Abstract
ABSTRACT: INTRODUCTION. Candida albicans is the most common inhabitant of the skin, mouth, vagina and gastro intestinal tract of human beings. One of the major reasons for the increase in Candida infection is the development of its resistant strains due to drugs used in the treatment of candidiasis. MATERIALS AND METHODS We studied 4027 samples collected from patients in various wards of the Emergency County Hospital Craiova, Romania between 2014-2015. The specimens were: pharyngeal exsudates, sputum, tracheal secretions, skin secretions, stools, ear secretions, urine, vaginal secretions. All the specimens were transported to the microbiology laboratory and cultured within 3 to 4 h of collection. Among the 4027 samples, 652 showed culture characteristics similar to Candida albicans.The samples were inoculated under sterile conditions using Sabouraud culture media, a medium designed to inhibit bacterial growth and allow the development of fungi. Antifungal Susceptibility Testing was performed by disc diffusion according to CLSI 2014 guidelines using: clotrimazole, ketoconazole, miconazole, econazole, amphotericine B, fluorocytozine, nistatin. RESULTS AND DISCUSSION In our study group the urocultures and dermatological products have a high infection rate, between 100% to 70%, in contrast, we find evidence of secretion ear (3.13%) and the throat swab (9.33%). Various resistant levels were detected against antifungal drugs but, complete resistance to 5 – Fluorocitozina (100%), and the organisms showed highly sensitive to Cotrimazol şi Ketoconazol (100%). In the case of Miconazol 256 (39, 26%), Econazol, 215 (32,98%), Amphotericinei B, 230(35,28%). Nystatin 329 (50,46%). CONCLUSIONSElucidating these mechanisms may provide new foundations for antifungal chemotherapy and can present an exciting challenge for the future investigations. Candida albicans infections are present and diverse clinical pathology.
Keywords: incidence, antifungal susceptibility, Candida albicans, Cotrimazol, Ketoconazol
Introduction
Candida species are the most common cause of fungal infections worldwide. Candida species are normal microbiota within the gastrointestinal tracts, respiratory tracts, vaginal area and the mouth and it is sexually transmitted diseases [2]. Candida is a yeast growth present in all females and is normally controlled by bacteria. Candida species differ in their antifungal susceptibility and virulence factors. The genus is composed of a heterogeneous group of organisms, and more than 17 different Candida species are known to be aetiological agents of human infections; however, more than 90% of invasive infections are caused by C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. krusei [3], C. dubliniensis, and C. lusitaniae. The yeast begins to invade and colonize the body tissues by releasing powerful chemicals into the bloodstream causing symptoms like: lethargy, chronic diarrhea, yeast vaginitis, bladder infections, muscle and joint pain, menstrual problems, constipation and severe depression. [15,16,17]The medical term for this overgrowth is candidiasis. Candidiasis is responsible for 90% of the cases of infectious vaginitis [4]. The most prevalent fungal pathogen of humans is Candida albicans which ranks as the fourth most common cause of hospital acquired infectious disease and is the primary cause of systemic candidiasis, with mortality rates approaching 50% [1]. Prolonged usage of antifungals in treating infections caused by C. albicans has led to the emergence of azole resistance. This acquired azole resistance in clinical isolates of C. albicans mostly results in cross-resistance to many unrelated drugs, a phenomenon termed multidrug resistance (MDR) [5,16,7]. MDR is a serious complication during treatment of opportunistic fungal infections which possess grave concern given the limited number of clinically useful antifungal drugs available [8,9]. The reasons for this increase in fungal infections are multifactorial: better clinical evaluation and diagnosis, greater survival for patients with malignancies, chronic diseases, increasing number of transplants, complex surgical procedures, catheters, implants and use of wide spectrum antibiotics.[14]Candida species have developed a multitude of mechanisms to survive exposure to antifungal drugs and some of them include an over expression or mutations. Thus identification of Candida up to species level along with antifungal susceptibility becomes very essential. [10,11]The accurate species identification of Candida is important for the treatment, as not all species respond to the same treatment because of the trouble of anti-fungal resistance.
Fungal etiology of some affections is a reality more and more common in medical practice, facilitated, because of the excessive use of antibiotics, often given in inadequate condition of sickness and taken in excessive doses, and also to the "abuse" of steroids, plus the high incidence of serious debilitating diseases (cancers, HIV infection, diabetes) which requires aggressive therapy or combination therapies that may have a negative effect by decreasing the overall body strength .[12,13]
Aim
The aim of the present study was to know the prevalence of Candida albicans from different specimens and to detect their antifungal susceptibility.
Materials and methods
We studied 4027 samples collected from patients in various wards of the Emergency County Hospital Craiova, Romania between 2014-2015. The specimens were : pharyngeal exsudates, sputum, tracheal secretions, skin secretions, stools, ear secretion, urine, vaginal secretions. All the specimens were transported immediately to the microbiology laboratory and cultured within 3 to 4 h of collection. Among the 4027 samples, 652 showed culture characteristics similar to Candida albicans.The samples were inoculated under sterile conditions using Sabouraud culture media in Petri plates, a medium designed to inhibit bacterial growth and allow the development of fungi ,incubated at room temperat 37°C. After 24 -48h of incubation, the culture plates were examined for the appearance, size, color and morphology of the colonies were recorded. Antifungal Susceptibility Testing was performed by disc diffusion according to CLSI 2014 guidelines using: Miconazole (25 mcg), Ketoconazole (10 mcg), Clotrimazole (10 mcg), Fluconazole (25 mcg), 5 – Fluorocitozina() Amphotericin-B (20 mcg), Econazole (10 mcg) and Nystatin (100 units) were tested. The zones measured only that is showing complete inhibition and the diameters of the zones recorded to the nearest millimeter.
Results and discussion
In this paper we studied 4027 pathological products in which we sought to isolate and identify Candida albicans.
Figure 1.
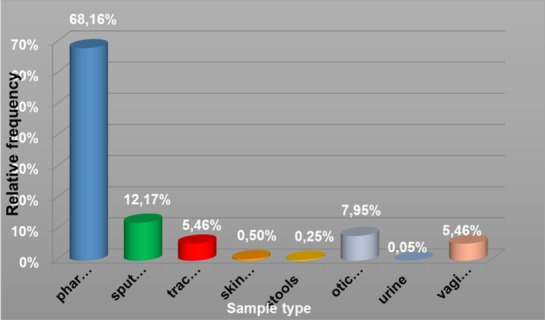
Figure 2.

Relative frequency of sample type
- 2745 from throat swab samples, of which 256 were positive for Candida albicans (9.33%);
- 490 sputum was positive for Candida albicans 220 (44 90%)
- Tracheobronchial secretions 220, 42 were positive (19.09%);
- 20 samples dermatological, 14 were positive for Candida albicans
- 10 coprocultures, 4 were positive (40%);
- 320 ear discharge, 10 were positive for Candida albicans (3.12%);
- 2 positive urine samples
- 220 vaginal discharges, 104 were positive (47.27%).
Fig. 1. Relative frequency of sample type
The presence of Candida albicans in different pathological products indicate the possibility of aggression to the different tissues and the important role of candidiasis etiology in many disease.
We have demonstrated that, there is a statistically significant difference between the highest proportions of positive samples identified for different types of samples ( p = 1.81 x 10-81 ≈0 < 0.001) . If for vaginal and dermatological products have a very high rate of infection , opposite secretion find evidence of ear drops (3.13 %) and the throat swab ( 9.33 %). (13; 14)
Figure 3.

Percent from positive samples
Figure 4.

Positivity index – samples type
The isolation of Candida albicans from sputum and tracheal secretions is an important part of diagnostic, especially in acute episodes in respiratory tract infections considering the therapy with antibiotics and/or corticosteroids that create good conditions for growing.
Next, we studied the antifungal susceptibility of Candida albicans strains isolated.The set of antifungal susceptibility testing of Candida albicans used were as follows:
Nystatin, Amphotericin - B, Ketoconazole, Miconazole, 5-Fluorocytosine, Clotrimazole,Econazole.
The 652 strains of Candida albicans isolates had different spectral sensitivity to the antifungals used (5).
Figure 5.
Antifungal susceptibility
Thus, Ketoconazole Clotrimazol- all the 652 strains were sensitive (100%). Our results are consistent with the literature
In our study group the urocultures and dermatological products have a high infection rate, 100 and 70%, in contrast, we find evidence of secretion ear (3.13%) and the throat swab (9.33%). Were resistant various levels detected against antifungal drugs but, complete resistance to 5 - fluorocytosine (100%), and the highly sensitive to Cotrimazol and Ketoconazole (100%). In the case of Miconazole 256 (39 26%), Econazole, 215 (32.98%), Amphotericin B, 230 (35.28%), Nystatin 329 (50.46%).
The most efficient antifungal antibiotics for Candida albicans were Clotrimazole and Ketoconazole, followed by Nistatin, Miconazole, Amphotericine B and Econazole, and 5 – Fluorocitozine was not effectiv. As result, it is necessary, during the isolation and identification of Candida albicans as an etiological agent, to do Antifungal Susceptibility Tests.
Conclusion
Candida albicans infections are present and diverse in clinical pathology.
The etiology of candidiasis (Candida albicans in sputum) should be considered primarily in dermatology and gynecological conditions (especially the vagina with Candida albicans vulvovaginal).
In patients with frequent episodes of chronic disease exacerbations requiring antibiotic therapy and / or corticosteroids with serious diseases that evolve with the exploration of immunosuppression required for detection of biological products and Candida albicans.
In children with antibiotics abuse, especially preschoolers, bowel disorders can be caused by Candida albicans.
In the elderly with repeated urinary infections treated with antibiotics for long periods, Candida albicans can cause urinary infections.
The most effective antifungal Candida albicans are the clotrimazole and ketoconazole.
Acknowledgments
All authors equally contributed in the research and drafting of this paper
References
- 1.Krasner RI. Concepts of microbial disease. In The Microbial Challenge: Human-Microbe Interactions, Washington, DC. American Society for Microbiology. 2002;6:103–120. [Google Scholar]
- 2.Prescott KA, Harley JM, Klein DA. Microbiology.7th edition. McGraw-Hill. Publication. New York USA. Resistant candidiasis. AIDS RES. HUM. Retroviruses. 2008;10:925–929. [Google Scholar]
- 3.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clinical Microbiol Reviews. 1998;11(2):382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrobial Agents and Chemotherapy. 2002;46(6):1704–1713. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Candidiasis. Available from: http://www.cdc.gov/fungal/diseases/candidiasis/index.html .
- 6.Pfaller MA, Diekema DJ. Epidemiology of Invasive Candidiasis: a Persistent Public Health Problem. Virulence. 2007;(2):119–128. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Public Health Agency of Canada. Candida albicans - Material Safety Data Sheets. Available from: http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/msds30e-eng.php .
- 8.Fanelloa S, Boucharab JP, Jousseta N, Delbosa V, LeFlohicc AM. Nosocomial Candida albicans acquisition in a geriatric unit: epidemiology and evidence for person-to-person transmission. Journal of Hospital Infection. 2001;47(1):46–52. doi: 10.1053/jhin.2000.0849. [DOI] [PubMed] [Google Scholar]
- 9.Franz R, Kelly SL, Lamb DC, Kelly DE, Ruhnke M, Morschhauser J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrobial Agents and Chemotherapy. 1998;42(12):3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson JB. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nature Reviews Microbiology. 2005;3(7):547–556. doi: 10.1038/nrmicro1179. [DOI] [PubMed] [Google Scholar]
- 11.Cowen LE, Steinbach WJ. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryotic Cell. 2008;7(5):747–764. doi: 10.1128/EC.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer AW, Kirby MDK, Sherris JC, Turck M. Antibiotic susceptibility testing by standardized single disc diffusion method. Am J Clin Pathol. 1996;45:493–496. [PubMed] [Google Scholar]
- 13.NCCLS. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline. Pennsylvania USA: Wayne; 2004. [Google Scholar]
- 14.Lai CC, Wang CY, Liu WL, Huang YT, Hsueh PR. Time to positivity of blood cultures of different Candida species causing fungaemia. J Med Microbiol. 2012;61:701–704. doi: 10.1099/jmm.0.038166-0. [DOI] [PubMed] [Google Scholar]
- 15.Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. Inflammatory response and clinical course of adult patients with nosocomial bloodstream infections caused by Candida spp. Clin Microbiol Infect. 2006;12:170–177. doi: 10.1111/j.1469-0691.2005.01318.x. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 17.Vidigal PG, Svidzinski TIE. Yeasts in the urinary and respiratory tracts: is it a fungal infection or not? J Bras Patol Med Lab. 2009;45:55–55. [Google Scholar]



