Abstract
Aims
A recent double‐blind placebo‐controlled crossover 70‐day trial demonstrated that a fixed combination of dextromethorphan and quinidine (DM/Q) improves speech and swallowing function in most patients with amyotrophic lateral sclerosis. In this study, a subset of participants, many of whom did not substantially improve while on DM/Q, were re‐evaluated using computer‐based speech analyses and expert clinician ratings of the overall severity of speech impairment.
Methods
Speech samples were recorded from the subset of 10 patients at four visits made at approximately 30‐day intervals. The recordings were analysed by automated computer‐based analysis of speech pausing patterns. Severity of speech impairment was rated by three experienced speech‐language pathologists using direct magnitude estimation. Scores on patient‐reported and clinician‐administered scales of bulbar motor involvement were obtained at each visit.
Results
The effects of DM/Q were detected on several of the objective speech measures, including total pause duration (s) (Cohen's d = 0.73, 95% confidence interval (CI) –1.70, 0.24), pause time (%) (d = 0.77, 95% CI –1.75, 0.21), and mean speech event duration (s) (d = 0.52, 95% CI –0.44, 1.47), but not on clinician ratings of speech or the speech components of the self‐report or clinician‐administered scales.
Conclusions
These findings suggest that even patients with modest improvement while on DM/Q may experience quantifiable improvements in speech when assessed using sensitive and objective measures. This study provides additional evidence of the positive impact of DM/Q on one or more of the neural systems that control bulbar motor function and production of speech.
Keywords: amyotrophic lateral sclerosis, bulbar function, combination, dextromethorphan, dysarthria, quinidine
What is Already Known about this Subject
There are currently no pharmacological treatments that have been approved for the treatment of impaired speech or swallowing in patients with amyotrophic lateral sclerosis (ALS); however, dextromethorphan/quinidine, a drug approved for the treatment of emotional lability in patients with ALS and kindred disorders, has recently been demonstrated to enhance bulbar function in a Phase II clinical trial.
What this Study Adds
The results of this study suggest that even patients who did not substantially improve while on dextromethorphan/quinidine, as determined by patient‐based and clinician‐administered assessments, showed quantifiable improvements in speech that could be detected using objective measures. Objective speech measures may be sensitive objective outcomes in therapeutic drug trials targeting bulbar motor function in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurological disease that causes degeneration of motor neurons. Among the devastating effects of ALS are progressive difficulties with speech, chewing and swallowing. After decades of clinical research, only two drugs have been approved by the US Food and Drug Administration for the treatment of ALS, neither of which have had beneficial effects on speech or swallowing in affected individuals. Edaravone, an antioxidant, was recently observed in a Phase III clinical trial (NCT01492686) to slow the rate of physical decline in a subgroup of patients with ALS 1. Riluzole was approved in 1995 for the treatment of ALS after it was demonstrated to increase life expectancy by several months 2. Riluzole inhibits the excitatory neurotransmitter glutamate, which may become dysregulated in ALS, leading to overexcitation and subsequent cell damage 3. Identification of drugs that mitigate glutamatergic‐induced excitotoxicity continues to be an active area in ALS research 4.
Since DM/Q, a combination product containing dextromethorphan (DM) and quinidine, was approved in 2011 for the treatment of emotional lability (pseudobulbar affect) in patients with a variety of neurological disorders, there have been many anecdotal reports of improvement in speech and swallowing in response to treatment 5. DM is the active ingredient in many over‐the‐counter cough suppressant medicines and has several known molecular mechanisms of action. The drug exerts molecular promiscuity in that it is a sigma‐1 receptor agonist, a http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 antagonist, a http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=76 antagonist, and an inhibitor of the serotonin and norepinephrine transporters 6. DM may have neuroprotective effects via such mechanisms 7, including attenuation of glutamate‐mediated excitotoxicity 8. Quinidine improves the bioavailability of DM by inhibiting cytochrome http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1329, a liver enzyme that O‐demethylates DM.
Anecdotal reports of improved bulbar function in patients treated with DM/Q prompted a short‐duration (70‐day), multicentre, randomized, double‐blind, crossover Phase II clinical trial to assess the effect of treatment on bulbar motor function in 60 patients with ALS 5. The primary efficacy endpoint in that trial was the score on the Center for Neurologic Study Bulbar Function Scale (CNS‐BFS) 5, a 21‐item patient‐report scale that assesses speech, swallowing, and salivation. CNS‐BFS scores can range from a low of 21 (no bulbar impairment) to a high of 112 (severe bulbar impairment). The results of that trial demonstrated that DM/Q had a positive effect on bulbar function, with an average improvement of 5.85 points on the CNS‐BFS, a finding that was corroborated by improved bulbar subscores on the ALS Functional Rating
Scale‐Revised (ALSFRS‐R) is a widely used clinician‐administered outcome measure 9. To our knowledge, no drug targeting ALS other than DM/Q has been demonstrated to improve speech or swallowing function. Therefore, the effects of DM/Q on bulbar motor function, speech and swallowing is a research priority.
Of particular relevance to this report is the sensitivity of measures used in the Phase II study to assess bulbar function. Although the effect on speech was robust as measured by the speech components of the CNS‐BFS, ALSFRS‐R and a visual analogue scale, statistically detectable improvements were not observed in any of the objective performance‐based measures of bulbar motor function (i.e. timed swallowing, chewing and speech tasks). The absence of improvement in these measures requires further inquiry because: (i) the evidence for a therapeutic effect will be strengthened if the perceived benefits are observed to translate into objective gains in bulbar motor performance; and (ii) although expert consensus regarding best practices for assessment of bulbar function is currently lacking 10, recent research has identified computer‐based measures of speech that are more responsive to bulbar motor involvement than the objective speech measures 11, 12, 13 used in the DM/Q study 5. The latter point is particularly germane to this report because it raises the possibility that relative nonresponders in the original trial, i.e. approximately 30% of all the participants may have experienced speech benefits that were undetected.
The current study aimed to determine if treatment with DM/Q produces measurable changes in speech using high‐precision, computer‐based analyses of the speech signal in a subset of patients in the earlier DM/Q trial. Quantitative speech testing was an exploratory outcome in the original trial. Digital speech recordings were obtained at all visits in only 10 of the 60 participants; therefore, the present analysis could only be undertaken in a limited number of the trial participants. While our findings further validate the positive effects of DM/Q on speech, more importantly, they inform ongoing efforts to identify responsive clinical endpoints for trials that target bulbar motor function 11. Drug discovery in ALS has been hampered by a lack of useful biomarkers 14, 15, so clinical endpoints with high responsiveness to change are needed for identification of candidate drug treatments that could be amplified by increased dosage and exposure or by addition of complementary agents 16.
Methods
Parent DM/Q trial
The parent Phase II DM/Q trial (NCT01806857) included data from 60 patients with ALS collected from seven sites. Eligible participants had a diagnosis of ALS according to the World Federation of Neurology El Escorial Criteria 17 and researcher‐judged bulbar dysfunction, specifically dysarthria and/or dysphagia.
Participants were deemed ineligible for inclusion if they showed evidence of cognitive impairment, had previously used DM/Q or if use of DM/Q was medically contraindicated. A full list of the inclusion/exclusion criteria applied in the DM/Q clinical trial is provided in the report by Smith et al. 5.
The parent study was a multicentre, randomized, double‐blind, crossover trial. Participants were randomly assigned to receive DM/Q (dextromethorphan hydrobromide 20 mg, quinidine sulphate 10 mg) or placebo for 30 days between visits 1 and 2 and were then crossed over to receive the alternative treatment for 30 days between visits 3 and 4. The study treatments were separated by a washout interval of 10–15 days.
Present study
In this study, speech recordings were obtained from 10 patients in the original cohort of 60 patients with a diagnosis of ALS 5. Demographic information and ratings of severity at the initial visit are shown in Table 1. The 10 study participants were selected based solely on the availability of speech recordings and were from two of the seven data collection sites involved in the parent study [Nebraska (n = 7) and Cleveland Clinic (n = 3)]. By chance, the subgroup included participants with less severe dysfunction at baseline (mean ALSFRS‐R 38.5 vs. 33.8, P = 0.05; abnormal speech evaluation 70% vs. 96%, P = 0.03) who were less likely to experience improvement in the primary endpoint of the parent study (CNS‐BFS total score –1.7 units, P = 0.63 vs. –6.4 units, P < 0.001). The trial was conducted in accordance with the provisions of the International Conference on Harmonisation Guidelines for Good Clinical Practice 18 and the Declaration of Helsinki 19. All participants at these sites consented to have their speech recorded during each of the four study visits.
Table 1.
Demographic information of participants
| Patient ID | Sex | Age at enrolment (years) | Onset | Time from symptom onset to enrolment (years, months) |
|---|---|---|---|---|
| 1 | M | 42 | Limb | 2, 5 |
| 2 | M | 65 | Bulbar | 2, 6 |
| 3 | M | 54 | Limb | 1, 2 |
| 4 | M | 62 | Bulbar | 0, 6 |
| 5 | M | 54 | Limb | 12, 0 |
| 6 | F | 64 | Bulbar | 1, 4 |
| 7 | F | 75 | Bulbar | 1, 5 |
| 8 | M | 38 | Limb | 4, 11 |
| 9 | F | 44 | Bulbar | 1, 9 |
| 10 | M | 77 | Limb | 0, 10 |
Outcome measures
Eleven outcome variables (Table 2) were measured at the beginning (day 1) and end (day 30) of each treatment period (active and placebo) during the trial. At all four data collection sessions, speech recordings were obtained while each participant read a short paragraph (the Rainbow Passage) 20. Speech samples were recorded using a high‐quality digital audio recording device (Zoom H1 Handy Recorder, Zoom, Hauppauge, NY, USA). The audio recordings were used to obtain clinician‐evaluated speech severity ratings and quantitative measures of speech pausing patterns.
Table 2.
Study outcome measures
| Outcome | Type of measurement | Derivation (quantitative measures only) |
|---|---|---|
| ALSFRS‐R Total | Clinician‐administered | ‐‐ |
| ALSFRS‐R Speech | Clinician‐administered | ‐‐ |
| CNS‐BFS Speech | Patient self‐report | ‐‐ |
| SLP severity rating | Clinician rating | ‐‐ |
| Total pause duration (s) | Quantitative speech | = SUM (duration of individual pause segments > 300 ms) |
| Number of pause events | Quantitative speech | = COUNT (individual pause segments > 300 ms) |
| Pause event duration (s) | Quantitative speech | = total pause duration (min)/No. of pause events |
| Speech event duration (s) | Quantitative speech | = total speech duration (min)/No. of speech events |
| Pause time (%) | Quantitative speech | = total pause time (min)/total response (speech + pause) duration (min) × 100 |
| Speaking rate (wpm) | Quantitative speech | = No. of total words/total response (speech + pause) duration (min) |
| Articulation rate (wpm) | Quantitative speech | No. of total words/total speech duration (min) |
ALSFRS‐R, ALS Functional Rating Scale Revised; CNS‐BFS, Center for Neurologic Study Bulbar Function Scale; SLP, speech‐language pathologist; wpm, words min–1
Clinical speech measures
The patient‐reported and clinician‐based measures considered in this study included the CNS‐BFS (speech subscore), ALSFRS‐R (total score and speech subscore), and speech severity ratings by speech‐language pathologists (SLPs). The CNS‐BFS is a patient self‐report scale that assesses three domains of bulbar motor function (i.e. speech, swallowing and salivation) 13. Each domain contains seven questions that are rated on scale of 1 (does not apply) to 5 (applies most of the time) for the swallowing and salivation domains, and 1 (does not apply) to 6 (unable to communicate by speaking) for the speech domain. The ALSFRS is a 12‐question survey that is the current gold standard assessment for ALS and is a valid and reliable measure of overall disease severity 9, 21, 22. A speech subscore was extracted based on the patient's answer to question 1 of the ALSFRS‐R, which rates overall speech performance on a scale of 4 (normal speech process) to 0 (loss of useful speech).
To obtain speech severity ratings, three SLPs provided dysarthria severity ratings by direct magnitude estimation for each of the four Rainbow Passage recordings produced by each patient with ALS 23. Direct magnitude estimation involves assigning a value to each speech sample that represents its perceived severity as a ratio relative to a reference sample known as a modulus. The modulus is typically chosen to represent a midpoint on the continuum of the construct being measured and is assigned a value of 100 23, 24, 25. Using this method, a sample judged to be twice as severe as the modulus would be rated as 200, and a sample judged to be half as severe as the modulus would be rated as 50. For the present study, the modulus chosen was a recording of the Rainbow Passage produced by a speaker who was not a subject in the study and was judged by the researchers to have moderate dysarthria.
The SLPs were instructed to listen to the first two sentences of the Rainbow Passage recordings from all four visits for each speaker before moving on to the next speaker to ensure that the raters were responding primarily to changes in speech across the sessions and not to the overall severity of speech impairment. The order of presentation of the speech recordings for the four time points was randomized for each speaker, and the SLPs were blinded to the visit number and treatment allocation at each visit. The order of the speakers was also randomized for each SLP. The SLPs were instructed to listen to the modulus once between each set of four speech samples to calibrate their ratings and could listen to each sample as many times as they deemed necessary before assigning a severity rating.
Quantitative speech measures
Prior to the analysis, the speech recordings were reviewed by a laboratory technician and filtered to attenuate ambient noise. An automated Speech Pause Analysis (SPA) programme 26 was used to algorithmically estimate speech and pause segments in the speech sample based on a minimum pause duration (300 ms) and a minimum speech duration (25 ms).
As shown in Figure 1, the SPA programme automatically demarcated pause and speech events and provided counts and duration values for each pause (>300 ms) and speech (>25 ms) event, as well as the summed duration of all pause and speech events. These data were used to derive the following five quantitative speech measures from each speech sample: number of pause events, total pause duration (s), percent pause time, (mean) duration of pause event (s), and mean duration of speech events (s). The accuracy of the automatic approach used to measure pauses in continuous speech has been validated against live operator performance 26, and the diagnostic accuracy of SPA measures has been also shown to be good for detecting bulbar motor involvement due to ALS 10. The SPA output was also used to derive two additional quantitative speech measures, i.e. speaking rate (i.e. number of words per total response duration) and articulation rate (i.e. number of words per total speech duration). Table 2 describes the derivation of each quantitative speech measure.
Figure 1.
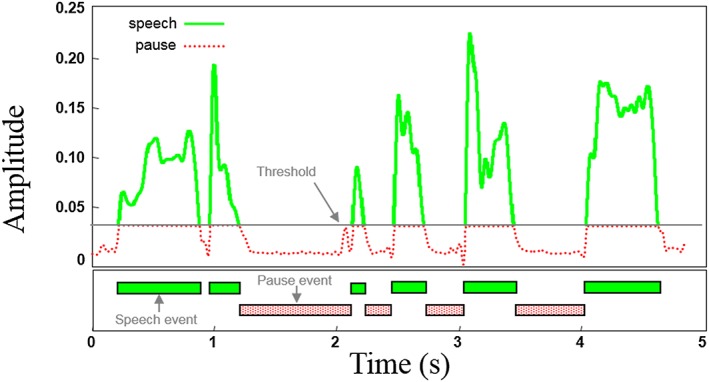
The maximum amplitude of a selected pause region was used to establish a threshold for separating speech from pause events. Amplitude values above the threshold mark boundaries for speech events and values below the threshold mark boundaries for speech events. Pause and speech event durations below 300 ms and 25 ms, respectively, were not included in the analysis. For each Rainbow Passage recording, five primary variables were extracted by Speech Pause Analysis, i.e. (mean) speech event duration, (mean) pause event duration, total pause duration, and percent pause time. The Speech Pause Analysis output was also used in combination with manual syllable counts to derive two additional variables, i.e. speaking rate and articulation rate
Statistical analysis
The Wilcoxon signed rank test was used to detect statistically significant differences in patients' scores between pretesting and post‐testing during the active treatment phase and the placebo phase for each of the 11 outcome measures. Nonparametric tests were used for group comparisons because of the small sample size and the non‐normal distribution of the change in scores for ordinal variables. Cohen's d effect sizes were computed to quantify the relative magnitude of change per variable in the active treatment phase versus the placebo phase. Effect sizes were calculated using the R effsize package 27. This study involved repeated measurements on a single sample, so effect size calculations were based on paired differences. For ordinal variables (ALSFRS‐R Total, ALSFRS‐R Speech, CNS‐BFS Speech), Cliff's d was computed instead of Cohen's d as a measure of effect size 28. Effect sizes for Cohen's d = 0.5 or Cliff's d = 0.33 were considered medium effect sizes and those with Cohen's d = 0.8 or Cliff's d = 0.474 or greater were considered large effect sizes 29.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 30, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 31, 32.
Results
Summary statistics for the mean pretreatment and post‐treatment values of each outcome measure in the active and placebo phases of the study are shown in Table 3, along with the results of pairwise significance testing for each variable. Table 3 also shows the effect sizes for the change in scores between pretreatment and post‐treatment during the active and placebo phases for each of the 11 outcome variables.
Table 3.
Descriptive statistics showing the average values for each variable at the pretreatment and post‐treatment time points for the active and placebo phases of the study. Cohen's d and Cliff's d effect sizes indicating the magnitude of the change during each phase of the trial are also reported (note: Cohen's d is larger than Cliff's d for the same magnitude of change)
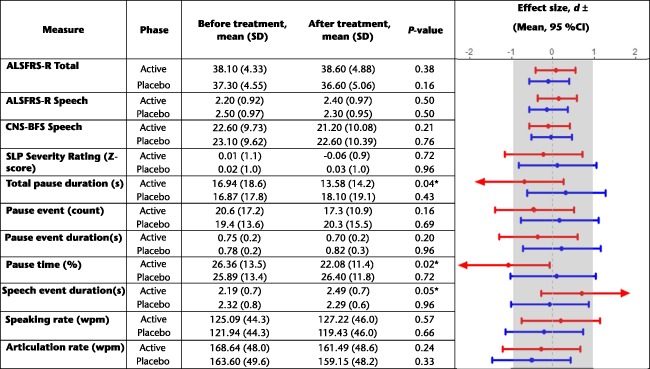
P < 0.05 denotes the statistical significance of pre vs. post scores across each of the study arms (active, placebo). ± Cohen's d effect sizes are reported for continuous variables (SLP severity rating, total pause duration, # pause events, pause event duration, speech event duration, percent pause time, speaking rate, articulation rate); Cliff's d effect sizes are reported for ordinal variables (ALSFRS‐R Total, ALRSFRS‐R Speech, CNS‐BFS Speech). Arrows indicate the direction of clinician‐judged improvement on a given measure, shown for statistically significant variables only. ALSFRS‐R, ALS Functional Rating Scale Revised; CI, confidence interval; CNS‐BFS, Center for Neurologic Study Bulbar Function Scale; SD, standard deviation; SLP, speech‐language pathologist; wpm, words min–1
Clinical speech measures
As shown in Table 3, the effect sizes for pre‐test to post‐test changes in both the active and placebo phases were small for all clinical speech measures including the clinician ratings of speech severity. None of the changes were statistically significant.
Quantitative speech measures
On average, participants showed a decrease in percent pause time during the active treatment phase and a minimal change during the placebo treatment phase. This change appeared to be driven by a decrease in total pause duration and an increase in the average duration of speech segments. Statistical comparisons of pretreatment and post‐test measurements for these variables showed medium–large effect sizes for changes in percent pause time (|d| = 1.08), total pause duration (|d| = 0.7), and speech event duration (|d|= 0.7) during the active treatment phase. The effect sizes for these three variables were small for changes between pretest and post‐test during the placebo phase (|d|= 0.09, 0.32 and 0.07, respectively). The Wilcoxon signed rank tests revealed statistically significant decreases in percent pause time (Z = –2.29, P = 0.02) and total pause duration (Z= –2.09, P = 0.04) as well as a significant increase in speech event duration (Z = –1.99, P = 0.047) during the active treatment phase. There was no significant change in any of these variables between pretesting and post‐testing during the placebo phase. The effect sizes for changes in the remaining four quantitative speech measures (i.e. number of pause events, pause event duration, speaking rate and articulation rate) between pretesting and post‐testing were small in both the active and placebo phases.
Discussion
Speech outcomes were analysed in a subset of 10 patients with ALS who were participants in a recent randomized controlled Phase II trial investigating the effects of DM/Q on bulbar motor function 1. Multiple speech endpoints were evaluated including objective measures of speech, and patient‐reported and clinician‐based measures. Statistically significant effects were detected for quantitative speech measures using an automated, computer‐based analysis of speech pause patterns 26. The observed changes in speech following treatment provide additional evidence for (i) the therapeutic effects of DM/Q on bulbar motor function in persons with ALS and (ii) the high responsiveness of quantitative speech analysis for detecting pharmacological effects on bulbar motor function.
The effects of DM/Q on speech included a decrease in the frequency of pauses and an associated increase in duration of uninterrupted speech. The suggestion that these changes are beneficial is supported by natural history studies demonstrating that bulbar motor deterioration is marked by an increase in both the number and duration of pauses in speech 26, 33, 34. Identifying the mechanisms of these speech improvements will be important for determining if the effects of DM/Q are widely distributed throughout the central nervous system or localized to bulbar motor pathways. Changes in pausing could arise from gains in frontotemporal control over affect and cognitive and linguistic processes 35, 37, 38 or reflect a more localized effect on the motor pathways that control speech breathing, voice and articulation 11, 26, 35. A recent study comparing speech pausing behaviour among different subtypes of ALS (e.g. those with primarily bulbar or respiratory involvement) suggest that speech pausing patterns were most aberrant in the subgroup with respiratory involvement 35. A more comprehensive speech battery that includes instrumental measures of bulbar motor performance 36 combined with neural imaging data is required to disentangle the relative contributions of cognitive, linguistic and motor processes to changes in speech pausing patterns in persons with ALS.
The absence of a DM/Q effect on patient‐reported and clinician‐based measures of speech in this subset of participants from the parent trial raises questions about the efficacy of these outcomes measures in ALS intervention trials with only modest sample sizes. Our prior work has also demonstrated the poor diagnostic accuracy of clinician‐based detection of early bulbar involvement with a false‐positive rate as high as 39% 10. Our expectation for detecting significant clinical effects on speech in the current study, however, was low because the subgroup of participants studied had, by chance, less severe bulbar dysfunction at baseline than many of the participants in the parent trial and as a group were less responsive to DM/Q treatment 5. These mixed findings between clinical and quantitative endpoints underscore the ongoing challenges with choosing endpoints for clinical trials that target bulbar motor functions such as speech and swallowing.
The need for improved surrogate markers of ALS to expedite drug discovery has been long recognized 14. Although clinically significant changes were not observed in speech, the finding that changes in speech could be detected using computer‐based analyses provide further support for the high responsiveness of these measurement tools for detecting subclinical changes in bulbar motor function 12. Therefore, the identification of these arguably subclinical changes in motor function are critical for improved drug discovery for the following reasons: (i) it suggests that even patients who were considered nonresponders in the parent trial received some physiological benefit from DM/Q; (ii) it justifies additional research to determine if the benefits can be amplified at increased dosages and exposure durations or when the drug is combined with other therapeutic agents; (iii) it helps validate the stratification of responders and nonresponders to the treatment; and (iv) it provides additional information about the biological selectivity of the drug across neural systems.
Overall, the findings of the present study and those of the parent clinical trial confirm the need for continued investigation of the potentially beneficial effects of DM/Q on bulbar motor function, speech and swallowing. Future studies should include larger cohorts and, if safety permits, higher doses and longer exposures. Additional research is also warranted to identify the mechanisms of improved speech and the characteristics of patients who respond to treatment.
The current findings also underscore the need to include quantitative speech motor testing in exploratory studies of drugs and biological interventions for ALS.
Competing Interests
G.L.P. and E.P.P. report consulting and/or speaker's fees from Avanir Pharmaceuticals (parent company Otsuka), the manufacturer of Nuedexta (the fixed combination product referred to herein). No other authors report materially relevant conflicts of interest.
This research was supported by NIH grants R01DC013547, R01DC017291, and K24 DC01631.
Green, J. R. , Allison, K. M. , Cordella, C. , Richburg, B. D. , Pattee, G. L. , Berry, J. D. , Macklin, E. A. , Pioro, E. P. , and Smith, R. A. (2018) Additional evidence for a therapeutic effect of dextromethorphan/quinidine on bulbar motor function in patients with amyotrophic lateral sclerosis: A quantitative speech analysis. Br J Clin Pharmacol, 84: 2849–2856. 10.1111/bcp.13745.
References
- 1. Tanaka M, Sakata T, Palumbo J, Akimoto M. A 24‐week, phase III, double‐blind, parallel‐group study of edaravone (MCI‐186) for treatment of amyotrophic lateral sclerosis (ALS)(P3. 189). Neurology 2016; 6 (Suppl. 16): P3–P189. [Google Scholar]
- 2. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med 1994; 330: 585–591. [DOI] [PubMed] [Google Scholar]
- 3. Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol 2011; 7: 613–630. [DOI] [PubMed] [Google Scholar]
- 4. Cheah BC, Vucic S, Krishnan A, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem 2010; 17: 1942–1959. [DOI] [PubMed] [Google Scholar]
- 5. Smith R, Pioro E, Myers K, Sirdofsky M, Goslin K, Meekins G, et al Enhanced bulbar function in amyotrophic lateral sclerosis: the Nuedexta treatment trial. Neurotherapeutics 2017; 14: 762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR. Pharmacology of dextromethorphan: relevance to dextromethorphan/quinidine (Nuedexta®) clinical use. Pharmacol Ther 2016; 164: 170–182. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen L, Thomas KL, Lucke‐Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR. Dextromethorphan: an update on its utility for neurological and neuropsychiatric disorders. Pharmacol Ther 2016; 159: 1–22. [DOI] [PubMed] [Google Scholar]
- 8. DeCoster MA, Klette KL, Knight ES, Tortella FC. σ receptor‐mediated neuroprotection against glutamate toxicity in primary rat neuronal cultures. Brain Res 1995; 671: 45–53. [DOI] [PubMed] [Google Scholar]
- 9. Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al The ALSFRSR: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 1999; 169: 13–21. [DOI] [PubMed] [Google Scholar]
- 10. Allison KM, Yunusova Y, Campbell TF, Wang J, Berry JD, Green JR. The diagnostic utility of patient‐report and speech‐language pathologists' ratings for detecting the early onset of bulbar symptoms due to ALS. Amyotroph Lateral Scler Front Degener 2017; 18: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Green JR, Yunusova Y, Kuruvilla MS, Wang J, Pattee GL, Synhorst L, et al Bulbar and speech motor assessment in ALS: challenges and future directions. Amyotroph Lateral Scler Frontotemporal Degener 2013; 14: 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plowman EK, Tabor LC, Wymer J, Pattee G. The evaluation of bulbar dysfunction in amyotrophic lateral sclerosis: survey of clinical practice patterns in the United States. Amyotroph Lateral Scler Front Degener 2017; 18: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith RA, Macklin EA, Myers KJ, Pattee GL, Goslin KL, Meekins GD, et al The assessment of bulbar function in amyotrophic lateral sclerosis: validation of a self‐report scale (CNS‐BFS). Eur J Neurol 2018; 25: 907–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berry JD, Cudkowicz ME. New considerations in the design of clinical trials for amyotrophic lateral sclerosis. Clin Investig (Lond) 2011; 1: 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicholson KA, Cudkowicz ME, Berry JD. Clinical trial designs in amyotrophic lateral sclerosis: does one design fit all? Neurotherapeutics 2015; 12: 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McElhiney M, Rabkin JG, Goetz R, Katz J, Miller RG, Forshew DA, et al Seeking a measure of clinically meaningful change in ALS. Amyotroph Lateral Scler Frontotemporal Degener 2014; 15: 398–405. [DOI] [PubMed] [Google Scholar]
- 17. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord 2000; 1: 293–299. [DOI] [PubMed] [Google Scholar]
- 18. Dixon JR. The international conference on harmonization good clinical practice guideline. Qual Assur Good Pract Regul Law 1999; 6: 65–74. [DOI] [PubMed] [Google Scholar]
- 19. World medical association declaration of Helsinki. JAMA 2013; 310: 2191. [DOI] [PubMed] [Google Scholar]
- 20. Fairbanks G. Voice and articulation drillbook, 2nd edn. New York: Harper & Row, 1960; 124–139. [Google Scholar]
- 21. Kaufmann P, Levy G, Montes J, Buchsbaum R, Barsdorf AI, Battista V, et al Excellent inter‐rater, intra‐rater, and telephone‐administered reliability of the ALSFRS‐R in a multicenter clinical trial. Amyotroph Lateral Scler 2007; 8: 42–46. [DOI] [PubMed] [Google Scholar]
- 22. Miano B, Stoddard G, Davis S, Bromberg M. Inter‐evaluator reliability of the ALS functional rating scale. Amyotroph Lateral Scler Other Mot Neuron Disord 2004; 5: 235–239. [DOI] [PubMed] [Google Scholar]
- 23. Weismer G, Laures JS. Direct magnitude estimates of speech intelligibility in dysarthria. J Speech Lang Hear Res 2002; 45: 421. [DOI] [PubMed] [Google Scholar]
- 24. Stevens S. Psychophysics: Introduction to its perceptual, neural, and social prospects. New York: John Wiley & Sons, 1975. [Google Scholar]
- 25. Eadie TL, Doyle PC. Direct magnitude estimation and interval scaling of pleasantness and severity in dysphonic and normal speakers. J Acoust Soc Am 2002; 112: 3014–3021. [DOI] [PubMed] [Google Scholar]
- 26. Green JR, Beukelman DR, Ball LJ. Algorithmic estimation of pauses in extended speech samples of dysarthric and typical speech. J Med Speech Lang Pathol 2004; 12: 149–154. [PMC free article] [PubMed] [Google Scholar]
- 27. Torchiano M, Torchiano M. Package “effsize”. 2017.
- 28. Hess MR, Kromrey JD. Robust confidence intervals for effect sizes: a comparative study of Cohen's d and Cliff's Delta under non‐normality and heterogeneous variances. In: Annual meeting of the American Educational Research Association; 2004, p. 12–6.
- 29. Cohen J. The Effect Size index: d In: Statistical Power Analysis for the Behavioral Sciences, 2nd edn. New York: Routledge, 1988; 20–26. [Google Scholar]
- 30. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexander SPH, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD, et al The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 2017; 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rong P, Yunusova Y, Wang J, Green JR. Predicting early bulbar decline in amyotrophic lateral sclerosis: a speech subsystem approach. Behav Neurol 2015; 2015: 183027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rong P, Yunusova Y, Wang J, Zinman L, Pattee G, Berry JD, et al Predicting speech intelligibility decline in amyotrophic lateral sclerosis based on the deterioration of individual speech subsystems. PLoS One 2016; 11: e0154971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yunusova Y, Graham NL, Shellikeri S, Phuong K, Kulkarni M, Rochon E, et al Profiling speech and pausing in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Kassubek J, editor. PLoS One 2016; 11: e0147573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yunusova Y, Green JR, Wang J, Pattee G, Zinman L. A protocol for comprehensive assessment of bulbar dysfunction in amyotrophic lateral sclerosis (ALS). J Vis Exp 2011; (48): e2422 10.3791/2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Campbell TF, Dollaghan CA. Expressive language recovery in severely brain‐injured children and adolescents. J Speech Hear Disord 1990; 55: 567–581. [DOI] [PubMed] [Google Scholar]
- 38. Cordella C, Dickerson BC, Quimby M, Yunusova Y, Green JR. Slowed articulation rate is a sensitive diagnostic marker for identifying non‐fluent primary progressive aphasia. Aphasiology 2017; 31: 241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]


