Abstract
Background
Vitiligo is characterized by a lack of pigmentation in the skin. To date, there are no studies that analyze the changes in gene expression in the skin of vitiligo patients in response to narrow-band ultraviolet B (nb-UVB) phototherapy treatment.
Objective
Explore the usefulness of new generation RNA sequencing in the identification of gene expression changes in the skin of vitiligo patients treated with nb-UVB phototherapy.
Methods
Four skin biopsies (4mm in diameter) were collected from 45 Mexican vitiligo vulgaris patients, 2 specimens before and 2 after treatment with nb-UVB phototherapy, obtained from pigmented and non-pigmented tissue. RNA extracted from the biopsies was analyzed using the Illumina TruSeq Targeted RNA Expression protocol to study the expression of genes that participate in pathways of skin homeostasis. The 2 groups were compared using Student's t-test and the Mann-Whitney U-test.
Results
The expression analysis identified differences in 12 genes included in this study after comparing the samples obtained before and after treatment: 5 genes involved in skin pigmentation, 2 genes involved in apoptosis, 2 genes involved in cell survival, 2 genes involved in oxidative stress responses and 1 gene involved in signal transduction mechanisms (p<0.05).
Study limitations
The small size of skin biopsies limits the amount of RNA obtained, the number of genes to be analyzed and the use of conventional techniques such as RT-qPCR.
Conclusion
We demonstrated usefulness of new generation RNA sequencing in the identification of gene expression changes, in addition to identifying new targets in the study of vitiligo.
Keywords: Gene expression, Phototherapy, Vitiligo
INTRODUCTION
Vitiligo belongs to a family of cutaneous diseases, characterized by a lack of pigmentation in the skin.1 This disease affects between 0.1% and 2% of the world's population; however, its incidence varies considerably among the populations or ethnic groups analyzed and is estimated to be 0.14% in Russia, between 1% and 2.5% in the United States and Japan,2 and high in Mexico (4%) and India (8.8%).1,3
The causes of vitiligo are complex and not yet fully understood. Several hypotheses and theories have been developed to explain the depigmentation and melanocyte destruction observed in this condition; however, these theories do not explain the full spectrum of this disease.4 Thus, the clinical data, embryonic origin of the melanocytes, association with autoimmune disorders, and presence of relatives affected with this disease, among other factors, should be considered, suggesting that vitiligo results from a combination of environmental, autoimmune and genetic factors, and several theories have been proposed.5,6
Studies worldwide have explored genes that are potentially involved in the development of vitiligo and other autoimmune diseases using gene expression analysis studies, candidate gene association studies, genetic linkage studies, and genomic association studies (GWAS).7 Gene expression studies performed on skin biopsies obtained from patients with vitiligo have enabled the analyses of changes in the expression patterns of several genes associated with immunomodulation,7 melanogenesis, and regulation of the development and survival of melanocytes.8-11
To date, no fully effective treatment for vitiligo has been identified. Techniques promoting repigmentation have been used, such as narrow-band ultraviolet B light (nb-UVB), which is currently considered the treatment of choice for vitiligo.12 This therapy uses a light spectrum of 311nm, presenting a penetrance to the basal layer of the epidermis, which induces local immunosuppression and stimulates the proliferation of melanocytes in the skin and in the external epithelial root sheath of the hair follicle, thereby stimulating melanogenesis and the production of melanocyte-stimulating hormone (MSH).12-14
Currently, there are no studies analyzing the changes in gene expression in the skin of subjects in response to the treatment of vitiligo, particularly the application of nb-UVB treatment. Using the currently available molecular tools, including reverse transcription polymerase chain reaction (RT-PCR) as well as transcript identification and gene expression quantification through RNA sequencing (RNA-seq) analysis, we can efficiently analyze the expression profiles of multiple genes present in cells and tissues under pathological and non-pathological human conditions, among other conditions.15-18 In particular, RNA-seq using next-generation sequencing (NGS) also facilitates a more accurate measurement of the levels of transcript expression, even the presence of isoforms in the analyzed samples.19 TruSeq RNA Targeted sequencing can effectively detect changes in patterns of gene expression by simultaneously analyzing a large variety of selected targets with custom designs, using smaller sample amounts, shorter processing times and lower costs than other technologies (qPCR and Microarray).20,21 This technology has previously been applied in the identification of expression profiles in other dermatological diseases, such as psoriasis and lupus erythematosus, suggesting that TruSeq RNA Targeted sequencing could be a useful tool for vitiligo.22-24 However, the discrimination power of this method has not been explored in vitiligo.
In a previous study conducted by our research group on skin biopsies obtained from a smaller number of untreated vitiligo patients and healthy controls, we detected changes in the expression of the genes involved in a wide variety of biological processes, including signaling, apoptosis, oxidative stress control, cell survival and pigmentation (unpublished data). Using the information from the gene expression profiles and the addition of other genetic targets identified by the previously mentioned methods, valuable information concerning gene expression patterns, clinical classification, and the effectiveness of the treatment could be obtained using RNA-seq.
Thus, the objective of the present study was to explore the use of RNA-seq technology for the identification of changes in the expression profiles of selected genes in skin biopsies obtained from vitiligo patients before and after treatment with nb-UVB; the present study used NGS TruSeq Targeted RNA Expression from Illumina.
METHODS
Patient Selection, interview and Informed Consent (CI) signature
This study included a total of 45 adult patients of both sexes, who were diagnosed with vitiligo vulgaris, defined as a condition with depigmentation between 10% and 80% of the body surface, and who were candidates for treatment with nb-UVB phototherapy, without having received treatments in the last 6 months. Patients who did not meet these selection criteria, with severe or poorly controlled systemic diseases, cancer, photosensitivity, infection or injury in the treated area were excluded. For female subjects, pregnant women were excluded. These patients were evaluated in two groups: 23 patients with active vitiligo vulgaris (AVV, in which the lesions grew or new lesions appeared in a period of 6 months) and 22 patients with stable vitiligo vulgaris (SVV, in which there was no growth or the appearance of new lesions in a period of 6 months).
Each subject participating in this study was asked to provide informed consent, after having the scope of the research protocol and the benefits and risks explained. The participants were interviewed and clinically evaluated by dermatologists to confirm the diagnosis of vitiligo. In addition, demographic information, personal data, and personal history of the disease (e.g., age of appearance of the first macula, number and distribution of lesions, and attributable causes) were obtained through a survey, and whether the patient received any previous treatment, the type of response observed, family history of vitiligo, association with other autoimmune diseases, potential exposure to stressful situations or psychological disorders, and measures of height and weight were also determined.
This protocol and the informed consent forms were approved by the Institutional Review Board and registered under the code DE13-001.
nb-UVB phototherapy treatment
Each patient was treated with nb-UVB phototherapy performed 2 times a week for a period of 6 months (for a total of 48 sessions) using a DAAVLIN Phototherapy Unit Model Spectra 311/350nm (Bryan, Ohio, USA) with a starting dose of 150mJ/cm2, with increments of 50mJ/cm2 every third session depending on the phototype of the patient, until the minimum erythema dose was reached. This dose was maintained once the response was obtained. If the patient presented any reaction, then this dose was decreased to the previous tolerated dose.
Treatment follow-up was documented through baseline iconography and at 1, 3 and 6 months after initiation of treatment. The clinical results (i.e., changes of repigmentation experienced by the patient after 6 months of treatment) were evaluated by 2 independent and double-blind investigators.
The iconography was obtained using a Sony Exmor DSC-HX1 9.1 megapixel camera (Shinagawa-ku, Tokyo, Japan).
Biological samples
Blood sample
Analyses to assess hematological biometry (BH), blood chemistry tests, antinuclear antibodies and thyroid profile were performed to rule out the presence of concomitant autoimmune diseases in the participants.
Skin biopsies
Skin biopsies, obtained by skin punch (4 mm diameter) of the pigmented areas and from lesions before and after treatment, were used for the mRNA extraction and subsequent gene expression analysis. In total, 4 biopsies were collected from each patient: 2 samples were collected during the protocol (one of non-affected skin and one of the vitiligo lesions) and 2 samples were collected at the end of phototherapy treatment (one of skin that repigmented after treatment and another of a vitiligo lesion that did not repigment at the end of treatment).
The biopsies were stabilized in RNAlater ® (Thermo Fisher Scientific, Inc. Waltham, MA, USA) according to the manufacturer's instructions and stored at -80ºC until the time of processing.
RNA extraction
RNA was extracted from the skin biopsies stored in RNAlater ® using the RNeasy Fibrous Tissue Mini Kit (Qiagen Inc. Valencia, CA, USA) according to the manufacturer's instructions.
Verification of RNA quantity and quality
The amount of total RNA was determined using spectrophotometric microvolume quantification using a NanoDrop 8000 (Thermo Fisher Scientific, Inc. Waltham, MA, USA) and confirmed using quantification according to the fluorometric method using the Qubit ® RNA-BR assays Kit for Qubit 2.0 (Thermo Fisher Scientific, Inc. Waltham, MA, USA). The quality of the RNA was determined through capillary electrophoresis using the Experion Automated Electrophoresis System (Bio-Rad, Hercules, CA, USA) through the determination of the ratio of 18S to 28S ribosomal subunits.
cDNA synthesis for sequencing
To perform this assay, reverse transcription was performed using 100 µg of total DNase-treated RNA extracted from patient biopsies, ProtoScript ® reverse transcriptase (New England Biolabs, Ipswich, MA, USA), and the TruSeq Targeted RNA Expression protocol from Illumina for NGS.
Analysis of gene expression
The cDNAs generated from the skin biopsies of patients before and after treatment were used to perform the expression analysis of a group of 29 genes involved in key pathways implicated in the pigmentation and homeostasis of the skin cells8-10,25-42 using the Illumina MiSeq ® Reagent Kit v3 (150 cycle) and the TruSeq Targeted RNA Custom Panel Kit (Chart 1).
Chart 1.
Genes included in TruSeq Targeted RNA Custom Panel Kit and the functions and routes involved in the development of vitiligo
| Pathway | Gene | Function |
|---|---|---|
| Pigmentation | DCT8,30 | Involved in regulating eumelanin and pheomelanin levels |
| MC1R 8 | Encodes the receptor protein for melanocyte-stimulating hormone (MSH); controls melanogenesis | |
| MC4R 8 | Encoded a protein that interacts with adrenocorticotropic and MSH hormones and is mediated by G proteins | |
| POMC8 | Encodes a preproprotein that undergoes extensive, tissue-specific, post-translational processing via cleavage. One peptide produced from the POMC protein is a-MSH involved in regulating the pigment-producing cells of the skin and hair (melanocytes), where it binds to melanocortin 1 receptor (MC1R) | |
| TYRP18,30 | Encodes a melanosomal enzyme that belongs to the tyrosinase family and plays an important role in the melanin biosynthetic pathway | |
| MLANA30 | Involved in melanosome biogenesis | |
| PHACTR227 | Phosphatase and actin regulator 2 | |
| Apoptosis | BAX25 | BCL2-associated X protein that acts as an anti- or pro-apoptotic regulator that is involved in a wide variety of cellular activities |
| BCL29,25 | Encodes an integral outer mitochondrial membrane protein that blocks apoptotic death | |
| BCL326 | Involved in the regulation of cell proliferation and contributes to transcriptional regulation of NFKB | |
| CASP325,29 | Is a member of the cysteine-aspartic acid protease (caspase) family; plays a central role in the execu- tion-phase of cell apoptosis | |
| CASP731 | Is a member of the cysteine-aspartic acid protease (caspase) family; plays a central role in the execu- tion-phase of cell apoptosis | |
| CASP829 | Is a member of the cysteine-aspartic acid protease (caspase) family; plays a central role in the execu- tion-phase of cell apoptosis. | |
| CASP1032 | Is a member of the cysteine-aspartic acid protease (caspase) family; plays a central role in the execu- tion-phase of cell apoptosis | |
| CFLAR28 | CASP8 and FADD-like apoptosis regulator, is a regulator of apoptosis and is structurally similar to caspase-8 | |
| FASLG35 | Fas ligand (TNF superfamily, member 6); induction of apoptosis triggered by binding to FAS | |
| TNF37 | Tumor necrosis factor, involved in the regulation of a wide spectra of biological processes, including cell proliferation, differentiation, apoptosis, lipid metabolism, and coagulation | |
| TNFRSF1A33 | One of the major receptors for the tumor necrosis factor-alpha; activates NF-kappa B, mediates apoptosis, and functions as a regulator of inflammation | |
| Oxidative stress | GGT134 | Involved in the maintenance of intracellular GSH levels. It is part of the cellular antioxidant defense mechanism |
| CCBL242 | Cysteine conjugate-beta lyase 2, involved in the regulation of oxidative stress | |
| GPD138 | Glycerol-3-phosphate dehydrogenase 1, involved in the redox metabolism | |
| TXN39 | Thioredoxin, inhibits oxidative stress and caspase 3 activity | |
| Cell survival | CAPN310 | A major intracellular protease involved in melanocyte survival |
| MITF9,30 | Involved in melanocyte survival, controls the expression of genes that relate melanin synthesis | |
| CDC5L36 | Involved in cell cycle control | |
| MAPK140 | Mitogen-activated protein kinase 1, involved in cell proliferation and differentiation | |
| Signal | CSNK1G340 | Participates in the Wnt signaling pathway |
| transduction | NFKB126 | Nuclear Factor Kappa B Subunit 1, is a transcription regulator that stimulates the expression of genes involved in a wide variety of biological functions |
| WNT7A41 | Wnt Family Member 7A, implicated in oncogenesis and several developmental processes | |
| Endogenous control / constitutive expression | TPT1 | Tumor protein, translationally controlled 1, involved in calcium binding and microtubule stabilization. Endogenous control / constitutive expression |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase. Endogenous control / constitutive expression |
Data obtained from RNA-seq were analyzed using the available TruSeq Targeted RNA tool BaseSpace (https://basespace.illumina.com). The results were exported and normalized to the total number of counts. The figures were generated using R statistical language (https://cran.r-project.org/).
Statistical analysis
The collected data were analyzed using SPSS software version 17.0 (SPSS, Inc. Chicago, IL, USA). For the demographic data and to define the variables, descriptive statistics were used for both the vitiligo injury group and the normal skin group. To examine normality, Kolmogorov-Smirnov and Shapiro-Wilk tests were performed. According to the type of distribution presented by the groups (normal or non-normal), the 2 groups were compared using Student's t-test and the Mann-Whitney U-test. A value of P ≤ 0.05 was considered significant.
RESULTS
Demographic analysis
We included 45 patients diagnosed with vitiligo from the State of Nuevo León, Mexico; the patients consulted the Dermatology department of the University Hospital Dr. José Eleuterio González. The samples were categorized according to the vitiligo activity in: 22 patients as stable type disease (14 males and 8 females) and in 23 patients as active type disease (10 male and 13 female). The mean age of the patients was 41.58 ± 14.36 years (minimum 19 years, maximum 73 years, 39.79 ± 12.18 years for male subjects and 40.00 ± 16.43 years for female subjects).
Response to treatment
After independent assessments by 2 dermatologists, 42 of the 45 subjects included in the expression analysis showed responses to treatment (93.33%, 20 Active [44.44%], 22 Stable [48.89%]). The three subjects who did not present repigmentation after treatment had active vitiligo (6.67%).
Expression analysis
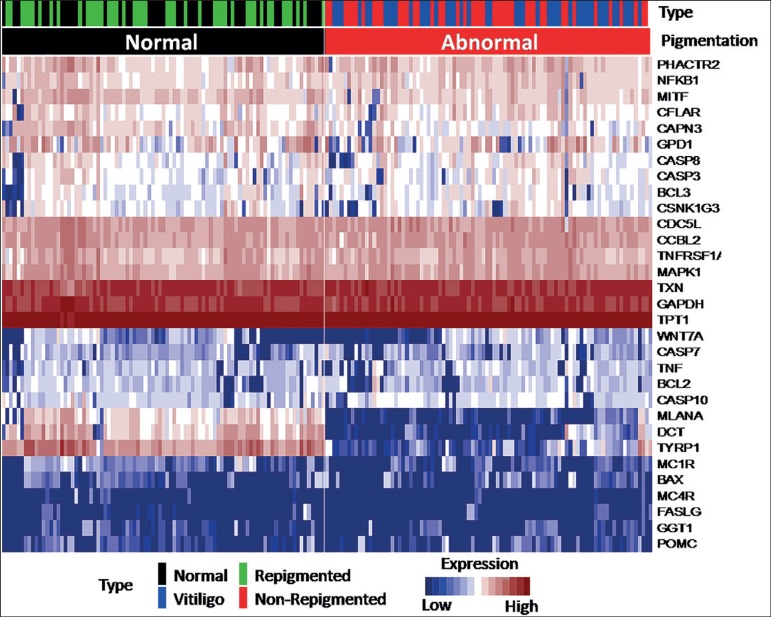
The heat maps generated after comparing the expression patterns from the samples obtained before and after treatment (Figure 1) enabled the identification of differences between the expression patterns of pigmented skin (not affected, and repigmented after treatment) and non-pigmented skin (vitiligo and tissue sample that did not repigment), organized into 2 main clades in the heat map.
Figure 1.
Heat map of expression patterns from biopsies obtained before and after treatment. In the figure, the vertical axis represents the gene expression patterns of the samples obtained for each gene (horizontal). Sample color codes indicate tissue with vitiligo (blue), unaffected skin (black) before treatment, repigmented skin (green) and vitiligo that did not repigment (red) after nb-UVB treatment. The red coloration within the heat map corresponds to over expression, while white corresponds to intermediate expression, and blue corresponds to sub expression
In addition, significant expression patterns were found in three pigment-related genes: DopachromeTautomerase (DCT), Melan-A (MLANA) and Tyrosinase-Related Protein (TYRP1) gene, which are over expressed in pigmented skin (p<0.05).
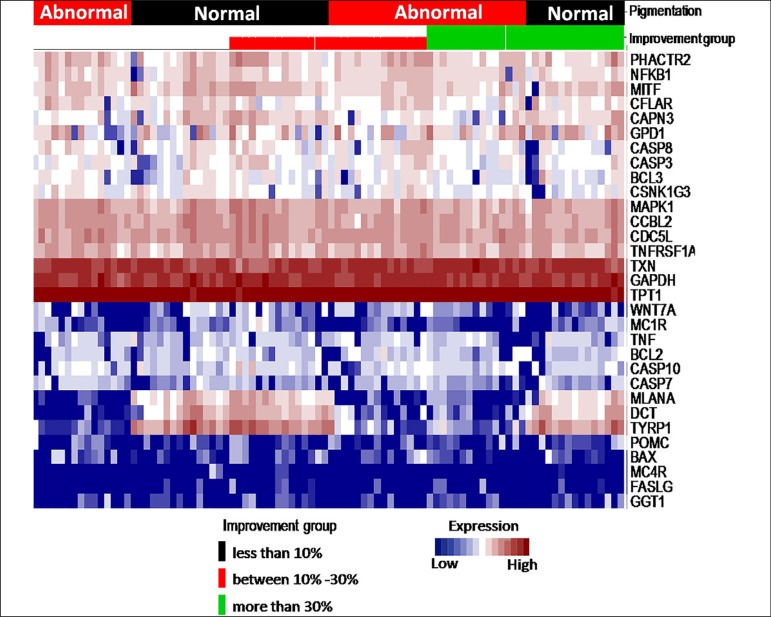
When grouping the samples according to the type and subtype of pigmentation (with pigment and without pigment), gender (female or male), age, type of vitiligo (active or stable), and degree of response to treatment (less than 10% improvement, 10%-30% improvement and more than 30% improvement), we observed the same patterns of over expression in DCT, MLANA and TYRP1 genes (related to skin pigmentation) (Figure 2).
Figure 2.
Heat map of expression patterns grouped according to vitiligo type and disease improvement. At the top of the heat map, the following study groups are represented: type and subtype of sample according to pigmentation (black and red), and improvement (black corresponding to less than 10% improvement, red corresponding to 10%-30% improvement and green corresponding to more than 30% improvement). The vertical axis represents the expression patterns of the samples obtained for each of the genes (horizontal). The red color within the heatmap corresponds to over expression, while white corresponds to intermediate expression, and blue corresponds to sub expression
No differences in the type of vitiligo were observed among skin samples from subjects with active or stable vitiligo.
Comparison of the skin expression patterns before and after treatment
Using Student's t-test, we compared the mean values for the expression patterns of the analyzed genes in pigmented and non-pigmented skin before and after treatment, and significant differences were observed in 12 genes related to the mechanisms of pigmentation, apoptosis, cell life, oxidative stress and cellular signaling mechanisms (Table 1).
Table 1.
Comparison of gene expression patterns in the skin biopsy of vitiligo patients before and after treatment
| Skin biopsy expression | ||||||
|---|---|---|---|---|---|---|
| Pathway | GENE | Unaffected skin (Means ± SD) | Vitiligo skin (affected) (Means ± SD) | Repigmented after treatment (Means ± SD) | Not Repigmented after treatment (Means ± SD) | P-value |
| Pigmentation | DCT | 2.965 ± 0.554 | 0.714 ± 0.947 | 2.729 ± 0.804 | 0.590 ± 0.897 | A**; C**; D**;F** |
| MC1R | 1.148 ± 0.822 | 0.602 ± 0742 | 1.013 ± 0.788 | 0.529 ± 0.621 | A**; C**; D**; F** | |
| MC4R | 0.113 ± 0.387 | 0.000 ± 0.000 | 0.083 ± 0.316 | 0.076 ± 0.252 | E* | |
| MLANA | 2.793 ± 0.505 | 0.476 ± 0.745 | 2.488 ± 0.653 | 0.454 ± 0.699 | A**; B*; C**;D**; F** | |
| TYRP1 | 3.544 ± 0.343 | 1.214 ± 0.920 | 3.451 ± 0.463 | 1.230 ± 0.983 | A**; C**; D**; F** | |
| Apoptosis | CASP3 | 2.361 ± 0.692 | 2.521 ± 0.302 | 2.335 ± 0.545 | 2.268 ± 0.703 | D*; E* |
| TNFRSF1A | 3.158 ± 0.310 | 3.179 ± 0.246 | 3.054 ± 0.303 | 3.136 ± 0.230 | D** | |
| Cell survival | CDC5L | 3.377 ± 0.199 | 3.373 ± 0.174 | 3.291 ± 0.190 | 3.359 ± 0.250 | B*; D* |
| MITF | 3.014 ± 0.522 | 2.862 ± 0.248 | 2.980 ± 0.238 | 2.643 ± 0.605 | C**; D*; E*; F** | |
| Cellular Oxidative Stress Response |
GPD1 | 2.624 ± 0.779 | 2.668 ± 0.753 | 2.805 ± 0.552 | 2.336 ± 0.907 | E*; F** |
| TXN | 4.203 ± 0.238 | 4.310 ± 0.128 | 4.289 ± 0.136 | 4.315 ± 0.132 | A**; B*;C** | |
| Signal transduction | CSNK1G3 | 2.252 ± 0.662 | 2.408 ± 0.365 | 2.238 ± 0.703 | 1.981 ± 0.944 | E** |
The data are shown as the means ± standard deviation. (A) Unaffected vs Vitiligo skin; (B) Unaffected vs Regimented; (C) Unaffected vs Not repigmented; (D)Vitiligo vs Repigmented; (E) Vitiligo vs Not repigmented; (F) Repigmented vs Not repigmented. (*) Denotes p<0.05, and (**) denotes p<0.01.
Among these genes, we observed statistically significant differences in DCT (involved in the regulation of eumelanin and pheomelanin levels), TYRP1 (involved in melanin biosynthesis), MC1R (melanocortin receptor 1 involved in the control of melanogenesis), and MLANA (involved in the biogenesis of melanosomes), particularly when comparing pigmented skin (not affected prior to treatment and repigmented skin) to skin that did not show pigment (vitiligo skin and skin that did not repigment after treatment), accounting for the degree of pigment observed in the respective skin types (p <0.05). However, in the MC4R gene (melanocortin receptor 4, which interacts with adrenocorticotropin and MSH in skin pigmentation), we only observed a statistically significant difference in the degree of expression in the vitiligo skin before treatment and not repigmented after treatment (p = 0.05), where vitiligo skin displayed reduced expression.
For genes involved in apoptosis mechanisms, we observed differences in two of the genes selected for this study (p <0.05), CASP3 (cysteine protease 3, processed by caspases 8, 9 and 10 and participating in the execution of the cell apoptosis signaling cascade, activating caspases 6, 7 and 9) and TNFRS1A (tumor necrosis factor receptor super family member 1A), which induces cell death signaling through caspase 8 (Table 1). Both genes were over expressed in the skin of patients with vitiligo, suggesting that the induction of an extrinsic mechanism of apoptosis is involved in the pathogenic mechanism of vitiligo.
However, the analysis of the expression patterns of the genes involved in melanocyte mechanisms (Table 1) indicates that two of the selected genes showed differential expression patterns between the skin types (p <0.05). The differential expression patterns of the CDC5L gene (involved in the control of the cell cycle) in unaffected and vitiligo versus repigmented skin after treatment were reduced in the repigmented skin. The expression of the MITF gene (that controls the expression of genes related to melanin synthesis and melanocyte survival) was higher for pigmented tissues (non-affected and repigmented after treatment) compared to vitiligo skin before treatment and in affected skin that did not repigment after treatment (Table 1).
When analyzing the genes involved in responses to oxidative stress (Table 1), the GPD1 gene (involved in the Redox metabolism of the cell) was over expressed in tissue that did not repigment after treatment. The TXN gene (involved in the inhibition of oxidative stress and activation of caspase 3) was over expressed in vitiligo skin and affected skin that did not repigment, accounting for the activation of mechanisms in response to apoptosis and oxidative stress generated by this cell death mechanism.
Finally, gene-related signaling pathways and signal translation mechanisms showed statistically significant differences when comparing the mean expression patterns observed for the CSNK1G3 gene when analyzing vitiligo and affected skin that did not repigment after treatment, which was reduced in the latter case (Table 1). The product of the CSNK1G3 gene is a serine/threonine kinase that phosphorylates several proteins involved in the Wnt pathway, participating in cellular processes such as DNA repair, cell division, nuclear localization and membrane transport: all active processes in the cells of the skin and potentially involved in the synthesis, transport and storage of the pigment.
DISCUSSION
In this research, a group of genes involved in skin pigmentation, apoptosis and cell survival, oxidative stress and signal transduction were selected to identify changes in the expression patterns of these genes in the skin of vitiligo patients undergoing treatment with nb-UVB phototherapy. Until recently, the relationship between the skin expression patterns of a group of genetic markers participating in the main routes involved in vitiligo, patients affected by this condition and responses to experienced standard treatments had not been documented.
For this purpose, nb-UVB phototherapy was applied for 6 months, which is considered the standard treatment for vitiligo vulgaris because of its effectiveness and safety.12
Evaluation by 2 dermatologists revealed that, consistent with patients undergoing treatment, 42 of the 45 subjects included in the expression analysis showed some degree of response to treatment. The three subjects who did not present repigmentation after treatment presented active vitiligo (6.67%). Kumar et al. (2009) and Chen et al. (2005)43,44 reported similar results, showing a high percentage of responses to this type of treatment. However, the latter study did not describe the type of vitiligo or disease activity presented by patients who showed no response to treatment.
TruSeq RNA Targeted sequencing, unlike other methodologies for gene expression analysis, offers the following advantages: i) effective detection of changes in the “transcriptome” or patterns of gene expression through the simultaneous analysis of a variety of targets, ii) reduced processing and analysis times, iii) the use of small sample sizes to maximize analyses in subjects affected by this condition compared to the patterns present in the genes of healthy subjects, and iv) affordable costs.20,21 Due to the limited biological sample size, the numerous genetic targets to be analyzed and the need for economic resources for its development, this methodology is presented as the best option for analysis, even over RT-qCR.
Using RNAseq technology for the analysis of gene expression, we observed differences in the expression patterns between the analyzed samples. The heat maps generated from the expression results obtained through the massive sequencing of Illumina TruSeq RNA (Figures 1 and 2) revealed similar expression patterns between the affected skin that repigmented after treatment and the unaffected skin of patients biopsied at the beginning of the study. Similar expression patterns were observed in the skin of vitiligo patients before treatment and affected skin that did not repigment after 6 months of phototherapy. Comparison of the different types of skin before and after treatment revealed the reduced expression of MLANA, DCT and TYRP1 genes, all of which are involved in skin pigmentation in non-pigmented skin compared to unaffected skin and repigmented skin after treatment. These results are consistent with those described in the PhD thesis (2012) of Salinas45 and results published by Regazzetti et al. (2015), who validated a group of genes using real-time PCR, reporting low levels of MLANA, DCT and TYRP1 expression in affected skin (vitiligo lesions, compared to perilesional skin and non-affected - pigmented - patients).30
Statistical analysis of the expression patterns obtained from the skin samples before and after treatment revealed alterations in the expression of genes related to skin pigmentation, apoptosis, cell survival, oxidative stress and signal transduction mechanisms.
Reduced gene expression patterns in non-pigmented skin were observed in 5 of the 7 genes included in this study, corresponding to MLANA, DCT and TYRP1, as previously mentioned, and MC1R and MC4R. These last two genes correspond to melanocortin receptors 1 and 4, which together with POMC form part of the hypothalamic-hypophyseal-adrenal axis in the skin, which acts as a coordinator and enforcer of stress responses.8
Kingo et al. (2007) published the results of an expression analysis performed on genes participating in the melanocortin system in skin biopsies obtained from non-pigmented lesions and pigmented (normal) skin from patients with vitiligo. These results were compared to the expression patterns of skin samples from healthy subjects, demonstrating that the expression of proopiomelanocortin and its receptors is reduced in the affected skin compared to the unaffected skin of patients with vitiligo and controls. Recently, Nagui et al. (2015) reported the reduced expression of MC1R and POMC genes in affected skin obtained from a vitiligo lesion after analyzing only MC1R, MC4R and POMC.11
In contrast, Kingo et al.8,9 analyzed the expression profiles of the genes involved in pigmentation in biopsies of affected and unaffected skin of vitiligo patients, compared to the expression levels observed in skin of normal control subjects, without immediate treatment, and our results correspond to the expression profile of a group of genes participating in 5 cell signaling pathways, including pigmentation, in biopsies from the affected and unaffected skin of patients obtained before and after nb-UVB treatment.
When analyzing the total RNA from each of the biopsies obtained from the patients, changes in the expression profiles were observed in most of the selected genes. In pigmentation, the genes that presented differential expression profiles before and after treatment were DCT, MC1R, MC4R, MLANA and TYRP1. Differences were detected after comparing the expression profiles of skin biopsies with pigment (non-affected skin and affected skin that repigmented after treatment) vs. non-pigmented biopsies (biopsies of vitiligo skin and biopsies of affected skin that did not repigment after treatment). In addition, there were no statistically significant differences between the biopsies with pigment, obtained before and after phototherapy application. The same phenomenon was observed among biopsies without pigment, indicating that, once the treatment was applied, the expression profile of the skin underwent changes favoring the manifestation of the final pigmented phenotype. In skin that recovered pigmentation, the expression patterns were similar or slightly higher in genes related to pigment.
The pigmentation gene expression results obtained in the present study reaffirm those of Kingo et al. (2007, 2008) on biopsies before treatment8,9 and are consistent with those of Nagui et al. (2015) in the pigment routes.11 However, we excluded the participation of POMC in this process because no statistically significant difference in the expression profile of the different biopsies analyzed was observed.
Two of the eleven analyzed genes involved in apoptosis presented statistically significant differences (p <0.05): cysteine protease 3 (CASP3, which is processed by caspases 8, 9 and 10 and participates in the execution of the signaling cascade of cellular apoptosis, activating caspases 6, 7 and 9) and tumor necrosis factor receptor family member 1A gene (TNFRS1A) (which induces cell death signaling through caspase 8). In both cases, these genes are slightly over expressed in affected vitiligo skin, indicating the induction of an extrinsic apoptoticpathway. Previously, Kumar R et al. (2011) reported an increase in CASP3 levels in melanocyte cultures obtained from samples of unstable vitiligo using cell cultures and immune fluorescence techniques.46
For the TNFRS1A gene, no alterations in the expression levels in the different skin types of vitiligo patients have been reported.
However, two of the four selected genes related to cell survival showed some pattern of differential expression between the skin types (p <0.05). Among these genes, the CDC5L gene, involved in cell cycle control, and the MITF gene, which controls the expression of genes related to melanin synthesis and melanocyte survival, showed higher expression patterns for pigmented tissues (not affected and repigmented) compared to affected vitiligo skin and skin that did not repigment after treatment. Kingo et al. (2008) published a similar observation for the MITF gene using real-time PCR expression analysis. In their study, MITF showed higher expression patterns in skin from healthy controls and the unaffected skin of vitiligo patients compared to skin affected with vitiligo lesions.9 A decrease in the expression pattern of the CDC5L gene was observed in skin that repigmented after treatment compared to affected vitiligo and unaffected skin before treatment. However, the participation of this gene in vitiligo has not yet been described.
For the genes involved in responses to oxidative stress, the GPD1 gene, involved in the oxidation-reduction metabolism of the cell (redox),38 was over expressed in tissue that repigmented after treatment. The TXN gene, involved in the inhibition of oxidative stress and activation of caspase 3, is over expressed in vitiligo and non-repigmented tissues, suggesting potential activation mechanisms in response to apoptosis and oxidative stress generated through mechanisms of cell death.
To date, only changes in the levels of TXN protein in the skin of vitiligo patients,39 and their relationship to the control of reactive oxygen species production and UV damage have been reported.47 The involvement of the GPD1 gene in the control of oxidative stress in vitiligo has not yet been explored.
Finally, the involvement of the WNT pathway in melanocyte differentiation and the development of vitiligo has recently been described.30 However, in our study, only statistically significant differences in the expression patterns observed for the CSNK1G3 gene were seen. The product of this gene is a serine/threonine kinase that phosphorylates a number of proteins involved in the WNT pathway, participating in important cellular processes, such as DNA repair, cell division, nucleus-cytoplasmic shuttling of transcription factors, and transport through the membrane. As shown in Table 1, an increase in the levels of vitiligo-affected skin expression may reflect, among other processes, the activation of DNA repair mechanisms in damaged melanocyte/keratinocyte cells. However, a decrease in the expression of this gene was observed in affected skin that did not repigment after treatment, which may account for a decrease in the cellular mechanisms underlying melanocyte differentiation and DNA repair in the damaged cells of skin affected with vitiligo.
CONCLUSION
The nb-UVB phototherapy produced significant changes in the expression patterns of the genes analyzed, primarily reflecting repigmentation and cellular apoptosis control.
Expression analysis revealed significant differences in the expression patterns of the DCT, MLANA and TYRP1 genes in pigmented skin (unaffected and affected then repigmented after treatment) compared to affected skin (vitiligo and non-pigmented skin after treatment). The statistical analysis of the expression patterns obtained using TruSeq Targeted RNA Expression technology from skin samples obtained before and after treatment showed changes in the expression profiles in selected genes in response to nb-UVB phototherapy, and some of these changes were comparable with those previously reported by other research groups, particularly in genes related to skin pigmentation.
In addition, the identification of novel targets genes, including TNFRS1A, GPD1 and CSNK1G3, implicated in apoptosis, oxidative stress control and signal transduction, suggests that these genes play a central role in many aspects of vitiligo development.
ACKNOWLEDGEMENTS
We would like to acknowledge MD. Osvaldo Vázquez-Martínez, MD. Alejandra Villarreal-Martínez and MD. Verónica Garza-Rodríguez for their assistance in patient selection and screening, skin biopsy obtaining, application of phototherapy treatment and data analysis. We would also like to thank Dr. Juan José Vilata-Corell and Dr. José Luis Alfonso-Sánchez for their unconditional support in the study and in data analysis, and PhD Eduardo de la Rosa-Moreno for his contributions in study design, gene selection, TruSeq RNA sample preparation, sequencing and data analysis. We would also like to thank all those who participated in this study for contributing to the proofreading the manuscript.
Funding Statement
Financial support: This project was funded by the “Support Program for Scientific and Technological Research” (PAICyT - UANL) with number CS879-11
Footnotes
Work conducted at the Dermatology Service, Hospital Universitario Dr. José Eleuterio González, Facultad de Medicina, Universidad Autónoma de Nuevo León, Nuevo León, México.
Financial support: This project was funded by the “Support Program for Scientific and Technological Research” (PAICyT - UANL) with number CS879-11, and from the Dermatology Service, Dr. José Eleuterio González University Hospital, UANL, MTY, Nuevo Leon, Mexico.
Conflict of interest: None.
AUTHORS' CONTRIBUTIONS
Jorge Ocampo-Candiani
0000-0002-0213-0031
Statistical analysis, Approval of the final version of the manuscript, Conception and planning of the study, Elaboration and writing of the manuscript, Obtaining, analyzing and interpreting the data, Effective participation in research orientation, Intellectual participation in propaedeutic and/or therapeutic conduct of the cases studied, Critical review of the literature, Critical review of the manuscript
Mauricio Salinas-Santander
0000-0002-5439-5787
Statistical analysis, Approval of the final version of the manuscript, Conception and planning of the study, Elaboration and writing of the manuscript, Obtaining, analyzing and interpreting the data, Effective participation in research orientation, Critical review of the literature, Critical review of the manuscript
Victor Trevino
0000-0002-7472-9844
Statistical analysis, Approval of the final version of the manuscript, Elaboration and writing of the manuscript, Obtaining, analyzing and interpreting the data, Critical review of the literature, Critical review of the manuscript
Rocio Ortiz-López
0000-0002-7783-026X
Approval of the final version of the manuscript, Conception and planning of the study, Effective participation in research orientation, Critical review of the literature, Critical review of the manuscript
Jorge Ocampo-Garza
0000-0002-1307-3407
Approval of the final version of the manuscript, Conception and planning of the study, Obtaining, analyzing and interpreting the data, Effective participation in research orientation, Critical review of the literatura
Celia Nohemi Sanchez-Dominguez
0000-0002-3444-9565
Approval of the final version of the manuscript, Elaboration and writing of the manuscript, Obtaining, analyzing and interpreting the data, Effective participation in research orientation, Critical review of the literature, Critical review of the manuscript
REFERENCES
- 1.Sehgal VN, Srivastava G. Vitiligo: compendium of clinico-epidemiological features. Indian J Dermatol Venereol Leprol. 2007;73:149–156. doi: 10.4103/0378-6323.32708. [DOI] [PubMed] [Google Scholar]
- 2.Steiner D, Bedin V, Brito MB, Tadeu RT, Steiner T. Vitiligo. An Bras Dermatol. 2004;79:335–351. [Google Scholar]
- 3.Parsad D, Dogra S, Kanwar AJ. Quality of life in patients with vitiligo. Health Qual Life Outcomes. 2003;1:58–58. doi: 10.1186/1477-7525-1-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spritz RA. The genetics of generalized vitiligo and associated autoimmune diseases. J Dermatol Sci. 2006;41:3–10. doi: 10.1016/j.jdermsci.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Spritz RA. The genetics of generalized vitiligo and associated autoimmune diseases. Pigment Cell Res. 2007;20:271–278. doi: 10.1111/j.1600-0749.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 6.Tazi-Ahnini R, McDonagh AJ, Wengraf DA, Lovewell TR, Vasilopoulos Y, Messenger AG, et al. The autoimmune regulator gene (AIRE) is strongly associated with vitiligo. Br J Dermatol. 2008;159:591–596. doi: 10.1111/j.1365-2133.2008.08718.x. [DOI] [PubMed] [Google Scholar]
- 7.Allam M, Riad H. Concise review of recent studies in vitiligo. Qatar Med J. 2013;2013:1–19. doi: 10.5339/qmj.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingo K, Aunin E, Karelson M, Philips MA, Ratsep R, Silm H, et al. Gene expression analysis of melanocortin system in vitiligo. J Dermatol Sci. 2007;48:113–122. doi: 10.1016/j.jdermsci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Kingo K, Aunin E, Karelson M, Ratsep R, Silm H, Vasar E, et al. Expressional changes in the intracellular melanogenesis pathways and their possible role in the pathogenesis of vitiligo. J Dermatol Sci. 2008;52:39–46. doi: 10.1016/j.jdermsci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Stromberg S, Bjorklund MG, Asplund A, Rimini R, Lundeberg J, Nilsson P, et al. Transcriptional profiling of melanocytes from patients with vitiligo vulgaris. Pigment Cell Melanoma Res. 2008;21:162–171. doi: 10.1111/j.1755-148X.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- 11.Nagui NA, Mahmoud SB, Abdel Hay RM, Hassieb MM, Rashed LA. Assessment of gene expression levels of proopiomelanocortin (POMC) and melanocortin-1 receptor (MC1R) in vitiligo. Australas J Dermatol. 2017;58:e36–e39. doi: 10.1111/ajd.12408. [DOI] [PubMed] [Google Scholar]
- 12.Majid I. Vitiligo Management: An Update. BJMP. 2010;3:a332–a332. [Google Scholar]
- 13.Westerhof W, Nieuweboer-Krobotova L. Treatment of vitiligo with UV-B radiation vs topical psoralen plus UV-A. Arch Dermatol. 1997;133:1525–1528. [PubMed] [Google Scholar]
- 14.Halder RM, Chappell JL. Vitiligo update. Semin Cutan Med Surg. 2009;28:86–92. doi: 10.1016/j.sder.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Chandrasekharappa SC, Lach FP, Kimble DC, Kamat A, Teer JK, Donovan FX, et al. Massively parallel sequencing, aCGH, and RNA-Seq technologies provide a comprehensive molecular diagnosis of Fanconi anemia. Blood. 2013;121:e138–e148. doi: 10.1182/blood-2012-12-474585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Hurley G, Busch C, Fagerberg L, Hallstrom BM, Stadler C, Tolf A, et al. Analysis of the Human Prostate-Specific Proteome Defined by Transcriptomics and Antibody-Based Profiling Identifies TMEM79 and ACOXL as Two Putative, Diagnostic Markers in Prostate Cancer. PLoS One. 2015;10:e0133449. doi: 10.1371/journal.pone.0133449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endsley MP, Moyle-Heyrman G, Karthikeyan S, Lantvit DD, Davis DA, Wei JJ, et al. Spontaneous Transformation of Murine Oviductal Epithelial Cells: A Model System to Investigate the Onset of Fallopian-Derived Tumors. Front Oncol. 2015;5:154–154. doi: 10.3389/fonc.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katayama S, Skoog T, Jouhilahti EM, Siitonen HA, Nuutila K, Tervaniemi MH, et al. Gene expression analysis of skin grafts and cultured keratinocytes using synthetic RNA normalization reveals Insights Into differentiation and growth control. BMC Genomics. 2015;16:476–476. doi: 10.1186/s12864-015-1671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu C, Fang Z, Hua X, Yang Y, Chen E, Cowley Jr AW, et al. deGPS is a powerful tool for detecting differential expression in RNA-sequencing studies. BMC Genomics. 2015;16:455–455. doi: 10.1186/s12864-015-1676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prokopec SD, Watson JD, Waggott DM, Smith AB, Wu AH, Okey AB, et al. Systematic evaluation of medium-throughput mRNA abundance platforms. RNA. 2013;19:51–62. doi: 10.1261/rna.034710.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swindell WR, Remmer HA, Sarkar MK, Xing X, Barnes DH, Wolterink L, et al. Proteogenomic analysis of psoriasis reveals discordant and concordant changes in mRNA and protein abundance. Genome Med. 2015;7:86–86. doi: 10.1186/s13073-015-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone RC, Du P, Feng D, Dhawan K, Ronnblom L, Eloranta ML, et al. RNA-Seq for enrichment and analysis of IRF5 transcript expression in SLE. PLoS One. 2013;8:e54487. doi: 10.1371/journal.pone.0054487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi L, Zhang Z, Yu AM, Wang W, Wei Z, Akhter E, et al. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS One. 2014;9:e93846. doi: 10.1371/journal.pone.0093846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baltadzhiev IG, Delchev SD. Changes of Bcl-2, Bax and Caspase-3 expression in the dermal microvascular endothelial cells and the epidermal layers of the eschar (tache noire) in patients with Mediterranean spotted fever. Folia Histochem Cytobiol. 2013;51:121–126. doi: 10.5603/FHC.2013.0019. [DOI] [PubMed] [Google Scholar]
- 26.Chang TP, Vancurova I. Bcl3 regulates pro-survival and pro-inflammatory gene expression in cutaneous T-cell lymphoma. Biochim Biophys Acta. 2014;1843:2620–2630. doi: 10.1016/j.bbamcr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein BA, Hubbard AE, Cutler A, Barcellos LF. An application of Random Forests to a genome-wide association dataset: methodological considerations & new findings. BMC Genet. 2010;11:49–49. doi: 10.1186/1471-2156-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar-Sinha C, Varambally S, Sreekumar A, Chinnaiyan AM. Molecular cross-talk between the TRAIL and interferon signaling pathways. J Biol Chem. 2002;277:575–585. doi: 10.1074/jbc.M107795200. [DOI] [PubMed] [Google Scholar]
- 29.Lee AY, Youm YH, Kim NH, Yang H, Choi WI. Keratinocytes in the depigmented epidermis of vitiligo are more vulnerable to trauma (suction) than keratinocytes in the normally pigmented epidermis, resulting in their apoptosis. Br J Dermatol. 2004;151:995–1003. doi: 10.1111/j.1365-2133.2004.06136.x. [DOI] [PubMed] [Google Scholar]
- 30.Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P, et al. Transcriptional Analysis of Vitiligo Skin Reveals the Alteration of WNT Pathway A Promising Target for Repigmenting Vitiligo Patients. J Invest Dermatol. 2015;135:3105–3114. doi: 10.1038/jid.2015.335. [DOI] [PubMed] [Google Scholar]
- 31.Shen C, Gao J, Sheng Y, Dou J, Zhou F, Zheng X, et al. Genetic Susceptibility to Vitiligo: GWAS Approaches for Identifying Vitiligo Susceptibility Genes and Loci. Front Genet. 2016;7:3–3. doi: 10.3389/fgene.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wybranska I, Polus A, Mikolajczyk M, Knapp A, Sliwa A, Zapala B. Apoptosis-related gene expression in glioblastoma (LN-18) and medulloblastoma (Daoy) cell lines. Hum Cell. 2013;26:137–148. doi: 10.1007/s13577-011-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alenzi FQ. The significance and occurrence of TNF receptor polymorphisms in the Saudi population. Saudi J Biol Sci. 2016;23:767–772. doi: 10.1016/j.sjbs.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karsli N, Akcali C, Ozgoztasi O, Kirtak N, Inaloz S. Role of oxidative stress in the pathogenesis of vitiligo with special emphasis on the antioxidant action of narrowband ultraviolet B phototherapy. J Int Med Res. 2014;42:799–805. doi: 10.1177/0300060513516294. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Sun D, Li C, Zhang Z, Gao L, Li K, et al. Functional polymorphisms of the FAS gene associated with risk of vitiligo in Chinese populations: a case-control analysis. J Invest Dermatol. 2008;128:2820–2824. doi: 10.1038/jid.2008.161. [DOI] [PubMed] [Google Scholar]
- 36.Martin JW, Chilton-MacNeill S, Koti M, van Wijnen AJ, Squire JA, Zielenska M. Digital expression profiling identities RUNX2, CDC5L, MDM2, RECQL4, and CDK4 as potential predictive biomarkers for neo-adjuvant chemotherapy response in paediatric osteosarcoma. PLoS One. 2014;9:e95843. doi: 10.1371/journal.pone.0095843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moretti S, Fabbri P, Baroni G, Berti S, Bani D, Berti E, et al. Keratinocyte dysfunction in vitiligo epidermis: cytokine microenvironment and correlation to keratinocyte apoptosis. Histol Histopathol. 2009;24:849–857. doi: 10.14670/HH-24.849. [DOI] [PubMed] [Google Scholar]
- 38.Mracek T, Drahota Z, Houstek J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim Biophys Acta. 2013;1827:401–410. doi: 10.1016/j.bbabio.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Schallreuter KU, Behrens-Williams S, Khaliq TP, Picksley SM, Peters EM, Marles LK, et al. Increased epidermal functioning wild-type p53 expression in vitiligo. ExpDermatol. 2003;12:268–277. doi: 10.1034/j.1600-0625.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 40.Verkaar F, van der Doelen AA, Smits JF, Blankesteijn WM, Zaman GJ. Inhibition of Wnt/beta-catenin signaling by p38 MAP kinase inhibitors is explained by crossreactivity with casein kinase Idelta/varepsilon. Chem Biol. 2011;18:485–494. doi: 10.1016/j.chembiol.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Yamada T, Hasegawa S, Inoue Y, Date Y, Yamamoto N, Mizutani H, et al. Wnt/beta-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol. 2013;133:2753–2762. doi: 10.1038/jid.2013.235. [DOI] [PubMed] [Google Scholar]
- 42.Yu P, Li Z, Zhang L, Tagle DA, Cai T. Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene. 2006;365:111–118. doi: 10.1016/j.gene.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 43.Kishan Kumar YH, Rao GR, Gopal KV, Shanti G, Rao KV. Evaluation of narrowband UVB phototherapy in 150 patients with vitiligo. Indian J Dermatol Venereol Leprol. 2009;75:162–166. doi: 10.4103/0378-6323.48662. [DOI] [PubMed] [Google Scholar]
- 44.Chen GY, Hsu MM, Tai HK, Chou TC, Tseng CL, Chang HY, et al. Narrow-band UVB treatment of vitiligo in Chinese. J Dermatol. 2005;32:793–800. doi: 10.1111/j.1346-8138.2005.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 45.Salinas-Santander M. Análisis del perfil de expresión de pacientes con vitiligo. Monterrey (NL): Universidad Autónoma de Nuevo León (UANL); 2012. tesis. [Google Scholar]
- 46.Kumar R, Parsad D, Kanwar AJ. Role of apoptosis and melanocytorrhagy: a comparative study of melanocyte adhesion in stable and unstable vitiligo. Br J Dermatol. 2011;164:187–191. doi: 10.1111/j.1365-2133.2010.10039.x. [DOI] [PubMed] [Google Scholar]
- 47.Schallreuter KU, Moore J, Wood JM, Beazley WD, Peters EM, Marles LK, et al. Epidermal H(2)O(2) accumulation alters tetrahydrobiopterin (6BH4) recycling in vitiligo: identification of a general mechanism in regulation of all 6BH4-dependent processes? J Invest Dermatol. 2001;116:167–174. doi: 10.1046/j.1523-1747.2001.00220.x. [DOI] [PubMed] [Google Scholar]