina et al., some patients with the metoprolol‐paroxetine combination were using other drugs (Table 1). These drugs also have no clinically significant interaction either with metoprolol or paroxetine, with an exception for the combination of acetylsalicylic acid with paroxetine 37, 38. This interaction increases the risk of gastrointestinal bleeding 38, 39, 40, 41, 42, 43. Therefore, it is recommended to prescribe proton pump inhibitors to manage this interaction 44, 45.
Quality assessment of included studies
The RCT studies did not give sufficient information about the procedure of randomization and allocation concealment. Both of the randomized studies were open trials, therefore, no blinding procedures were used. For the pre‐post study without reference group, there is a low risk of bias based on the question list of the assessment tool. However, the study has only limited sample size, and due to the lack of a control group, the validity of the results might be questioned. The quality assessment of the quasi‐experimental study revealed a low risk of bias. The presence of the reference group in this study design was expected to increase validity of the results. However, the treatment and control groups in the study were not comparable because these concerned different clinical conditions. The treatment group consisted of patients with AMI developing depression, whereas the control group only consisted of patients with AMI. Lastly, both observational studies carried a low risk of bias. All the case reports demonstrated the characteristics, clinical conditions, the medical history, the intervention and the adverse events sufficiently. However, none of them described the family history of the patient including their genetic information. The complete results of the quality assessment of included studies can be seen in Table 3.
Table 3.
Assessments of the methodological quality of the included studies
| Risk of Bias Tools | Reference | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JBI Critical Appraisal Checklist for Case Reports | O. Onalan et al. 33 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||
| T. Walley et al. 19 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | ||||||
| F. König et al. 18 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||||
| JBI Critical Appraisal Checklist for RCTs | S. Stout et al. 27 | Unclear | Unclear | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| R. Parker et al. 28 | Unclear | Unclear | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| NHLBI Critical Appraisal Checklist for before‐after studies with no control group | A. Hemeryck et al. 29 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | NA | |
| JBI Critical Appraisal Checklist for Quasi‐Experimental Study | K. Goryachkina et al. 30 | Yes | No | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| JBI Critical Appraisal Checklist for Case Control Study | P.A. Kurdyak et al. 31 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||
| JBI Critical Appraisal Checklist for Cohort Study | M.A. Bahar et al. 32 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes |
Discussion
Here we present a systematic review that addressed the PK, PD and clinical relevance of the metoprolol and paroxetine/fluoxetine interaction. We found nine studies that provided information on the impact of this DDI. The experimental studies and case reports indicated that the DDI may cause important clinical effects. However, the results from the observational studies were conflicting. The only case control study showed that metoprolol‐paroxetine/fluoxetine was not significantly associated with bradycardia. Meanwhile, a cohort study indicated that the combination is significantly associated with an early discontinuation of metoprolol. The current conflicting evidence arising from these single studies regarding the clinical effect of the interaction may have become one of the underlying reasons for the frequent co‐administration of the combination in clinical practice 8.
Due to the limited number of cases and the lack of pharmacokinetic data, the case reports seem not to provide sufficient evidence to make a clear statement about the clinical relevance of the combination 18, 19, 33. However, conclusions from the case reports were further supported by the outcomes of experimental studies 27, 28, 29. Paroxetine was reported to significantly increase metoprolol exposure (three to five‐fold increase of [S]‐metoprolol AUC) and reduce the heart rate and systolic blood pressure of patients, both in rest and exercise state. Although no experimental studies used fluoxetine, we assume that the impact of the interaction is comparable. Paroxetine and fluoxetine have an equipotent inhibitory capacity on CYP2D6 metabolic activity (Ki value = 0.15 microM and 0.60 microM, respectively) 16. Moreover, the major metabolite of fluoxetine, norfluoxetine, was also reported to have an equal inhibitory potency on CYP2D6 (Ki = 0.43 microM) 16. The combination may therefore trigger clinically relevant adverse events.
The loss of stereoselective metabolism of metoprolol was also found in the experimental studies. CYP2D6 has been shown to preferentially metabolise the inactive [R]‐enantiomer 30. Therefore, the inhibition of metoprolol metabolism by paroxetine/fluoxetine might cause [R]‐enantiomer concentrations to increase more than those of the [S]‐enantiomer, as suggested by the decreased [S]/[R] AUC ratios 27, 28, 29. Since the [R]‐enantiomer has a lower affinity and selectivity for the β‐1 receptor, a relative increase of this enantiomer might lead to a loss of cardio‐selectivity 27. However, only few metoprolol related non‐cardiac side‐effects were reported, with the exception of fatigue, nausea, drowsiness, sleepiness, and diarrhea, which might be also related to paroxetine pharmacodynamic effects 27, 28, 29. Metoprolol is available in IR and ER preparations. There were no clinically significant differences in the interaction with paroxetine between the IR and ER preparations.
The conflicting impact of the combination is illustrated by the observational studies 31, 32. Kurdyak et al. reported that no increased risk of bradycardia in patients with the combination. However, the study has several limitations because it has no data on PK, drug dose, and heart rates. Moreover, the study did not control for mild inhibitory effects of citalopram and fluvoxamine, which were included in the reference group, on CYP2D6 16, 34, 35, 46, 47, 48. It may influence the outcome of interaction especially in a senior population because of physiological changes due to the aging process. Additionally, citalopram has been associated with an increased risk of bradycardia particularly in the elderly population 49, 50, 51, 52, 53. The risk might be even higher in elderly patients with cardiovascular problems who are treated with metoprolol. Finally, the statistical power of the study was relatively low which led to a very wide confidence interval.
Bahar et al. tried to avoid the confounding effect of the weak inhibitory activity of citalopram on CYP2D6 and its potential bradycardia inducing effect by offering an alternative comparison mirtazapine‐metoprolol 32. Mirtazapine is an atypical antidepressant which has no interaction with metoprolol 34, 54. They found that the metoprolol and paroxetine/fluoxetine combination confers a significant 43% higher risk of early discontinuation of metoprolol, but not dose adjustment of metoprolol. It seems that medical doctors tend to stop the use of metoprolol instead of adjusting its dose when there is an emergence of metoprolol‐related side effects. However, their study is not without limitation. They used a prescription database which does not have any PK and PD information on metoprolol. They only used proxy outcomes to indicate the emergence of metoprolol‐related side effects. A prescription database only records the information from the prescription which may not reflect the real situation of the patients, for example, whether they take their drugs as prescribed. Another limitation was that they only used the name of drugs for treating certain diseases as a proxy for the comorbidities which may cause the early discontinuation of metoprolol.
Both observational studies have also another important limitation. They did not have any information regarding the phenotype of CYP2D6 which may influence the magnitude of the DDI 32. Metoprolol metabolism is greatly dependent on the CYP2D6 phenotype status 55, 56. Therefore, the potential effect on certain risk populations might be ignored, as can be seen from a study by Goryachkina et al. They reported that two of their patients, who had one non‐active CYP2D6 allele, experienced postural hypotension and excessive bradycardia during the use of the metoprolol‐paroxetine combination, and therefore required a dose adjustment of metoprolol. Meanwhile, other participants with the normal metabolizer (NM) genotype of CYP2D6 did not experience the side effects 30. It has been reported that patients with less active CYP2D6 enzyme are more prone to experiencing a phenoconversion to poor metabolizer (PM) CYP2D6 than patients with a fully active enzyme after administration of a strong CYP2D6 inhibitor. Moreover, apart from its potent CYP2D6 inhibitory capacity, paroxetine itself is also metabolized by CYP2D6. Therefore, the concentration of paroxetine that is available to inhibit the activity of a lower metabolic activity of CYP2D6 is higher in the patients with CYP2D6 intermediate metabolizer (IM) than NM genotype 57.
The identification of susceptible patient populations should be the first step towards clinical management guidelines. Patients with deviating genotypes such as PM, IM or ultra‐rapid metabolizer (UM) genotypes for CYP2D6 might experience a different magnitude of DDI compared to those with NM 58. Therefore, genotype and phenotype information of the patients are important factors to be considered in the management of DDI. The prevalence of deviating CYP2D6 genotypes in Caucasians is 3 to 5% UM, 10 to 17% IM and 5 to 10% PM 59, 60. In addition, patients that are more susceptible for the side effects of increased β‐blockade, such as elderly patients with bronchospastic disease or with a poor left ventricular systolic function have been suggested to be at higher risk for the interaction 28. Bahar et al. also reported that female elderly patients with the interacting combination have a significantly 62% higher risk for early discontinuation of metoprolol compared to those without the DDI. This difference was not observed in male elderly patients. The authors explained that it might be caused by differences in the body mass index and the rate of CYP2D6 metabolic activity between men and women. However, the data regarding the latter is not clear 32.
Furthermore, other safer SSRIs with comparable effectiveness could be used instead of paroxetine/fluoxetine in depressed patients that are on metoprolol 61. Patients that are started on concomitant metoprolol and paroxetine/fluoxetine therapy should be carefully monitored, at least during the first few weeks of the treatment, to allow timely intervention if a significant hypotension or bradycardia starts to occur.
The strength of our study is that we used a systematic search strategy to include all published articles without any language restrictions in four databases. Therefore, we included all published evidence available regarding the clinical impact of the combination. The limitation of this current study is that we cannot perform meta‐analysis of the data since the studies had different characteristics. Hence, we only provide a description of the result of the included studies, and quantification of effects was based on single overall study results.
In conclusion, despite the conflicting evidence, most of the studies indicate that the DDI have a significant clinical impact. More research is required to determine the clinical impact of difference in CYP2D6 phenotype on the magnitude of metoprolol‐paroxetine/fluoxetine combination. The studies should include more patients with genotype and phenotype information and should have complete PK and PD data. Since alternative and safe antidepressants and beta‐blockers are available, it is prudent to avoid the concurrent use of metoprolol and paroxetine/fluoxetine. Nonetheless, if the prescription of the combination is unavoidable, a does adjustment or a close monitoring of metoprolol‐related side effects is necessary.
Competing Interests
M.A.B., E.H., and B.W. are authors of one article included in this systematic review. No other competing interests are declared.
M.A.B. has obtained a DIKTI scholarship from the Ministry of Research, Technology and Higher Education of Indonesia.
Supporting information
Data S1 Search strategy
Data S2 Critical Appraisal Checklist
Bahar, M. A. , Kamp, J. , Borgsteede, S. D. , Hak, E. , and Wilffert, B. (2018) The impact of CYP2D6 mediated drug–drug interaction: a systematic review on a combination of metoprolol and paroxetine/fluoxetine. Br J Clin Pharmacol, 84: 2704–2715. 10.1111/bcp.13741.
References
- 1. Joynt KE, Whellan DJ, O'Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry 2003; 54: 248–261. [DOI] [PubMed] [Google Scholar]
- 2. Garcia Vicente E, Del Villar Sordo V, Garcia Y, Garcia EL. Post‐myocardial infarction depression. An Med Interna 2007; 24: 346–351. [DOI] [PubMed] [Google Scholar]
- 3. Strik JJ, Honig A, Maes M. Depression and myocardial infarction: relationship between heart and mind. Prog Neuropsychopharmacol Biol Psychiatry 2001; 25: 879–892. [DOI] [PubMed] [Google Scholar]
- 4. Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, et al Depression, heart rate variability, and acute myocardial infarction. Circulation 2001; 104: 2024–2028. [DOI] [PubMed] [Google Scholar]
- 5. Herlitz J, Wikstrand J, Denny M, Fenster P, Heywood T, Masszi G, et al Effects of metoprolol CR/XL on mortality and hospitalizations in patients with heart failure and history of hypertension. J Card Fail 2002; 8: 8–14. [DOI] [PubMed] [Google Scholar]
- 6. Goldstein S, Fagerberg B, Hjalmarson Å, Kjekshus J, Waagstein F, Wedel H, et al Metoprolol controlled release/extended release in patients with severe heart failure: analysis of the experience in the MERIT‐HF study. J Am Coll Cardiol 2001; 38: 932–938. [DOI] [PubMed] [Google Scholar]
- 7. Wiersma T, Verduijn M, Bouma M, Goudswaard A. NHG houdt voorkeur voor metoprolol. Huisarts Wet 2008; 51: 283–286. [Google Scholar]
- 8. Bahar MA, Hak E, Bos JHJ, Borgsteede SD, Wilffert B. The burden and management of cytochrome P450 2D6 (CYP2D6)‐mediated drug‐drug interaction (DDI): co‐medication of metoprolol and paroxetine or fluoxetine in the elderly. Pharmacoepidemiol Drug Saf 2017; 26: 752–765. [DOI] [PubMed] [Google Scholar]
- 9. BPAC . Beta‐blockers for cardiovascular conditions: one size does not fit all patients 2017; 2018: 1.
- 10. Medlicott R. Over‐reliance in cardiovascular treatment – a supply risk that needs to change 2017; 2018: 1.
- 11. Jiang W, Davidson JR. Antidepressant therapy in patients with ischemic heart disease. Am Heart J 2005; 150: 871–881. [DOI] [PubMed] [Google Scholar]
- 12. Mavrides N, Nemeroff C. Treatment of depression in cardiovascular disease. Depress Anxiety 2013; 30: 328–341. [DOI] [PubMed] [Google Scholar]
- 13. Molden E, Garcia B, Braathen P, Eggen A. Co‐prescription of cytochrome (P450) 2D6/3A4 inhibitor‐substrate pairs in clinical practice. A retrospective analysis of data from Norwegian primary pharmacies. Eur J Clin Pharmacol 2005; 61: 119–125. [DOI] [PubMed] [Google Scholar]
- 14. Otton SV, Crewe HK, Lennard MS, Tucker GT, Woods HF. Use of quinidine inhibition to define the role of the sparteine/debrisoquine cytochrome P450 in metoprolol oxidation by human liver microsomes. J Pharmacol Exp Ther 1988; 247: 242–247. [PubMed] [Google Scholar]
- 15. Johnson JA, Burlew BS. Metoprolol metabolism via cytochrome P4502D6 in ethnic populations. Drug Metab Dispos 1996; 24: 350–355. [PubMed] [Google Scholar]
- 16. Crewe HK, Lennard MS, Tucker GT, Woods FR, Haddock RE. The effect of selective serotonin re‐uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol 1992; 34: 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Belpaire FM, Wijnant P, Temmerman A, Rasmussen BB, Brøsen K. The oxidative metabolism of metoprolol in human liver microsomes: Inhibition by the selective serotonin reuptake inhibitors. Eur J Clin Pharmacol 1998; 54: 261–264. [DOI] [PubMed] [Google Scholar]
- 18. Konig F, Hafele M, Hauger B, Loble M, Wossner S, Wolfersdorf M. Bradycardia after beginning therapy with metoprolol and paroxetine. Psychiatr Prax 1996; 23: 244–245. [PubMed] [Google Scholar]
- 19. Walley T, Pirmohamed M, Proudlove C, Maxwell D. Interaction of metoprolol and fluoxetine [23]. Lancet 1993; 341: 967–968. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute 2017.
- 22. NHLBI R. International . Quality assessment tool for before‐after (pre‐post) studies with no control group, 2014.
- 23. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2017; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, et al The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 2017; 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petrie A, Sabin C. Medical statistics at a glance. John Wiley & Sons, 2013. [Google Scholar]
- 27. Stout SM, Nielsen J, Welage LS, Shea M, Brook R, Kerber K, et al Influence of Metoprolol Dosage Release Formulation on the Pharmacokinetic Drug Interaction With Paroxetine. J Clin Pharmacol 2011; 51: 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parker RB, Soberman JE. Effects of Paroxetine on the Pharmacokinetics and Pharmacodynamics of Immediate‐Release and Extended‐Release Metoprolol. Pharmacotherapy 2011; 31: 630–641. [DOI] [PubMed] [Google Scholar]
- 29. Hemeryck A, Lefebvre R, De Vriendt C, Belpaire F. Paroxetine affects metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin Pharmacol Ther 2000; 67: 283–291. [DOI] [PubMed] [Google Scholar]
- 30. Goryachkina K, Burbello A, Boldueva S, Babak S, Bergman U, Bertilsson L. Inhibition of metoprolol metabolism and potentiation of its effects by paroxetine in routinely treated patients with acute myocardial infarction (AMI). Eur J Clin Pharmacol 2008; 64: 275–282. [DOI] [PubMed] [Google Scholar]
- 31. Kurdyak PA, Manno M, Gomes T, Mamdani MM, Juurlink DN. Antidepressants, metoprolol and the risk of bradycardia. Ther Adv Psychopharmacol 2012; 2: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bahar MA, Wang Y, Bos JH, Wilffert B, Hak E. Discontinuation and dose adjustment of metoprolol after metoprolol‐paroxetine/fluoxetine co‐prescription in Dutch elderly. Pharmacoepidemiol Drug Saf 2018; 27.6: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Onalan O, Cumurcu BE, Bekar L. Complete atrioventricular block associated with concomitant use of metoprolol and paroxetine. Mayo Clin Proc 2008; 83: 595–599. [DOI] [PubMed] [Google Scholar]
- 34. Molden E, Spigset O. Interactions between metoprolol and antidepressants. Tidsskr Nor Laegeforen 2011; 131: 1777–1779. [DOI] [PubMed] [Google Scholar]
- 35. Lane RM. Pharmacokinetic drug interaction potential of selective serotonin reuptake inhibitors. Int Clin Psychopharmacol 1996; 11 (Suppl. 5): 31–61. [DOI] [PubMed] [Google Scholar]
- 36. Hawley C, Roberts A, Walker M. Tolerability of combined treatment with lithium and paroxetine: 19 cases treated under open conditions. J Psychopharmacol 1994; 8: 266–267. [DOI] [PubMed] [Google Scholar]
- 37. Hansten PD, Horn JR. Drug interactions: analysis and management. St. Louis, Missouri: Wolters Kluwer Health, 2014. [Google Scholar]
- 38. Loke Y, Trivedi A, Singh S. Meta‐analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non‐steroidal anti‐inflammatory drugs. Aliment Pharmacol Ther 2008; 27: 31–40. [DOI] [PubMed] [Google Scholar]
- 39. Rodríguez LAG, Martín‐Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long‐term low‐dose aspirin: a systematic review of observational studies. PLoS One 2016; 11: e0160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Abajo FJ, Rodriguez LA, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case‐control study. BMJ 1999; 319: 1106–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dalton SO, Johansen C, Mellemkjær L, Sørensen HT, Nørgård B, Olsen JH. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population‐based cohort study. Arch Intern Med 2003; 163: 59–64. [DOI] [PubMed] [Google Scholar]
- 42. de Jong JC, van den Berg PB, Tobi H, de Jong‐van den Berg LT. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol 2003; 55: 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laporte S, Chapelle C, Caillet P, Beyens M, Bellet F, Delavenne X, et al Bleeding risk under selective serotonin reuptake inhibitor (SSRI) antidepressants: a meta‐analysis of observational studies. Pharmacol Res 2017; 118: 19–32. [DOI] [PubMed] [Google Scholar]
- 44. Jiang H, Chen H, Hu X, Yu Z, Yang W, Deng M, et al Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2015; 13: 42 50.e3. [DOI] [PubMed] [Google Scholar]
- 45. Targownik LE, Bolton JM, Metge CJ, Leung S, Sareen J. Selective serotonin reuptake inhibitors are associated with a modest increase in the risk of upper gastrointestinal bleeding. Am J Gastroenterol 2009; 104: 1475–1482. [DOI] [PubMed] [Google Scholar]
- 46. Alfaro CL, Lam YF, Simpson J, Ereshefsky L. CYP2D6 status of extensive metabolizers after multiple‐dose fluoxetine, fluvoxamine, paroxetine, or sertraline. J Clin Psychopharmacol 1999; 19: 155–163. [DOI] [PubMed] [Google Scholar]
- 47. Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. Clin Pharmacokinet 1997; 32: 1–21. [DOI] [PubMed] [Google Scholar]
- 48. Spina E, Campo GM, Avenoso A, Pollicino MA, Caputi AP. Interaction between fluvoxamine and imipramine/desipramine in four patients. Ther Drug Monit 1992; 14: 194–196. [DOI] [PubMed] [Google Scholar]
- 49. Barak Y, Swartz M, Levy D, Weizman R. Age‐related differences in the side effect profile of citalopram. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27: 545–548. [DOI] [PubMed] [Google Scholar]
- 50. Brucculeri M, Kaplan J, Lande L. Reversal of Citalopram‐Induced Junctional Bradycardia with Intravenous Sodium Bicarbonate. Pharmacotherapy 2005; 25: 119–122. [DOI] [PubMed] [Google Scholar]
- 51. Favre M, Sztajzel J, Bertschy G. Bradycardia during citalopram treatment: a case report. Pharmacol Res 1999; 39: 149–150. [DOI] [PubMed] [Google Scholar]
- 52. Isbister GK, Prior FH, Foy A. Citalopram‐induced bradycardia and presyncope. Ann Pharmacother 2001; 35: 1552–1555. [DOI] [PubMed] [Google Scholar]
- 53. Padala KP, Padala PR, Wengel SP. Dose‐dependent bradycardia with citalopram in an elderly patient. Prim Care Companion J Clin Psychiatry 2010; 12: PCC.09100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Owen JR, Nemeroff CB. New antidepressants and the cytochrome P450 system: focus on venlafaxine, nefazodone, and mirtazapine. Depress Anxiety 1998; 7: 24–32. [PubMed] [Google Scholar]
- 55. Lennard MS, Silas JH, Freestone S, Ramsay LE, Tucker GT, Woods HF. Oxidation phenotype—a major determinant of metoprolol metabolism and response. N Engl J Med 1982; 307: 1558–1560. [DOI] [PubMed] [Google Scholar]
- 56. Lennard M, Tucker G, Silas J, Freestone S, Ramsay L, Woods H. Differential stereoselective metabolism of metoprolol in extensive and poor debrisoquin metabolizers. Clin Pharmacol Ther 1983; 34: 732–737. [DOI] [PubMed] [Google Scholar]
- 57. Storelli F, Matthey A, Lenglet S, Thomas A, Desmeules J, Daali Y. Impact of CYP2D6 functional allelic variations on phenoconversion and drug–drug interactions. Clin Pharmacol Ther 2017; 104: 148–157. [DOI] [PubMed] [Google Scholar]
- 58. Bahar MA, Setiawan D, Hak E, Wilffert B. Pharmacogenetics of drug–drug interaction and drug–drug–gene interaction: a systematic review on CYP2C9, CYP2C19 and CYP2D6. Pharmacogenomics 2017; 18: 701–739. [DOI] [PubMed] [Google Scholar]
- 59. Zhou S. Polymorphism of human cytochrome P450 2D6 and its clinical significance. Clin Pharmacokinet 2009; 48: 761–804. [DOI] [PubMed] [Google Scholar]
- 60. Sachse C, Brockmoller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 1997; 60: 284–295. [PMC free article] [PubMed] [Google Scholar]
- 61. Preskorn SH, Greenblatt DJ, Flockhart D, Luo Y, Perloff ES, Harmatz JS, et al Comparison of duloxetine, escitalopram, and sertraline effects on cytochrome P450 2D6 function in healthy volunteers. J Clin Psychopharmacol 2007; 27: 28–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Search strategy
Data S2 Critical Appraisal Checklist


