Abstract
OA is a multifactorial and progressive disease with no cure yet. Substantial efforts have been made and several biochemical and genetic markers have been reported, but neither alone nor in combination is adequate to identify early OA changes or determine disease progression with sufficient predictive values. Recent advances in metabolomics and its application to the study of OA have led to elucidation of involvement of several metabolic pathways and new specific metabolic markers for OA. Some of these metabolic pathways affect amino acid metabolism, including branched chain amino acids and arginine, and phospholipid metabolism involving conversion of phosphatidylcholine to lysophosphatidylcholine. These metabolic markers appear to be clinically actionable and may potentially improve the clinical management of OA patients. In this article, we review the recent studies of metabolomics of OA, discuss those novel metabolic markers and their potential clinical utility, and indicate future research directions in the field.
Keywords: osteoarthritis, metabolomics, biomarkers, branched chain amino acids, arginine, phosphatidylcholine
Rheumatology key messages
Blood concentration of branched chain amino acids is increased in OA but decreased in RA.
Arginine deficiency is associated with OA and blood arginine concentration could be used for monitoring the OA process.
The conversion pathway of phosphatidylcholine to lysophosphatidylcholine is overactivated in OA.
Introduction
OA is the most common form of arthritis, affecting about 10% of the world’s population aged 60 years or older [1]. It is the major source of joint pain and disability [2], and imposes a substantial socioeconomic burden on society estimated to be between 1.0 and 2.5% of gross domestic product [3]. The disease course of OA is variable and there is a paucity of biomarkers with which to objectively assess disease activity or predict radiographic progression. MRI has the ability to visualize simultaneously all relevant joint tissues (cartilage, menisci, ligaments and bone marrow) and has been increasingly used in OA research [4]. MRI, with its ability to discriminate articular tissues, holds potential as a tool for whole organ imaging of the joint, and an MRI global knee joint score has been developed as an aid for diagnosis [5]. However, the parameters for early diagnosis and clinical trials are still unclear and the high costs per joint scan prohibit its application in routine clinical joint examination. The clinician is left to rely on the patient’s global assessment and plain radiographs in clinical decision-making. Thus, there is a pressing need for meaningful biomarkers that can not only improve early diagnosis, but also assess disease activity and progression. At present, there are no approved disease-modifying OA drugs, and the primary goal of treatment is pain management.
OA biochemical and genetic markers
The identification of biomarkers as predictors of the early changes and progression of OA has been the focus of considerable research effort, but to date there are no established molecular markers that reliably predict early disease change and progression for use in clinical practice [6]. Large-scale meta-analysis examining studies into the three most studied cartilage-derived biomarkers, namely urinary C-terminal telopeptide (uCTX-II), serum cartilage oligomeric protein and serum MMP degraded type II collagen, by receiver operating characteristics (ROC) analysis showed that these biomarkers offered minimal clinical utility in predicting knee OA. The area under the curve (AUC) was 0.646 with age, sex and BMI as knee OA predictors, compared with an AUC of 0.668 when including those three most studied biomarkers [7]. Adding all the genetic markers with P < 10−7, based on a large genome-wide meta-analysis of different studies of knee OA in the Caucasian population, added only marginally to the prediction of knee OA incidence based on use of age, sex and BMI in an elderly population [8]. Hence, there is an enormous need for better biomarkers that can be used to identify patients at high risk, to detect early changes in disease and to act as clinical surrogates that can be used to evaluate the response to lifestyle modification and disease-modifying drugs for OA [9]. Reviews on other biochemical markers and genetic factors for OA can be found elsewhere [10, 11], and the current review focuses on deranged metabolism and impact on metabolite concentrations in a metabolomics approach to diagnosis and management of OA.
Metabolomics
Metabolomics involves the systematic study of small-molecule metabolites in a biological system. Metabolites are the intermediates and end products of cellular biochemical processes. Their steady-state levels can be regarded as the ultimate response of biological systems to genotype, phenotype and environment. Advances in technology have allowed relatively large numbers of small-molecule metabolites from body fluids or tissues to be detected and measured quantitatively in a single step and promise immense potential for early diagnosis, for therapy monitoring and for understanding the pathogenesis of many diseases [12]. The most commonly used methods in metabolomics are NMR-based and mass spectroscopy (MS)-based. Extensive reviews on use and applications of both NMR- and MS-based metabolomics techniques can be found elsewhere [13, 14].
Metabolomics of animal OA models
High-resolution NMR is a reproducible and reliable non-destructive analytical method. Early work on metabolomics of OA involved use of NMR-based methods and was particularly focused on animal models. Damyanovich et al. [15] studied SFs of 14 canine surgery-induced knee OA and 14 canine normal knees using 1 H NMR spectroscopy. Through analysis of NMR spectra of osteoarthritic SF they documented increased concentrations of lactate, pyruvate, lipoprotein-associated fatty acids and glycerol, as well as 3-D-hydroxybutyrate, reduced levels of glucose, and elevated levels of N-acetyl-glycoproteins, acetate, creatine/creatinine and glutamine/glutamate compared with healthy normal canine SF. An increase was also observed in the concentrations of the amino acids alanine and isoleucine. Similar results were also observed in a study of 25 OA SF samples and 8 normal ones from 22 horses [16]. In a study of urine samples of osteoarthritic male Hartley guinea pigs, Lamers et al. [17] identified an NMR fingerprint that reflected the osteoarthritic changes in guinea pigs. The metabolites that comprised the specific fingerprint pointed to the involvement of energy and purine metabolism as being of major importance in OA [15, 18]. Borel et al. [19] used high-resolution magic angle spinning (HRMAS) 1 H NMR spectroscopy to characterize the articular osteoarthritic cartilage in a meniscectomized guinea pig model and suggested that the major OA-induced 1 H HRMAS NMR changes involved an increase of the N-acetyl peak of proteoglycans (at day 20 after meniscectomy) and a decrease after day 60 as the pathology evolved. They also observed a sharp increase in methylene resonances of chondrocyte membrane lipids from day 90, which the authors concluded was a marker of apoptosis. These animal OA model studies suggest that 1 H NMR spectroscopy could be useful in understanding alterations in joint metabolism consequent to arthritic diseases and helpful in identifying potential markers of OA.
Metabolomics of population-based OA studies
A small number of population-based OA studies using NMR-based metabolomics have been reported. Williamson et al. [20] first studied the levels of a range of components measured by 1 H NMR in samples of SF taken from three groups of patients comprising 10 with OA, 18 with RA and 11 with traumatic effusions. They found that patients with traumatic effusions had high levels of saturated triglycerides, while those with OA had low levels, but fatty acid chain lengths of the triglycerides found in OA SF appear to be shorter than those for the other groups. Lamers et al. [21] studied urine samples of 47 non-OA controls and 45 individuals with radiographic OA of the knees or hips. They showed that urine NMR spectra can discriminate OA cases and controls in both males and females, and the metabolic profiles largely resembled the profile identified in the guinea pig model [17]. They also demonstrate a high correlation between Kellgren–Lawrence (KL) score and the metabolite profile with r2 = 0.82–0.93. While a number of NMR signals as parts per million chemical shift were reported in the paper, the authors concluded that those signals were most likely to be associated with altered energy utilization and histidine metabolism. By integrating 1 H NMR and GC-MS results of the SF samples obtained from 55 OA patients and 13 normal human cadaveric knee joints, Mickiewica et al. [22] found 11 metabolites were statistically important for the separation between OA and normal, and these metabolites were mostly related to energy metabolism. We recently studied 80 SF samples obtained from total joint replacement patients due to primary OA using a MS-based targeted metabolomics approach and liquid chromatography–tandem mass spectrometry, and found that the OA patients can be clearly classified into two distinct groups, with one group having a significantly higher concentration of acylcarnitines, but lower concentration of free carnitine, suggesting alterations in mitochondrial fatty acid oxidation [23]. Together, these studies suggest that altered energy metabolism is involved in at least a subgroup of OA patients.
Drawbacks of the NMR-based method are difficulties in identification of individual metabolites, especially those of relatively low concentration, and the restriction of detecting only metabolites at medium-to-high levels of abundance [24]. On the other hand, MS-based metabolomics provides highly selective and sensitive quantitative analyses with the potential to identify individual metabolites [25]. Recent application of MS-based metabolomics in OA has allowed identification of several specific novel and promising metabolic markers for OA.
Most promising metabolic markers and metabolic pathways for OA
Branched chain amino acids
Branched chain amino acids (BCAAs) (valine, leucine and isoleucine) are essential amino acids making up approximately one-third of skeletal muscle protein, and are important fuels for energy metabolism. A study of 199 female knee OA patients defined as radiographic OA and 399 female controls selected from the UK using a MS-based targeted metabolomics approach found that the BCAA to histidine ratio was associated with knee OA [26]. This ratio was increased by 0.21–0.24 in the knee OA patients compared with the controls. These findings were later confirmed in an independent Canadian cohort from Newfoundland and Labrador and extended to the male population [27]. Using ROC analysis, the study demonstrated that the plasma BCAA to histidine ratio has an AUC of 0.76 in discriminating knee OA patients from controls. However, the ratio was not predictive of total knee joint replacement in a longitudinal cohort with 10 years’ follow-up [27]. This may suggest the BCAA to histidine ratio is a diagnostic marker, reflecting metabolic processes occurring with OA that are not necessarily pathogenic themselves and hence not predictive of progression. While details concerning the mechanism of involvement remain to be elucidated, a sheep OA model showed that altered BCAA metabolism was observed in an anterior cruciate ligament transaction (ACLT)-induced OA model but not other models [28]. This suggests that the ratio may be associated with anterior cruciate ligament injury-related knee OA. Anterior cruciate ligament injury is associated with a substantially increased risk for development of future knee OA in the patellofemoral and tibiofemoral joints [29]. Thus, while these observations require further validation, further exploration of the role of the BCAA to histidine ratio as a potential tool for monitoring anterior cruciate ligament injury patients for OA risk is also warranted.
A number of epidemiological studies have indicated that elevated circulating levels of BCAAs are associated with poor metabolic health and linked to the metabolic syndrome and cardiovascular disease [30–32]. Longitudinal studies report that increased blood levels of BCAAs are predictive of future insulin resistance or type 2 diabetes [33, 34]. A study comparing serum and adipose tissue of healthy and metabolically unhealthy obese individuals found that BCAA levels were significantly correlated with the unhealthy phenotype and that gene expression of proteins involved in BCAA catabolism and the tricarboxylic acid cycle were less down-regulated in the samples from healthy subjects [35]. Obesity has long been recognized as a strong risk factor for knee OA [36] and emerging evidence suggests that OA is also associated with other obesity-related metabolic abnormalities, particularly type 2 diabetes, and cardiovascular diseases [37, 38], leading to a notion of metabolic OA [39] or metabolic syndrome-associated OA [40]. While a causal relationship has not been established, the epidemiological association between OA and obesity related-metabolic diseases might be explained in part by, or share commonality with, disease processes resulting in unique alterations in BCAA metabolism in addition to that recently proposed by Huang et al. [41] where low grade inflammation mediated by lipopolysaccharide might explain the association between obesity, metabolic syndrome and OA. BCAA levels are lower in patients affected by RA [42] and could be a marker for monitoring responses to etanercept in RA patients [43]. These observations might suggest the usefulness of measuring serum BCAA levels in initial differential diagnosis between OA and RA.
A causal relationship between BCAAs and OA has not been established. As essential amino acids, BCAAs must be provided in adequate amounts in the diet to support normal growth and metabolism. Provided there are no obvious dietary differences between OA and unaffected individuals, increased levels of BCAAs in OA patients could be due to either blocked catabolism of BCAAs or increased release from the breakdown of proteins. BCAA catabolism begins with two common steps. The first is a transamination reaction catalysed by branched chain aminotransferase (BCAT). This enzyme is found intracellularly as two isoforms—mitochondrial BCATm and cytosolic BCATc. Transamination is followed by the flux-generating step catalysed by the branched chain keto acid dehydrogenase complex [44]. It is mutations in the branched chain keto acid dehydrogenase enzyme complex that causes the rare inborn error of metabolism called maple syrup urine disease (MSUD). Children affected by MSUD have elevated levels of BCAAs and corresponding alpha-keto acids from transamination. The clinical features include lethargy, weight loss, metabolic derangement and progressive neurological signs of altered hypotonia and hypertonia [45]. Unlike other rare monogenic disorders such as aneurysm-osteoarthritis syndrome caused by mutations in the SMAD3 gene, and alkaptonuria caused by the homogentisate 1,2-dioxygenase gene (HGD), in which early OA development is one of the features in those affected [46, 47], there is no report yet of MSUD-affected individuals having an early OA manifestation. However, a study of human osteoarthritic synovium showed a lower level of the first-step transamination catabolites of BCAAs (4-methyl-2-oxopentanoate, 3-methyl-2-oxobutyrate and 3-methyl-2-oxovalerate) in media from the late-stage OA group than early or no-OA group [48], suggesting BCAA catabolism might be altered in OA by less transamination. A study showing enhancement of the resonance at 0.85 ppm of the 1 H HRMAS NMR spectra of the OA-affected cartilage sample attributable to an increase in leucine and isoleucine [19] also suggests a relationship between altered BCAA metabolism and OA joint pathology.
Arginine
Using a targeted metabolomics approach and two-stage study design (discovery and replication) with a total of 138 end-stage knee OA patients and 121 OA-free controls, we discovered a significant reduction of plasma concentration of arginine of 31% in OA patients compared with controls [49]. The reference interval for plasma arginine concentration in healthy adults is ∼80–120 μM [50]. ROC analysis of plasma arginine levels in identifying end-stage knee OA patients indicated excellent diagnostic value with AUC of 0.984. With an optimal cutoff of 57 μM, low plasma arginine showed 98.3% sensitivity and 89% specificity for OA [49]. Similar results were also found in an Italian cohort of 32 patients with idiopathic end-stage OA of the knee and 31 age- and sex-matched controls [51]. Plasma concentration was reduced by 24% in OA patients in that study. Furthermore, the Italian study demonstrated that the arginine/asymmetric dimethylarginine ratio was significantly lower in the OA patients than in the control group, suggesting a poor availability of nitric oxide (NO) in the SF of the OA patients, which may contribute to the progression of OA [51]. NO is a vasodilator produced by conversion of arginine to citrulline by NO synthase [52]. Increased flux through this pathway would result in increased NO formation and increased citrulline levels. Our study showed no evidence of this based on measurement of plasma citrulline, which was present at comparable levels in OA patients and controls [43]. In fact, the results from the Italian study indicated that NO production was diminishing along with the reduced arginine concentration in OA patients [51], supporting our hypothesis. Furthermore, arginine can be synthesized in the body from citrulline by the sequential action of cytosolic enzymes also involved in the urea cycle in the liver. The normal level of citrulline indicates that endogenous synthesis of arginine is normal in OA patients [49] and hence increased catabolic loss, or loss to protein or another biosynthetic process, may be responsible for the reduced arginine in OA patients. Arginine is catabolized to ornithine by arginase in the liver to allow urea production. Ornithine can form a metabolic precursor for proline, a key amino acid especially enriched in collagen. In our study [49], we found that the concentration of ornithine was significantly increased in OA patients compared with controls and the ratio of arginine to ornithine was substantially lower in OA patients than controls. This suggests that the reduced arginine concentration in OA is possibly due to decreased synthesis of arginine from ornithine through ornithine transcarbamylase and argininosuccinase activity and/or increased ornithine production via arginase activity primarily in the liver (Fig. 1). While there is no longitudinal study yet that investigates a potential relationship between arginine and OA, an ACLT OA model of rabbit showed that the concentration of arginine was decreased after ACLT and the post-ACLT arginine concentration was negatively associated with severity of OA histological assessment score [53], suggesting that the reduced arginine concentration is due to the OA process and progression. When cartilage is damaged, the body would initiate the repair process, and the arginine–proline metabolic pathway may be overactivated to produce proline for collagen synthesis. Alternatively, relatively selective utilization of arginine by other metabolic or biosynthetic processes cannot be excluded. Nevertheless, alterations in arginine levels are significant and of potential value in diagnostic workup of OA disease.
Fig. 1.
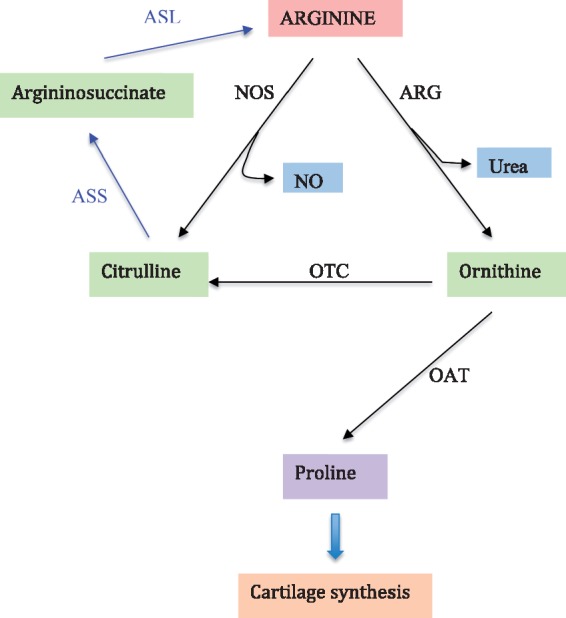
Schematic overview of metabolic relationship of arginine to proline and subsequent cartilage synthesis
ASL: argininosuccinate lyase; ASS: argininosuccinate synthetase; ARG: arginase; NOS: nitric oxide synthase; OAT: ornithine aminotransferase; OTC: ornithine transcarbamylase.
Phospholipid metabolism and phosphatidylcholine to lysophosphatidylcholine metabolic pathway
Kosinska et al. [54] utilized a lipidomics approach with the electrospray ionization tandem mass spectrometry method and studied SF samples obtained from 17 patients with early OA, 13 patients with late OA, 18 patients with RA and 9 controls obtained post-mortem with no history of joint disease. They identified the presence of the following phospholipid classes in the SF: phosphatidylcholine (PC), lysophosphatidylcholine (lysoPC), phosphatidylethanolamine, phosphatidylethanolamine-based plasmalogens, phosphatidylglycerol, phsphatidylserine, sphingomyelin and ceramide. PC is the predominant phospholipid class, accounting for ∼67% of all phospholipids. Compared with the median PC concentration in control SF (135 nmol/ml), the median PC concentration was increased 2.7-fold in early OA SF (372 nmol/ml; P = 0.0043), 5.4-fold in late OA SF (737 nmol/ml; P ⩽ 0.001) and 3.9-fold in RA SF (527 nmol/ml; P ⩽ 0.001). Using a targeted metabolomics approach and two-stage study design (discovery and replication), we studied both SF and plasma samples from knee OA patients, knee OA patients with diabetes and healthy controls [55]. We identified and confirmed two specific PCs, belonging to an ether lipid subgroup of phospholipids that gives rise to inflammatory mediators such as platelet activating factor, to be associated with knee OA, namely, PC acyl-alkyl C34:3 and PC acyl-alkyl C36:3. Both have three carbon–carbon double bonds associated with constituent fatty acids but one has 34 carbons (attributable to fatty acid composition) and the other has 36 carbons in its fatty acid chains. The concentrations of these two PCs in OA patients were significantly lower than that in controls. Further, we found that the concentrations of these two PCs were also reduced in diabetes patients compared with controls and the effects were additive; for example, knee OA patients with diabetes had lowest concentrations of the two PCs than knee OA patients alone as well as diabetes patients alone. However, the effect direction was opposite to the study of Kosinska et al. [54]. The reason for this conflict is unclear, but the controls in Kosinska et al. [54] were much younger, with a median age of 22 years, and the SF samples were not aspirated in vivo but up to 3 days after death, giving ample opportunity for post-mortem changes.
Independent associations between the two PCs and knee OA and diabetes and the additive effect in knee OA patients with diabetes suggest that the potential mechanism for the association may be different in OA and diabetes. The altered PC metabolism might be responsible in part for the epidemiological association between OA and diabetes. Recently, we hypothesized that the increased advanced glycation end products due to high glucose levels might be responsible for the altered PC metabolism in knee OA patients with diabetes and found that both methylglyoxal (MG) and free MG-derived hydroimidazolone (MG-H1) were significantly increased in the SF samples of knee OA patients with diabetes compared with those without, and the SF concentration, particularly MG-H1, was negatively and significantly correlated with the two PCs [56].
When metabolite ratios as proxies for enzymatic reaction were examined in OA patients, we identified that the ratio of lysoPCs to PCs was significantly increased in OA patients compared with controls [27]. The optimal cut-off value for diagnosis was 0.09, which was used to categorize subjects into two groups in a longitudinal cohort collected in Australia [27], and we found that the subjects with lysoPC to PC ratio of ⩾0.09 were 2.3 times more likely to undergo total knee joint replacement due to OA in a 10-year follow-up. Using the radiographic assessment in a Dutch cohort, Castro-Petez et al. [57] also found that the lysoPC to PC ratio, particularly lysoPCs with 16 and 18 carbons in their chains to PCs with four double bonds and 36 or 38 carbons, was increased in early OA patients, consistent with what we found for end stage OA. Increased lysoPCs to PCs in OA patients suggests that the metabolic pathway for conversion of PCs to lysoPCs catalysed by phospholipase A2 (PLA2) is more highly active. Pruzanski et al. [58] described a higher concentration of PLA2 in cartilage than in synovium, suggesting cartilage is the source of PLA2, and OA patients had a higher PLA2 concentration than RA patients. However, the study did not have normal controls and did not distinguish different forms of PLA2, which includes several unrelated families with common enzymatic activity, such as secreted, cytosolic and lipoprotein-associated PLA2 with each having several isoforms. Recently, we examined the gene expression levels of multiple forms of PLA2 in cartilage samples obtained from hip replacement patients due to primary OA with hip fracture patients as controls, and found that the expression of the PLA2 G5 was substantially increased in OA-affected cartilage compared with controls. The increased PLA2 G5 expression was significantly associated with an increased expression of TNF-α (G. Zhai, unpublished data). The findings were supported by an in vitro study [59] in which multiple PLA2 isoforms, especially PLA2 G5, were highly expressed in OA chondrocytes by stimulating pro-inflammatory factors, particularly TNF-α and IL-1β. This is interesting, as in an open label study of patients with erosive hand OA, intra-articular TNF-α inhibitor infliximab injections of affected joints produced a reduction in radiographic score compared with saline injections [60]. However, other studies with the subcutaneous TNF-α inhibitor adalumimab have failed to demonstrate reduction of pain [61, 62], possibly due to outcomes chosen, small number of patients treated and the short duration of the treatment.
Thus, one could speculate that the inflammatory process, either as a consequence of OA development or an initial factor, leads to increased activation of the PC to lysoPC metabolic pathway (Fig. 2) in OA patients, which, on release of long chain polyunsaturated fatty acids, such as arachidonate, leads to downstream formation of eicosanoids leading to OA joint symptoms, as certain eicosanoids mediate pain. Further, some eicosanoids such as prostaglandin E2 and proinflammatory cytokines such as TNF-α, IL-1β and IL-6 may also play a role in cartilage degradation [63].
Fig. 2.
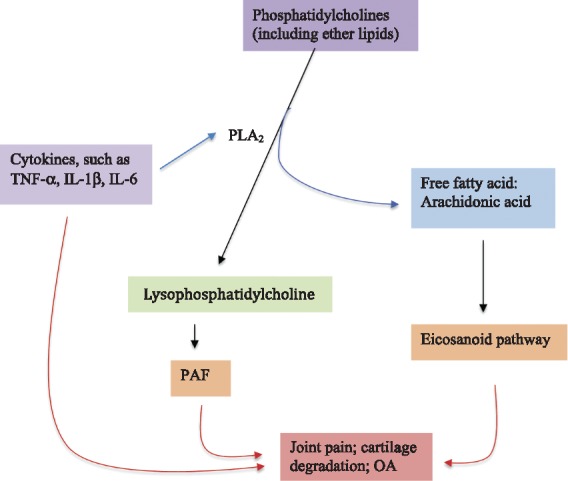
Schematic overview of phosphatidylcholines to lysophosphatidylcholine metabolic pathway in relation to OA
Phosphatidylcholines include both acyl-acyl phosphatidylcholine and acyl-alkyl phosphatidylcholine, where the latter is an ether lipid and potential precursor for synthesis of platelet activating factor. Proinflammatory cytokines such as TNF-α, IL-1β and IL-6 have their effects either through overactivating the expression of PLA2 or independently on cartilage damage and joint pain. PAF: platelet-activating factor; PLA2: phospholipase A2.
Therapeutic potentials of these metabolic pathways
The primary target for OA at the present time is pain reduction. Structural modification is not the standard of care. Investigational studies to retard structural damage have included inhibition of the inflammatory pathways (e.g. TNF-α, IL-1β, MMP and inducible NO inhibition), alteration of cartilage metabolism and anabolism (e.g. BMP and platelet-rich plasma) and bone remodelling (e.g. calcitonin and bisphosphonates). The efficacy of these experimental agents is beyond the scope of this review.
Over-the-counter medications are common in the management of OA. Glucosamine and chondroitin either alone or in combination are widely used, but there are conflicting results regarding their efficacy. A statistically significant response was achieved in a small subset of patients, and the effect size was very small. Despite this, the willingness of the public to self-administer over-the-counter medications is enormous. The identification of both the arginine and the BCAA metabolic pathway in OA provides novel targets for nutraceutics. Arginine supplements are already on the market and promoted for cardiovascular health by enhancing NO bioavailability [64]. Exogenous supplementation of arginine may also be useful for burns, severe wounds, infections, insufficient circulation, intensive physical activity or sterility. In recent years, attention was paid to the use of L-arginine supplementation by athletes during intensive sport activity, to enhance tissue growth and general performance, to potentiate the ergogenic potential and muscle tolerance to high intensity work and the gas exchange threshold [65]. One study showed that consuming a combination supplement of β-hydroxy-β-methyl butyrate, arginine and glutamine may suppress the loss of muscle strength after total knee joint replacement [66]. A study also showed that decreased consumption of BCAAs could improve metabolic health [67]. Taken together, while it needs to be carefully vetted, arginine supplementation and restriction of BCAA intake might provide novel nutracutic approaches to OA management.
In addition, cyclooxygenase and lipoxygenase inhibitors have been used in OA patients for relieving pain, but with variable effects and significant adverse effects. Cyclooxygenase and lipoxygenase pathways are responsible for formation of individual eicosanoids, which are downstream from hydrolysis of the SN-2 fatty acid from PC to form lysoPC. Thus, the PC to lysoPC metabolic pathway could present novel targets for developing new drugs for OA, particularly, targeting on a specific PLA2, G5.
Conclusion and future direction
The current literature data suggest that three metabolic pathways may be of special significance in the pathogenesis of OA, namely those affecting BCAAs, arginine and PC to lysoPC metabolism. Table 1 summarized the key studies investigating the three most promising metabolic pathways and Fig. 3 summarizes the relationship between OA and these metabolic pathways. While increased BCAA concentration could be an OA diagnostic marker, arginine concentrations could be used for monitoring the OA process and progression. The PC to lysoPC metabolic pathway may play a role in mediating joint symptoms, particularly joint pain. While these findings require further confirmation in independent studies, selectively altering these three metabolic pathways may have great potential as a focus for new OA treatments. Carefully designed clinical trials should be conducted to investigate the disease-modifying effects of arginine supplementation or dietary restriction of BCAAs on OA while PLA2 G5 should be further examined as a potential druggable target for developing OA therapy. As pointed out by a recent review [68], such an effort could be facilitated when combining metabolomics with other omics such as genomics, which can enrich our understanding of pathogenesis and improve diagnosis, prognosis and disease management.
Table 1.
Key studies investigating the three most promising metabolic pathways
| Study design | Study population | Specimen | Sample size | Findings | References |
|---|---|---|---|---|---|
| Cross-sectional (discovery and replication) | Human, British females | Serum | 598 | BCAA to histidine ratio | Zhai et al. [26] |
| Cross-sectional (discovery and replication); longitudinal | Human, Canadian and Australian population | Plasma | 415 | BCAA to histidine ratio; lysoPC to PC ratio | Zhang et al. [27] |
| Animal OA model | Sheep | Serum | 72 | BCAAs | Maher et al. [28] |
| In vitro | Cultured human synovial tissue | OA synovium | 22 | Collagen, BCAA, energy and tryptophan metabolism | Adams et al. [48] |
| Cross-sectional (discovery and replication) | Human, Canadian population | Plasma | 256 | Arginine | Zhang et al. [49] |
| Cross-sectional | Human, Italian population | Plasma and SF | 63 | Arginine | Pascale et al. [51] |
| Animal OA model | Rabbits | Plasma | 12 | Arginine | Ohnishi et al. [53] |
| Cross-sectional | Human, German population | SF | 57 | PC metabolism | Kosinska et al. [54] |
| Cross-sectional (discovery and replication) | Human, Canadian population | Plasma and SF | 227 | PC metabolism | Zhang et al. [55] |
| Cross-sectional | Human, Dutch females | Plasma | 59 | LysoPC to PC ratio | Castro-Perez et al. [57] |
BCAA: branched chain amino acid; lysoPC: lysophosphatidylcholine; PC: phosphatidylcholine.
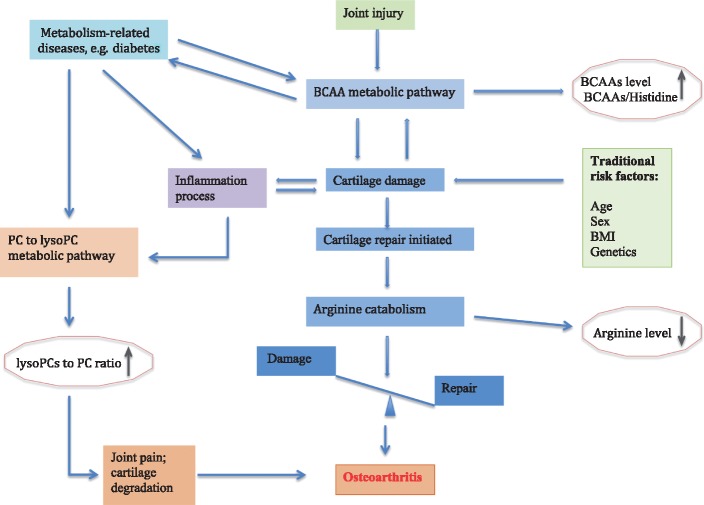
Fig. 3.
Hypothesized relationship between OA development and three promising metabolic pathways and related markers
BCAA metabolic pathway is altered because of either joint injury or articular cartilage pathology, whereas arginine catabolism is increased to activate arginine–proline pathway for cartilage repair or alternatively relatively selective utilization of arginine by other metabolic or biosynthetic process concurrent with OA process. Inflammatory factors either as an OA initiation factor or caused by OA disease process activate PC to lysoPC pathway, which mediates joint pain. Metabolism-related diseases such as diabetes alter both BCAA and PC to lysoPC metabolic pathways or mediate low-grade inflammation via lipopolysaccharide to contribute to OA, or alternatively both OA and diabetes share the same pathway. BCAA: branched chain amino acid; lysoPC: lysophosphatidylcholine; PC: phosphatidylcholine.
Funding: This work was supported by Canadian Institutes of Health Research (FRN153298; FRN143058; and FRN132178).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. WHO Scientific Group on the Burden of Musculoskeletal Conditions at the Start of the New Millennium. The Burden of Musculoskeletal Conditions at the Start of the New Millennium. World Health Organ Tech Rep Ser; 2003;919:i-x,1-218, back cover. [PubMed] [Google Scholar]
- 2. Vos T, Flaxman AD, Naghavi M. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hiligsmann M, Cooper C, Arden N. et al. Health economics in the field of osteoarthritis: an expert's consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2013;43:303–13. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Wluka AE, Jones G, Ding C, Cicuttini FM.. Use magnetic resonance imaging to assess articular cartilage. Ther Adv Musculoskelet Dis 2012;4:77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peterfy CG, Guermazi A, Zaim S. et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12:177–90. [DOI] [PubMed] [Google Scholar]
- 6. Lotz M, Martel-Pelletier J, Christiansen C. et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis 2013;72:1756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valdes AM, Meulenbelt I, Chassaing E. et al. Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthritis Cartilage 2014;22:683–9. [DOI] [PubMed] [Google Scholar]
- 8. Kerkhof HJ, Bierma-Zeinstra SM, Arden NK. et al. Prediction model for knee osteoarthritis incidence, including clinical, genetic and biochemical risk factors. Ann Rheum Dis 2014;73:2116–21. [DOI] [PubMed] [Google Scholar]
- 9. Blanco FJ. Osteoarthritis year in review 2014: we need more biochemical biomarkers in qualification phase. Osteoarthritis Cartilage 2014;22:2025–32. [DOI] [PubMed] [Google Scholar]
- 10. Hunter DJ, Nevitt M, Losina E, Kraus V.. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol 2014;28:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Meurs JB. Osteoarthritis year in review 2016: genetics, genomics and epigenetics. Osteoarthritis Cartilage 2017;25:181–9. [DOI] [PubMed] [Google Scholar]
- 12. Gowda GA, Zhang S, Gu H. et al. Metabolomics-based methods for early disease diagnostics. Expert Rev Mol Diagn 2008;8:617–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson CH, Ivanisevic J, Siuzdak G.. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 2016;17:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Markley JL, Bruschweiler R, Edison AS. et al. The future of NMR-based metabolomics. Curr Opin Biotechnol 2017;43:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Damyanovich AZ, Staples JR, Marshall KW.. 1H NMR investigation of changes in the metabolic profile of synovial fluid in bilateral canine osteoarthritis with unilateral joint denervation. Osteoarthritis Cartilage 1999;7:165–72. [DOI] [PubMed] [Google Scholar]
- 16. Lacitignola L, Fanizzi FP, Francioso E, Crovace A.. 1H NMR investigation of normal and osteo-arthritic synovial fluid in the horse. Vet Comp Orthop Traumatol 2008;21:85–8. [DOI] [PubMed] [Google Scholar]
- 17. Lamers RJ, DeGroot J, Spies-Faber EJ. et al. Identification of disease- and nutrient-related metabolic fingerprints in osteoarthritic guinea pigs. J Nutr 2003;133:1776–80. [DOI] [PubMed] [Google Scholar]
- 18. Herbert KE, Scott DL, Perrett D.. Nucleosides and bases in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin Sci 1988;74:97–9. [DOI] [PubMed] [Google Scholar]
- 19. Borel M, Pastoureau P, Papon J. et al. Longitudinal profiling of articular cartilage degradation in osteoarthritis by high-resolution magic angle spinning 1H NMR spectroscopy: experimental study in the meniscectomized guinea pig model. J Proteome Res 2009;8:2594–600. [DOI] [PubMed] [Google Scholar]
- 20. Williamson MP, Humm G, Crisp AJ.. 1H nuclear magnetic resonance investigation of synovial fluid components in osteoarthritis, rheumatoid arthritis and traumatic effusions. Br J Rheumatol 1989;28:23–7. [DOI] [PubMed] [Google Scholar]
- 21. Lamers RJ, van Nesselrooij JH, Kraus VB. et al. Identification of an urinary metabolite profile associated with osteoarthritis. Osteoarthritis Cartilage 2005;13:762–8. [DOI] [PubMed] [Google Scholar]
- 22. Mickiewicz B, Kelly JJ, Ludwig TE. et al. Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. J Orthop Res 2015;33:1631–8. [DOI] [PubMed] [Google Scholar]
- 23. Zhang W, Likhodii S, Zhang Y. et al. Classification of osteoarthritis phenotypes by metabolomics analysis. BMJ Open 2014;4:e006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan TW, Lane AN.. Applications of NMR spectroscopy to systems biochemistry. Prog Nucl Magn Reson Spectrosc 2016;92–93:18–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lei Z, Huhman DV, Sumner LW.. Mass spectrometry strategies in metabolomics. J Biol Chem 2011;286:25435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhai G, Wang-Sattler R, Hart DJ. et al. Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis. Ann Rheum Dis 2010;69:1227–31. [DOI] [PubMed] [Google Scholar]
- 27. Zhang W, Sun G, Aitken D. et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology 2016;55:1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maher AD, Coles C, White J. et al. 1H NMR spectroscopy of serum reveals unique metabolic fingerprints associated with subtypes of surgically induced osteoarthritis in sheep. J Proteome Res 2012;11:4261–8. [DOI] [PubMed] [Google Scholar]
- 29. Neuman P, Kostogiannis I, Friden T. et al. Patellofemoral osteoarthritis 15 years after anterior cruciate ligament injury – a prospective cohort study. Osteoarthritis Cartilage 2009;17:284–90. [DOI] [PubMed] [Google Scholar]
- 30. Newgard CB, An J, Bain JR. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Batch BC, Shah SH, Newgard CB. et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 2013;62:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang Y, Zhou M, Sun H, Wang Y.. Branched-chain amino acid metabolism in heart disease: an epiphenomenon or a real culprit? Cardiovasc Res 2011;90:220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang TJ, Larson MG, Vasan RS. et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCormack SE, Shaham O, McCarthy MA. et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes 2013;8:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Badoud F, Lam KP, DiBattista A. et al. Serum and adipose tissue amino acid homeostasis in the metabolically healthy obese. J Proteome Res 2014;13:3455–66. [DOI] [PubMed] [Google Scholar]
- 36. Berenbaum F, Sellam J.. Obesity and osteoarthritis: what are the links? Joint Bone Spine 2008;75:667–8. [DOI] [PubMed] [Google Scholar]
- 37. Schett G, Kleyer A, Perricone C. et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 2013;36:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshimura N, Muraki S, Oka H. et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 2012;20:1217–26. [DOI] [PubMed] [Google Scholar]
- 39. Kluzek S, Newton JL, Arden NK.. Is osteoarthritis a metabolic disorder? Br Med Bull 2015;115:111–21. [DOI] [PubMed] [Google Scholar]
- 40. Courties A, Sellam J, Berenbaum F.. Metabolic syndrome-associated osteoarthritis. Curr Opin Rheumatol 2017;29:214–22. [DOI] [PubMed] [Google Scholar]
- 41. Huang Z, Kraus VB.. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev Rheumatol 2016;12:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zabek A, Swierkot J, Malak A. et al. Application of 1H NMR-based serum metabolomic studies for monitoring female patients with rheumatoid arthritis. J Pharm Biomed Anal 2016;117:544–50. [DOI] [PubMed] [Google Scholar]
- 43. Priori R, Casadei L, Valerio M. et al. 1H-NMR-based metabolomic study for identifying serum profiles associated with the response to etanercept in patients with rheumatoid arthritis. PLoS One 2015;10:e0138537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brosnan JT, Brosnan ME.. Branched-chain amino acids: enzyme and substrate regulation. J Nutr 2006;136:207S–11S. [DOI] [PubMed] [Google Scholar]
- 45. Burrage LC, Nagamani SC, Campeau PM, Lee BH.. Branched-chain amino acid metabolism: from rare Mendelian diseases to more common disorders. Human Mol Genet 2014;23:R1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aref-Eshghi E, Zhang Y, Hart D. et al. SMAD3 is associated with the total burden of radiographic osteoarthritis: the Chingford study. PLoS One 2014;9:e97786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zatkova A. An update on molecular genetics of Alkaptonuria (AKU). J Inherit Metab Dis 2011;34:1127–36. [DOI] [PubMed] [Google Scholar]
- 48. Adams SB Jr, Setton LA, Kensicki E. et al. Global metabolic profiling of human osteoarthritic synovium. Osteoarthritis Cartilage 2012;20:64–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang W, Sun G, Likhodii S. et al. Metabolomic analysis of human plasma reveals that arginine is depleted in knee osteoarthritis patients. Osteoarthritis Cartilage 2016;24:827–34. [DOI] [PubMed] [Google Scholar]
- 50. Morris SM., Jr. Arginine metabolism: boundaries of our knowledge. J Nutr 2007;137:1602S–9S. [DOI] [PubMed] [Google Scholar]
- 51. Pascale V, Pascale W, Lavanga V. et al. L-arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in plasma and synovial fluid of patients with knee osteoarthritis. Med Sci Monit 2013;19:1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morris SM., Jr. Arginine: beyond protein. Am J Clin Nutr 2006;83:508S–12S. [DOI] [PubMed] [Google Scholar]
- 53. Ohnishi A, Osaki T, Matahira Y. et al. Correlation of plasma amino acid concentrations and chondroprotective effects of glucosamine and fish collagen peptide on the development of osteoarthritis. J Vet Med Sci 2013;75:497–502. [DOI] [PubMed] [Google Scholar]
- 54. Kosinska MK, Liebisch G, Lochnit G. et al. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum 2013;65:2323–33. [DOI] [PubMed] [Google Scholar]
- 55. Zhang W, Sun G, Likhodii S. et al. Metabolomic analysis of human synovial fluid and plasma reveals that phosphatidylcholine metabolism is associated with both osteoarthritis and diabetes mellitus. Metabolomics 2015;12:24. [Google Scholar]
- 56. Zhang W, Randell EW, Sun G. et al. Hyperglycemia-related advanced glycation end-products is associated with the altered phosphatidylcholine metabolism in osteoarthritis patients with diabetes. PLoS One 2017;12:e0184105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Castro-Perez JM, Kamphorst J, DeGroot J. et al. Comprehensive LC-MS E lipidomic analysis using a shotgun approach and its application to biomarker detection and identification in osteoarthritis patients. J Proteome Res 2010;9:2377–89. [DOI] [PubMed] [Google Scholar]
- 58. Pruzanski W, Bogoch E, Stefanski E, Wloch M, Vadas P.. Enzymatic activity and distribution of phospholipase A2 in human cartilage. Life Sci 1991;48:2457–62. [DOI] [PubMed] [Google Scholar]
- 59. Leistad L, Feuerherm AJ, Faxvaag A, Johansen B.. Multiple phospholipase A2 enzymes participate in the inflammatory process in osteoarthritic cartilage. Scand J Rheumatol 2011;40:308–16. [DOI] [PubMed] [Google Scholar]
- 60. Fioravanti A, Fabbroni M, Cerase A, Galeazzi M.. Treatment of erosive osteoarthritis of the hands by intra-articular infliximab injections: a pilot study. Rheumatol Int 2009;29:961–5. [DOI] [PubMed] [Google Scholar]
- 61. Magnano MD, Chakravarty EF, Broudy C. et al. A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J Rheumatol 2007;34:1323–7. [PubMed] [Google Scholar]
- 62. Chevalier X, Ravaud P, Maheu E. et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: a randomised, multicentre, double-blind, placebo-controlled trial. Ann Rheum Dis 2015;74:1697–705. [DOI] [PubMed] [Google Scholar]
- 63. Wang P, Guan PP, Guo C. et al. Fluid shear stress-induced osteoarthritis: roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. FASEB J 2013;27:4664–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Morita M, Hayashi T, Ochiai M. et al. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem Biophys Res Commun 2014;454:53–7. [DOI] [PubMed] [Google Scholar]
- 65. Hristina K, Langerholc T, Trapecar M.. Novel metabolic roles of L-arginine in body energy metabolism and possible clinical applications. J Nutr Health Aging 2014;18:213–8. [DOI] [PubMed] [Google Scholar]
- 66. Nishizaki K, Ikegami H, Tanaka Y, Imai R, Matsumura H.. Effects of supplementation with a combination of beta-hydroxy-beta-methyl butyrate, L-arginine, and L-glutamine on postoperative recovery of quadriceps muscle strength after total knee arthroplasty. Asia Pac J Clin Nutr 2015;24:412–20. [DOI] [PubMed] [Google Scholar]
- 67. Fontana L, Cummings NE, Arriola Apelo SI. et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep 2016;16:520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Menni C, Zierer J, Valdes AM, Spector TD.. Mixing omics: combining genetics and metabolomics to study rheumatic diseases. Nat Rev Rheumatol 2017;13:174–81. [DOI] [PubMed] [Google Scholar]