Abstract
Osteoarthritis (OA) affects elderly population worldwide and endoplasmic reticulum (ER) stress is known to be positively correlated with OA development. Previous reports prove the cytoprotective effects of baicalin on chondrocytes, whereas the mechanisms are hardly reported. Hence, we aimed to investigate the links between OA, ER stress, and baicalin. Chondrocytes from patients with OA were subjected to H2O2 treatment with or without baicalin pretreatment, and cell viability was assessed via Cell Counting Kit-8. Messenger RNA (mRNA) amounts of apoptosis-related genes (Bax, Bcl-2, and Caspase-3), extracellular matrix (ECM)-related genes (Collange I, Collange II, Aggrecan, and Sox9) and ER stress hallmarks (binding immunoglobulin protein [BiP] C/EBP homologous protein [CHOP]) were evaluated via quantitative real-time PCR. Bax, Bcl-2, BiP, and CHOP protein levels were analyzed via Western blot. Baicalin suppressed the changes in cell viability and apoptosis-related gene expressions caused by H2O2. Reactive oxygen species and glutathione/oxidized glutathione assay showed that H2O2 enhanced oxidative stress. Baicalin suppressed H2O2-induced downregulation of mRNA expression of ECM-related genes. Moreover, baicalin reduced H2O2-stimulated increase in oxidative stress and the expression of ER stress hallmarks. Endoplasmic reticulum stress inducer abolished the protective activities, whereas ER stress inhibitor did not exhibit extra protective effects. Baicalin pretreatment protected patient-derived chondrocytes from H2O2 through ER stress inhibition.
Keywords: baicalin, osteoarthritis, H2O2, ER stress, chondrocytes from OA patients
Introduction
Osteoarthritis (OA), a prevalent degenerative disease, is often caused by heredity, aging, overweight, or other factors.1–3 Chondrocyte loss and progressive cartilage degradation are proved to be the key features of OA.4 Chondrocytes, the only cells in articular cartilage (AC), are in charge of maintaining the homeostasis of AC via modulating extracellular matrix (ECM) anabolism and catabolism.5 Because of the positive relationship between the severity of OA and the degree of chondrocyte apoptosis, preventing chondrocyte apoptosis is regarded to be a powerful way to relieve OA.
Medication treatment is one of the common therapeutic strategies for OA. In recent year, traditional Chinese herbs have been demonstrated to possess high clinical value because of their powerful treatment effects and minimal general toxicity. Baicalin, a principal flavonoid mainly isolated from roots of Scutellaria baicalensis, has been proved to possess various functions, such as anti-inflammation, anti-infection, antitumor, and antioxidant activities.6–9 Moreover, increasing literature studies proved that baicalin administration had anti-OA roles. For example, Chen et al demonstrated that baicalin exhibited protective activities in both human OA chondrocytes and OA animal model.1 Yang et al showed that baicalin protected CHON-001 cells from inflammation.10 Pan et al indicated that oxidative stress was an important factor during OA progress and baicalin protected chondrocytes from oxidative stress caused via H2O2.11 Nevertheless, the related mechanisms are still unclear.
Endoplasmic reticulum (ER) stress is known to have major effects during OA progress. Hence, we wonder whether the anti-OA activities of baicalin are mediated by decreasing ER stress. To answer the above question, chondrocytes from patients with OA were treated via H2O2 with or without baicalin pretreatment. Chondrocyte viability, reactive oxygen species (ROS) level, glutathione/oxidized glutathione (GSH/GSSG) ratio, expressions of apoptosis-related genes (Bax, Bcl-2, and Caspase-3) and ER stress hallmarks (binding immunoglobulin protein, BiP; and C/EBP homologous protein, CHOP), and messenger RNA (mRNA) levels of ECM-related genes (Collange I, Collange II, Aggrecan, and Sox9) were analyzed. To our delight, baicalin has cytoprotective effects on H2O2-treated human OA chondrocytes, which was consistent with previous literature studies. Moreover, the above experiment data also suggested that baicalin’s protection may be linked to the suppression of ER stress. Subsequently, tauroursodeoxycholic acid dihydrate (TUDCA) or tunicamycin (Tm), the inhibitor and inducer of ER stress, was used in combination with baicalin before H2O2 treatment. Cell Counting Kit-8 (CCK-8) and Western blot assay further confirmed the direct suppressive activities of baicalin against ER stress.
Methods and Materials
Ethical Statement
The experimental protocols were approved by the Ethics Committee of Tianjin Hospital. Cartilages of patients with OA were collected after knee replacement surgery and all patients provided informed written consent.
Osteoarthritis Patient-Derived Chondrocyte Culture
The culture of human OA chondrocytes was performed via a previously reported method.12 We subdivided chondrocytes into 5 groups: control group, H2O2 group (incubation with 0.5 mM H2O2 for 4 hours at day 4), H2O2 + baicalin group (pretreatment via 100 μM baicalin for 1 day before H2O2 administration), H2O2 + baicalin + Tm group (pretreatment with 2.5 μg/mL Tm and 100 μM baicalin for 1 day before H2O2 treatment) and H2O2 + baicalin + TUDCA group (pretreatment with 100 μM TUDCA and 100 μM baicalin for 1 day before H2O2 treatment).
Western Blot
Primary antibodies against Bax, Bcl-2, BiP, and CHOP were bought from Abcam(Cambridge, MA). Chondrocytes were lysed via Radio-Immunoprecipitation Assay lysis buffer. After being centrifuged, proteins were quantified and then subjected to sodium salt-polyacrylamide gel electrophoresis. After blocking, membranes were immunoblotted via primary antibodies, followed by the 2-hour incubation via horse radish peroxidase-conjugated secondary antibodies. Membranes were analyzed via enhanced chemiluminescence detection reagents.
Oxidative Stress Assessment
Reactive oxygen species level of chondrocytes was analyzed via 2,7-Dichlorodi -hydrofluorescein diacetate, and GSH/GSSG assay kit (Byotime) was employed to examine the GSH/GSSG ratio.
Quantitative Real-Time PCR
Total RNA was prepared through TRIzol Reagent (Invitrogen, Pleasanton, CA). MRNA abundances of Bax, Bcl-2, Caspase-3, Collange I, Collange II, Aggrecan, Sox9, BiP, and CHOP were analyzed via SYBR-Green Master Mix (TaKaRa, Dalian, China). Primer sequences are listed below:
-
Bax-fwd: 5′-CCCGAGAGGTCTTTTTCCGAG-3′
Bax-rev: 5′-CCAGCCCATGATGGTTCTGAT-3′
Bcl-2-fwd: 5′-GTCTTCGCTGCGGAGATCAT-3′
Bcl-2-rev: 5′-CATTCCGATATACGCTGGGAC-3′
Collange I-fwd: 5′-GTTCAGCTTTGTGGACCTCCG-3′
Collange I-rev: 5′-GCAGTTCTTGGTCTCGTCAC-3′
Collange II-fwd: 5′-CCACACTCAATCCCTCAAC-3′
Collange II-rev: 5′-GCTGCTCCACCAGTTCTTC-3′
Caspase-3-fwd: 5′-TGTGGCATTGAGACAGAC-3′
Caspase-3-rev: 5′-CACTTGCCATACAAACTA-3′
Aggrecan-fwd: 5′-CCCCTGCTATTTCATCGACCC-3′
Aggrecan-rev: 5′-GACACACGGCTCCACTTGAT-3′
Sox9-fwd: 5′-AGCGAACGCACATCAAGAC-3′
Sox9-rev: 5′-CTGTAGGCGATCTGTTGGGG-3′
BiP-fwd: 5′-AAAGAAGACGGGCAAAGATGT-3′
BiP-rev: 5′-TGCTTGATGCTGAGAAGACAG-3′
CHOP-fwd: 5′-GGAAACAGAGTGGTCATTCCC-3′
CHOP-rev: 5′-CTGCTTGAGCCG TTCATTCTC-3′
CCK-8 Assay
104 chondrocytes were seeded in the 96-well plate and treated with CCK-8 at 37°C. After 4 hours, optical density 490 nm was measured.
Statistical Analysis
Results were presented as means (SD). All analyses were conducted via SPSS software 12.0 and significant differences between treatment groups were examined via 1-way analysis of variance analysis. P < .05 was regarded statistically significant.
Results
Dose Response of Baicalin on Cell Survival
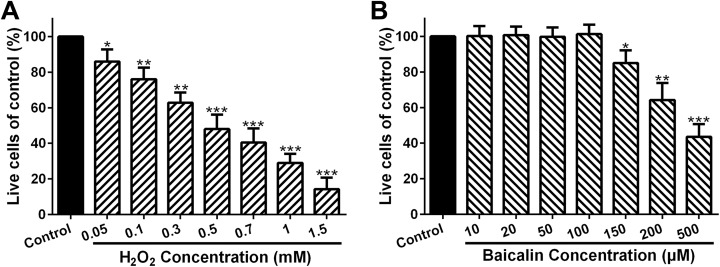
Chondrocytes were subjected to the indicated concentrations of H2O2 for 4 hours. Figure 1A indicated that H2O2 dose dependently restricted chondrocyte viability. Moreover, the survival rate of chondrocytes in 0.5 mM H2O2 group decreased by ∼53.57% in comparison to the control, which suggested that 0.5 mM H2O2 had an extremely significant cytotoxicity (Figure 1A; P < .001). At 0.7, 1, and 1.5 mM H2O2, the cell survival rate was reduced by 60.7%, 71.4%, and 85.7% in comparison with the control, respectively (Figure 1A; P < .001). On the other hand, chondrocytes were incubated via 10 to 500 μM baicalin for 1 day, and Figure 1B showed that 10 to 100 μM baicalin exhibited little influence on cell viability, whereas 150 μM (P < .05), 200 μM (P < .01), or 500 μM (P < .001) baicalin significantly inhibited the viability of chondrocytes. Hence, 0.5 mM H2O2 and 100 μM baicalin were chosen for further analysis.
Figure 1.
Dose-dependent cytotoxicity of H2O2 and baicalin. Effects of H2O2 (A) and baicalin (B) at indicated concentrations on chondrocyte viability. *P < .05, **P < .01, and ***P < .001.
Baicalin Decreased Oxidative Stress After H2O2 Treatment
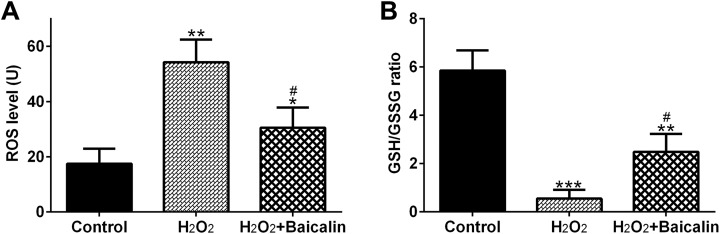
To analyze the effects of baicalin on H2O2-treated chondrocytes, chondrocytes were treated with 100 μM baicalin for 2 hours, followed by a 4-hour incubation of 0.5 mM H2O2. Figure 2A showed that the ROS level in H2O2 group was increased by 221.42% (P < .01) in comparison with the control, whereas baicalin pretreatment decreased the ROS level to 55.56% in comparison to the H2O2 group (P < .05).
Figure 2.
Baicalin inhibited H2O2-mediated oxidative stress. Reactive oxygen species level (A) and glutathione/oxidized glutathione (GSH/GSSG) ratio (B) in human osteoarthritis (OA) chondrocytes. *P < .05, **P < .01, and ***P < .001 in comparison with control, # P < .05 in comparison with H2O2 group.
In addition, Figure 2B demonstrated that H2O2 decreased the GSH/GSSG ratio to 10%, compared to the control group (P < .001). However, baicalin partially recovered the decrease of GSH/GSSG ratio induced by H2O2 treatment (Figure 2B; P < .05).
Baicalin Pretreatment Improved Chondrocyte Viability and Restricted Cell Apoptosis Under H2O2 Treatment
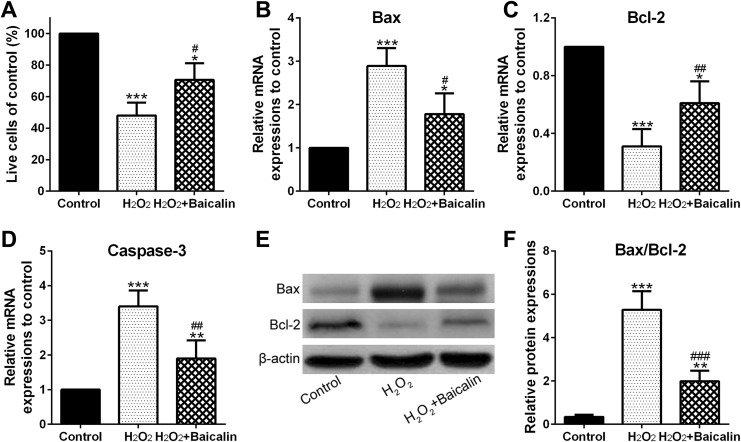
CCK-8 assay showed that H2O2 treatment inhibited cell viability to 47.5% compared to the control (Figure 3A; P < .001), whereas Figure 3A also showed that baicalin significantly improved the viability of chondrocytes to 142.31%, compared to the H2O2 group (P < .05). In the other hand, quantitative real-time PCR (qPCR) indicated that H2O2 improved the messenger RNA (mRNA) expression of Bax (Figure 3B; P < .001) and Caspase-3 (Figure 3C; P < .001) and inhibited Bcl-2 mRNA expression (Figure 3D; P < .001), whereas baicalin inhibited these phenomena (Figure 3B–D; P < .05, P < .01, and P < .01 for Bax, Bcl-2, and Caspase-3, respectively). In addition, Figure 3E showed that baicalin inhibited H2O2-stimulated upregulation of Bax and suppression of Bcl-2 at the protein level. Moreover, Figure 3F demonstrated that baicalin significantly decreased H2O2-medicated increase in the Bax/Bcl-2 ratio (P < .001 compared to H2O2 group).
Figure 3.
Baicalin pretreatment increased chondrocyte viability under H2O2 treatment. A, Cell viability was examined via CCK-8. B-D, Bax (B), Bcl-2 (C), and Caspase-3 (D) messenger RNA (mRNA) abundances were measured by qPCR. E, Western blot analysis of Bax and Bcl-2 protein expression. F, Relative expressions of Bax/Bcl-2. *P < .05, **P < .01, and ***P < .001 in comparison with control, # P < .05, ## P < .01, ### P < .001 in comparison with H2O2 group.
Baicalin Pretreatment Protected ECM of Human OA Chondrocytes Under H2O2 Treatment
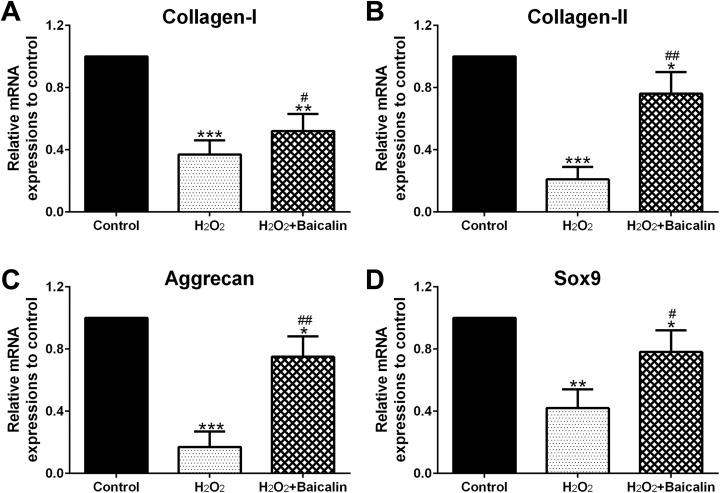
Figure 4A to D indicated that H2O2 dramatically restricted the mRNA expression of Collange I (P < .001), Collange II (P < .001), Aggrecan (P < .001), and Sox9 (P < .01), compared to the control group. In contrast, baicalin pretreatment protected ECM of H2O2-treated chondrocytes, as shown by suppressing the inhibitory effect of H2O2 on the above gene expression (Figure 4A–D; P < .05 for Collange I and Sox9, P < .01 for Collange II and Aggrecan, compared to H2O2 group).
Figure 4.
Baicalin pretreatment protected extracellular matrix (ECM) of human osteoarthritis (OA) chondrocytes under H2O2 treatment. Collange I (A), Collange II (B), Aggrecan (C), and Sox9 (D) messenger RNA (mRNA) expressions were measured by qPCR. *P < .05, **P < .01, and ***P < .001 in comparison with control, # P < .05, ## P < .01 in comparison with H2O2 group.
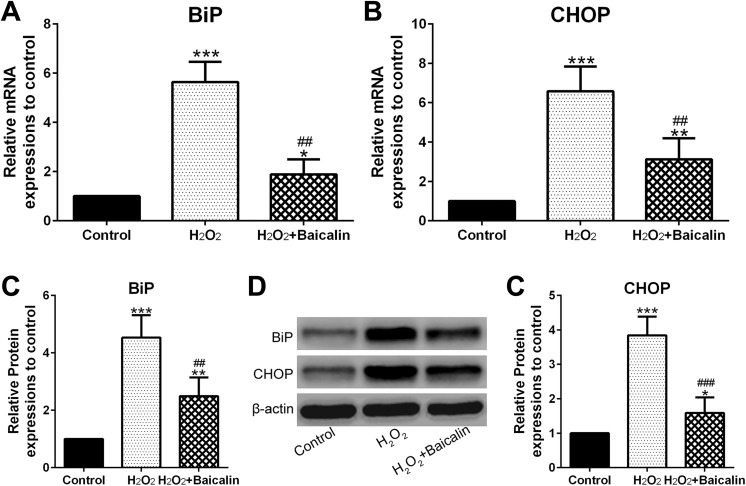
Baicalin Pretreatment Reduced H2O2-Mediated ER Stress
Figure 5A and B showed that H2O2 increased BiP and CHOP mRNA level to 520% and 600% in comparison to the control group, respectively. However, BiP and CHOP mRNA levels in H2O2 + baicalin group are much lower than those in the H2O2 group (Figure 5A and B; P < .01). In addition, Figure 5C to E indicated that baicalin suppressed H2O2-stimulated increase in BiP (P < .01) and CHOP (P < .001) protein expression.
Figure 5.
Baicalin reduced H2O2-mediated endoplasmic reticulum (ER) stress. A and B, BiP (A) and CHOP (B) messenger RNA (mRNA) abundances were measured by qPCR. D, Western blot analysis of BiP and CHOP protein expressions. C, and (E) were the statistical data of BiP (C) and CHOP (E) expression shown in (D). *P < .05, **P < .01, and ***P < .001 in comparison with control, ## P < .01, ### P < .001 in comparison with H2O2 group.
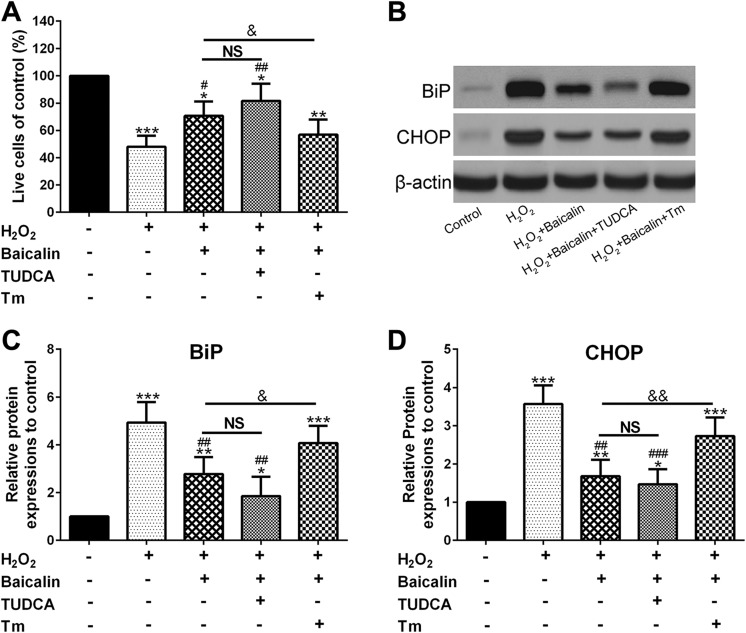
Baicalin Protected Human OA Chondrocytes From H2O2 Stress by Inhibiting ER Stress
To investigate the correlation between the protective effect of baicalin and ER stress change, TUDCA, or Tm was employed in combination with baicalin before H2O2 administration and cell viability, and the expression of ER stress hallmarks were analyzed. Figure 6A indicated that Tm significantly abolished baicalin’s protection on the viability of chondrocytes (P < .05), whereas the viability of chondrocytes in the H2O2 + baicalin + TUDCA group had no significant change compared with those in the H2O2 + baicalin group. In addition, Figure 6B–D proved that Tm significantly abolished baicalin-induced decrease in BiP (P < .05) and CHOP (P < .01) expression, whereas baicalin plus TUDCA didn’t exhibit extra activities on BiP and CHOP expression in comparison with baicalin alone. Hence, these data implied that baicalin protected chondrocytes from H2O2 through ER stress attenuation.
Figure 6.
Baicalin protected human osteoarthritis (OA) chondrocytes from H2O2 by inhibiting endoplasmic reticulum (ER) stress. A, Cell viability was determined via CCK-8. B, Western blot assay of BiP and CHOP protein expression. C and D, were the statistical data of BiP (C) and CHOP (D) expression shown in (B). *P < .05, **P < .01, and ***P < .001 compared to control, # P < .05, ## P < .01, ### P < .001 compared to H2O2 group. & P < .05, && P < .01 the comparison of H2O2 + baicalin group and H2O2 + baicalin + Tm group. NS means no significance between H2O2 + baicalin group and H2O2 + baicalin + TUDCA group. TUDCA indicates tauroursodeoxycholic acid dihydrate.
Discussion
Osteoarthritis is a progressive disorder of bones and joints linked to intricate mechanisms which involve subchondral bone sclerosis and the degradation of AC. Baicalin is a well-documented natural compound that has therapeutic effects on various diseases, such as autoimmune diseases, obesity, stomach disease, brain injury, neurodegenerative disease, and so on.13–17 Moreover, baicalin has been proved to have anti-OA activities in vivo and in vitro.1 To get more information about the related mechanisms of baicalin’s anti-OA roles, chondrocytes derived from patients with OA were treated via 0.5 mM H2O2 to mimic OA and we found that baicalin protected human OA chondrocytes against the cytotoxicity of H2O2 through abrogating the inhibition of chondrocyte viability and inhibiting H2O2-mediated expression change of apoptosis-associated gene (Bcl-2, Bax, and Caspase-3). In addition, the activities of baicalin on chondrocytes were further explored through the assessment of mRNA abundances of ECM-related genes, such as Collange I, Collange II, Aggrecan, and Sox9. Quantitative real-time PCR data demonstrated that baicalin administration partially attenuated the decrease in the expression of the above ECM-related genes, which suggested that baicalin may also have important roles in cartilage matrix deposition.
Oxidative stress caused by H2O2 administration is known to generate the initial injury to chondrocytes. The measurement of ROS level and GSH/GSSG ratio indicated that baicalin administration reduced the increase in intracellular oxidative stress of H2O2-treated chondrocytes, as shown by decreased ROS level and increased GSH/GSSG ratio. On the other hand, it is generally accepted that oxidative stress is one of the primary factors which can lead to ER stress.18,19 Moreover, increasing reports proved that ER stress in chondrocytes exists in the AC of patients with OA, and there was a positive relationship between ER stress and the apoptosis of chondrocytes.20,21
Endoplasmic reticulum is an important organelle involved in protein synthesis, modification, and secretion.22 Physiological or pathological processes, which lead to the misfolded/unfolded protein accumulation in the ER, are known as ER stress.23 Previous reports demonstrate that the anti-OA effects of several naturally occurring products are directly linked with the attenuation of ER stress. For example, panax quinquefolium saponin suppressed ER stress-induced apoptosis of chondrocytes and then alleviated OA development in OA rats.24 Taurine had cytoprotective effects on H2O2-stimulated chondrocytes via suppressing ER stress.12 5,7,3′,4′-tetramethoxyflavone had chondroprotective roles via blocking ER stress-mediated apoptosis.25 Moreover, recent studies already proved the regulatory activities of baicalin on ER’s function. For instance, baicalin suppressed ER stress mediated by H2O2 and subsequently exhibited cytoprotective effects on HK-2 cells.22 Therefore, we wondered if baicalin’s protection was associated with ER stress attenuation.
Here, qPCR and Western blot were used to assess BiP and CHOP expression, and the results indicated that baicalin pretreatment significantly restricted H2O2-stimulated BiP and CHOP expression. BiP (also called GRP78)26 is a representative intra-ER chaperone.27 It is generally believed that BiP expression level is positively associated with the degree of ER stress.27 Nugent et al showed that GRP78 expression was enhanced in cartilages of patients with advanced OA, whereas GRP78 expression wasn’t detected in non-OA cartilage.28 CHOP, also called GADD153, is an indicator of ER stress-induced apoptosis. Previous studies show that downregulation of CHOP expression inhibits cell apoptosis, whereas elevated CHOP expression fosters apoptosis.29,30 Therefore, the above data implied that baicalin may attenuate ER stress in human OA chondrocytes.
Furthermore, Tm and TUDCA were employed to examine the protective roles of baicalin against ER stress. Tm, an effective ER stress inducer, is an antibiotic which can specifically suppress the synthesis of N-linked glycans and subsequently lead to misfolding of proteins.31 Tauroursodeoxycholic acid dihydrate, a powerful ER stress inhibitor, could effectively attenuate ER stress and restrict cell apoptosis.32,33 The data showed that Tm significantly abolished baicalin-mediated protection, as shown by decreased cell viability and enhanced Bip and CHOP expression. In addition, baicalin plus TUDCA didn’t exhibit enhanced protective activities compared to baicalin alone, suggesting that baicalin treatment effectively relived H2O2-stimulated ER stress. Taken together, the above results proved that baicalin administration conferred protection against H2O2 via attenuating ER stress.
There are 2 main limitations in the current study. First, the conclusions were drawn based on the in vitro results. Although the cells derived from the patients could mimic the pathological condition to some extent, this is not exactly the same with the pathological condition in the OA. Second, H2O2 treatment was used to cause oxidative stress to the cells in the current study, which differs from the real physiological or pathological condition as well.
In summary, the current study showed that baicalin protected human OA chondrocytes from H2O2-stimulated cytotoxicity through attenuating ER stress, as shown by enhanced cell viability, the downregulation of Bax, Caspase-3, and ER stress markers, and the upregulation of Bcl-2 and ECM-related genes. Therefore, our findings suggested that baicalin may be an effective therapeutic medicine for relieving the impairment of cartilage caused by OA. On the other hand, Zhang et al demonstrated that baicalin ameliorated ER stress via the TXNIP/NLRP3 pathway in hepatocytes.34 According to Yu et al, baicalin fostered ER-stress-mediated apoptosis via the activation of the ATF6 signaling pathway in hepatocellular carcinoma cells.35 However, baicalin’s target in human OA chondrocytes still needs to be further explored.
Conclusions
We found that baicalin protected human OA chondrocytes from H2O2 via attenuating ER stress.
Footnotes
Authors’ Note: J.C., Y.Z., T.W., and B.L. contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chen C, Zhang C, Cai L, et al. Baicalin suppresses IL-1beta-induced expression of inflammatory cytokines via blocking NF-kappaB in human osteoarthritis chondrocytes and shows protective effect in mice osteoarthritis models. Int Immunopharmacol. 2017;52:218–226. [DOI] [PubMed] [Google Scholar]
- 2. Li ZC, Xiao J, Peng JL, et al. Functional annotation of rheumatoid arthritis and osteoarthritis associated genes by integrative genome-wide gene expression profiling analysis. PLoS One. 2014;9(2):e85784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi X, Ye H, Yao X, Gao Y. The involvement and possible mechanism of NR4A1 in chondrocyte apoptosis during osteoarthritis. Am J Transl Res. 2017;9(2):746–754. [PMC free article] [PubMed] [Google Scholar]
- 4. Uehara Y, Hirose J, Yamabe S, et al. Endoplasmic reticulum stress-induced apoptosis contributes to articular cartilage degeneration via C/EBP homologous protein. Osteoarthritis Cartilage. 2014;22(7):1007–1017. [DOI] [PubMed] [Google Scholar]
- 5. Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15(1):27–34. [DOI] [PubMed] [Google Scholar]
- 6. Cheng F, Lu Y, Zhong X, et al. Baicalin’s therapeutic time window of neuroprotection during transient focal cerebral ischemia and its antioxidative effects in vitro and in vivo. Evid Based Complement Alternat Med. 2013;2013:120261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui L, Feng L, Zhang ZH, Jia XB. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-kappaB pathway activation. Int Immunopharmacol. 2014;23(1):294–303. [DOI] [PubMed] [Google Scholar]
- 8. Shin SH, Bak SS, Kim MK, Sung YK, Kim JC. Baicalin, a flavonoid, affects the activity of human dermal papilla cells and promotes anagen induction in mice. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(5):583–586. [DOI] [PubMed] [Google Scholar]
- 9. Wu J, Hu D, Wang KX. Study of Scutellaria baicalensis and Baicalin against antimicrobial susceptibility of Helicobacter pylori strains in vitro [in Chinese]. Zhong Yao Cai. 2008;31(5):707–710. [PubMed] [Google Scholar]
- 10. Yang X, Zhang Q, Gao Z, Yu C, Zhang L. Baicalin alleviates IL-1beta-induced inflammatory injury via down-regulating miR-126 in chondrocytes. Biomed Pharmacother. 2018;99:184–190. [DOI] [PubMed] [Google Scholar]
- 11. Pan Y, Chen D, Lu Q, et al. Baicalin prevents the apoptosis of endplate chondrocytes by inhibiting the oxidative stress induced by H2O2. Mol Med Rep. 2017;16(3):2985–2991. [DOI] [PubMed] [Google Scholar]
- 12. Bian Y, Wang H, Sun S. Taurine alleviates endoplasmic reticulum stress in the chondrocytes from patients with osteoarthritis. Redox Rep. 2018;23(1):118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai J, Liang K, Zhao S, et al. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc Natl Acad Sci U S A. 2018;115(26):E5896–E5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi X, Fu Y, Zhang S, Ding H, Chen J. Baicalin attenuates subarachnoid hemorrhagic brain injury by modulating blood-brain barrier disruption, inflammation, and oxidative damage in mice. Oxid Med Cell Longev. 2017;2017:1401790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Neuroprotective and cognitive enhancement potentials of baicalin: a review. Brain Sci. 2018;8(6):E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu J, Liu J, Yue G, et al. Therapeutic effect of the natural compounds baicalein and baicalin on autoimmune diseases. Brain Sci. 2018;18(1):1149–1154. [DOI] [PubMed] [Google Scholar]
- 17. Zhang T, Yang D, Meng X. Baicalin protects against gastroduodenal ulcers via the modulation of Nrf2 expression: experimental, biochemical, and histological analyses. Pharmacol Rep. 2017;69(6):1154–1158. [DOI] [PubMed] [Google Scholar]
- 18. Banerjee A, Banerjee V, Czinn S, Blanchard T. Increased reactive oxygen species levels cause ER stress and cytotoxicity in andrographolide treated colon cancer cells. Oncotarget. 2017;8(16):26142–26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med. 2010;3(1):33–40. [PMC free article] [PubMed] [Google Scholar]
- 20. Honsawek S, Tanavalee A, Sakdinakiattikoon M, Chayanupatkul M, Yuktanandana P. Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin Biochem. 2009;42(9):808–812. [DOI] [PubMed] [Google Scholar]
- 21. Lin P, Weng X, Liu F, et al. Bushen Zhuangjin decoction inhibits TM-induced chondrocyte apoptosis mediated by endoplasmic reticulum stress. Int J Mol Med. 2015;36(6):1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin M, Li L, Zhang Y, et al. Baicalin ameliorates H2O2 induced cytotoxicity in HK-2 cells through the inhibition of ER stress and the activation of Nrf2 signaling. Int J Mol Sci. 2014;15(7):12507–12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda). 2007;22:193–201. [DOI] [PubMed] [Google Scholar]
- 24. Xie JJ, Chen J, Guo SK, et al. Panax quinquefolium saponin inhibits endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and attenuates the progression of osteoarthritis in rat. Biomed Pharmacother. 2018;97:886–894. [DOI] [PubMed] [Google Scholar]
- 25. Yuan X, Li L, Shi W, et al. TMF protects chondrocytes from ER stress-induced apoptosis by down-regulating GSK-3beta. Biomed Pharmacother. 2017;89:1262–1268. [DOI] [PubMed] [Google Scholar]
- 26. Rasheed Z, Haqqi TM. Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2alpha, p38-MAPK and NF-kappaB in advanced glycation end products stimulated human chondrocytes. Biochim Biophys Acta. 2012;1823(12):2179–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cavener DR, Gupta S, McGrath BC. PERK in beta cell biology and insulin biogenesis. Trends Endocrinol Metab. 2010;21(12):714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nugent AE, Speicher DM, Gradisar I, et al. Advanced osteoarthritis in humans is associated with altered collagen VI expression and upregulation of ER-stress markers Grp78 and bag-1. J Histochem Cytochem. 2009;57(10):923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Du Y, Wang M, Liu X, et al. Araloside C prevents hypoxia/reoxygenation-induced endoplasmic reticulum stress via increasing heat shock protein 90 in h9c2 cardiomyocytes. Front Pharmacol. 2018;9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gu LL, Shen ZL, Li YL, Bao YQ, Lu H. Oxymatrine causes hepatotoxicity by promoting the phosphorylation of JNK and induction of endoplasmic reticulum stress mediated by ROS in LO2 cells. Mol Cells. 2018;41(5):401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guha P, Kaptan E, Gade P, Kalvakolanu DV, Ahmed H. Tunicamycin induced endoplasmic reticulum stress promotes apoptosis of prostate cancer cells by activating mTORC1. Oncotarget. 2017;8(40):68191–68207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoon YM, Lee JH, Yun SP, et al. Tauroursodeoxycholic acid reduces ER stress by regulating of Akt-dependent cellular prion protein. Sci Rep. 2016;6:39838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Y, Qu P, Ma X, et al. Tauroursodeoxycholic acid (TUDCA) alleviates endoplasmic reticulum stress of nuclear donor cells under serum starvation. PLoS One. 2018;13(5):e0196785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang J, Zhang H, Deng X, Zhang Y, Xu K. Baicalin protects AML-12 cells from lipotoxicity via the suppression of ER stress and TXNIP/NLRP3 inflammasome activation. Chem Biol Interact. 2017;278:189–196. [DOI] [PubMed] [Google Scholar]
- 35. Yu Z, Luo X, Wang C, et al. Baicalin promoted site-2 protease and not site-1 protease in endoplasmic reticulum stress-induced apoptosis of human hepatocellular carcinoma cells. FEBS Open Bio. 2016;6(11):1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]