Abstract
Humanin (HN) is a 24-residue peptide identified from the brain of a patient with Alzheimer's disease (AD). HN has been found to protect against neuronal insult caused by Aβ peptides or transfection of familial AD mutant genes. In order to elucidate the molecular mechanisms of HN neuroprotection, we explored the effects of HN on the association of Bax or Bid with lipid bilayers and their oligomerization in the membrane. By using single-molecule fluorescence and Förster resonance energy transfer techniques, we showed that Bax was mainly present as monomers, dimers and tetramers in lipid bilayers, while truncated Bid (tBid) enhanced the membrane association and tetramerization of Bax. HN (100 nmol/L) inhibited the self-association and tBid-activated association of Bax with the bilayers, and significantly decreased the proportion of Bax in tetramers. Furthermore, HN inhibited Bid translocation to lipid bilayers. HN could bind with Bax and Bid either in solution or in the membrane. However, HN could not pull the proteins out of the membrane. Based on these results, we propose that HN binds to Bax and cBid in solution and inhibits their translocation to the membrane. Meanwhile, HN interacts with the membrane-bound Bax and tBid, preventing the recruitment of cytosolic Bax and its oligomerization in the membrane. In this way, HN inhibits Bax pore formation in mitochondrial outer membrane and suppresses cytochrome c release and mitochondria-dependent apoptosis.
Keywords: Alzheimer's disease, humanin, Bcl-2 family, mitochondrial outer membrane, single-molecule fluorescence
Introduction
Alzheimer's disease (AD) is the most prevalent neurodegenerative disorder and causes a gradual impairment in memory, learning, communication and behavioral function. Currently, the primary therapeutic strategy for AD is to use cholinesterase inhibitors (ChEIs) or an N-methyl-D-aspartate receptor (NMDAR) inhibitor to augment cholinergic neurotransmission. These inhibitors can improve the impaired cognition and behavioral function of patients. However, they only delay the progression of AD but are insufficient to reduce the neuronal damage or reverse the pathology of the disease1. To establish a novel disease-modifying therapy, much effort has been focused on elucidating the mechanisms of neuronal death in AD, which unveiled multiple pathogenic factors such as the production of aggregation-prone β-amyloid peptides (ie, Aβ42, Aβ40), the formation of fibrillar tangles of Tau and the polymorphism of APOE42. Based on the amyloid hypothesis, chemical drugs to reduce Aβ production or prevent their aggregation as well as antibodies to eliminate Aβ from the central nervous system have been developed in the past years3. Clinical trials on some of these agents have been completed; however, the outcomes were not encouraging.
Considering that AD pathology may be found in the brains of older persons without dementia4, and the carriers of familial AD-causative genes do not have any AD symptoms in early age, there must be endogenous factors capable of rescuing neuron cells from the death induced by AD-specific insults. In this regard, Hoshimoto et al identified a novel gene encoding a 24-residue peptide termed humanin (HN) by a death-trap screening with a cDNA library from an occipital lobe of an AD-patient5. HN not only protects neuron cells from the insult of Aβ peptides but also suppresses neuronal death induced by transfection of familial AD mutant genes6,7. Consistently, administration of a more potent HN derivative S14G-HN ameliorated the amnesia caused by scopolamine or Aβ in rodents8,9. S14G-HN also improved cognition and reduced Aβ accumulation in transgenic AD model mice10.
HN exerts its protective actions through receptors on the cell membrane and proteins in the cytoplasm. Upon HN binding, a pertussis toxin (PTX)-sensitive G protein-coupled receptor FPRL-1 has been shown to be activated, which induces calcium flux and ERK activation11,12. HN may also act on a trimeric receptor complex composed of CNTFR, WSX-1 and gp130. Stimulation of the receptor complex induces the activation of STAT313. In addition, insulin-like growth factor (IGF)-binding protein 3 (IGFBP-3) and three of the Bcl-2 family members (ie, Bax, Bid, Bim) have been shown to bind HN14,15,16,17. As Aβ stimulates astrocytes to release IGFBP-3, the protein level of IGFBP-3 has been shown to be highly up-regulated in the temporal cortices of AD patients18. Additionally, the mRNA level of Bax in the rat hippocampus was increased after Aβ42-induced neurotoxicity19. HN interacts with IGFBP-3 and these Bcl-2 family proteins to antagonize their pro-apoptotic activities.
Bcl-2 family proteins are central regulators of mitochondria-dependent apoptosis and are classified as anti-apoptotic proteins and pro-apoptotic proteins by their functions. Anti-apoptotic proteins such as Bcl-2, Bcl-xL and Mcl-1 have four Bcl-2 homology (BH) domains (ie, BH1–BH4). Pro-apoptotic proteins can be further classified as multi-domain proteins such as Bax, Bak and Bok (with BH1–BH3 domains), and BH3-only proteins such as Bid, Bim, Bad and Beclin 1. The integrity of the mitochondrial membrane is controlled primarily by a balance between the activities of anti-apoptotic and pro-apoptotic proteins.
Experiments with bax/bak doubly deficient murine embryonic fibroblasts showed that Bax and Bak serve as effectors for mitochondria-dependent apoptosis20. Most BH3-only proteins function as sensitizers of anti-apoptotic proteins, while Bid and Bim can also directly activate Bax and Bak. Activation of Bax and Bak is associated with their homo-oligomerization and pore formation in the mitochondrial outer membrane (MOM)21. Apoptogenic factors such as cytochrome c, AIF and SMAC/Diablo are released through Bax or Bak pores into the cytosol, which is followed by caspase activation and cell death.
Since HN was found to bind to Bax, Bid and Bim and attenuate the Bax-induced cytochrome c release from isolated mitochondria14, intracellular HN is proposed to prevent the translocation of Bax to the mitochondrial membrane and thus suppress apoptosis. However, the inhibitory mechanism of HN on the association of Bax with the membrane has not been fully explained. Here, by using total internal reflection fluorescence (TIRF) and Förster resonance energy transfer (FRET) techniques, we studied the interaction of HN with Bax and Bid in solution and in the membrane and how HN affects the translocation and oligomerization of Bax and Bid in the lipid membrane. Our study provides mechanistic insights into the neuroprotective activity of HN, which may help unveil the biological functions of HN and develop novel drug therapies for AD.
Materials and methods
Protein expression and purification
The expression plasmids for full-length Bax and Bid were constructed by ligating the cDNA of Bax or Bid between the Nde I/Sap I sites of pTYB1 or Nde I/BamH I sites of pET-15b, respectively. An intein tag was fused to the C-terminus of Bax. A His-tag was fused to the N-terminus of Bid, and the Tyr54-Asp60 residues of Bid were replaced with a tobacco etch virus protease (TEV) cleavage site (ie, DENLYFQ). To label the proteins with Cy3 or Cy5 fluorescent dye, we constructed expression plasmids for Bax (C126S) and Bid (C3S, C15S, C28S, S76C) mutants. E coli BL21 (DE3) containing the expression plasmid of Bax, Bax (C126S), Bid or Bid (C3S, C15S, C28S, S76C) were grown at 37 °C in LB culture medium until the OD600 reached 0.6–0.8. The protein expression of Bax or its mutant was induced with 0.4 mmol/L isopropyl β-D-1-thiogalactopyranoside (IPTG) at 30 °C for 3 h, whereas the protein expression of Bid or its mutant was induced with 1 mmol/L IPTG at 16 °C for 12 h. Cells were harvested by centrifugation and lysed by sonication on ice in lysis buffer (20 mmol/L Tris, 500 mmol/L NaCl, 1 mmol/L PMSF, pH 8.0). The cell extract was collected by centrifugation.
Cell extract containing Bax or its mutant Bax (C126S) was loaded onto chitin affinity resin (New England Biolabs Inc, Catalog No S6651L). The resin was washed with 20 column volumes of storage buffer (20 mmol/L Tris, 500 mmol/L NaCl, pH 8.0). After incubation with 50 mmol/L DTT at 16 °C for 40 h, the intein tag was removed. The protein was eluted with 5 column volumes of storage buffer and further purified by size-exclusion chromatography with Superdex 75 (GE Healthcare).
Bid or Bid (C3S, C15S, C28S, S76C) was loaded onto Ni-NTA resin (QIAGEN, Catalog No 30210). The resin was washed with 20 column volumes of wash buffer (5 mmol/L imidazole, 20 mmol/L Tris, 200 mmol/L NaCl, pH 8.0). The protein was then eluted with elution buffer (500 mmol/L imidazole, 20 mmol/L Tris, 200 mmol/L NaCl, pH 8.0). Further, Bid was purified by size-exclusion chromatography (Superdex 75) with storage buffer.
In apoptotic cells, full-length Bid was cleaved into p7 and p15 fragments by caspase-8 at Thr54-Asp60. The p7 and p15 fragments were bound together through a hydrophobic interaction to form cBid. To obtain cBid, Bid or the dye-labelled Bid mutant was cleaved by TEV at room temperature for 12 h, and the product was stored in the storage buffer above. The purification quality of the proteins was analyzed by SDS-PAGE (Supplementary Figure S1).
Protein labeling with Cy3 or Cy5 dye
Here, 1 mg/mL Bax (C126S) or Bid (C3S, C15S, C28S, S76C) was incubated with 100 M excess of TCEP and 10 M excess of Cy3 (GE Healthcare, PA23031) or Cy5 (GE Healthcare, PA25031) in PBS at 4 °C for 12 h. The unconjugated dye was removed by dialysis and size-exclusion chromatography (Superdex 75) with the storage buffer. The labeling efficiency (ie, [dye]/[protein]) was calculated by the absorbances at 280 nm (EBax: 39 580 M−1·cm−1, EBid: 9 970 M−1·cm−1), 550 nm (ECy3: 150 000 M−1·cm−1) and 649 nm (ECy5: 250 000 M−1·cm−1). The labeling efficiency for Bax (C126S) or cBid (C3S, C15S, C28S, S76C) was ∼60%.
Preparation of lipid bilayers
Coverslips (Fischer Scientific, Catalog No 12-548-5M) were cleaned with freshly prepared Piranha solution (3:1 mixture of sulfuric acid and 30% hydrogen peroxide) for 1 h at 95 °C. Then, the coverslips were rigorously washed with Milli-Q water and freeze-dried. A sample chamber was assembled according to the protocol as described in the reference22. Lipids consisting of 93% 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) (Avanti Polar Lipids, Catalog No 850355) and 7% cardiolipin (Sigma-Aldrich, Catalog No C0563) were prepared in chloroform. After evaporation with nitrogen, the lipids were hydrated in PBS to 10 mg/mL and treated with 10 cycles of freeze-thaw. Then, 1 μL of lipids was diluted to 400 μL with PBS and incubated in the sample chamber for one hour. The supported lipid bilayers were formed on the glass coverslip in the chamber. The sample chamber was washed with PBS three times to remove the excess lipids.
Single-molecule fluorescence measurement
For one hour, 100, 200, or 400 pmol/L Cy3-labelled Bax (C126S) or 2, 4, or 8 pmol/L Cy3-labelled cBid (C3S, C15S, C28S, S76C) was incubated with lipid bilayers in PBS containing 0.1 mg/mL BSA at room temperature. Here, 100 pmol/L Cy3-Bax (C126S) and 50 and 500 pmol/L cBid were incubated with lipid bilayers to determine whether cBid affected the translocation of Bax to the membrane. Then, 100 nmol/L HN was added to the above experiments to study the effect of HN on the translocation of Bax or cBid. The sample chamber was washed three times with PBS to remove the excess proteins. Imaging buffer (2 mmol/L Trolox, 165 U/mL glucose oxidase, 217 U/mL catalase, 0.4% β-D-glucose, in PBS, pH 7.4) was injected into the chamber before the single-molecule fluorescence measurement. All experiments were performed on an inverted microscope (Olympus IX81) with a 100×/1.49 oil objective (Olympus). The samples were first focused with 2 mW of a 532-nm laser from a laser power supply (CrystaLaser, CL-2005). Then, the samples were irradiated with 30 mW of a 532-nm laser, and the images were acquired by an EMCCD camera (iXonX3 Andor Technology) as described previously23. ImageJ (National Institutes of Health, version 1.46)24 was used to count the fluorescent spots (Supplementary Figure S2). The spots were selected with the “find maximal” function. The number of selected spots was shown with the “ROI manager” function. For photobleaching experiments, three data sets of 1000 frames (512×512 pixels) were collected with a time resolution of 100 ms per frame. Analysis of the photobleaching steps was performed using ImageJ and PIF software (version 1.1.2)25. The fluorescent spots were pre-selected in ImageJ. Appropriate parameters were chosen in PIF to select a comparable number of spots as in ImageJ (Supplementary Figure S2). Spots that were too close to one another or not bleached were abandoned automatically. As shown in Supplementary Figure S3, the trajectory of the selected spots was first analyzed by PIF. Then, the processed images of all the spots were visually checked, and the steps of the spots were counted manually (Supplementary Figure S4).
To determine whether HN could pull Bax or tBid out of the membrane, 100 pmol/L Cy3-Bax (C126S) and 500 pmol/L cBid or 2 pmol/L Cy3-cBid (C3S, C15S, C28S, S76C) was incubated with lipid bilayers in PBS containing 0.1 mg/mL BSA for one hour at room temperature. The sample chamber was washed with PBS three times to remove excess proteins. Then, the lipid bilayers were incubated with 100 nmol/L HN for one hour at room temperature. The samples were washed and imaged as above.
Förster resonance energy transfer experiment
For the FRET experiments, the Cy3-labelled protein was the donor, and the Cy5-labelled protein was the acceptor. Here, 0.5, 1, or 2 nmol/L Cy5-Bax (C126S) or 0.25, 0.5, or 1 nmol/L Cy5-cBid (C3S, C15S, C28S, S76C) was incubated with 1 nmol/L Cy3-labelled HN or Cy3 dye at 25 °C in PBS for 10 min; 0.5, 1, or 2 nmol/L Cy5-Bax (C126S) or 0.25, 0.5, or 1 nmol/L Cy5-cBid (C3S, C15S, C28S, S76C) was incubated with 1 mg/mL liposomes (93% DPPC+7% cardiolipin) at 25 °C in PBS for 30 min and then incubated with 1 nmol/L Cy3-HN (Sangon-Peptide Biotech Co, Ltd, China) or Cy3 dye at 25 °C in PBS for 10 min. The FRET efficiency was evaluated by measuring the fluorescence at 600 nm with the excitation at 532 nm on a microplate reader (TECAN, infinite M200 Pro). The fluorescence intensity of different samples containing the donor and the acceptor (FD+A), only donor (FD), only acceptor (FA) or without donor/acceptor (ie, F0) were measured. The FRET efficiency was evaluated by the values of (FD+A-FA)/(FD-F0), wherein (FD+A-FA) and (FD-F0) denote the emission of the donor with or without the accepter. If FRET exists, the absorption of the accepter will decrease FD+A-FA but not FD-F0; therefore, high FRET efficiency corresponds to low values of (FD+A-FA)/(FD-F0).
Then, 1 nmol/L Cy3-Bax (C126S) was incubated with 0.2 or 5 μmol/L HN, 4 nmol/L Cy5- cBid (C3S, C15S, C28S, S76C) and 1 mg/mL liposomes (93% DPPC+7% cardiolipin) in PBS for 4 h. The FRET efficiency was evaluated by measuring the Cy5 fluorescence at 670 nm with the excitation at 532 nm. If FRET exists, the fluorescence emission of Cy5 will increase.
Correction of the distribution with protein labeling efficiency
The number of protein molecules in the aggregates was determined by counting the photobleaching steps of the fluorescent spots. Ideally, all protein molecules were labelled with Cy3 (ie, the labeling efficiency was 100%). Under the intense irradiation of the 532-nm laser beam, one instance of fluorescence quenching would be observed with one Cy3-labelled molecule. Therefore, the number of the quenching events would be the number of molecules at the fluorescent spot. However, in reality, the Cy3-labeling efficiency is usually lower than 100%, which means that some molecules in the aggregates are possibly unlabeled (Supplementary Figure S5). Therefore, the photobleaching steps would not necessarily indicate the number of protein molecules. In our single-molecule fluorescence measurement experiments, only Cy3-labelled protein molecules were quenched by laser beam, whereas unlabeled protein molecules without fluorescence were ignored. Therefore, the number of photobleaching was indicative of the actual number of Cy3-labelled protein molecules. Considering that the possibility of a N-mer aggregate with m Cy3-labelled molecules follows a binomial distribution (equation 1).
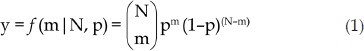 |
wherein p is the Cy3-labeling efficiency, N is the number of all molecules in the aggregate, m is the number of Cy3-labelled molecules in the N-mer aggregate (0≤m≤N) (please note that m can be zero because the aggregate may not contain any labelled molecules), the fractions of protein aggregates should follow the equation set 2, wherein h'k and hk denote the fraction of aggregates with k Cy3-labelled molecules and the fraction of k-mer aggregates, respectively (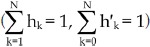 ).
).
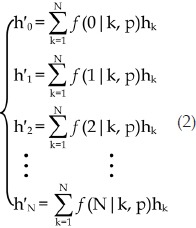 |
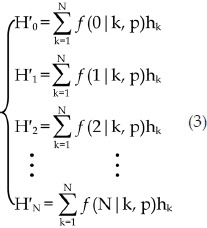 |
If we multiply both sides of equation 2 by the number of all protein aggregates, equation 3 can be derived, wherein H'k and Hk are the number of aggregates with k Cy3-labelled molecules and the number of k-mer aggregates, respectively. The number of all protein aggregates should be equal to 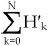 or
or 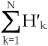 . Since through the photobleaching experiments we only know the number of aggregates with k Cy3-labelled molecules (H′k, 1≤k≤N), we may calculate Hk (1≤k≤N) by fitting H′k (0≤k≤N) with the equation 3, where
. Since through the photobleaching experiments we only know the number of aggregates with k Cy3-labelled molecules (H′k, 1≤k≤N), we may calculate Hk (1≤k≤N) by fitting H′k (0≤k≤N) with the equation 3, where 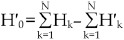 . Therefore, the corrected fraction of m-mer aggregates, hm, can be calculated with the equation of
. Therefore, the corrected fraction of m-mer aggregates, hm, can be calculated with the equation of 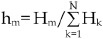 (1≤m≤N). The calculation was performed with the aid of an in-house computer program (Supplementary Figure S6).
(1≤m≤N). The calculation was performed with the aid of an in-house computer program (Supplementary Figure S6).
Results
Bax oligomerizes in lipid bilayers, while cBid prompts the process
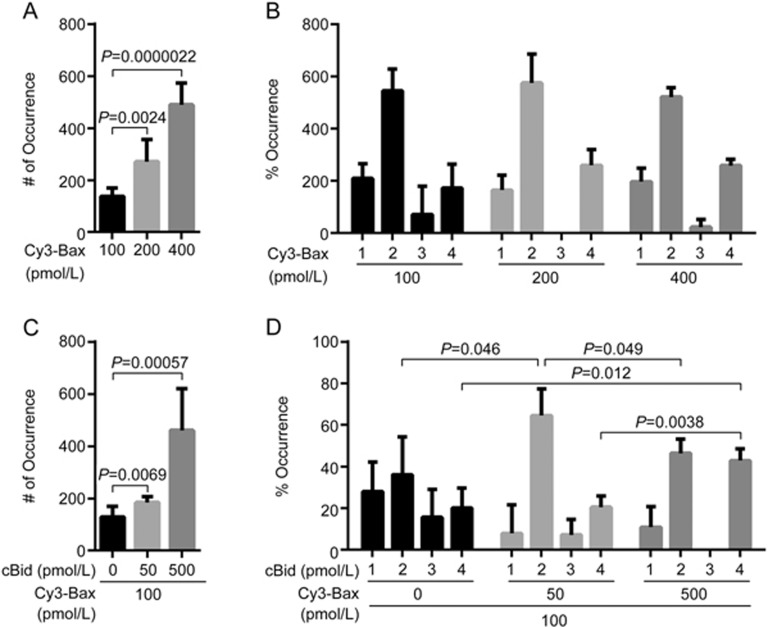
Bax constantly translocates to mitochondria and back to the cytosol in healthy cells26. In response to apoptotic stimuli, full-length Bid is cleaved by caspase-8 into cBid, which is composed of p7 and p15 fragments27. The two fragments remain bound together in solution. However, the longer p15 fragment, known as truncated Bid (tBid), is separated from the p7 fragment upon interaction with the mitochondrial membrane. The membrane-bound tBid recruits cytosolic Bax and induces a conformational change of Bax, leading to its insertion and oligomerization in the membrane28. Bax contains two cysteines, Cys62 and Cys126. As the membrane insertion regions of Bax are α5, α6 and α9 helices29, labeling of Cys126 at the α5 helix may interfere with Bax insertion in the membrane. To study the translocation and oligomerization of Bax in lipid bilayers with single-molecule fluorescence techniques, Cys126 was mutated to serine whereas Cys62 in the α2 helix was labelled with Cy3 dye. The circular dichroism (CD) spectra (Supplementary Figure S7), fluorescence polarization (FP) assays (Supplementary Figure S8) and liposome permeabilization assays (Supplementary Figure S9) showed that the mutation did not change the conformation of Bax, its affinity with HN or its function in liposome permeabilization. In the absence of cBid, the number of fluorescent spots of Cy3-labelled Bax (C126S) translocated to lipid bilayers increased in a concentration-dependent manner (Figure 1A). Bax (C126S) was mainly present as monomers, dimers and tetramers in the membrane (Figure 1B). In the presence of cBid, the number of fluorescent Cy3-Bax (C126S) spots in lipid bilayers increased along with the concentration of cBid (Figure 1C). Bax (C126S) was mainly present as dimers and tetramers. At 50 pmol/L of cBid, the proportion of Bax in dimers increased (P=0.046), while the tetrameric proportion remained unchanged. Notably, the proportion of Bax in dimers decreased (P=0.049), but the tetrameric proportion increased (P=0.0038) in the presence of 500 pmol/L cBid (Figure 1D). These observations suggest that the formation of Bax dimers is the rate-limiting step of its oligomerization. cBid prompts the translocation and oligomerization of Bax in the membrane.
Figure 1.
Translocation and oligomerization of Bax in lipid bilayers with or without cBid. (A) Numbers of fluorescent spots (# of Occurrence) in the images of 100, 200 and 400 pmol/L Cy3-labelled Bax (C126S) in DPPC lipid bilayers with cardiolipin (93% DPPC and 7% cardiolipin). Error bars indicate the standard errors, n=6. Data analyzed by an unpaired one-tailed t test. (B) Percentage of occurrence (% Occurrence) of different oligomeric species in the images (A). Error bars indicate the standard errors, n=3. (C) Numbers of fluorescent spots (# of Occurrence) in the images of 100 pmol/L Cy3-labelled Bax (C126S) with 50 and 500 pmol/L cBid in DPPC lipid bilayers with cardiolipin (93% DPPC and 7% cardiolipin). Error bars indicate the standard errors, n=6. Data analyzed by an unpaired one-tailed t test. (D) Percentage of occurrence (% Occurrence) of different oligomeric species in the images (C). Error bars indicate the standard errors, n=3. Data analyzed by an unpaired one-tailed t test.
HN inhibits the translocation of Bax to the membrane and its oligomerization
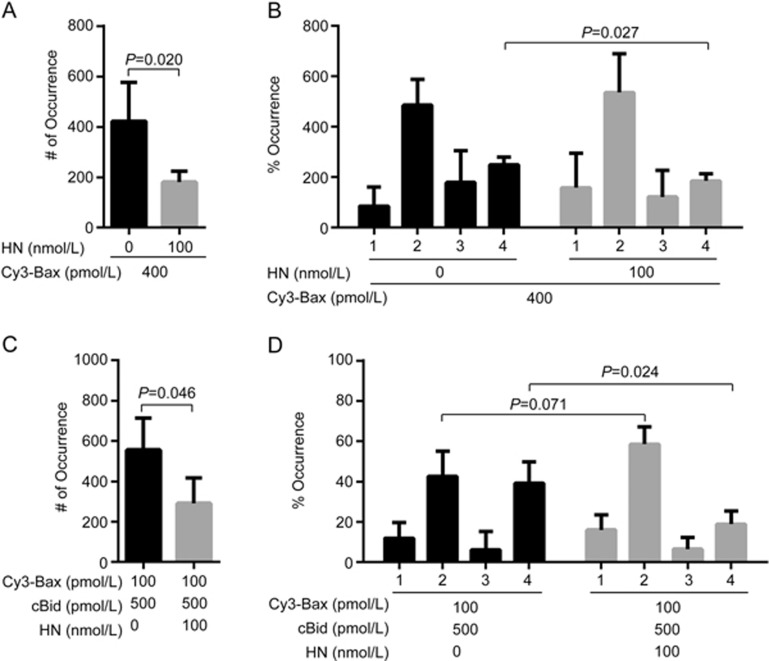
HN binds with Bax and suppresses apoptosis14. Since the oligomerization of Bax in MOM leads to cytochrome c release, we studied the effect of HN on Bax translocation and oligomerization in lipid bilayers. In the absence of cBid, HN decreased the number of Cy3-Bax (C126S) fluorescent spots translocated to lipid bilayers (P=0.020) (Figure 2A, Supplementary Figure S10), suggesting that HN inhibited the translocation of Bax (C126S). Analysis of the photobleaching steps of Cy3-Bax (C126S) in lipid bilayers showed that 100 nmol/L HN appeared to decrease the tetrameric proportion of Bax (C126S) (P=0.027) (Figure 2B). In the presence of 500 pmol/L cBid, 100 nmol/L HN also decreased the number of 100 pmol/L Cy3-Bax (C126S) fluorescent spots translocated to lipid bilayers (P=0.046) (Figure 2C, Supplementary Figure S10). The proportion of tetrameric Bax (C126S) in bilayers was significantly decreased by HN (P=0.024) (Figure 2D). Most Bax (C126S) was maintained in the dimeric form. Clearly, these results demonstrated that HN suppressed Bax translocation to lipid bilayers in the absence and presence of cBid. Meanwhile, HN inhibited the oligomerization of Bax in the membrane.
Figure 2.
HN inhibits the translocation and oligomerization of Bax incubated with or without cBid. (A) Numbers of fluorescent spots (# of Occurrence) in the images of 400 pmol/L Cy3-labelled Bax (C126S) incubated with 100 nmol/L HN in DPPC lipid bilayers with cardiolipin (93% DPPC and 7% cardiolipin). Error bars indicate the standard errors, n=6. Data analyzed by an unpaired one-tailed t test. (B) Percentage of occurrence (% Occurrence) of different oligomeric species in the images (A). Error bars indicate the standard errors, n=3. Data analyzed by an unpaired one-tailed t test. (C) Numbers of fluorescent spots (# of Occurrence) in the images of 100 pmol/L Cy3-labelled Bax (C126S) with 500 pmol/L cBid and 100 nmol/L HN in DPPC lipid bilayers with cardiolipin (93% DPPC and 7% cardiolipin). Error bars indicate the standard errors, n=6. Data analyzed by an unpaired one-tailed t test. (D) Percentage of occurrence (% Occurrence) of different oligomeric species in the images (C). Error bars indicate the standard errors, n=3. Data analyzed by an unpaired one-tailed t test.
HN inhibits the translocation of tBid to the membrane and its oligomerization
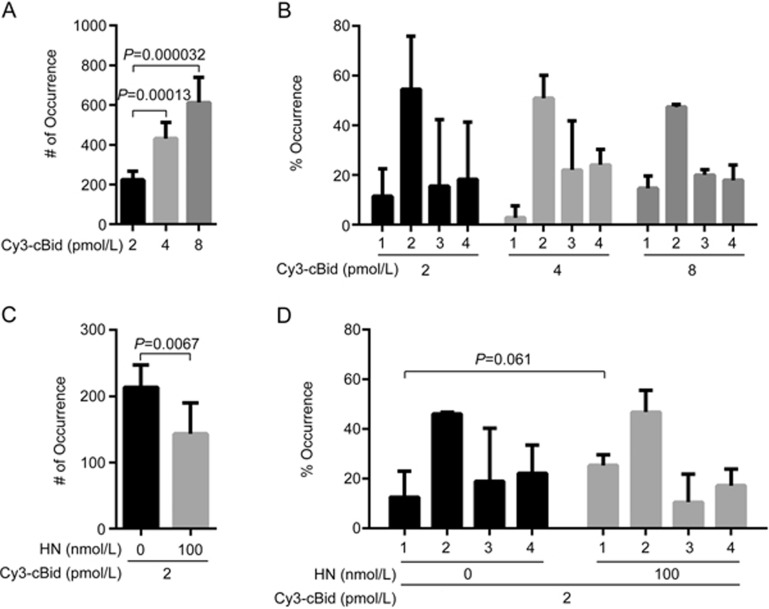
Membrane-bound tBid interacts with cytosolic Bax and initiates Bax pore formation in the membrane28. A tBid mutant that was unable to induce the dimerization of either Bax or Bak was found to form homotrimers in a variety of cell lines and induce apoptosis, suggesting that tBid itself can oligomerize in the MOM and induce cytochrome c release30. Thus, we further explored the effect of HN on the translocation and oligomerization of tBid in lipid bilayers. Wild-type cBid contains three cysteines at the p7 fragment (ie, Cys3, Cys15 and Cys28). As the membrane insertion regions of cBid are α4-α7 helices at the p15 fragment31, mutations of Cys3, Cys15 and Cys28 to serine and Ser76 to cysteine at the α2-α3 loop of the p15 fragment should not interfere with tBid insertion in the membrane. Therefore, the residues of Cys3, Cys15 and Cys28 were mutated to serine. Ser76 at the p15 fragment was mutated to cysteine so that it could be labelled with the Cy3 dye. The CD spectra (Supplementary Figure S7), FP assays (Supplementary Figure S8) and liposome permeabilization assays (Supplementary Figure S9) showed that the mutations did not change the conformation of Bid, its affinity with HN or its function in liposome permeabilization. In the absence of HN, the number of Cy3-labelled cBid (C3S, C15S, C28S, S76C) fluorescent spots translocated to lipid bilayers increased in a concentration-dependent manner (Figure 3A, Supplementary Figure S11). cBid (C3S, C15S, C28S, S76C) was present as monomers, dimers, trimers and tetramers in the membrane (Figure 3B). HN at 100 nmol/L decreased the number of 2 pmol/L Cy3-cBid (C3S, C15S, C28S, S76C) fluorescent spots in lipid bilayers (P=0.0067) (Figure 3C, Supplementary Figure S11). The proportion of tBid monomers in lipid bilayers was increased by HN (P=0.061) (Figure 3D). These data revealed that HN inhibits the translocation of cBid and its oligomerization in the membrane.
Figure 3.
HN inhibits the translocation and oligomerization of cBid in lipid bilayers. (A) Numbers of fluorescent spots (# of Occurrence) in the images of 2, 4 and 8 pmol/L Cy3-labelled cBid (C3S, C15S, C28S, S76C) in DPPC lipid bilayers with cardiolipin (93% DPPC and 7% cardiolipin). Error bars indicate the standard errors, n=6. Data analyzed by an unpaired one-tailed t test. (B) Percentage of occurrence (% Occurrence) of different oligomeric species in the images (A). Error bars indicate the standard errors, n=3. (C) Numbers of fluorescent spots (# of Occurrence) in the images of 2 pmol/L Cy3-labelled cBid (C3S, C15S, C28S, S76C) with 100 nmol/L HN in DPPC lipid bilayers with cardiolipin (93% DPPC and 7% cardiolipin). Error bars indicate the standard errors, n=6. Data analyzed by an unpaired one-tailed t test. (D) Percentage of occurrence (% Occurrence) of different oligomeric species in the images (C). Error bars indicate the standard errors, n=3. Data analyzed by an unpaired one-tailed t test.
HN binds to Bax and cBid in solution and in the membrane
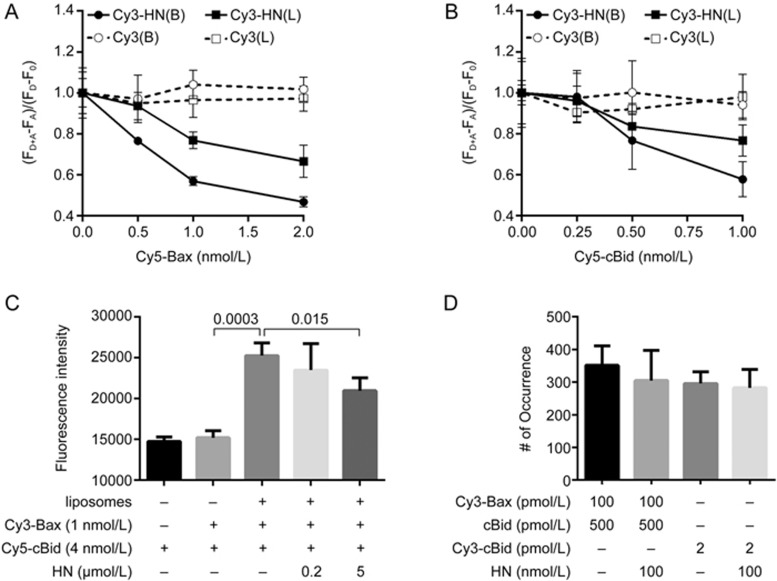
Previous fluorescence polarization (FP) assays showed that HN binds Bax or Bid in solution14,15. To verify the interactions by using the FRET technique, we labelled HN with Cy3 dye. The amino acid sequence of HN is MAPRGFSCLLLLTSEIDLPVKRRA. As the Pro3-Pro19 residues are the neuroprotective core domain, and Cys8 is critical for its interaction with Bax or Bid14,15,32, the only lysine at the C-terminus of HN (ie, Lys21) was labelled with Cy3. Cy5-Bax (C126S) was incubated with Cy3-labelled HN in the absence of liposomes. Consistent with the FP assay, we observed the FRET between Cy5-Bax (C126S) and Cy3-HN. (FD+A-FA)/(FD-F0) decreased along with the increasing concentration of Cy5-Bax (C126S) (Figure 4A). This result also proved that the Lys21 labeling did not affect the interaction of HN with Bax. Similarly, Cy5-labelled cBid (C3S, C15S, C28S, S76C) was incubated with Cy3-HN in the absence of liposomes. We observed the FRET between Cy5-cBid (C3S, C15S, C28S, S76C) and Cy3-HN as well. (FD+A-FA)/(FD-F0) decreased along with the increasing concentration of Cy5-cBid (C3S, C15S, C28S, S76C) (Figure 4B). Furthermore, we examined the binding activity of HN to the membrane-bound Bax or tBid. In vitro, Bax may associate with liposomes in the presence of cardiolipin33. Flotation assays showed that liposomes alone do not cause the membrane insertion of Bax34. In contrast, the interaction of cBid with liposomes triggers the separation of p15 (tBid) and p7 fragments. tBid fully binds to liposomes and inserts into membranes31. To ensure the membrane-bound efficiency of Bax or cBid, 2 nmol/L Cy5-Bax (C126S) or Cy5-cBid (C3S, C15S, C28S, S76C) was incubated with 1 mg/mL liposomes. The solution was separated from the liposomes by ultrafiltration. We measured the fluorescence of the liposomes and the filtrate at 670 nm (ie, the Cy5 fluorescence) and found that the membrane-bound efficiency was ∼99% (Supplementary Figure S12). This proved that Cy5-Bax (C126S) and Cy5-cBid (C3S, C15S, C28S, S76C) associate with the liposomes at this concentration. After that, 1 nmol/L of Cy3-HN was incubated with various concentrations of Cy5-Bax (C126S) or Cy5-labelled tBid-bound liposomes. The samples were irradiated with 532 nm of light. (FD+A-FA)/(FD-F0) decreased with the increasing concentrations of the Cy5-labelled proteins (Figure 4A, 4B), showing that HN also binds Bax or tBid in liposomes. To further confirm that the FRET between HN and Bax or tBid was due to the specific interaction of HN with Bax or tBid, we used 1 nmol/L of the Cy3 dye instead of Cy3-HN as the control. We incubated Cy5-Bax (C126S) or Cy5-cBid (C3S, C15S, C28S, S76C) with the Cy3 dye in the absence or presence of liposomes. We did not observe FRET between Cy5-Bax (C126S) and the Cy3 dye nor between Cy5-cBid (C3S, C15S, C28S, S76C) and the Cy3 dye. Clearly, this demonstrated that the FRET between HN and Bax or tBid truly reflects a specific interaction of HN with the proteins.
Figure 4.
HN interacts with membrane-bound Bax or tBid and inhibits the interaction between Bax and tBid in the membrane. (A) Cy5-labelled Bax (C126S) at 0.5, 1, or 2 nmol/L or membrane-bound Cy5-labelled Bax (C126S) was incubated with 1 nmol/L Cy3-labelled HN or Cy3 dye. In the presence of Cy3-labelled HN or Cy3 dye, FD+A and FD denote the fluorescence of Cy3 at 600 nm with or without Cy5-Bax (C126S). In the absence of Cy3-labelled HN or Cy3 dye, FA and F0 denote the fluorescence of the background at 600 nm with or without Cy5-Bax (C126S). (FD+A-FA) and (FD-F0) denote the emission of Cy3-labelled HN or Cy3 dye at 600 nm with or without Cy5-Bax (C126S). The samples in the buffer were labelled with (B), whereas the samples in liposomes were labelled with (L). Error bars indicate the standard errors, n=3. (B) Cy5-labelled cBid (C3S, C15S, C28S, S76C) at 0.25, 0.5, or 1 nmol/L or membrane-bound Cy5-labelled cBid (C3S, C15S, C28S, S76C) was incubated with 1 nmol/L Cy3-labelled HN or Cy3 dye. In the presence of Cy3-labelled HN or Cy3 dye, FD+A and FD denote the fluorescence of Cy3 at 600 nm with or without Cy5-labelled cBid (C3S, C15S, C28S, S76C). In the absence of Cy3-labelled HN or Cy3 dye, FA and F0 denote the fluorescence of the background at 600 nm with or without Cy5-Bax (C126S). (FD+A-FA) and (FD-F0) denote the emission of Cy3-labelled HN or Cy3 dye at 600 nm with or without Cy5-labelled cBid (C3S, C15S, C28S, S76C). The samples in the buffer were labelled with (B), whereas the samples in liposomes were labelled with (L). Error bars indicate the standard errors, n=3. (C) Cy3-labelled Bax (C126S) at 1 nmol/L was incubated with 0.2 or 5 μmol/L HN, 4 nmol/L Cy5-labelled cBid (C3S, C15S, C28S, S76C) and 1 mg/mL liposomes (93% DPPC and 7% cardiolipin). The fluorescence was measure at the Cy3 excitation wavelength of 532 nm and the Cy5 emission wavelength of 670 nm. Error bars indicate the standard errors, n=3. Data analyzed by an unpaired one-tailed t test. (D) Numbers of fluorescent spots (# of Occurrence) in the images of 100 pmol/L Cy3-labelled Bax (C126S) and 500 pmol/L cBid or 2 pmol/L Cy3-labelled cBid (C3S, C15S, C28S, S76C) in DPPC lipid bilayers with cardiolipin (93% DPPC and 7% cardiolipin) with or without incubation with 100 nmol/L HN. Error bars indicate the standard errors, n=6.
HN prevents the interaction of tBid and Bax in liposomes
Membrane-bound tBid can recruit cytosolic Bax to the MOM. As shown above, HN binds with Bax and tBid in solution and in the membrane. Naturally, we asked whether HN can affect the interaction of Bax by being recruited and activated by tBid and Bax. To answer this question, we incubated 1 nmol/L Cy3- Bax (C126S) with 1 mg/mL and 4 nmol/L Cy5-cBid (C3S, C15S, C28S, S76C) liposomes in the absence or presence of HN. The samples were irradiated with Cy3 excitation light at 532 nm. If Cy5-cBid (C3S, C15S, C28S, S76C) binds with Cy3-Bax (C126S), we would observe the fluorescence emission of Cy5 at 670 nm due to the FRET between Cy3-Cy5. As shown in Figure 4C, cBid (C3S, C15S, C28S, S76C) did not bind with Bax (C126S) in the absence of liposomes. The addition of liposomes significantly increased the Cy5 fluorescence (P=0.0003), indicating that cBid (C3S, C15S, C28S, S76C) binds with Bax (C126S) in the membrane. In contrast, the Cy5 fluorescence decreased with increasing concentrations of HN (P=0.015). These results revealed that HN prevents the interaction of Bax and tBid in liposomes.
HN cannot pull Bax or tBid out of the membrane
Bcl-xL is an anti-apoptotic protein that can retrotranslocate Bax from the mitochondria into the cytosol26. Consistently, our previous studies showed that Bcl-xL may translocate Bax from lipid bilayers to solution23. Subburaj et al also demonstrated that Bcl-xL is able to disassemble Bax oligomers and decrease the proportion of Bax tetramer in the lipid bilayers35. As HN interacts with Bax and tBid in the membrane (Figure 4A, 4B), we further investigated whether, similar to Bcl-xL, HN could pull Bax or cBid out of the lipid bilayers. Here, 100 pmol/L Cy3-Bax (C126S) and 500 pmol/L cBid or 2 pmol/L Cy3-cBid (C3S, C15S, C28S, S76C) was incubated with lipid bilayers. Then, the membrane-bound protein was incubated with or without 100 nmol/L HN. As shown in Figure 4D, the number of Cy3-Bax (C126S) or Cy3-cBid (C3S, C15S, C28S, S76C) fluorescent spots with or without HN was not significantly different, indicating that HN cannot pull Bax and tBid out of the membrane.
Discussion
HN is an endogenous cytosolic peptide that is able to protect neuron cells from death induced by Aβ peptide insult or transfection of familial AD mutant genes. Co-immunoprecipitation (co-IP) experiments and fluorescence polarization assays showed that HN binds Bax or Bid. HN suppressed the cytochrome c release induced by Bax but not by Bak14. To investigate the Bax-related neuroprotection mechanism of HN, we simulated the mitochondrial membrane environment with lipid bilayers consisting of 93% DPPC and 7% cardiolipin. By using single-molecule fluorescence techniques, we studied the effects of HN on the association of Bax or Bid with lipid bilayers and their oligomerization in the membrane.
Bax is a key regulator of apoptosis that presents mainly in the cytosol of healthy cells36. It dynamically translocates to the MOM and back to the cytosol. tBid initiates Bax pore formation in the MOM of apoptotic cells. Our photobleaching experiments showed that Bax presents as monomers, dimers and tetramers in lipid bilayers, while tBid enhances its membrane association and tetramerization. The distribution of Bax oligomers revealed by our studies coincides with the double electron-electron resonance (DEER) experiments showing that membrane-embedded Bax is organized as assemblies of dimers37. We also found that HN suppressed the self-association and tBid-activated association of Bax with lipid bilayers. Meanwhile, the proportion of Bax tetramer in the bilayers was reduced by HN. Consistently, previous studies proved that most of the Bax protein remained in the cytosol of SF268 cells, which contain high levels of endogenous HN. When the expression of HN was knocked down, staurosporine-induced Bax translocation to the membrane was enhanced, indicating that HN suppresses the translocation of Bax to mitochondria14.
In addition, our results showed that there were more 8 pmol/L Cy3-cBid fluorescent spots in lipid bilayers than 400 pmol/L Cy3-Bax spots (Figure 1A, 3A), suggesting that tBid bound to the lipid membrane more efficiently than Bax. Since tBid is separated from the p7 fragment of cBid upon interaction with the lipid membrane and can interact with Bax and activate its translocation to the membrane, the easy association of tBid with the membrane should facilitate the activation of Bax. tBid at such concentrations presented as monomers, dimers, trimers and tetramers in the bilayers. Previous confocal experiments showed that the mobile species of tBid on the membrane were monomers to trimers, whereas the membrane-inserted tBid was mainly distributed as tetramers and higher oligomers38. Our experiments showed that HN inhibited cBid translocation to lipid bilayers. HN increased the monomeric proportion of tBid, suggesting that HN inhibits the oligomerization and insertion of tBid in the membrane.
The inhibitory effect of HN on the oligomerization of Bax and tBid revealed by our single-molecule studies is also in line with previous Western-blot and co-IP experiment results. Zhai et al incubated tBid, Bax, and HN with isolated mitochondria15. The mitochondrial membranes were then solubilized in 2% CHAPS. The oligomerization of Bax in membranes was analyzed by gel-sieve chromatography and Western-blot, showing that tBid promotes Bax oligomerization, whereas HN inhibits the tBid-induced oligomerization as well as the self-oligomerization of Bax. In addition, GFP or GFP-fused HN were expressed together with myc-tagged Bid or myc-tagged tBid in HEK293T cells. The Co-IP results showed that GFP-fused HN bound to both Bid and tBid, and the interaction inhibited the self-oligomerization of tBid.
In addition, consistent with previous FP assays14,15, our FRET experiments showed that HN bound Bax and cBid in solution. The binding of HN with Bax or cBid should constrain the proteins to latent conformations14 and suppress their association with the membrane. In addition, we found that HN bound to Bax or tBid in the membrane. However, HN could not pull the proteins out of the membrane.
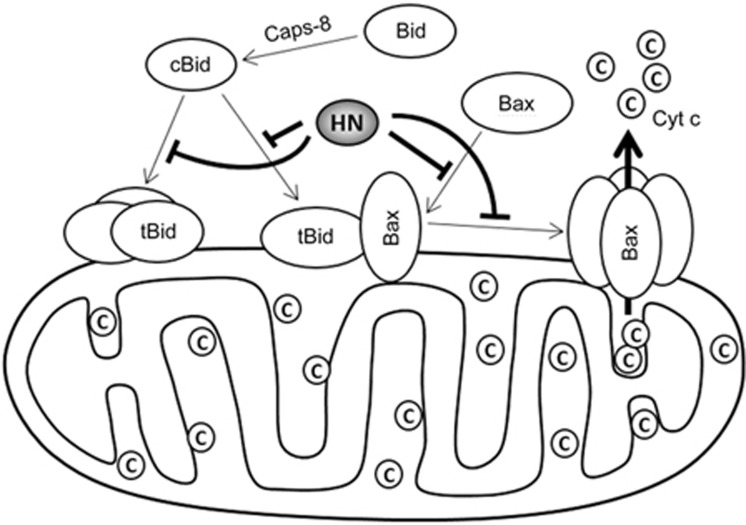
Based on the above results, we propose a mechanism by which HN suppresses the Bax-induced cytochrome c release (Figure 5). In apoptotic cells, Bid is truncated by caspase-8 into cBid, which can readily translocate to the MOM. The BH3 domain of tBid in membrane-bound conformations remains accessible to aqueous solution, whereas the α4-α7 helices are inserted into membrane31. The α2-α5 helices of membrane-bound Bax, which contain the BH3 domain, are also above the membrane37. The BH3 domain of membrane-bound tBid or Bax may interact with cytosolic Bax39,40,41,42, inducing its conformational change and association with the membrane. Our experiments and the FP assays showed that HN binds to Bax or Bid in solution. NMR chemical shift mapping experiments demonstrated that HN-derived peptides bind to a region at the surface of Bid, which includes residues from the BH3 domain43. Such interactions should restrain cBid or Bax to inactive conformations and prevent their association with the membrane. In addition, HN also interacts with membrane-bound Bax and tBid. As HN not only inhibits the self-oligomerization but also the tBid-activated oligomerization of Bax in the membrane and the BH3 domain, and the α9 helix of membrane-bound Bax are two interfaces for its homo-oligomerization29,37,44,45, HN likely binds at the BH3 domains of Bax and tBid to prevent the recruitment and assembly of Bax in the membrane. In summary, HN suppresses Bax pore formation through two mechanisms. On one hand, HN directly binds to Bax and cBid in solution and inhibits their conformational change and translocation to the membrane. On the other hand, HN interacts with membrane-bound Bax and tBid, preventing the recruitment of cytosolic Bax and its oligomerization in the membrane.
Figure 5.
Inhibitory mechanism of HN on Bax-induced cytochrome c release.
Author contribution
Ze-wei MA performed the experiments, analyzed the data and wrote the paper; Dong-xiang LIU designed the project and wrote the paper.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Grant 21472206).
Footnotes
Supplementary information is available on the website of Acta Pharmacologica Sinica.
Supplementary Information
Supplementary Figure S1–S12
References
- Larson EB, Kukull WA, Katzman RL. Cognitive impairment: dementia and Alzheimer's disease. Annu Rev Public Health 1992; 13: 431–49. [DOI] [PubMed] [Google Scholar]
- Peskind ER. Neurobiology of Alzheimer's disease. J Clin Psychiatry 1996; 57: 5–8. [PubMed] [Google Scholar]
- Panza F, Solfrizzi V, Frisardi V, Imbimbo BP, Capurso C, D'Introno A, et al. Beyond the neurotransmitter-focused approach in treating Alzheimer's disease: drugs targeting beta-amyloid and tau protein. Aging Clin Exp Res 2009; 21: 386–406. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006; 66: 1837–44. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Ito Y, Niikura T, Shao Z, Hata M, Oyama F, et al. Mechanisms of neuroprotection by a novel rescue factor humanin from Swedish mutant amyloid precursor protein. Biochem Biophys Res Commun 2001; 283: 460–8. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci U S A 2001; 98: 6336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, et al. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer's disease-relevant insults. J Neurosci 2001; 21: 9235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya T, Ukai M. [Gly(14)]-Humanin improved the learning and memory impairment induced by scopolamine in vivo. Br J Pharmacol 2001; 134: 1597–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima H, Kawasumi M, Chiba T, Yamada M, Yamashita K, Nawa M, et al. A humanin derivative, S14G-HN, prevents amyloid-beta-induced memory impairment in mice. J Neurosci Res 2005; 79: 714–23. [DOI] [PubMed] [Google Scholar]
- Niikura T, Sidahmed E, Hirata-Fukae C, Aisen PS, Matsuoka Y. A humanin derivative reduces amyloid beta accumulation and ameliorates memory deficit in triple transgenic mice. PLoS One 2011; 6: e16259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Habata Y, Hosoya M, Nishi K, Fujii R, Kobayashi M, et al. N-Formylated humanin activates both formyl peptide receptor-like 1 and 2. Biochem Biophys Res Commun 2004; 324: 255–61. [DOI] [PubMed] [Google Scholar]
- Ying G, Iribarren P, Zhou Y, Gong W, Zhang N, Yu ZX, et al. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J Immunol 2004; 172: 7078–85. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol Biol Cell 2009; 20: 2864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 2003; 423: 456–61. [DOI] [PubMed] [Google Scholar]
- Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J Biol Chem 2005; 280: 15815–24. [DOI] [PubMed] [Google Scholar]
- Luciano F, Zhai D, Zhu X, Bailly-Maitre B, Ricci JE, Satterthwait AC, et al. Cytoprotective peptide humanin binds and inhibits proapoptotic Bcl-2/Bax family protein BimEL. J Biol Chem 2005; 280: 15825–35. [DOI] [PubMed] [Google Scholar]
- Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, et al. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci U S A 2003; 100: 13042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Uemura K, Asada M, Maesako M, Akiyama H, Shimohama S, et al. The participation of insulin-like growth factor-binding protein 3 released by astrocytes in the pathology of Alzheimer's disease. Mol Brain 2015; 8: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin A, Soddu E, Turunc Bayrakdar E, Uyanikgil Y, Kanit L, Armagan G, et al. Neuroprotective effects of engineered polymeric nasal microspheres containing hydroxypropyl-beta-cyclodextrin on beta-Amyloid (1-42)-induced toxicity. J Pharm Sci 2016; 105: 2372–80. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001; 292: 727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 2000; 7: 1166–73. [DOI] [PubMed] [Google Scholar]
- Roy R, Hohng S, Ha T. A practical guide to single-molecule FRET. Nat Methods 2008; 5: 507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Yang J, Liu D. Integration and oligomerization of Bax protein in lipid bilayers characterized by single molecule fluorescence study. J Biol Chem 2014; 289: 31708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbury CL. Data analysis for total internal reflection fluorescence microscopy. Cold Spring Harb Protoc 2016; 2016: pdb.prot085571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire H, Aurousseau MR, Bowie D, Blunck R. Automating single subunit counting of membrane proteins in mammalian cells. J Biol Chem 2012; 287: 35912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, et al. Bcl-xL retrotranslocates Bax from the mitochondria into the cytosol. Cell 2011; 145: 104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu C J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94: 491–501. [DOI] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 2008; 135: 1074–84. [DOI] [PubMed] [Google Scholar]
- Gahl RF, He Y, Yu S, Tjandra N. Conformational rearrangements in the pro-apoptotic protein, Bax, as it inserts into mitochondria: a cellular death switch. J Biol Chem 2014; 289: 32871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg M, Sarig R, Zaltsman Y, Frumkin D, Grammatikakis N, Reuveny E, et al. tBID Homooligomerizes in the mitochondrial membrane to induce apoptosis. J Biol Chem 2002; 277: 12237–45. [DOI] [PubMed] [Google Scholar]
- Shamas-Din A, Bindner S, Zhu W, Zaltsman Y, Campbell C, Gross A, et al. tBid undergoes multiple conformational changes at the membrane required for Bax activation. J Biol Chem 2013; 288: 22111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Hashimoto Y, Niikura T, Nishimoto I. Identification of essential amino acids in Humanin, a neuroprotective factor against Alzheimer's disease-relevant insults. Peptides 2003; 24: 585–95. [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande S, Montessuit S, Martinou JC. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ 2008; 15: 929–37. [DOI] [PubMed] [Google Scholar]
- Yethon JA, Epand RF, Leber B, Epand RM, Andrews DW. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J Biol Chem 2003; 278: 48935–41. [DOI] [PubMed] [Google Scholar]
- Subburaj Y, Cosentino K, Axmann M, Pedrueza-Villalmanzo E, Hermann E, Bleicken S, et al. Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nat Commun 2015; 6: 8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem 1998; 273: 10777–83. [DOI] [PubMed] [Google Scholar]
- Bleicken S, Jeschke G, Stegmueller C, Salvador-Gallego R, García-Sáez AJ, Bordignon E. Structural model of active Bax at the membrane. Mol Cell 2014; 56: 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar S, Kurylowicz M, Hirmiz N, Manan Y, Friaa O, Shamas-Din A, et al. The proapoptotic protein tBid forms both superficially bound and membrane-inserted oligomers. Biophys J 2014; 106: 2085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C, Dlugosz PJ, Peng J, Zhang Z, Lapolla SM, Plafker SM, et al. Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J Biol Chem 2006; 281: 14764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Zhang J, O'Neill KL, Gurumurthy CB, Quadros RM, Tu Y, et al. Cleavage by caspase 8 and mitochondrial membrane association activate the BH3-only protein Bid during TRAIL-induced apoptosis. J Biol Chem 2016; 291: 11843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez F, Pariselli F, Jalmar O, Dupaigne P, Sureau F, Dellinger M, et al. Mechanistic issues of the interaction of the hairpin-forming domain of tBid with mitochondrial cardiolipin. PLoS One 2010; 5: e9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockings C, Anwari K, Ninnis RL, Brouwer J, O'Hely M, Evangelista M, et al. Bid chimeras indicate that most BH3-only proteins can directly activate Bak and Bax, and show no preference for Bak versus Bax. Cell Death Dis 2015; 6: e1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Zhai D, Zhou X, Satterthwait A, Reed JC, Marassi FM. Mapping the specific cytoprotective interaction of humanin with the pro-apoptotic protein Bid. Chem Biol Drug Des 2007; 70: 383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar P, Westphal D, Dewson G, Ma S, Hockings C, Fairlie W, et al. Bax crystal structures reveal how BH3 domains activate Bax and Nucleate its oligomerization to induce apoptosis. Cell 2013; 152: 519–31. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Subramaniam S, Kale J, Liao C, Huang B, Brahmbhatt H, et al. BH3-in-groove dimerization initiates and helix 9 dimerization expands Bax pore assembly in membranes. EMBO J 2016; 35: 208–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1–S12