Abstract
Massive metformin overdose can cause metabolic acidosis with hyperlactatemia. A 55‐year‐old woman presented 5 h after multidrug overdose, including 132 g extended‐release metformin. Continuous venovenous haemodiafiltration (CVVHDF) and noradrenaline were commenced due to metabolic acidosis (pH 7.0, lactate 17 mmol l–1) and shock. Despite 3 h of CVVHDF, her acidosis worsened (pH 6.83, lactate 24 mmol l–1). Intermittent haemodialysis (IHD) improved acidosis (pH 7.13, lactate 26 mmol l–1) but again worsened (pH 6.91, lactate 30 mmol l–1) with CVVHDF recommencement. IHD (12 h), CVVHDF (26 h) and vasopressor support for 7 days resulted in survival. Measured metformin concentrations were extremely high with a peak of 292 μg ml–1 at 8 h postingestion. IHD, but not CVVHDF in this case, was associated with improvement in metabolic acidosis and hyperlactataemia. Pharmacokinetic analysis of metformin concentrations found a reduced apparent oral clearance of 8.2 l h–1 and a half‐life of approximately 30 h. During IHD, the apparent oral clearance increased to 22.2 l h–1 with an approximate half‐life of 10 h. The impact of prolonged oral absorption from a pharmacobezoar and redistribution of metformin from peripheral sites (including erythrocytes) on the pharmacokinetic profile cannot be determined from the data available.
Keywords: extracorporeal elimination, metabolic acidosis, metformin, overdose
Introduction
Large http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4779 ingestions can cause severe metabolic acidosis with hyperlactataemia but differ from metformin‐associated lactic acidosis because they usually occur in patients without concurrent acute pathology. We present a case of massive metformin overdose that survived despite developing a severe metabolic acidosis with a high lactate concentration. Multiple serum metformin concentrations were collected throughout her admission. The aim of this case report was to characterize the pharmacokinetics of metformin in a patient who took an intentional overdose of greater than 100 g.
Case report
A 55‐year‐old (80 kg) woman presented 5 h postingestion of metformin extended release 132 g (132 × 1 g), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6318 290 mg, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2713 24 g, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4798 20 g, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2954 150 mg, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6367 120 mg and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7203 37.5 mg. This was on a background of type 2 diabetes mellitus and hypertension.
On presentation, she was vomiting, Glasgow Coma Scale 14, heart rate 88 beats min–1, respiratory rate 24 breaths min–1 and systolic blood pressure 144 mmHg. Her initial arterial blood gas (ABG) showed a pH 7.18 [reference range (RR): 7.35–7.45], serum bicarbonate concentration (HCO3) 14 mmol l–1 (RR: 22–26 mmol l–1), lactate 11.6 mmol l–1 (RR: <2 mmol l–1), base excess –14 (RR: –2 to +2) and creatinine 107 μmol l–1 (RR: 40–80 μmol l–1). Over the next 2 h, she became progressively hypotensive despite intravenous fluids, and noradrenaline was commenced (Figure 1A). Continuous venovenous haemodiafiltration (CVVHDF) was commenced 8 h postingestion due to worsening acidosis (ABG: pH 7.0, HCO3 5 mmol l–1, lactate 17 mmol l–1; Figure 1B). CVVHDF was performed due to availability, using a Primaflex Dialysis System (filter size 0.9 m2) at a blood flow rate of 200 ml min–1, dialysate flow rate of 2400 ml h–1 (lactate free solution) and ultrafiltration of 1600 ml h–1. As the patient was haemodynamically unstable, she could not tolerate higher blood flow rates.
Figure 1.
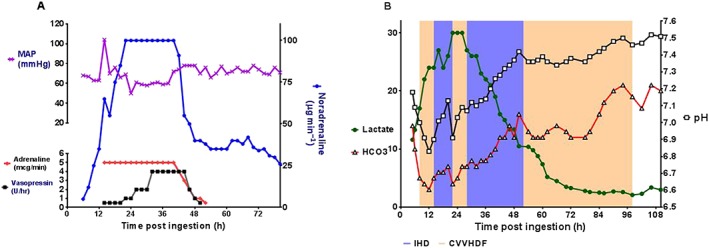
(A) Mean arterial pressure (MAP) and inotrope (noradrenaline, adrenaline and vasopressin) requirements during admission. (B) Lactate (mmol l−1), pH and serum bicarbonate (mmol l−1) concentrations during her admission in relation to intermittent haemodialysis (IHD: blue shaded area) and continuous venovenous haemodiafiltration (CVVHDF: pink shaded area)
Despite 3 h of CVVHDF, the patient deteriorated with worsening hypotension requiring high‐dose vasopressors [http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=505 (90 μg min–1), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4450 (5 μg min–1) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2168] (Figure 1A) and increasing acidosis (ABG: pH 6.83, HCO3 3 mmol l–1, lactate 24 mmol l–1). At 14 h postingestion, her urine output decreased and she became anuric (Figure S1). She was intubated at this stage, activated charcoal was administered and whole bowel irrigation commenced, with a good result of large white tablet matter in the effluent stool.
At 14 h post‐ingestion, she was changed to intermittent haemodialysis (IHD; blood flow rate of 250 ml min–1 and dialysate flow rate of 500 ml min–1) due to severe refractory metabolic acidosis. After 4 h of IHD, her pH improved from 6.9 to 7.13, HCO3 from 5 to 7 mmol l–1 and lactate remained stable at 26 mmol l–1 (Figure 1B). A http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4507 infusion was commenced at 18 h post‐ingestion at 50–100 mmol h–1 for 4 h. At 22 h postingestion, she was recommenced on CVVHDF, with increasing acidosis (pH 6.91, HCO3 4 mmol l–1, lactate 30 mmol l–1) and so was recommenced on IHD. This was associated with improvement in her metabolic acidosis (pH 7.12, HCO3 6 mmol l–1, lactate 26 mmol l–1). IHD was performed continually for 12 h until she improved (pH 7.42, HCO3 16 mmol l–1, lactate 10.5 mmol l–1, creatinine 72 μmol l–1); this was followed by 26 h of CVVHDF. She required vasopressors for 7 days and was extubated on day 9. She had ongoing anuric renal failure after cessation of CVVHDF with a rising creatinine to 470 μmol l–1 and required further management with IHD (Figure S1). This improved over the next month, and she made a full recovery with her creatinine returning to baseline (creatinine 74 μmol l–1).
Methods
Serum metformin concentrations were collected throughout her admission. A liquid chromatography mass spectrometry method was developed for quantification of serum metformin concentrations. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5484 was selected as the internal standard (IS). A 50‐μl aliquot of blank serum/patient serum was added to 50 μl of IS (pregabalin 5 μg ml–1) and 50 μl of each standards or quality controls (in methanol); then 200 μl acetonitrile was added. The mixtures were vortex mixed and then centrifuged at 13 000 g for 5 min at 4°C. A 2‐μl sample of the supernatant were analysed by hydrophilic interaction liquid chromatography–mass spectrometry. Detection was performed using a MDS Sciex API3000 triple quadrupole mass spectrometer (Applied Biosystems Inc., Foster City, CA, USA) that was operated in positive ion mode with turbo electrospray ionization sources (Applied Biosystems Inc.).
Chromatographic separation was achieved using a Kinetex 2.6 μm hydrophilic interaction liquid chromatography (Phenomenex) 50 × 2.1 mm using isocratic flow and 15% solvent A (20 m mol l–1 ammonium acetate) and 85% solvent B (20 mmol l–1 ammonium acetate in 95:5 acetonitrile:water). The total run time was 5 min with a total flow rate of 0.2 ml min–1. The detection was made with electrospray ionization operating at positive ion mode, and the tandem spectrometer was operated in the multiple reaction monitoring (MRM) mode. The mass spectrometric conditions were optimized for metformin and IS by continuous infusion of the standard solutions (1 μg min–1) using a Harvard infusion pump. This assay was validated over the range 1.5–100 μg ml–1 for metformin in human serum.
The precision and accuracy of quality controls (at 40, 20 and 6.25 μg ml–1) for both intraday and interday measurements were within 15%.
Pharmacokinetic analysis was conducted using NONMEM (v7.3), using the first‐order conditional estimation method with interaction. An Intel‐5 processor and a GNU Fortran 95 compiler (GCC 4.6.0) were employed for the analysis. One and two compartment pharmacokinetic models with first‐order absorption and elimination were explored, as well as the influence on IHD on metformin apparent clearance (CL/F). The data were not sufficient to characterize the influence CVVHDF on metformin pharmacokinetics. A time lag on drug absorption was also investigated. The data were insufficient to support other models for drug absorption (e.g. zero order). Models for residual unexplained error included additive, exponential and combined, with both additive and exponential components.
Model selection was based on a likelihood ratio test in which a decrease in the objective function value of 3.84 units (χ2, P < 0.05) with one degree of freedom was considered a better fit for nested models and graphical goodness of fit plots. Models were evaluated primarily by visual inspection of the model prediction overlaid on the observed data for the individual.
Written consent was given by the patient for metformin concentration measurements and publication of her case.
Results
Eight serum metformin concentrations were collected at 5, 8, 16, 22, 27, 33, 56 and 80 h postingestion (Figure 2A,B). The peak concentration was 292 μg ml–1 (RR therapeutic dose: 1.5–3.0 μg ml–1) approximately 8 h postingestion. A one‐compartment model with first‐order absorption, a mixed IHD and non‐IHD clearance and a time lag on absorption provided the best fit to the data (Figure 2A). The parameter estimates for the final model are presented in Table 1. Metformin CL/F was found to be 8.2 l h–1 when the patient was not receiving IHD, with an approximate half‐life of about 30 h. During IHD, the CL/F increased to 22.2 l h–1 with an approximate half‐life of 10 h. Note that the estimation of half‐life is a rough approximation based on clearance and volume values and using the slope of the natural log of metformin concentrations (Figure 2B). Changes in blood volume that occur during IHD or CVVHDF would be expected to impact the determination of half‐life but were not accounted for here.
Figure 2.
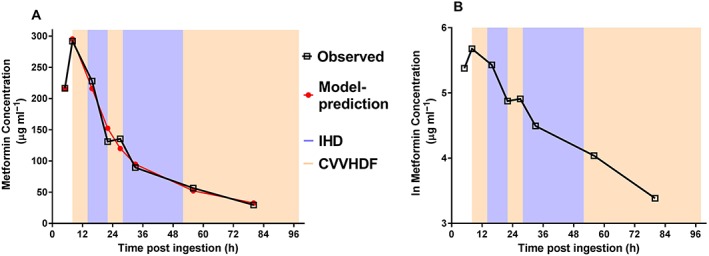
Plots of the observed and predicted metformin concentrations vs. time in relation to intermittent haemodialysis (IHD: blue shaded area) and continuous venovenous haemodiafiltration (CVVHDF: pink shaded area) as (A) untransformed data and (B) natural log of the data
Table 1.
Parameter estimates for the metformin pharmacokinetic model
| Parameter | Parameter estimates |
|---|---|
| CL/F (l h –1 ) Non‐IHD | 8.2 |
| CL/F (l h –1 ) IHD | 22.2 |
| V/F (l) | 370 |
| k a (h–1) | 0.5 |
| tlag (h) | 3.1 |
| σadd (mg l –1 ) | 32.9 |
| σprop (CV) | 0.001 (fix) |
| t 1/2 (h) non‐IHD | Approx. 30 h |
| t 1/2 (h) IHD | Approx. 10 h |
CV, coefficient of variance; σ prop, proportional residual error; σ add, additive residual error; tlag, absorption time lag parameter
Note that the estimation of half‐life (t 1/2) is a rough approximation based on apparent clearance (CL/F) and volume (V/F) values. Changes in blood volume that occur during intermittent haemodialysis (IHD) or continuous venovenous haemodiafiltration were not accounted for.
Discussion
Metformin is a biguanide oral antihyperglycaemic drug used primarily for type 2 diabetes mellitus 1. It is a small (molecular weight 129.16 g mol–1) water‐soluble compound that does not bind to plasma proteins 2. It is predominately eliminated as unchanged drug by active transport processes (predominately http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1020) in the kidney, and the clearance greatly exceeds glomerular filtration rate 3. Its clearance is reduced with renal impairment. In acute overdoses, minor gastrointestinal symptoms and hyperlactataemia are the commonest effects 4. With large ingestions (typically >50 g), patients may develop severe toxicity characterized by severe metabolic acidosis, hyperlactatemia and hypotension 4. Metformin overdose can produce both an increased production and decreased hepatic metabolism of lactate.
Much of the data published on metformin are cases of metformin associated lactic acidosis, with acute overdose less commonly reported. Dell'Aglio et al. 5 performed a systemic review of 22 acute metformin ingestions from the literature and found that patients with serum pH <6.9 or lactate >25 mmol l–1 had a mortality rate of 83%. Furthermore, those with metformin concentration >50 μg ml–1 had a 38% mortality 5. In this case, the lowest pH was 6.83, maximum lactate 30 mmol l–1 and peak metformin concentration 292 μg ml–1. Hence this patient had a high risk of mortality and one of the highest metformin concentrations reported in the literature.
In this case, lactate concentrations did not appear to be affected by treatment with CVVHD or IHD, with concentrations fluctuating regardless of dialysis modality (Figure 1B). The small changes in lactate concentration were probably due to the overwhelming production of lactate, which was far greater than lactate clearance by either dialysis modality. However, IHD resulted in more rapid correction of acidaemia (hydrogen ion concentration) as evidenced by improvement in both pH and HCO3 concentrations once IHD was commenced (Figure 1B). The small change seen in lactate concentration with extracorporeal treatment is consistent with the limited data from the literature. Lactate is a low molecular weight molecule (90 Da) like urea, and its clearance is probably similar to other small solutes 6. There are few studies on lactate clearance via renal replacement therapy. Levraut et al. 7 looked at lactate clearance during continuous renal replacement therapy and compared it with endogenous lactate clearance. They found that median endogenous lactate clearance was 1379 ml min–1, while the median filter lactate clearance was 24.2 ml min–1, which was <3% of total lactate removal 7. In comparison, IHD has a clearance of small solutes that is 150–200 ml min–1, which represents approximately 20% of endogenous lactate clearance 6. This offers a likely explanation of why lactate concentrations stabilized in this case, but did not fall during IHD, as clearance of lactate is still much lower than endogenous clearance during states of high lactate production.
This patient had extremely high metformin concentrations peaking at 292 μg ml–1 8 h postingestion and a concentration of 29.5 μg ml–1 (10× therapeutic range) 80 h postingestion after 3 days of extracorporeal treatment. Our estimated metformin IHD CL/F and half‐life values of 22.2 l h–1 and about 10 h, respectively, are larger than those reported in the literature from overdose and nonoverdose cases. While somewhat variable due to the haemodialysis set‐up used, published cases report metformin CL/F and half‐life values during IHD of 4 to 14 l h–1 and 4 to 7 h, respectively 8, 9, 10, 11. The reason for the discrepancy is unclear. Half‐life estimates are approximate and may be inaccurate since the change in blood volume that occurs during IHD was not accounted for here. In addition, we note that the estimate of IHD CL/F in the overdose case presented here is contingent on two plasma concentrations measured at 22 and 27 h postingestion (Figure 2A,B) being an accurate reflection of metformin behaviour during and after IHD. Visual inspection of the natural log plot (Figure 2B) of metformin concentrations suggests an increased elimination during the first IHD session (14 to 22 h postingestion) with a possible post‐IHD rebound at 27 h postingestion. During the second IHD session (27 to 52 h postingestion), visual inspection suggests that the elimination is not consistent across the dialysis period, raising the concern that the data point at 27 h postingestion may be aberrant, possibly due to process error (e.g. error in timing or labelling of the sample and assay error).
When not receiving IHD, the metformin CL/F was estimated to be 8.2 l h–1 with an approximate half‐life of about 30 h. The CL/F is substantially reduced compared to the reported values of approximately 70 l h–1 after therapeutic administration 2. The model also included a lag time, which was considerably longer than previously reported values 3. We propose that the lag time parameter accounts for the uncertainty in the time that the dose (overdose) was taken. Previous population pharmacokinetic analyses of overdose patients have used lag time to account for uncertainty in dose–time 12.
The current Extracorporeal Treatment in Poisoning recommendations conclude that metformin is moderately dialysable. However, this varies according to kidney function and dialysis modality utilized. Furthermore, this is mainly based on cases where renal clearance of metformin is substantially decreased due to renal impairment. Extracorporeal treatment is recommended in severe metformin poisoning including a lactate concentration >20 mmol l–1, pH ≤7.0, shock, failure of standard supportive measures and decreased level of consciousness. IHD is recommended as the preferred method of extracorporeal treatment, because it is superior in terms of its correction of acidaemia, removal of metformin and lactic acid 8, 13. From our case, it appears that the major benefit of IHD is better correction of refractory severe acidaemia and not clearance of lactate.
We present a case of massive metformin overdose with an extremely high metformin concentration that survived with good supportive care. Pharmacokinetic modelling in this case showed a dramatically reduced apparent oral clearance and a prolonged half‐life. IHD increased the clearance of metformin, consistent with other reports of metformin overdose. CVVHDF did not correct the severe metabolic acidosis, and hence IHD was used. The case highlights that IHD is more effective in treating severe metabolic acidosis with high metformin and lactate concentrations, when compared with CVVHDF secondary to acute massive metformin ingestion. Patients may require prolonged IHD in massive metformin overdose, especially those with renal impairment or persistent severe acidosis.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 14, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 15.
Competing Interests
There are no competing interests to declare. The authors alone are responsible for the content and writing of the paper.
G.K.I. is funded by an NHMRC Senior Research Fellowship ID1061041. M.S.R. is funded by an NHMRC Senior Principal Research Fellowship ID1002611. This research was partially supported by an NHMRC Program Grant 1055176.
Supporting information
Figure S1 Plot of the creatinine concentrations and urine output vs. time including the time when continuous venovenous haemodiafiltration (CVVHDF; orange shading) and intermittent haemodialysis (IHD; blue shading) were undertaken
Chiew, A. L. , Wright, D. F. B. , Dobos, N. M. , McArdle, K. , Mostafa, A. A. , Newth, A. , Roberts, M. S. , and Isbister, G. K. (2018) ‘Massive’ metformin overdose. Br J Clin Pharmacol, 84: 2923–2927. 10.1111/bcp.13582.
References
- 1. Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci 2012; 122: 253–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al Clinical pharmacokinetics of metformin. Clin Pharmacokinet 2011; 50: 81–98. [DOI] [PubMed] [Google Scholar]
- 3. Duong JK, Kumar SS, Kirkpatrick CM, Greenup LC, Arora M, Lee TC, et al Population pharmacokinetics of metformin in healthy subjects and patients with type 2 diabetes mellitus: simulation of doses according to renal function. Clin Pharmacokinet 2013; 52: 373–384. [DOI] [PubMed] [Google Scholar]
- 4. McNamara K, Isbister GK. Hyperlactataemia and clinical severity of acute metformin overdose. Intern Med 2015; 45: 402–408. [DOI] [PubMed] [Google Scholar]
- 5. Dell'Aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD, Morgan BW. Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med 2009; 54: 818–823. [DOI] [PubMed] [Google Scholar]
- 6. Bellomo R. Bench‐to‐bedside review: lactate and the kidney. Crit Care 2002; 6: 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levraut J, Ciebiera JP, Jambou P, Ichai C, Labib Y, Grimaud D. Effect of continuous venovenous hemofiltration with dialysis on lactate clearance in critically ill patients. Crit Care Med 1997; 25: 58–62. [DOI] [PubMed] [Google Scholar]
- 8. Calello DP, Liu KD, Wiegand TJ, Roberts DM, Lavergne V, Gosselin S, et al Extracorporeal treatment for metformin poisoning: systematic review and recommendations from the extracorporeal treatments in poisoning workgroup. Crit Care Med 2015; 43: 1716–1730. [DOI] [PubMed] [Google Scholar]
- 9. Smith FC, Kumar SS, Furlong TJ, Gangaram SV, Greenfield JR, Stocker SL, et al Pharmacokinetics of metformin in patients receiving regular hemodiafiltration. Am J Kidney Dis 2016; 68: 990–992. [DOI] [PubMed] [Google Scholar]
- 10. Ayoub P, Hetu PO, Cormier M, Benoit A, Palumbo A, Dube MC, et al Toxicokinetics of metformin during hemodialysis. Kidney Int Rep 2017; 2: 759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lalau JDAM, Morinière P, Coevoet B, Debussche X, Westeel PF, Fournier A, et al Hemodialysis in the treatment of lactic acidosis in diabetics treated by metformin: a study of metformin elimination. Int J Clin Pharmacol Ther Toxicol 1989; 27: 285–288. [PubMed] [Google Scholar]
- 12. Cooper JM, Duffull SB, Saiao AS, Isbister GK. The pharmacokinetics of sertraline in overdose and the effect of activated charcoal. Br J Clin Pharmacol 2015; 79: 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lemyze M, Baudry JF, Collet F, Guinard N. Life threatening lactic acidosis. BMJ 2010; 340: c857. [DOI] [PubMed] [Google Scholar]
- 14. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, et al The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 2017; 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Plot of the creatinine concentrations and urine output vs. time including the time when continuous venovenous haemodiafiltration (CVVHDF; orange shading) and intermittent haemodialysis (IHD; blue shading) were undertaken


