Abstract
Due to their immunoregulatory properties, several specialized cell subsets, including regulatory T (Treg), invariant natural killer T (iNKT) and regulatory B (Breg) cells, are involved in the pathogenesis of non-Hodgkin lymphoma (NHL). However, the interaction between various cells remains to be elucidated. The aim of the present study was to evaluate the levels of Treg, iNKT and Breg cell subsets and their interrelationships in the peripheral blood (PB) and bone marrow (BM) of patients with B-cell NHL who received rituximab-based regimens and achieved a complete remission. A total of 20 patients and 20 healthy age- and sex-matched controls were prospectively enrolled for investigation of Treg, iNKT and Breg cell subsets in PB and BM by flow cytometry and cell culture. Prior to administration of combination chemotherapy with rituximab, the patients had lower levels of Breg cells and, to a lesser degree, Treg cells, but not iNKT cells, in PB compared with controls. Compartmental differences in the levels of Treg and Breg cell subsets, but not iNKT cells, were observed between PB and BM, suggesting an increase in trafficking through the blood of these regulatory cell subsets to the marrow. Following complete remission, the levels of circulating Treg, iNKT and Breg cell subsets increased. The levels of Treg cells were not significantly associated with iNKT and Breg cell subsets, although negative correlations were observed. Taken together, these results may provide new insights into the potential role of regulatory cell subsets in patients with B-cell NHL. However, whether the observed differences between PB and BM may affect clinical outcomes requires further investigation.
Keywords: regulatory, T cells, B cells, invariant natural killer T cells, lymphoma, blood, marrow
Introduction
In recent years, several immune cells endowed with potent regulatory properties have been identified. The best-known are the regulatory T (Treg) cells, which serve a critical role in modulating immune responses in a variety of conditions (1). By analogy to Treg cells, a small subset of B-cells, termed regulatory B (Breg) cells, was identified and found to exert its immunosuppressive effects via the production of interleukin-10 (IL-10), an inhibitory cytokine also utilized by Treg cells (2). In addition to their interaction with Treg cells, Breg cells promote activation of invariant natural killer (iNKT) cells that also display immunoregulatory properties and share several functional hallmarks with Treg cells (3–5). This cross-talk among Treg, Breg and iNKT cell subsets indicates a link between innate and adaptive immunity in order to elicit strong immune responses to protect the body from diseases.
While their immunogenic role is well-established, some reports revealed that Breg cells may also promote tumorigenesis and may limit the therapeutic efficacy of B-cell depletion therapies with rituximab, a monoclonal antibody directed against the CD20 antigen that is commonly used in the treatment of patients with B-cell malignancies (6–8). Interestingly, even small number of residual murine Breg cells was able to inhibit rituximab activity, and only their total removal was associated with optimal clearance of malignant B-cells (8). Collectively, these findings support the hypothesis that preferential depletion of Breg cells may enhance tumor-specific immune responses and control tumor growth. Thus, it is necessary to elucidate the various immunological barriers in order to optimize and increase the efficacy of antitumor responses.
Despite the fact that an increasing number of patients with B-cell malignancies achieve complete remission (CR) following rituximab-based therapies, a significant number relapse or are refractory to these treatments. There is a need for novel markers that have the potential to identify those patients who are more prone to relapse and modify their therapy to improve outcomes. In this context, Breg cells may serve as a potential novel candidate.
To date, limited data on the levels of Treg, iNKT and Breg cell subsets and their interrelationships have been reported in patients who receive rituximab-based regimens and achieve CR. In addition, little is known on the levels of these regulatory cell subsets in the bone marrow (BM), which constitutes a key site providing important information for the diagnosis and follow-up of patients with B-cell malignancies. Therefore, there remains the question of whether Treg, iNKT and Breg cell subsets change following therapy in rituximab-treated patients who achieve CR. The aim of the present study was to investigate the levels of Treg, iNKT and Breg cell subsets and their interrelationships in the peripheral blood (PB) and BM of patients who received rituximab-based regimens for the treatment of B-cell non-Hodgkin lymphoma (B-NHL) at diagnosis and after achieving CR.
Patients and methods
Patients
Patients with B-NHL were recruited at the Sultan Qaboos University (SQU) Hospital, Sultanate of Oman. All patients were previously treated with rituximab in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP), or other chemotherapeutic agents as part of standard protocols. Each case was reviewed and the final diagnosis was made according to the 2016 World Health Organization classification of tumors of the hematopoietic and lymphoid tissues (9). All patients had sustained CR for at least 2 months prior to sampling. Age and sex-matched healthy controls were also recruited for comparison. This study was performed according to the principles outlined in the Declaration of Helsinki and approved by the Institutional Research Ethics Committee of the SQU (Sultanate of Oman). All the study participants signed an informed consent form prior to enrollment.
Cell isolation
PB samples were drawn into vacutainer tubes containing ethylenediaminetetraacetic acid as anticoagulant (BD Biosciences, San Jose, CA, USA) and processed within 2 h of collection. PB mononuclear cells (PBMC) were separated by Ficoll-Hypaque density centrifugation, washed and re-suspended in phosphate-buffered saline (PBS) containing heat-inactivated fetal calf serum (FCS). The freshly processed PBMC samples were either stained with appropriate monoclonal antibodies or stored in liquid nitrogen until used. Similarly, mononuclear cells from BM samples were prepared by Ficoll-Hypaque density centrifugation and stored until used.
Monoclonal antibodies
Specific human monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE-CF594, PECy-7, allophycocyanin (APC), APC-H7 and peridin-chlorophyll-protein (PerCP) were used to identify cellular antigens. 5 µl of anti-CD3-APC/H7 (cat. no. 560176), 7.5 µl of anti-CD4-APC (cat. no. 555349) 7.5 µl of anti-CD8-PerCp (cat. no. 347314), 9 µl of anti-CD19-APC (cat. no. 555415), 7.5 µl of anti-CD24-FITC (cat. no. 560992), 5 µl of anti-CD25-PE-CF594 (cat. no. 562403), 5 µl of anti-CD38-PE-CF594 (cat. no. 562288), 5 µl of anti-CD127-PECy-7 (cat. no. 560822), 12.5 µl of anti-FoxP3-PE (cat. no. 560046), 12.5 µl of anti-IL-10-PE (cat. no. 562035), and 10 µl of anti-iNKT-PE (cat. no. 562051) antibodies were obtained from BD Biosciences (Franklin Lakes, NJ, USA).
Surface and intracellular staining
Immunostaining was performed as recently reported (10). Briefly, in a 96-well polystyrene plate, appropriate amounts of each surface monoclonal antibody and 2×106 of PBMC were incubated in the dark for 30 min at 4°C. The cells were then washed twice with PBS containing 1% FCS and fixed with Cytofix™ buffer (BD Biosciences). Subsequently, the Cytoperm™ Plus buffer was used for permeabilization (BD Biosciences). Cells were washed and stained intracellularly with anti-FoxP3 antibody, then fixed for cell acquisition by flow cytometry. For Breg cells, the PBMC were first stimulated and cultured for 48 h. They were then stained extracellularly, fixed with Cytofix™ buffer and permeabilized with Cytoperm™ Plus buffer (BD Biosciences). The cells were then stained intracellularly with anti-IL-10 antibody and fixed for analysis by flow cytometry.
Immunophenotypic analysis
The LSR Fortessa and FACSDIVA™ Software version 8.0.1 (BD Biosciences) were used for cell acquisition and analysis, respectively, as previously reported (11). In brief, the sensitivity of fluorescence detectors was set and monitored using calibrated beads according to the manufacturer's recommendations (BD Biosciences). Fluorescence voltages and compensation values were determined using a combination of CS&T beads and CompBeads (BD Biosciences). Lymphocytes were first identified according to their light-scattering properties and then analyzed for expression of specific markers. Treg cells were defined as CD3+CD4+CD25hiCD127loFoxP3+, iNKT cells as CD3+CD4+6B11+ and Breg cells as CD19+CD24hiCD38hiIL-10+. For each sample, a minimum of 50×106 events in live cell gate were accumulated. To discriminate live from dead cells, the LIVE/DEAD Stain kit (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used in all experiments.
Statistical analysis
Quantitative data were expressed as means ± standard deviations. Differences in the levels of Treg, Breg, and iNKT cell subsets in PB and BM were evaluated using Wilcoxon's test for paired samples and Mann-Whitney U test for unpaired samples, as data were not normally distributed. Correlations among regulatory cell subsets, study parameters and clinical data were evaluated by Spearman's correlation test. All tests were two-tailed with an α level of 0.05, which was considered as significant. Analyses were performed using the GraphPad PRISM 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Patient characteristics
A total of 20 patients with B-NHL in CR following treatment with rituximab and chemotherapy and 20 healthy age- and sex-matched healthy controls were enrolled. The baseline characteristics of the participants at study enrollment are presented in Table I. Overall, the sociodemographic data were similar between patients and controls. The majority of the enrolled patients were diagnosed with diffuse large B-cell lymphoma (DLBCL). In order to study as homogeneous a group of patients as possible, all DLBCL patients received R-CHOP, while patients with follicular lymphoma received rituximab with cyclophosphamide, vincristine and prednisone (R-CVR). Full doses of cytostatic agents were administered to all patients. Of note, none of the patients received bendamustine-based chemotherapy, given its potential impact on immune reconstitution. As expected, hemoglobin levels were slightly lower in patients compared with those in healthy controls. Both patients and healthy controls were negative for infections with human immunodeficiency virus, hepatitis C virus (anti-HCV antibody) and hepatitis B virus (HBVsAg). All patients were disease-free for at least 8 weeks prior to sampling, with a mean length of time of 3±1 months (range, 2–4 months). The patients received between 6 and 8 cycles of rituximab-based treatment, which was well-tolerated.
Table I.
Baseline characteristics of study participants.
| Characteristics | Patients (n=20) | Healthy controls (n=20) |
|---|---|---|
| Age (years) | 53±5 (18–69) | 54±3 (18–68) |
| Male sex, n (%) | 14 (70) | 14 (70) |
| Haemoglobin (g/dl) | 11±3 (9–14) | 14±2 (13–15) |
| Lactate dehydrogenase (U/l) | 268±47 (128–239) | ND |
| Lymphocyte/monocyte ratio | 4±1.9 (0.92–8.1) | 5±1.4 (1.6–8.1) |
| Diagnosis, n (%) | NA | |
| Diffuse large B-cell lymphoma | 16 (80) | |
| Follicular lymphoma | 4 (20) | |
| B symptoms, n (%) | NA | |
| Present | 6 (30) | |
| Extranodal lesions, n (%) | NA | |
| Present | 4 (20) | |
| Bone marrow involvement, n (%) | NA | |
| Present | 5 (25) | |
| International prognostic index, n (%) | NA | |
| 0–1 | 6 (30) | |
| 2 | 4 (20) | |
| 3 | 3 (15) | |
| 4–5 | 7 (35) |
Results are shown as mean ± standard deviation (range). NA, not applicable; ND, not determined.
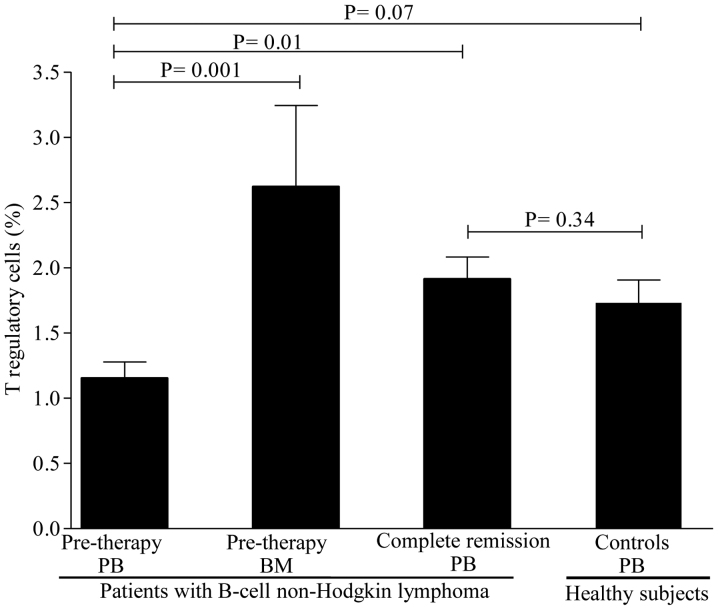
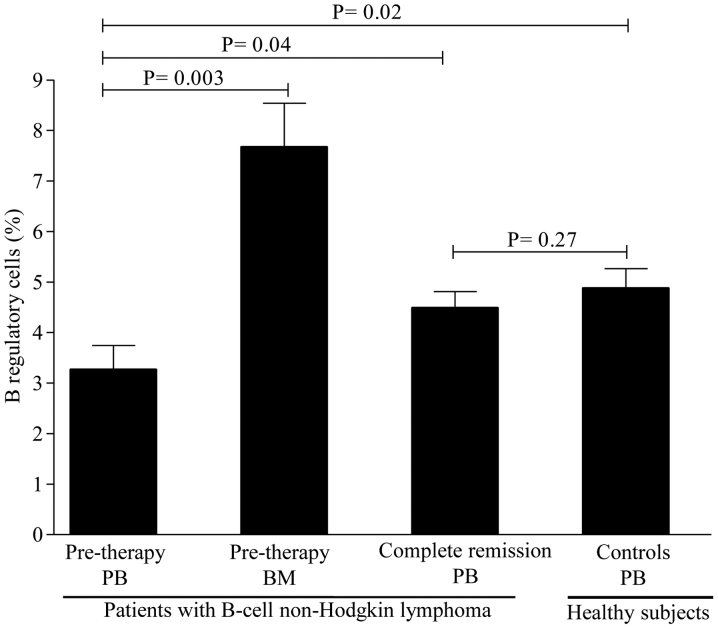
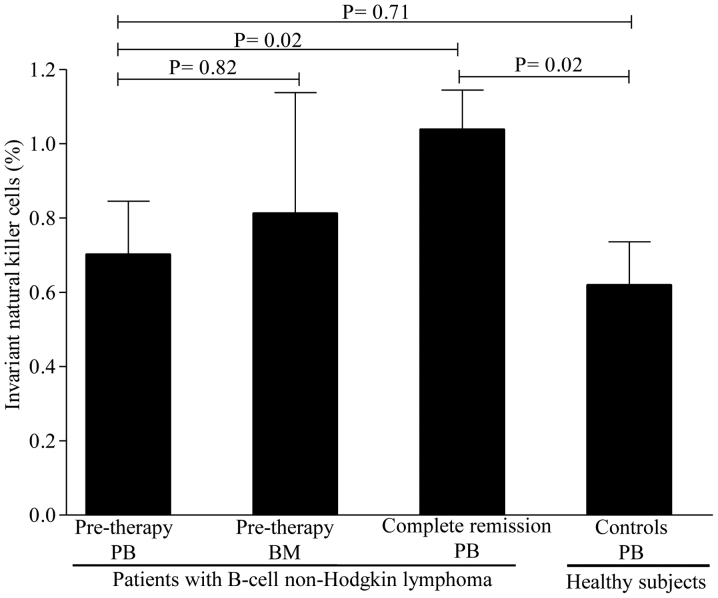
Levels of circulating regulatory cell subsets prior to therapy. To determine whether the levels of regulatory cell subsets differ between patients and healthy controls, we first compared their levels prior to rituximab-based regimen administration. As shown in Fig. 1, the levels of Treg cells were lower in patients compared with those in healthy controls, but the difference did not reach statistical significance. A mean value of Treg cells of 1.15±0.12% (range, 0.39–2.10%) was observed in patients compared with 1.73±0.17% (range, 0.88–3.30%) in healthy controls (P=0.07). In contrast to Treg cells, the levels of Breg cells were substantially lower in patients [3.26±0.48% (range, 0.11–6.7%)] compared with healthy controls [4.97±0.39% (range, 2.10–7.3%)] (P=0.02; Fig. 2). Interestingly, the levels of iNKT cells were similar between patients [0.71±0.14% (range, 0.11–1.9%)] and healthy controls [0.61±0.11% (range, 0.11–1.90%)] (P=0.71; Fig. 3). Collectively, these data suggest that patients with B-cell NHL displayed a pronounced reduction in Breg cells and, to a lesser degree, Treg cells, as compared to controls, but with comparable levels of iNKT cells.
Figure 1.
Levels of regulatory T cells in the peripheral blood and bone marrow of patients with B-cell non-Hodgkin lymphoma and healthy controls. The mean ± standard error of the mean and P-values are shown. PB, peripheral blood; BM, bone marrow.
Figure 2.
Levels of regulatory B cells in the peripheral blood and bone marrow of patients with B-cell non-Hodgkin lymphoma and healthy controls. The mean ± standard error of the mean and P-values are shown. PB, peripheral blood; BM, bone marrow.
Figure 3.
Levels of invariant natural killer T cells in the peripheral blood and bone marrow of patients with B-cell non-Hodgkin lymphoma and healthy controls. The mean ± standard error of the mean and P-values are shown. PB, peripheral blood; BM, bone marrow.
Levels of regulatory cell subsets in the BM
As the BM acts as a reservoir for regulatory cells, whether the levels of Treg, Breg and iNKT cells differ between BM and PB was next investigated. The levels of Treg cells were higher in the BM [2.62±0.62% (range, 1.21–4.80%)] compared with those in the PB [1.15±0.12% (range, 0.39–2.10%)] (P=0.001) as evidenced by the 1-fold increase in BM levels (Fig. 1). Similarly, the levels of Breg cells were markedly higher in the BM [7.67±0.86% (range, 5.60–10.50%)] compared with those in the PB [3.26±0.48% (range, 0.11–6.7%)], with a nearly 2-fold increase in BM levels (Fig. 2). The levels of iNKT cells, however, were similar between BM and PB (Fig. 3). Of note, no differences were observed in the levels of regulatory cells in patients with or without BM involvement, B symptoms, or displaying >1 extranodal sites (P=0.51). Similarly, no statistically significant differences in regulatory cells were identified between patients with favorable and poor prognostic markers (P=0.77). Taken together, these findings suggest that the Treg and Breg cell subsets, but not the iNKT cell subset, are retained in the BM compared with the PB.
Levels of circulating regulatory cell subsets after CR
To study the impact of rituximab-based regimens on regulatory cell subsets, their levels were assessed in patients achieving CR. A significant increase was observed in the levels of Treg cells [from 1.15±0.12% (range, 0.39–2.10%) to 1.81±0.16% (range, 0.68–3.10%); P=0.01] after CR (Fig. 1). However, only a slight increase was observed in the levels of Breg cells [from 3.26±0.48% (range, 0.11–6.7%) to 4.63±0.45% (range, 1.50–9.30); P=0.04] after CR (Fig. 2). In contrast to Breg cells, a marked increase in iNKT cells was noticed in almost all patients [from a pre-therapy level of 0.71±0.14% (range, 0.11–1.9%) to 1.10±0.11% (0.41–2.30); P=0.02] after CR (Fig. 3). Of note, regulatory cell subsets at CR reached the levels of normal or nearly normal values in the majority of the patients. In addition, there were no significant differences in the levels of regulatory cells between patients who were in CR for <3 months and those in CR for >3 months (P=0.37). Overall, these results suggest that Breg cells only slowly repopulated the PB, while the Treg and iNKT cell subsets recovered rapidly after CR.
Associations among regulatory cell subsets
We next assessed the associations among the levels of regulatory cell subsets and study parameters. Interestingly, the levels of Breg cells were negatively associated with both Treg cells (Rho = −0.29; P=0.13) and iNKT cells (Rho = −0.31; P=0.10), but the differences did not reach statistical significance. The majority of the other correlations among study variables were not statistically significant (data not shown). Of note, the levels of Breg cells were positively correlated with the length of time after CR (Rho = 0.37; P=0.08), but this did not reach statistical significance.
Discussion
The pathophysiology of B-cell NHL involves impaired mechanisms of cellular regulation that control the maintenance of normal homeostasis. Hence, probing the regulatory activities at a single-cell level may improve our understanding of the disease and eventually result in more specific and effective therapeutic measures. The interactions among regulatory cells in B-cell NHL have not yet been fully elucidated. This prospective study aimed to investigate the levels of Treg, Breg and iNKT cell subsets and their interrelationships in the PB and BM of patients with B-cell NHL who were treated with rituximab-based regimens and achieved a CR.
Thus far, published data on the levels of circulating Treg cells in newly diagnosed patients with NHL have yielded conflicting results. While some studies reported that the pre-therapy levels of Treg cells in patients with B-cell NHL are higher in PB compared with those of healthy individuals, other studies reported the opposite, or no association (12–17). In the present study, the levels of circulating Treg cells tended to be lower in patients compared with healthy controls prior to the initiation of rituximab-based treatment, but their levels were markedly increased after CR. These findings appear to differ from those studies reporting increased pre-therapy levels of circulating Treg cells in patients with NHL compared with healthy controls. Several possible explanations may account for these differences. First, they may be due to an increase in the migration of Treg cells from the PB to the BM. This hypothesis is supported by our findings showing that the levels of Treg cells are higher in the BM compared with the PB. It is also supported by earlier studies showing increased homing of Treg cells into the tumor sites compared with other compartments (14,15). Second, these findings may be explained by the fact that Treg cell measurements are subject to marked immunophenotyping variability across diverse studies where different markers are being used. In the present study, five markers were employed simultaneously to accurately define Treg cell phenotype. In fact, the combination of these markers was shown to better reflect Treg cell functions, which are enriched within the CD25hiCD127loFoxP3+ gated population (18). Finally, it is possible that the studied populations are somehow heterogeneous, as they differ in several factors, including sociodemographic and histological subtypes of NHL. It has been demonstrated that patients with unhealthy habits, such as smoking and alcohol abuse, or those suffering from extreme stress, exhibited high levels of Treg cells in the PB (19).
The findings of the present study also extended to investigation of the levels of Breg cells in patients with B-cell NHL. In a recent study, Qiu et al (20) reported a long-lasting overrepresentation of circulating CD19+CD20-CD27hiIL-s10-producing B cells in R-CHOP-treated patients with DLBCL who were in remission. Although Qiu et al used different cellular makers to phenotypically define Breg cells, our results confirmed their findings, showing increased levels of Breg cells after achieving CR, but with a slow repopulation of the PB. This may be due in part to the fact that rituximab treatment induced depletion of most CD20-expressing cells, despite the fact that Breg cells express low levels of the CD20 antigen and may escape depletion therapies, as described in murine models (6–8). Indirectly, our data are in line with this observation, as a positive association between the levels of Breg cells and length of time after CR was observed, although this did not reach statistical significance. Additionally, increased levels of Breg cells were found in the BM compared with the PB prior to starting rituximab-based treatment. The observed compartmental differences in the levels of Breg cells may suggest mobilization and homing of these cells from the PB to the BM to exert their regulatory effects. This hypothesis is supported by studies reporting that BM acts as a reservoir for immune cells, including Breg and Treg cells (21,22).
Previous studies in animal models have demonstrated that iNKT cells are endowed with potent antitumor activity in different types of cancer, including B-cell NHL (23). However, only a limited number of studies have focused on their levels in patients with B-cell NHL who achieved CR following rituximab-based therapy. In an earlier study, Yoneda et al (24) reported that patients with malignant lymphoma who achieved a CR exhibited comparable absolute numbers of circulating Vα24+ NKT cells, which are equivalent to iNKT cells, to those of healthy individuals. In another study, Hus et al (25) found lower levels of circulating iNKT cells in patients with B-cell NHL prior to chemotherapy compared with healthy controls, and these levels markedly increased after the completion of R-CHOP. Our results are quite similar, except that the pre-therapy levels of iNKT cells were not different from those in healthy controls in the present study. In addition, it was demonstrated that the levels of iNKT cells were comparable between PB and BM, suggesting a balance between the two compartments.
One of the objectives of the present study was to evaluate the interrelationships among the regulatory cell subsets, as these cells are known to interact with each other to establish a potent immunoregulatory environment to control tumor growth. Associations among circulating regulatory cell subsets and standard disease markers in patients with B-cell NHL has been evaluated by few studies with conflicting results (12–17,20). The findings of the present study revealed that the levels of circulating Treg cells were not significantly correlated with Breg or iNKT cells, either pre- or post-rituximab-based therapy. Similarly, weak and insignificant associations were observed between the levels of regulatory cell subsets and clinical parameters, including lymphocyte-to-monocyte ratio, which was recently identified as an independent prognostic factor in patients with B-cell NHL (26). The lack of significant correlations may be explained in part by the fact that the levels of regulatory cell subsets in the PB do not accurately reflect their intratumoral counterparts. Whether the associations among circulating regulatory cell subsets are truly insignificants requires further investigation, along with co-culture assays using sorted cells to better mimic their interactions in ex vivo studies.
There were certain limitations to the present study. The sample size was relatively small, but it reflects the routine care in our clinical settings. This may have affected the statistical power to discriminate the effects of tested correlations between the analyzed groups. However, the low level of statistical dispersion of the results suggests that increasing the number of patients would not have had a major effect on the significance of the obtained results. Another limitation is that all enrolled patients were in CR; therefore, it was not possible to evaluate the effects of regulatory cell subsets as factors predictive of response. In addition, due to the limited sample availability, functional cellular assays were not performed. It would be useful to perform such studies to investigate whether the Treg, Breg and iNKT cell subsets are also functionally impaired in future studies.
In conclusion, the present study demonstrated that patients with B-cell NHL who receive rituximab-based regimens and achieve CR display lower pre-therapy levels of Breg cells and, to a lesser degree, Treg cells, but not iNKT cells in the PB when compared with healthy individuals. Compartmental differences in the levels of Treg and Breg cells, but not iNKT cells, exist between PB and BM, suggesting increased trafficking through the blood of these regulatory cell subsets to the marrow. Despite the fact that the levels of circulating Breg, Treg and iNKT cells increased after CR in almost all patients, no significant associations were identified. Overall, these results provide new insights into the role of regulatory cell subsets in patients with B-cell NHL. Further investigation into the interrelationships among these regulatory cells may help to elucidate the mechanisms underlying antitumor control.
Acknowledgements
The authors would like to thank all participants and the staff from the Department of Microbiology and Immunology for the technical assistance.
Funding
The present study was supported by a grant from the Sultan Qaboos University (grant no. IG/MED/HAEM/14/01).
Availability of data and materials
The datasets generated and/or analyzed during the present study are not publicly available due to SQU regulations, but are available from the corresponding author on reasonable request.
Authors' contributions
MB designed, performed and analyzed data and wrote the manuscript. ZQ, NA, HH and AB performed the experiments and analyzed the data. IB, HK, RQ, VP and KF recruited participants, collected clinical data and critically reviewed the manuscript.
Ethics approval and consent to participate
This study was performed according to the principles outlined in the Declaration of Helsinki and approved by the Institutional Research Ethic Committee of the Sultan Qaboos University (SQU), Sultanate of Oman. Further approval was obtained from the hospital management before accessing the patient medical records. All study participants signed an informed consent form prior to enrollment.
Patient consent for publication
Consents, verbal and written, were obtained from all the participants.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 2.Mauri C, Menon M. Human regulatory B cells in health and disease: Therapeutic potential. J Clin Invest. 2017;127:772–779. doi: 10.1172/JCI85113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5:173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 4.Oleinika K, Rosser EC, Matei DE, Nistala K, Bosma A, Drozdov I, Mauri C. CD1d-dependent immune suppression mediated by regulatory B cells through modulations of iNKT cells. Nat Commun. 2018;9:684. doi: 10.1038/s41467-018-02911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vomhof-DeKrey EE, Yates J, Hägglöf T, Lanthier P, Amiel E, Veerapen N, Besra GS, Karlsson MC, Leadbetter EA. Cognate interaction with iNKT cells expands IL-10-producing B regulatory cells. Proc Natl Acad Sci USA. 2015;112:12474–12479. doi: 10.1073/pnas.1504790112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP, Gress RE, Chan AC, Hesdorffer C, Biragyn A. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res. 2013;73:2127–2138. doi: 10.1158/0008-5472.CAN-12-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest. 2011;121:4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulassel MR, Al-Ghonimi M, Al-Balushi B, Al-Naamani A, Al-Qarni Z, Wali Y, Elshinawy M, Al-Shezawi M, Khan H, Nazir H, et al. Regulatory B Cells Are Functionally Impaired in Patients Having Hemophilia A With Inhibitors. Clin Appl Thromb Hemost. 2018;24:618–624. doi: 10.1177/1076029617702244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulassel MR, Mercier F, Gilmore N, Routy JP. Immunophenotypic patterns of CD8+ T cell subsets expressing CD8α and IL-7Rα in viremic, aviremic and slow progressor HIV-1-infected subjects. Clin Immunol. 2007;124:149–157. doi: 10.1016/j.clim.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Gunduz E, Sermet S, Musmul A. Peripheral blood regulatory T cell levels are correlated with some poor prognostic markers in newly diagnosed lymphoma patients. Cytometry B Clin Cytom. 2016;90:449–454. doi: 10.1002/cyto.b.21330. [DOI] [PubMed] [Google Scholar]
- 13.Lin H, Sun XF, Zhen ZJ, Xia Y, Ling JY, Huang HQ, Xia ZJ, Lin TY. Correlation between peripheral blood CD4+CD25high CD127low regulatory T cell and clinical characteristics of patients with non-Hodgkin's lymphoma. Chin J Cancer. 2009;28:1186–1192. doi: 10.5732/cjc.009.10180. [DOI] [PubMed] [Google Scholar]
- 14.Han Y, Wu J, Bi L, Xiong S, Gao S, Yin L, Jiang L, Chen C, Yu K, Zhang S. Malignant B cells induce the conversion of CD4+CD25- T cells to regulatory T cells in B-cell non-Hodgkin lymphoma. PLoS One. 2011;6:e28649. doi: 10.1371/journal.pone.0028649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111:5359–5370. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 16.Cao J, Guo P, Xiao S, Fu X. Relationship of regulatory T cell level in peripheral blood with curative efficacy and prognosis of patients with non-Hodgkin's lymphoma. Int J Clin Exp Pathol. 2016;9:11876–11882. [Google Scholar]
- 17.Chang C, Wu SY, Kang YW, Lin KP, Chen TY, Medeiros LJ, Chang KC. High levels of regulatory T cells in blood are a poor prognostic factor in patients with diffuse large B-cell lymphoma. Am J Clin Pathol. 2015;144:935–944. doi: 10.1309/AJCPUJGMVV6ZF4GG. [DOI] [PubMed] [Google Scholar]
- 18.Mohr A, Malhotra R, Mayer G, Gorochov G, Miyara M. Human FOXP3+ T regulatory cell heterogeneity. Clin Transl Immunology. 2018;7:e1005. doi: 10.1002/cti2.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Ke XY. The four types of Tregs in malignant lymphomas. J Hematol Oncol. 2011;4:50. doi: 10.1186/1756-8722-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu H, Li J, Feng Z, Yuan J, Lu J, Hu X, Gao L, Lv S, Yang J, Chen L. CD19(+) CD20(−) CD27(hi) IL-s10-producing B cells are overrepresented in R-CHOP-treated DLBCL patients in complete remission. Clin Exp Pharmacol Physiol. 2016;43:795–801. doi: 10.1111/1440-1681.12603. [DOI] [PubMed] [Google Scholar]
- 21.Pierini A, Nishikii H, Baker J, Kimura T, Kwon HS, Pan Y, Chen Y, Alvarez M, Strober W, Velardi A, et al. Foxp3+ regulatory T cells maintain the bone marrow microenvironment for B cell lymphopoiesis. Nat Commun. 2017;8:15068. doi: 10.1038/ncomms15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, Wang G, Zou W. Bone marrow and the control of immunity. Cell Mol Immunol. 2012;9:11–19. doi: 10.1038/cmi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol. 2011;140:119–129. doi: 10.1016/j.clim.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoneda K, Morii T, Nieda M, Tsukaguchi N, Amano I, Tanaka H, Yagi H, Narita N, Kimura H. The peripheral blood Vα24+ NKT cell numbers decrease in patients with haematopoietic malignancy. Leuk Res. 2005;29:147–152. doi: 10.1016/j.leukres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Hus I, Bojarska-Junak A, Kamińska M, Dobrzyńska-Rutkowska A, Szatan K, Szymczyk A, Kukiełka-Budny B, Szczepanek D, Roliński J. Imbalance in circulatory iNKT, Th17 and T regulatory cell frequencies in patients with B-cell non-Hodgkin's lymphoma. Oncol Lett. 2017;14:7957–7964. doi: 10.3892/ol.2017.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan-Li L, Kang-Sheng G, Yue-Yin P, Yang J, Zhi-Min Z. The lower peripheral blood lymphocyte/monocyte ratio assessed during routine follow-up after standard first-line chemotherapy is a risk factor for predicting relapse in patients with diffuse large B-cell lymphoma. Leuk Res. 2014;38:323–328. doi: 10.1016/j.leukres.2013.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are not publicly available due to SQU regulations, but are available from the corresponding author on reasonable request.