Abstract
Aims
Drug prescription is difficult to manage in patients with chronic kidney disease (CKD). We assessed the prevalence and determinants of inappropriate drug prescriptions (whether contraindications or inappropriately high doses) with regard to kidney function in patients with CKD under nephrology care. We also assessed the impact of the equation used to estimate GFR on the prevalence estimates.
Methods
The CKD‐REIN cohort includes 3033 outpatients with CKD (eGFR between 15 and 60 ml min−1 1.73 m−2). We examined the daily doses of pharmacological agents prescribed at study entry. Inappropriate prescription was defined as the reported prescription of either a contraindicated drug or an indicated drug at an inappropriately high dose level with regard to the patient's GFR, as estimated with the CKD‐EPI equation, the de‐indexed CKD‐EPI equation, or the Cockcroft–Gault (CG) equation. Multivariate logistic regression was used to assess the determinants of inappropriate prescription risk.
Results
At baseline, patients' median [interquartile range] number of drugs prescribed per patient was 8 [5–10]. Half of the patients had been prescribed at least one inappropriate drug. Anti‐gout, cardiovascular agents and antidiabetic agents accounted for most of the inappropriate prescriptions. The percentage of inappropriate prescriptions varied from one GFR equation to another: 52% when using the CKD‐EPI equation, 47% when using the de‐indexed CKD‐EPI equation and 41% with the CG equation. A multiple logistic regression analysis showed significantly higher odds ratios [95% confidence interval] for inappropriate prescriptions in male patients (1.28 [1.07; 1.53]), patients with diabetes (1.34 [1.06; 1.70]), those with a high BMI (1.58 [1.25; 1.99]), and those with a low GFR (10.2 [6.02; 17.3]). The risk of having at least one inappropriate prescription increased with the number of drugs per patient (P for trend < 0.0001) and therefore the odds ratio was 5.88 [4.17; 8.28] for those who received at least 11 prescribed medications compared to those who received fewer than 5.
Conclusion
Our results emphasize the complexity of drug management for CKD patients, for whom inappropriate prescription appears to be common.
Keywords: chronic kidney disease, drug utilization, pharmacoepidemiology, prescribing
What is Already Known about this Subject
Pharmacokinetics and pharmacodynamics are greatly altered by GFR decline.
The dosage of many drugs may need to be reduced in patients with poor kidney function, in order to avoid drug accumulation and toxicity.
What this Study Adds
This study is the first to focus on drug prescriptions and their adjustment to the kidney function in a large and representative cohort of CKD patients monitored by nephrologist.
We reported a high proportion of study participants (52%) receiving one or more inappropriate prescriptions (a contraindicated or inappropriately high dose drug).
The choice of CKD equation led to marked differences in the proportion of inappropriate prescriptions and this choice could have an impact on patient safety.
Introduction
Chronic kidney disease (CKD) is associated with multiple comorbidities and a particularly high level of polymedication 1. Medication is often difficult to manage, since the decline in the glomerular filtration rate (GFR) greatly alters the pharmacokinetics and pharmacodynamics of kidney excreted drugs 2, 3. Indeed, the dosage of many drugs may need to be reduced in patients with poor kidney function, in order to avoid drug accumulation, toxicity and the accelerated progression of CKD 4. Optimal drug management should be an important target for preserving kidney function. Indeed, a recent Clinical Practice Guideline for the evaluation and management of CKD (issued by the Kidney Disease Improving Global Outcomes, KDIGO) 5 contains the following Grade 1A (the highest) recommendation: ‘We recommend that prescribers should take GFR into account when drug dosing’. However, putting this recommendation into practice is challenging in view of (i) differences between the available guidelines on dose adjustment, and (ii) the lack of standardized guidelines on estimating kidney function for drug dosing.
Most studies of drug prescriptions and dose adjustments with regard to kidney function have been performed in elderly patients. In the literature, the proportion of patients with inappropriate prescriptions with regard to kidney function varied from 13 to 53% 6, 7, 8, 9; these disparities may be due to heterogeneity in the study design (retrospective design based on reimbursement claims vs. prospective studies, for example), the study population and/or the equation used to estimate kidney function. Data regarding drug prescriptions and their adjustment to kidney function are scarce in patients consulting in a nephrology department and have been infrequently evaluated in prospective cohort studies. Furthermore, few studies reported on both medication dosing and the level of kidney function. The few published reports in CKD patients were only designed to evaluate specific drug classes 10, 11.
When adapting drug doses, selection of the most appropriate tool for assessing the GFR is a challenge. The Cockcroft–Gault (CG) equation is most frequently applied during the development of many drugs. However, the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation is now implemented worldwide for classifying and monitoring CKD patients; it is indexed to body surface area and so it might be necessary to de‐index this equation in CKD patients requiring dose adaptation 12, 13.
Hence, the main objective of the present study was to investigate the prevalence and determinants of inappropriate drug prescriptions (whether contraindications or inappropriately high doses) with regard to kidney function in patients with CKD under nephrology care. We also assessed the impact of the equation used to estimate eGFR on the prevalence estimates.
Methods
Study design and participants
CKD‐REIN (Chronic Kidney Disease ‐ Renal Epidemiology and Information Network) is a representative, prospective cohort study carried out in 40 nephrology outpatient facilities in France. Enrolled patients were at least 18 years of age, under nephrology care with a confirmed diagnosis of CKD stages 3–5 (estimated GFR (eGFR) <60 ml min−1 1.73 m−2, no previous chronic dialysis or kidney transplantation). A total of 3033 patients were included. Details of the study protocol have been published elsewhere 14, 15. The study protocol was approved by the institutional review board at the French National Institute of Health and medical research (INSERM; reference: IRB00003888). The study was registered at http://ClinicalTrials.gov (NCT03381950).
Data collection and routine laboratory measurements
Patient‐level forms were completed by trained clinical research associates (CRAs) on the basis of a patient interview and the inspection of medical records. Detailed definitions of comorbidities have been published elsewhere 15. Self‐administered patient questionnaires were used to assess medication adherence on the Girerd score 16 and frequency of consultation to family physicians and specialist physicians.
Patients were asked to bring all their drug prescriptions (regardless of the prescribing physician) for the three months immediately preceding the interview with a CRA. The CRAs used an electronic case report form (linked to the international Anatomical Therapeutic and Chemical (ATC) 17 thesaurus) to enter standardized ATC codes. This specifically designed system enabled (i) coding by non‐medical staff, (ii) documentation of a defined daily dose (DDD), and (iii) easier data mining. For each drug prescription, the trade name, international non‐proprietary name, ATC class, unit dose, DDD, pharmaceutical formulation, and administration route can be easily extracted from our database.
The patient's height and weight were measured during the enrolment visit. Body surface area (BSA) was calculated using the Dubois equation 18. Routine serum creatinine values were collected from hospital and local laboratories and GFR was estimated with the CKD‐EPI creatinine equation 19. Kidney function was also estimated using the Cockcroft–Gault (CG) equation 20 for creatinine clearance (CrCl). We calculated a GFR de‐indexed from BSA.
Definition of inappropriate drug prescriptions with regard to the kidney function
We analysed the appropriateness of all drug prescriptions in the three months immediately preceding study enrolment. First, two pharmacists (S.M.L. and S.L.) studied a variety of documents in order to choose the most exhaustive source of dosing recommendations or contraindications with regard to kidney function. Few of the 779 substance codes (prescribed to included patients) had precise international recommendations on dose adaptation. Summaries of product characteristics (SPCs, approved by the regulatory authorities and updated annually) constituted the most detailed source of recommendations 21. The SPC corresponds to the Physician's Desk Reference and should be used as the reference for prescribing as it constitutes formal evidence in legal proceedings. Each drug's SPC was reviewed to extract any dosing guidelines or contraindications with regard to kidney function (Table S1A). For each substance, we defined a threshold above which the drug was contraindicated or required dose adjustment in proportion to the patient's kidney function. As a sensitivity analysis, we examined antidiabetic agents, for which detailed international and European guidelines have been published in KDOQI 2012 22 (Table S1B) and European Renal Best Practice 23 (Table S1C). We combined these two sets of guidelines with the SPCs, in order to define CKD‐related dose adaptations for these drug classes.
Inappropriate prescription was defined as the reported prescription of either a contraindicated drug or an indicated drug prescribed at an inappropriately high dose level with regard to the patient's GFR. In the main analysis, we used the CKD‐EPI creatinine equation to estimate the GFR. We performed sensitivity analysis to evaluate the proportion of inappropriate prescriptions for the CG equation and the de‐indexed CKD‐EPI creatinine equation.
Statistical analyses
Baseline characteristics were described for patients with at least one drug prescription as a whole and for CKD stage sub‐groups (defined according to the CKD‐EPI creatinine equation), and were expressed as the mean ± standard deviation (SD), the median [interquartile range (IQR)] or the number (percentage). In 3011 patients with at least one prescription, proportions of patients with at least one inappropriate prescription were assessed overall and by drug class, according to the GFR estimated by the CKD‐EPI creatinine equation.
The numbers of contraindicated and inappropriately high dose drug prescriptions, and the proportions of patients with at least one inappropriate prescription, were compared for the three equations used to estimate kidney function (CKD‐EPI, CG, and de‐indexed CKD‐EPI) in 2941 patients with data on their weight and height. Next, we studied the level of agreement in evaluating appropriateness between the different equations by calculating Cohen's kappa. A value of <0.6 was defined as poor agreement, 0.6 to <0.8 was defined as moderate agreement, 0.8 to <0.9 was defined as good agreement and ≥0.9 was defined as excellent agreement.
Lastly, multivariate logistic regression was used to assess the determinants of inappropriate prescription risk, i.e. exposure to at least one inappropriate prescription (expressed as the odds ratio (OR) [95% confidence interval (CI)]). Selection of the variables included in the multivariate models was based on a literature review and a p‐value below 0.2 in a univariate analysis. In view of missing data for some variables, we performed multiple imputations of 10 datasets with the fully conditional specification method 24. The imputation model included all the variables in Table 3 and baseline eGFR and serum creatinine values. Logistic regression was performed on each complete dataset and the estimated ORs were combined according to Rubin's rules. Statistical analyses were performed using SAS software (version 9.4, SAS Institute Inc., Cary, NC) and R software (version 3.3.1, Foundation for Statistical Computing, Vienna, Austria).
Table 3.
Factors associated with having at least one inappropriate prescription when renal function was estimated with the CKD‐EPI equation
| Variable | Crude OR [95% CI] | P‐value | Partially adjusted OR [95% CI] a | P‐value | Fully‐adjusted OR [95% CI] b | P‐value |
|---|---|---|---|---|---|---|
| Sociodemographic factors | ||||||
| Men | 1.10 [0.95–1.28] | 0.197 | 1.17 [0.98–1.39] | 0.08 | 1.28 [1.07–1.53] | 0.007 |
| Age (years) | 0.04 | 0.11 | 0.008 | |||
| 65–74 vs. <65 | 1.23 [1.04–1.46] | 0.95 [0.77–1.15] | 0.86 [0.69–1.06] | |||
| ≥75 vs. <65 | 1.19 [1.00–1.42] | 0.80 [0.65–0.99] | 0.70 [0.55–0.87] | |||
| High education level | 0.81 [0.69–0.94] | 0.004 | 1.06 [0.89–1.26] | 0.54 | 1.14 [0.95–1.37] | 0.15 |
| Clinical indicators | ||||||
| BMI (kg/m 2 ) | <.0001 | <.0001 | 0.0007 | |||
| 25–29 vs. <25 | 1.44 [1.20–1.73] | 1.33 [1.08–1.63] | 1.25 [1.01–1.55] | |||
| ≥30 vs. <25 | 2.25 [1.87–2.71] | 1.80 [1.44–2.25] | 1.58 [1.25–1.99] | |||
| CKD stage | <.0001 | <.0001 | <.0001 | |||
| Stage 3B vs. 2 and 3A | 1.64 [1.30–2.05] | 1.58 [1.25–2.00] | 1.53 [1.20–1.95] | |||
| >Stage 4 vs. 2 and 3A | 7.15 [5.68–8.99] | 7.67 [6.04–9.74] | 6.91 [5.37–8.91] | |||
| Stage 5 vs. 2 and 3A | 11.6 [7.12–19.1] | 12.4 [7.45–20.5] | 10.2 [6.02–17.3] | |||
| Comorbidities | ||||||
| Hypertension | 2.16 [1.67–2.81] | <.0001 | 1.46 [1.08–1.96] | 0.01 | 1.28 [0.93–1.75] | 0.12 |
| Dyslipidemia | 1.90 [1.60–2.26] | <.0001 | 1.56 [1.27–1.92] | <.0001 | 1.20 [0.96–1.50] | 0.10 |
| Diabetes | 1.88 [1.62–2.17] | <.0001 | 1.52 [1.27–1.82] | <.0001 | 1.34 [1.06–1.70] | 0.01 |
| Cardiovascular comorbidities | 0.0002 | 0.62 | 0.08 | |||
| 1–3 vs. none | 1.36 [1.17–1.58] | 1.07 [0.89–1.28] | 0.86 [0.70–1.05] | |||
| 4–6 vs. none | 1.40 [0.98–2.00] | 0.91 [0.60–1.38] | 0.63 [0.41–0.98] | |||
| Clinical care indicators | ||||||
| Visits to the GP | 0.09 | 0.70 | ||||
| 3 or 4 visits vs. ≤2 | 1.10 [0.86–1.41] | 1.09 [0.81–1.47] | ||||
| 4+ visits vs. ≤2 | 1.25 [1.00–1.56] | 1.06 [0.81–1.39] | ||||
| Visits to the nephrologist | <.0001 | 0.10 | ||||
| 3 or 4 visits vs. ≤2 | 1.74 [1.44–2.10] | 1.13 [0.90–1.43] | ||||
| 4+ visits vs. ≤2 | 1.45 [1.13–1.86] | 0.81 [0.59–1.10] | ||||
| Visits to the endocrinologist | 0.01 | 0.08 | ||||
| 1 or 2 visits vs. 0 | 1.20 [0.99–1.47] | 0.72 [0.53–0.98] | ||||
| 3 or 4 visits vs. 0 | 1.60 [1.13–2.26] | 0.83 [0.52–1.33] | ||||
| 4+ visits vs. 0 | 1.27 [0.81–2.00] | 0.66 [0.38–1.17] | ||||
| Visits to the cardiologist | 0.02 | 0.49 | ||||
| 1 or 2 visits vs. 0 | 1.26 [1.05–1.51] | 0.87 [0.69–1.11] | ||||
| 3 or 4 visits vs. 0 | 1.51 [1.08–2.11] | 0.86 [0.56–1.31] | ||||
| 4+ visits vs. 0 | 1.18 [0.77–1.82] | 0.77 [0.45–1.30] | ||||
| Factors linked to medications | ||||||
| Number of medications | <.0001 | <.0001 | ||||
| 5 or 6 vs. <5 | 2.16 [1.67–2.78] | 2.00 [1.50–2.66] | ||||
| 7 or 8 vs. <5 | 3.67 [2.87–4.68] | 3.13 [2.33–4.21] | ||||
| 9 or 10 vs. <5 | 3.82 [2.97–4.91] | 3.21 [2.33–4.42] | ||||
| ≥11 vs. <5 | 6.99 [5.47–8.92] | 5.88 [4.17–8.28] | ||||
| Poor observance | 1.49 [1.28–1.73] | <.0001 | 1.11 [0.93–1.32] | 0.24 | ||
Adjusted for sociodemographic factors, clinical indicators and comorbidities.
Adjusted for all variables
BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; GP, general practitioner; OR, odds ratio
Results
Characteristics of the population at baseline
Among the 3033 patients in the CKD‐REIN cohort, 3011 had at least one drug prescription. The participants' characteristics are summarized in Table 1 and are presented among 3011 patients with at least one drug prescription. At baseline, 2% patients were at CKD stage 2 (according to the CKD‐EPI equation), 16% were at stage 3A, 37% were at stage 3B, 41% were at stage 4, and 4% were at stage 5. The median [IQR] age was 69 [61; 76]. Thirty‐six per cent of the patients had a body mass index (BMI) between 25 and 30 kg m−2, and 36% had a BMI greater than 30 kg m−2. Most of patients had a history of hypertension, and 43% were diabetic. More than 65% of the patients reported visiting their nephrologist once to twice a year. Almost 60% of the patients said that they had visited their family physician more than four times during the year preceding inclusion in the cohort (Table 1). The number of visits to family physicians and nephrologists increased with the CKD stage (Table 1).
Table 1.
Patient characteristics for patients with at least one drug prescription as a function of the CKD stage
| All | Baseline stage (CKD‐EPI) | |||
|---|---|---|---|---|
| Stages 2 and 3A | Stage 3B | Stages 4 and 5 | ||
| n | 3011 | 526 | 1126 | 1359 |
| Men | 66% | 71% | 66% | 63% |
| Age, years | 69 [61–76] | 66 [57–72] | 69 [62–76] | 70 [62–78] |
| <65 | 35% | 46% | 32% | 32% |
| 65–74 | 35% | 35% | 37% | 33% |
| ≥75 | 30% | 19% | 30% | 35% |
| Educational level | ||||
| Below high school diploma | 64% | 57% | 63% | 67% |
| High school diploma or higher | 36% | 43% | 37% | 33% |
| Medical history | ||||
| Hypertension | 91% | 86% | 92% | 92% |
| Diabetes | 43% | 39% | 44% | 44% |
| Dyslipidemia | 75% | 72% | 76% | 76% |
| Cardiovascular disease | 54% | 50% | 53% | 56% |
| Acute kidney injury | 24% | 16% | 24% | 26% |
| Body mass index, kg m −2 | 29 ± 6 | 28 ± 6 | 29 ± 6 | 29 ± 6 |
| <25 | 28% | 31% | 26% | 28% |
| 25–29 | 36% | 39% | 37% | 35% |
| ≥30 | 36% | 30% | 36% | 37% |
| Body surface area (m 2 ) | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 |
| Renal function estimation | ||||
| CKD‐EPI (ml min −1 1.73 m −2 ) | 33 ± 12 | 52 ± 6 | 37 ± 4 | 22 ± 5 |
| CKD‐EPI de‐indexed (ml min −1 ) | 36 ± 14 | 58 ± 10 | 41 ± 7 | 24 ± 6 |
| Cockcroft–Gault (ml min −1 ) | 41 ± 16 | 61 ± 16 | 45 ± 12 | 29 ± 9 |
| Number of medications | 8 [5–10] | 6 [4–9] | 7 [5–10] | 8 [6–11] |
| <5 | 19% | 29% | 22% | 13% |
| 5 or 6 | 17% | 22% | 18% | 15% |
| 7 or 8 | 21% | 17% | 21% | 22% |
| 9 to 10 | 18% | 17% | 16% | 20% |
| >10 | 25% | 15% | 23% | 30% |
| Cardiovascular comorbidities score | ||||
| None | 53% | 58% | 55% | 50% |
| 1–3 | 42% | 39% | 41% | 45% |
| 4–6 | 4% | 3% | 4% | 6% |
| Number of visits to physicians during the year before inclusion in the cohort | ||||
| Family physician | ||||
| ≤2 visits | 15% | 15% | 15% | 15% |
| 3 or 4 visits | 28% | 31% | 27% | 28% |
| More than 4 visits | 57% | 54% | 58% | 57% |
| Nephrologist | ||||
| ≤2 visits | 66% | 77% | 75% | 53% |
| 3 or 4 visits | 24% | 16% | 18% | 32% |
| More than 4 visits | 11% | 7% | 8% | 14% |
| Endocrinologist or diabetologist | ||||
| No visit | 70% | 70% | 67% | 72% |
| 1 or 2 visits | 21% | 19% | 25% | 18% |
| 3 or 4 visits | 6% | 8% | 5% | 6% |
| More than 4 visits | 3% | 3% | 2% | 4% |
| Cardiologist | ||||
| No visit | 32% | 33% | 31% | 32% |
| 1 or 2 visits | 57% | 58% | 57% | 57% |
| 3 or 4 visits | 8% | 6% | 8% | 8% |
| More than 4 visits | 3% | 2% | 4% | 4% |
Data are quoted as the mean ± SD, the median [interquartile range] or %.
Characteristics of drug prescriptions
The median [IQR] number of prescribed medications per patient was 8 [5; 10], and 2182 (72%) patients had been prescribed six or more drugs. The median number of prescribed drugs per patient was positively correlated with the severity of CKD, rising from 6 [4; 9] in stages 2 and 3A to 8 [6; 11] in stages 4 and 5 (Table 1). Among the 3011 patients with at least one drug prescription, the four most frequently prescribed classes were cardiovascular agents, antidiabetic agents, drugs for acid‐related gastrointestinal disorders and anti‐gout preparations (Tables 2 and S2).
Table 2.
Description of inappropriate drug prescriptions by drug class, overall, and by CKD stage
| STAGE | ||||
|---|---|---|---|---|
| TOTAL | Stages 2 and 3A | Stage 3B | Stages 4 and 5 | |
| n = 3011 | n = 526 | n = 1126 | n = 1359 | |
| Antihypertensives, n | 2820 | 467 | 1052 | 1301 |
| At least one contraindicated drug | 13.4% | 1.7% | 1.4% | 27.3% |
| At least one inappropriately high dose drug | 16.2% | 12.6% | 15.9% | 17.7% |
| At least one inappropriate prescription | 27.3% | 14.1% | 16.9% | 40.5% |
| Lipid‐modifying agents, n | 1900 | 309 | 722 | 869 |
| At least one contraindicated drug | 12.2% | 0.0% | 0.4% | 26.2% |
| At least one inappropriately high dose drug | 2.7% | 3.2% | 2.4% | 2.8% |
| At least one inappropriate prescription | 14.8% | 3.2% | 2.8% | 29.0% |
| Antithrombotic agents, n | 1804 | 272 | 674 | 858 |
| At least one contraindicated drug | 6.1% | 0.0% | 0.0% | 12.8% |
| At least one inappropriately high dose drug | 0.0% | 0.4% | 0.0% | 0.0% |
| At least one inappropriate prescription | 6.2% | 0.4% | 0.0% | 12.8% |
| Drugs used in diabetes, n | 1085 | 178 | 418 | 489 |
| At least one contraindicated drug | 7.6% | 0.0% | 0.0% | 16.8% |
| At least one inappropriately high dose drug | 13.7% | 16.9% | 21.5% | 5.9% |
| At least one inappropriate prescription | 20.8% | 16.9% | 21.5% | 21.7% |
| Drugs for acid‐related disorders, n | 1041 | 150 | 394 | 497 |
| At least one contraindicated drug | 0.4% | 0.0% | 0.0% | 0.8% |
| At least one inappropriately high dose drug | 0.5% | 0.7% | 0.3% | 0.6% |
| At least one inappropriate prescription | 0.9% | 0.7% | 0.3% | 1.4% |
| Anti‐gout preparations, n | 1025 | 129 | 351 | 545 |
| At least one contraindicated drug | 2.7% | 0.0% | 0.0% | 5.1% |
| At least one inappropriately high dose drug | 38.6% | 15.5% | 28.2% | 50.8% |
| At least one inappropriate prescription | 40.5% | 15.5% | 28.2% | 54.3% |
| Analgesics, n | 717 | 117 | 277 | 323 |
| At least one contraindicated drug | 6.6% | 0.0% | 0.0% | 14.6% |
| At least one inappropriately high dose drug | 3.1% | 0.0% | 0.7% | 6.2% |
| At least one inappropriate prescription | 9.6% | 0.0% | 0.7% | 20.7% |
| Psycholeptics, n | 501 | 84 | 180 | 237 |
| At least one contraindicated drug | 0.4% | 0.0% | 0.0% | 0.8% |
| At least one inappropriately high dose drug | 8.8% | 8.3% | 8.3% | 9.3% |
| At least one inappropriate prescription | 9.0% | 8.3% | 8.3% | 9.7% |
| Mineral supplements, n | 480 | 62 | 135 | 283 |
| At least one contraindicated drug | 40.2% | 38.7% | 48.1% | 36.7% |
| At least one inappropriately high dose drug | 0.0% | 0.0% | 0.0% | 0.0% |
| At least one inappropriate prescription | 40.2% | 38.7% | 48.1% | 36.7% |
| Cardiac therapy, n | 432 | 63 | 160 | 209 |
| At least one contraindicated drug | 1.9% | 0.0% | 0.0% | 3.8% |
| At least one inappropriately high dose drug | 2.5% | 0.0% | 0.0% | 5.3% |
| At least one inappropriate prescription | 4.2% | 0.0% | 0.0% | 8.6% |
| Urologicals, n | 345 | 64 | 116 | 165 |
| At least one contraindicated drug | 14.2% | 0.0% | 0.0% | 29.7% |
| At least one inappropriately high dose drug | 0.0% | 0.0% | 0.0% | 0.0% |
| At least one inappropriate prescription | 14.2% | 0.0% | 0.0% | 29.7% |
| Psychoanaleptics, n | 241 | 40 | 90 | 111 |
| At least one contraindicated drug | 4.1% | 0.0% | 0.0% | 9.0% |
| At least one inappropriately high dose drug | 5.0% | 0.0% | 10.0% | 2.7% |
| At least one inappropriate prescription | 9.1% | 0.0% | 10.0% | 11.7% |
| Antiepileptics, n | 188 | 25 | 72 | 91 |
| At least one contraindicated drug | 0.0% | 0.0% | 0.0% | 0.0% |
| At least one inappropriately high dose drug | 14.9% | 8.0% | 6.9% | 23.1% |
| At least one inappropriate prescription | 14.9% | 8.0% | 6.9% | 23.1% |
| Antibacterials for systemic use, n | 182 | 33 | 67 | 82 |
| At least one contraindicated drug | 1.6% | 0.0% | 1.5% | 2.4% |
| At least one inappropriately high dose drug | 14.3% | 3.0% | 6.0% | 25.6% |
| At least one inappropriate prescription | 15.9% | 3.0% | 7.5% | 28.0% |
| Immunosuppressants, n | 167 | 36 | 61 | 70 |
| At least one contraindicated drug | 2.4% | 2.8% | 0.0% | 4.3% |
| At least one inappropriately high dose drug | 1.8% | 0.0% | 1.6% | 2.9% |
| At least one inappropriate prescription | 4.2% | 2.8% | 1.6% | 7.1% |
| Antiinflammatory and antirheumatic products, n | 43 | 12 | 14 | 17 |
| At least one contraindicated drug | 20.9% | 0.0% | 0.0% | 52.9% |
| At least one inappropriately high dose drug | 0.0% | 0.0% | 0.0% | 0.0% |
| At least one inappropriate prescription | 20.9% | 0.0% | 0.0% | 52.9% |
For each drug class and each CKD group, we reported the number of treated patients and calculated the proportion of patients with at least one contraindicated drug, at least one inappropriately high dose drug, and at least one inappropriate prescription (i.e. contraindicated or inappropriately high dose drug).
Prevalence of contraindicated and inappropriately high dose drug prescriptions
When using the CKD‐EPI equation to evaluate kidney function, 31% of the patients had been prescribed at least one contraindicated drug, and 35% had been prescribed at least one drug at an inappropriately high dose level with regard to their eGFR. The proportion of inappropriate prescriptions was greater in patients at CKD stages 4 and 5. Indeed, when considering patients with an eGFR below 30 ml min−1 1.73 m−2, 57% had been prescribed at least one contraindicated drug, and 42% had received a prescription for an inappropriately high dose level (Figure 1).
Figure 1.
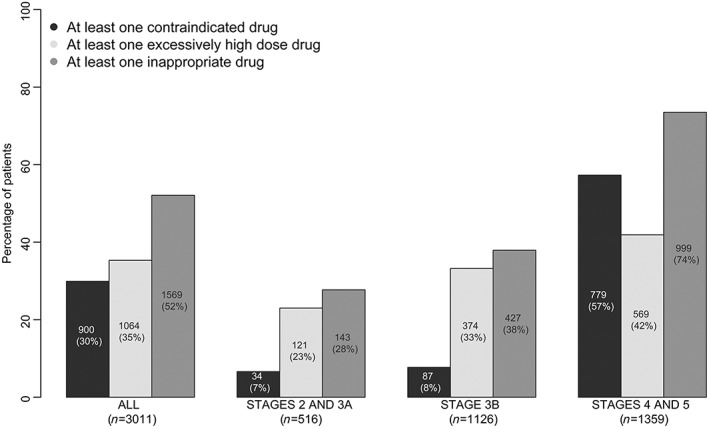
Percentage of patients with at least one contraindicated prescription, at least one inappropriately high dose prescription, or at least one inappropriate prescription (i.e. a contraindicated or inappropriately high dose drug), by CKD stage (assessed using the CKD‐EPI equation)
Overall, 52% of treated patients presented at least one inappropriate prescription, and 22% had received two or more inappropriate prescriptions (Figure S1A). Sixty‐five per cent of the patients had not been prescribed any drugs at an inappropriately high dose level (Figure S1B). Seventy per cent of the patients had not been prescribed any contraindicated drugs (Figure S1C).
The proportions of patients with inappropriate prescriptions among drug classes, by eGFR, are summarized in Table 2. Among drug classes prescribed in more than one third of patients, the highest proportion of inappropriate prescriptions were observed for anti‐gout, cardiovascular system and antidiabetic drugs, with respectively 41%, 27% and 21% of patients treated with these drugs having at least one inappropriate prescription.
Three drugs (allopurinol, ramipril and perindopril) accounted for more than half of the inappropriately high dose prescriptions (n = 701/1299) (Table S3). Nevertheless, when removing allopurinol, ramipril and perindopril prescriptions, for which benefit may have been considered higher than risk by prescribers, the proportion of patients with inappropriate prescription remained high (41%). For the 777 patients receiving the urate‐lowering agent allopurinol, 51% of the prescriptions were at an inappropriately high dose level. Forty‐one per cent of the colchicine prescriptions concerned patients with an eGFR below 30 ml min−1 1.73 m−2 (n = 26/64). The number of prescriptions of non‐steroidal anti‐inflammatory drugs in patients with an eGFR below 30 ml min−1 1.73 m−2 was relatively low (n = 9), as was the number of prescriptions of salicylic acid derivatives (used as an analgesic and/or antipyretic, i.e. >160 mg day−1) (n = 29). In addition, 40% of the prescriptions for oral antidiabetic agents were inappropriate (n = 253/641). The most frequently employed drug was metformin; 17% of metformin prescriptions corresponded to contraindications, and 33% corresponded to inappropriately high doses with regard to the patient's eGFR. It is noteworthy that the proportion of patients with inappropriate prescription of antidiabetic agents was similar when the SPC, KDOQI or European Renal Best Practice recommendations were applied (Figure S2).
Prevalence of inappropriate prescription according the CG, CKD‐EPI and de‐indexed CKD‐EPI equations
The proportions of patients with at least one contraindicated prescription or at least one inappropriately high dose prescription differed significantly according to the equation used to estimate kidney function (P < 0.0001). The CKD‐EPI equation yielded higher proportions (52%) than the CG equation (41%) and the de‐indexed CKD‐EPI equation (47%) (Figure 2 and Figure S3).
Figure 2.
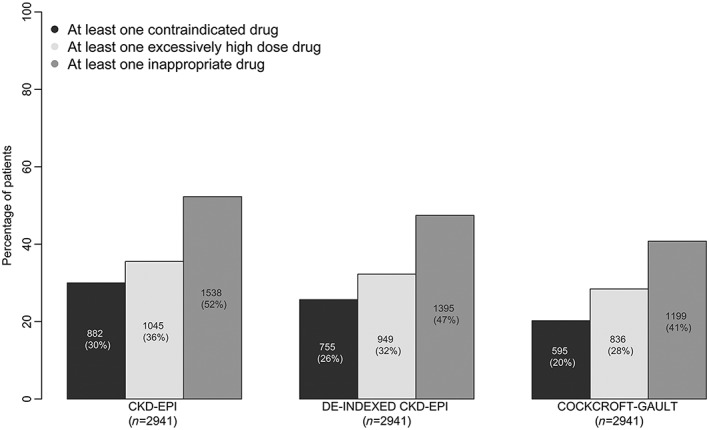
Percentage of patients with at least one contraindicated prescription, at least one inappropriately high dose prescription or at least one inappropriate prescription (i.e. a contraindicated or inappropriately high dose drug) by CKD stage according to the CKD‐EPI equation, the de‐indexed CKD‐EPI equation and the Cockcroft–Gault equation. The analyses were restricted to the 2941 patients with data on weight and height
Of the 1285 prescriptions considered to be contraindicated when the kidney function was calculated with the CKD‐EPI equation, 557 were not considered to be contraindicated with regard to the CG equation (kappaCG vs. CKD‐EPI = 0.69) (Table S4A). The level of agreement between the CG equation and the de‐indexed CKD‐EPI equation was higher (kappaCG vs. De‐indexed CKD‐EPI = 0.81).
When considering the 1275 inappropriately high dose prescriptions when the kidney function was calculated with the CKD‐EPI equation, 337 were not considered to be inappropriately high dose prescriptions with regard to the CG equation (kappa = 0.82) (Table S4B).
Determinants of inappropriate drug prescription
As expected, the OR for the risk of an inappropriate prescription rose with the CKD stage (P for trend < 0.0001) (Table 3). When compared with patients at CKD stage 2 or 3A, those at stage 5 had a 10.2‐fold higher risk of having at least one inappropriate prescription. Furthermore, this risk increased with the number of medications per patient (P for trend < 0.0001). Men, patients with diabetes, and overweight patients had a greater risk of having at least one inappropriate prescription; the ORs [95% CI] were respectively 1.28 [1.07; 1.53], 1.34 [1.06; 1.70] and 1.58 [1.25; 1.99]. In contrast, older age was associated with a lower risk. The number of visits to the nephrologist or to other specialists or to the family physician was not associated with the percentage of inappropriate prescriptions, and there was no interaction with CKD stage (data not shown). It is noteworthy that similar predictors were identified when the kidney function was defined according to the CG equation or the de‐indexed CKD‐EPI equation. However, the predictive values for CKD stage were lower than those observed when the kidney function was defined according to CKD‐EPI creatinine equation (data not shown).
Discussion
The present study is the first to focus on drug prescriptions and their adjustment to the kidney function in a large and representative cohort of CKD patients monitored by nephrologist. We confirmed that polymedication is common among non‐dialysed patients with CKD: 72% of the study participants had been prescribed more than five drugs. A high proportion of study participants (52%) had received one or more inappropriate prescriptions (i.e. a contraindicated or inappropriately high dose drug) with regard to the eGFR. The number of prescribed drugs appeared to be the main determinant of inappropriate prescription. Our data highlight the complexity of prescription in the CKD setting, due in part to the lack of up‐to‐date guidelines on dose level adjustments in patients with impaired kidney function. Lastly, we observed that the choice of the CKD equation led to marked differences in the proportion of inappropriate prescriptions, with the CG equation yielding the lowest prevalence of inappropriate prescriptions.
A high prevalence of inappropriate prescriptions has already been documented in elderly patients with altered kidney function. In a French cohort of elderly patients 6, 53% of the individuals with an eGFR between 30 and 59 ml min−1 1.73 m−2 were exposed to a risk of inappropriate drug use. However, 14% of the patients in the latter study had an eGFR below 60 ml min−1 1.73 m−2, and only 25 patients had an eGFR below 30 ml min−1 1.73 m−2. Furthermore, drug dose levels were not recorded, and so only the potential risk of inappropriate or inappropriately high dose drug use could be estimated. In the USA, a retrospective study of older veterans 9 showed that 13% of the individuals with a CrCl rate between 30 and 49 ml min−1 and 29% of the individuals with a CrCl rate between 15 and 29 ml min−1 were prescribed at least one drug that was contraindicated or used at an inappropriately high dose with regard to their kidney function. In an Australian study of patients aged 65 and over 8, 28% of the participants received inappropriate medications with regard to their kidney function. These two reports were retrospective (i.e. based on reimbursement database) and focused on a prespecified list of drugs.
The majority of published data on inappropriate prescriptions concerns elderly patients with impaired renal function. We extended these investigations to patients with a confirmed diagnosis of CKD and an accurate, prospective record of drug dose levels. In the CKD‐REIN cohort, 31% of the patients had been prescribed at least one contraindicated drug and 35% had been prescribed at least one inappropriately high dose drug with regard to their eGFR. The high proportion of patients receiving inappropriate prescriptions (with regard to SPCs) suggests that clinicians do not always adopt the guidelines issued by health authorities. In fact, CKD patients are often excluded from participating in large clinical trials. Information on the adjustment of drug dosing, particularly the contraindicated part, is often imprecise or even inaccessible, and is rarely validated by clinical trial results. Indeed, old drugs that have not been tested in patients with CKD are systematically contraindicated in this population. Hence, if a physician believes that a guideline is not valid, he/she will be less likely to adopt and implement it; the prescription of contraindicated drugs will be not uncommon if he/she thinks that the risk/benefit ratio is still favourable for the patient or if there are no alternative treatments. In contrast to recommendations on contraindications, recommendations on dose adaptations seem to be less controversial because they are justified by the risk of accumulation of drugs excreted by the kidneys. However, our results suggest that prescribers are not aware of or do not apply many of these adjustment guidelines.
Hyperuricaemia is a frequent complication in CKD patients; in the present study, 34% of the participants received at least one uricosuric agent (most frequently allopurinol). The dose of allopurinol must be adjusted as a function of the eGFR because the elimination half‐life of oxypurinol (the active metabolite) is significantly longer in patients with kidney failure. Furthermore, the frequency of allopurinol‐associated adverse events is higher in this type of patient 25, 26. In the present study, half of all the allopurinol prescriptions (i.e. in 395 patients) were classified as an inappropriately high dose of this hypouricemic agent. It is noteworthy that other researchers have also reported on inappropriate prescriptions of allopurinol 9, 27, 28, 29. Although the latter studies (predominantly in elderly patients) were performed with other designs and other reference guidelines, the results nevertheless suggest that prescribing physicians are not aware of the need to adjust the allopurinol dose level as a function of the eGFR.
Diabetes is one of the leading causes of CKD worldwide 1. In the present study, more than a third of patients were being treated for diabetes. A large proportion of antidiabetic agents are excreted by kidney, and the accumulation of these drugs can lead to adverse events. These particularities have prompted international learned societies to issue guidelines on dose adjustments 22, 23. In the present study, application of the SPCs, the European guidelines and the international guidelines gave approximately the same proportion of patients with at least one inappropriate prescription of an antidiabetic agent. This observation suggests that our present findings are not restricted to France and may have international implications. Indeed, the proportion of inappropriate prescriptions in the CKD‐REIN cohort was high – as seen in studies of elderly adults that referred to other guidelines (e.g. LexiComp®, pharmaceutical compendia and other drug guideline databases).
Unsurprisingly, a high number of prescribed drugs was the main determinant of inappropriate prescriptions in the present study. The oldest patients were less likely to have inappropriate prescriptions, suggesting that physicians take more account of kidney function when prescribing drugs to elderly patients. In contrast to a literature report 9, the number of consultations with a nephrologist did not appear to protect against inappropriate prescriptions. However, all the patients were being followed up in a nephrology department in the CKD‐REIN study, leading to difficulty in comparing the two studies. They were more likely to consult a family physician: 57% of the patients reported having seen their family physician more than 4 times a year, whereas only 35% reported seeing a nephrologist more than 3 times a year. These findings suggest that the family physician represents an important prescriber in this population, hence the need to reinforce the dissemination of information and guidelines on drug management in patients with CKD – particularly for family physicians.
The prevalence of inappropriate prescriptions depends strongly on the equation used to estimate kidney function. Indeed, we found that according the equation used, the proportion of inappropriate, inappropriately high dose and contraindicated drugs varied significantly. Upon inclusion in the present study, the mean ± SD eGFR was 33 ± 12 ml min−1 1.73 m−2 (according to the CKD‐EPI equation) and the mean ± SD CrCl rate was 41 ± 16 ml min−1 (according to the CG equation). This discrepancy might be due to a relatively high BMI in the present cohort, which may have greatly influenced the proportion of inappropriate prescriptions. The proportion of patients with at least one contraindicated drug was 20% when kidney function was evaluated with the CG equation and 30% when kidney function was evaluated with the CKD‐EPI equation. Most of the guidelines on adjusting drug dose levels to kidney function have used the CG equation 30, 31. However, nephrologists also classify and monitor CKD patients with reference to the CKD‐EPI equation, which is acknowledged to produce the best estimation of actual kidney function 5, 19. It would have been useful for us to know whether physicians use the two equations in distinct ways or whether they use the CKD‐EPI equation to calculate the eGFR and then adjust the drug dose accordingly. A main concern here is the indexation of the CKD‐EPI equation with regard to the BSA (1.73 m−2 for an average‐sized person). Thirty‐six per cent of the patients in the CKD‐REIN cohort had a BMI of more than 30 kg m−2, and thus had a BSA of more than 1.73 m−2. Hence, the use of the de‐indexed CKD‐EPI equation to adjust drug dose levels appears to be a good compromise. This might be especially true for CKD patients with a high BMI; we found that the proportion of inappropriate prescriptions changed when the patient's kidney function was evaluated with the de‐indexed CKD‐EPI equation.
The present study had several major strengths. It constitutes the ever first study of inappropriate drug prescriptions in a large (n > 3000) cohort of nephrology department outpatients with a confirmed diagnosis of CKD. Indeed, previous studies of inappropriate drug prescriptions focused on elderly adults 6, 7, 8, 9. Our prospective data collection and especially a detailed survey of prescribed drugs and dose levels also constitute a major strength.
The study also had some limitations. Only prescription medications were analysed here; in fact, some over‐the‐counter drugs are also not recommended in patients with impaired kidney function. We performed the analyses using only three of the many equations available; other available equations include the Berlin Initiative Study equation 32, the results of which could have been slightly different. Lastly, we analysed prescriptions in the three months preceding study inclusion; this might have underestimated the proportion of inappropriate prescriptions in some drug classes (e.g. antibiotics). Moreover, data on chronic treatments (i.e. long‐term prescriptions) can be collected with more reliability than data on short‐term prescriptions. Linking the CKD‐REIN database to a reimbursement dataset should provide us with better insight in these cases 33.
Conclusion
The CKD‐REIN study provided us with a good opportunity to evaluate inappropriate drug prescriptions in a large, representative, French cohort of CKD patients being followed up in a nephrology clinic. Of the 3011 patients receiving medication, 52% had at least one inappropriate prescription (i.e. a prescription of a contraindicated drug or a drug that was prescribed at an inappropriately high dose level with regard to the patient's kidney function). This high proportion emphasizes the complexity of drug prescribing in CKD, given the many different prescription guidelines and the various equations used to estimate kidney function. It remains to be determined whether these inappropriate prescriptions impact clinical outcomes (i.e. iatrogenic events, hospitalizations and mortality).
Contributors
S.M.L., S.L., Z.A.M., M.M. and B.S. designed the present project. S.M.L., S.L., M.M. analysed the data. S.M.L., S.L., Z.A.M., M.M. and B.S. contributed to the interpretation of the results. S.M.L., S.L., Z.A.M. wrote the first draft paper. S.M.L., S.L., Z.A.M, M.M., B.S., C.J., C.C., D.F., M.L., L.F., C.A., E.S. and B.M.R. provided critical feedback and helped shape the research, analysis and the final draft of the manuscript. B.S. was the Principal Investigator.
Competing Interests
CKD‐REIN is funded by the Agence Nationale de la Recherche through the 2010 ‘Cohortes‐Investissements d'Avenir’ program and by the 2010 national Programme Hospitalier de Recherche Clinique. CKD‐REIN is also supported through a public‐private partnership with Amgen, Fresenius Medical Care, GlaxoSmithKline (GSK), since 2012, Lilly France since 2013, and Otsuka Pharmaceutical since 2015, Baxter and Merck Sharp & Dohme‐Chibret (MSD France) from 2012 to 2017, and Sanofi‐Genzyme from 2012 to 2015. Inserm Transfert set up and has managed this partnership since 2011. A specific ‘Drug optimization in CKD Patients’ project was funded by the French National Agency for Medicines and Health Products Safety (ANSM). It should be noted that the authors of the present article were solely responsible for interpretation of the data; the ANSM was not involved.
We acknowledge the CKD‐REIN study coordination staff for their efforts in setting up the CKDREIN cohort: Marie Metzger, Elodie Speyer, Céline Lange, Sophie Renault, Reine Ketchemin and all the clinical research associates.
All legal authorizations were obtained including those from the Comité consultatif sur le traitement de l'information en matière de recherche dans le domaine de la santé (CCTIRS No. 12.360), the Commission nationale de l'informatique et des libertés (CNIL No. DR‐2012‐469), and from the Kremlin‐Bicêtre Comité de protection des personnes (CPP No. IDRCB 2012‐A00902‐41). CKD‐REIN biological collection is registered in the management application of the COnservation D' Eléments du COrps Humain (CODECOCH No. 2012‐1624). The Institut national de la santé et de la recherche médical (Inserm) Institutional Review Board approved the study protocol (IRB00003888).
Supporting information
Figure S1 Appropriateness of prescriptions, expressed as the number of inappropriate (i.e. contraindicated or inappropriately high dose drug), inappropriately high dose or contraindicated drugs per patient (n = 3011 patients receiving at least one medication). (A) Number of inappropriate prescriptions per patient (B) Number of inappropriately high dose prescriptions per patient. (C) Number of contraindicated prescriptions per patient
Figure S2 Percentage of patients with at least one contraindicated antidiabetic prescription, at least one inappropriately high dose antidiabetic prescription, or at least one inappropriate antidiabetic prescription, according to the summary of product characteristics (SPC), the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines [22] and the European Renal Best Practice (ERBP) guidelines [23]
Figure S3 Percentages of contraindicated, inappropriately high dose, or inappropriate prescriptions (i.e. contraindicated or inappropriately high dose), by CKD stage. (A) Using the CKD‐EPI equation to estimate renal function. (B) Using the de‐indexed CKD‐EPI equation to estimate renal function. (C) Using the Cockcroft‐Gault equation to estimate renal function
These analyses were restricted to the 2941 patients with data on weight and height
Table S1 (A) Prescribing guidelines for all medications examined in the study (according to the French SPCs, as assessed from January 2017 to March 2017) (B) Prescribing guidelines for antidiabetic agents examined in the study (according to the ERBP 2015 recommendations [22]) (C) Prescribing guidelines for antidiabetic agents examined in the study (according to the KDOQI 2012 recommendations [23])
Table S2 Details of drug prescriptions at baseline according to CKD stages and therapeutic classes
Table S3 Inappropriate prescriptions by ATC class (using SPCs as the reference)
Table S4 Agreement between the CKD‐EPI or de‐indexed CKD‐EPI equations and Cockcroft‐Gault equation (A) Agreement in evaluating contraindications (CIs) (B) Agreement in evaluating inappropriately high doses (IHDs)
Appendix A. CKD‐REIN clinical sites and investigators, by region
Alsace: Prs T. Hannedouche and B. Moulin (CHU, Strasbourg), Dr A. Klein (CH Colmar); Aquitaine: Pr C. Combe (CHU, Bordeaux), Dr J.P. Bourdenx (Clinique St Augustin, Bordeaux), Dr A. Keller, Dr C. Delclaux (CH, Libourne), Dr B. Vendrely (Clinique St Martin, Pessac), Dr B. Deroure (Clinique Delay, Bayonne), Dr A. Lacraz (CH, Bayonne); Basse Normandie: Dr T. Lobbedez (CHU, Caen), Dr I. Landru (CH, Lisieux); Ile de France: Pr Z. Massy (CHU, Boulogne – Billancourt), Pr P. Lang (CHU, Créteil), Dr X. Belenfant (CH, Montreuil), Pr E. Thervet (CHU, Paris), Dr P. Urena (Clinique du Landy, St Ouen), Dr M. Delahousse (Hôpital Foch, Suresnes); Languedoc – Roussillon: Dr C. Vela (CH, Perpignan); Limousin: Dr M. Essig (CHU, Limoges); Lorraine: Dr H. Sekhri, Dr M. Smati (CH, Epinal); Dr M. Jamali, Dr B. Hacq (Clinique Louis Pasteur, Essey‐les‐Nancy), Dr V. Panescu, Dr M. Bellou (Polyclinique de Gentilly, Nancy), Pr Luc Frimat (CHU, Vandœuvre‐les‐Nancy); Midi‐Pyrénées: Pr N Kamar (CHU, Toulouse); Nord‐Pas‐de‐Calais: Prs C. Noël et F. Glowacki (CHU, Lille), Dr N. Maisonneuve (CH, Valenciennes), Dr R. Azar (CH, Dunkerque), Dr M. Hoffmann (Hôpital privé La Louvière, Lille); Pays‐de‐la Loire: Pr M. Hourmant (CHU, Nantes), Dr A. Testa (Centre de dialyse, Rezé), Dr D. Besnier (CH, St Nazaire); Picardie: Pr G. Choukroun (CHU, Amiens), Dr G. Lambrey (CH, Beauvais); Provence‐Alpes ‐ Côte d'Azur: Pr S. Burtey (CHU, Marseille), Dr G. Lebrun (CH, Aix‐en‐Provence), Dr E. Magnant (Polyclinique du Parc Rambot, Aix‐en‐Provence); Rhône‐Alpes: Pr M. Laville, Pr D. Fouque (CHU, Lyon‐Sud) et L. Juillard (CHU Edouard Herriot, Lyon), Dr C. Chazot (Centre de rein artificiel Tassin Charcot, Ste Foy‐les‐Lyon), Pr P. Zaoui (CHU, Grenoble), Dr F. Kuentz (Centre de santé rénale, Grenoble).
Laville, S. M. , Metzger, M. , Stengel, B. , Jacquelinet, C. , Combe, C. , Fouque, D. , Laville, M. , Frimat, L. , Ayav, C. , Speyer, E. , Robinson, B. M. , Massy, Z. A. , and Liabeuf, S. (2018) Evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD‐REIN cohort. Br J Clin Pharmacol, 84: 2811–2823. 10.1111/bcp.13738.
References
- 1. Levey AS, Coresh J. Chronic kidney disease. The Lancet 2012; 379: 165–180. [DOI] [PubMed] [Google Scholar]
- 2. Hassan Y, Al‐Ramahi R, Abd Aziz N, Ghazali R. Drug use and dosing in chronic kidney disease. Ann Acad Med Singapore 2009; 38: 1095–1103. [PubMed] [Google Scholar]
- 3. Nolin TD. A synopsis of clinical pharmacokinetic alterations in advanced CKD. Semin Dial 2015; 28: 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munar MY, Munar MY, Signh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician 2007; 75: 1487–1496. [PubMed] [Google Scholar]
- 5. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 1–150. [Google Scholar]
- 6. Breton G, Froissart M, Janus N, Launay‐Vacher V, Berr C, Tzourio C, et al Inappropriate drug use and mortality in community‐dwelling elderly with impaired kidney function—the Three‐City population‐based study. Nephrol Dial Transplant 2011; 26: 2852–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gheewala PA, Peterson GM, Curtain CM, Nishtala PS, Hannan PJ, Castelino RL. Impact of the pharmacist medication review services on drug‐related problems and potentially inappropriate prescribing of renally cleared medications in residents of aged care facilities. Drugs Aging 2014; 31: 825–835. [DOI] [PubMed] [Google Scholar]
- 8. Khanal A, Peterson GM, Castelino RL, Jose MD. Potentially inappropriate prescribing of renally cleared drugs in elderly patients in community and aged care settings. Drugs Aging 2015; 32: 391–400. [DOI] [PubMed] [Google Scholar]
- 9. Chang F, O'Hare AM, Miao Y, Steinman MA. Use of renally inappropriate medications in older veterans: a national study. J Am Geriatr Soc 2015; 63: 2290–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muller C, Dimitrov Y, Imhoff O, Richter S, Ott J, Krummel T, et al Oral antidiabetics use among diabetic type 2 patients with chronic kidney disease. Do nephrologists take account of recommendations? J Diabetes Complications 2016; 30: 675–680. [DOI] [PubMed] [Google Scholar]
- 11. Verhave JC, Troyanov S, Mongeau F, Fradette L, Bouchard J, Awadalla P, et al Prevalence, awareness, and management of CKD and cardiovascular risk factors in publicly funded health care. Clin J Am Soc Nephrol 2014; 9: 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delanaye P, Flamant M, Cavalier É, Guerber F, Vallotton T, Moranne O, et al Adaptation posologique des médicaments et fonction rénale: quel(s) estimateur(s) faut‐il choisir? Néphrologie Thérapeutique 2016; 12: 18–31. [DOI] [PubMed] [Google Scholar]
- 13. Delanaye P, Guerber F, Scheen A, Ellam T, Bouquegneau A, Guergour D, et al Discrepancies between the Cockcroft–Gault and Chronic Kidney Disease Epidemiology (CKD‐EPI) equations: implications for refining drug dosage adjustment strategies. Clin Pharmacokinet 2017; 56: 193–205. [DOI] [PubMed] [Google Scholar]
- 14. Stengel B, Combe C, Jacquelinet C, Briançon S, Fouque D, Laville M, et al The French Chronic Kidney Disease‐Renal Epidemiology and Information Network (CKD‐REIN) cohort study. Nephrol Dial Transplant 2014; 29: 1500–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stengel B, Metzger M, Combe C, Jacquelinet C, Briançon S, Ayav C, et al Risk profile, quality of life and care of patients with moderate and advanced CKD. The French Chronic Kidney Disease – Renal Epidemiology and Information Network (CKD‐REIN) Cohort Study. Nephrol Dial Transplant 2018. 10.1093/ndt/gfy058. [DOI] [Google Scholar]
- 16. Girerd X, Hanon O, Anagnostopoulos K, Ciupek C, Mourad JJ, Consoli S. Assessment of antihypertensive compliance using a self‐administered questionnaire: development and use in a hypertension clinic. Presse Medicale 2001; 30: 1044–1048. [PubMed] [Google Scholar]
- 17. WHO Collaborating Centre for Drug Statistics Methodology, Oslo . Guidelines for ATC classification and DDD assignment 2016, 20th Edition [online]. 2016. Available at: http://www.whocc.no/atc_ddd_index (last accessed March 2017).
- 18. Du Bois D, Du Bois EF. Clinical calorimetry: tenth paper—a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 1916; XVII: 863–871. [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, et al A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 21. VIDAL: Base de données médicamenteuse pour les prescipteurs libéraux [online]. VIDAL. Available at: https://www.vidal.fr/ (last accessed July 2017).
- 22. National Kidney Foundation . KDOQI Clinical Practice Guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012; 60: 850–886. [DOI] [PubMed] [Google Scholar]
- 23. Guideline Development Group . Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol Dial Transplant 2015; 30 (Suppl. 2): ii1–ii142. [DOI] [PubMed] [Google Scholar]
- 24. Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007; 16: 219–242. [DOI] [PubMed] [Google Scholar]
- 25. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity: description and guidelines for prevention in patients with renal insufficiency. Am J Med 1984; 76: 47–56. [DOI] [PubMed] [Google Scholar]
- 26. Chen I‐H, Kuo M‐C, Hwang S‐J, Chang J‐M, Chen H‐C. Allopurinol‐induced severe hypersensitivity with acute renal failure. Kaohsiung J Med Sci 2005; 21: 228–232. [DOI] [PubMed] [Google Scholar]
- 27. Hoffmann F, Boeschen D, Dörks M, Herget‐Rosenthal S, Petersen J, Schmiemann G. Renal insufficiency and medication in nursing home residents. Dtsch Ärztebl Int 2016; 113: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Getachew H, Tadesse Y, Shibeshi W. Drug dosage adjustment in hospitalized patients with renal impairment at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. BMC Nephrol 2015; 16: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papaioannou A, Clarke J‐A, Campbell G, Bédard M. Assessment of adherence to renal dosing guidelines in long‐term care facilities. J Am Geriatr Soc 2000; 48: 1470–1473. [DOI] [PubMed] [Google Scholar]
- 30. Spruill WJ, Wade WE, Cobb HH. Continuing the use of the Cockcroft–Gault equation for drug dosing in patients with impaired renal function. Clin Pharmacol Ther 2009; 86: 468–470. [DOI] [PubMed] [Google Scholar]
- 31. Dowling TC, Matzke GR, Murphy JE, Burckart GJ. Evaluation of renal drug dosing: prescribing information and clinical pharmacist approaches. Pharmacotherapy 2010; 30: 776–786. [DOI] [PubMed] [Google Scholar]
- 32. Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, et al Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012; 157: 471–481. [DOI] [PubMed] [Google Scholar]
- 33. Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merlière Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique 2010; 58: 286–290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Appropriateness of prescriptions, expressed as the number of inappropriate (i.e. contraindicated or inappropriately high dose drug), inappropriately high dose or contraindicated drugs per patient (n = 3011 patients receiving at least one medication). (A) Number of inappropriate prescriptions per patient (B) Number of inappropriately high dose prescriptions per patient. (C) Number of contraindicated prescriptions per patient
Figure S2 Percentage of patients with at least one contraindicated antidiabetic prescription, at least one inappropriately high dose antidiabetic prescription, or at least one inappropriate antidiabetic prescription, according to the summary of product characteristics (SPC), the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines [22] and the European Renal Best Practice (ERBP) guidelines [23]
Figure S3 Percentages of contraindicated, inappropriately high dose, or inappropriate prescriptions (i.e. contraindicated or inappropriately high dose), by CKD stage. (A) Using the CKD‐EPI equation to estimate renal function. (B) Using the de‐indexed CKD‐EPI equation to estimate renal function. (C) Using the Cockcroft‐Gault equation to estimate renal function
These analyses were restricted to the 2941 patients with data on weight and height
Table S1 (A) Prescribing guidelines for all medications examined in the study (according to the French SPCs, as assessed from January 2017 to March 2017) (B) Prescribing guidelines for antidiabetic agents examined in the study (according to the ERBP 2015 recommendations [22]) (C) Prescribing guidelines for antidiabetic agents examined in the study (according to the KDOQI 2012 recommendations [23])
Table S2 Details of drug prescriptions at baseline according to CKD stages and therapeutic classes
Table S3 Inappropriate prescriptions by ATC class (using SPCs as the reference)
Table S4 Agreement between the CKD‐EPI or de‐indexed CKD‐EPI equations and Cockcroft‐Gault equation (A) Agreement in evaluating contraindications (CIs) (B) Agreement in evaluating inappropriately high doses (IHDs)


