Abstract
Objectives
To examine whether gout is an independent risk factor for total joint replacement (TJR) and whether urate-lowering treatment (ULT) reduces this risk.
Methods
Using the Taiwan National Health Insurance database and the UK Clinical Practice Research Datalink, 74 560 Taiwan patients and 34 505 UK patients with incident gout were identified and age and sex matched to people without gout. Cox proportional hazards models and condition logistic regression were used to examine the risk of TJR in gout patients and the association between cumulative defined daily dose (cDDD) of ULT and TJR.
Results
The prevalence rates of TJR in the patients at the time of diagnosis of gout and in people without gout were 1.16% vs 0.82% in Taiwan and 2.61% vs 1.76% in the UK. After a gout diagnosis, the incidence of TJR was higher in the patients with gout compared with those without (3.23 vs 1.91 cases/1000 person-years in Taiwan and 6.87 vs 4.61 cases/1000 person-years in the UK), with adjusted HRs of 1.56 (95% CI 1.45, 1.68) in Taiwan and 1.14 (1.05, 1.22) in the UK. Compared with patients with gout with <28 cDDD ULT, the adjusted ORs for TJR were 0.89 (95% CI 0.77, 1.03) for 28–90 cDDD, 1.03 (0.85, 1.24) for 90–180 cDDD and 1.12 (0.94, 1.34) for >180 cDDD ULT in Taiwan. In the UK, the respective ORs were 1.09 (0.83, 1.42), 0.93 (0.68, 1.27) and 1.08 (0.94, 1.24).
Conclusion
This population-based study provides evidence from two nation populations that gout confers significant TJR risk, which was not reduced by current ULT.
Keywords: gout, urate-lowering treatment, total joint replacement
Rheumatology key messages
Gout patients have a higher risk of total hip and knee replacement at diagnosis.
The incidence of total joint replacement after a gout diagnosis was higher than in those without gout.
Current urate-lowering treatment did not reduce the risk of total joint replacement in gout patients.
Introduction
Clinically, gout is characterized by recurrent acute attacks of synovitis, chronic arthritis, subcutaneous urate crystal concretions (tophi) and an increased risk of urolithiasis [1]. It is also associated with higher mortality [2] and many co-morbidities [3–8]. Gout results from chronic hyperuricaemia, which leads to the deposition of monosodium urate (MSU) crystals, mainly in and around peripheral joints, that can damage joints irreversibly. As with OA and other arthropathies, gout may result in the need for total joint replacement (TJR) to restore function and reduce pain. Mechanistic explanations for the irreversible joint damage associated with gout primarily focus on the interaction between MSU crystals and joint tissues, especially cartilage, bone and synovium [9–11]. However, few studies have investigated the association between irreversible joint damage in gout, which may result in an increased need for total hip replacement (THR) and total knee replacement (TKR). One previous study reported the co-localization of joints affected by acute attacks of gout and OA [12], and we recently examined the risk of co-morbidities associated with gout and found a higher prevalence of OA in people with gout [13]. The positive association between gout and OA, the single most important risk factor for TJR, could potentially lead to an increased risk of TJR in patients with gout [14].
Effective urate-lowering treatment (ULT) reverses hyperuricaemia [15] and may help dissolve away MSU crystals, which theoretically may retard the progression of joint damage and subsequently reduce the risk of TJR in patients with gout. However, the current use of ULT is suboptimal, with only a third of patients being prescribed ULT, usually at a fixed dose without titration to a target serum uric acid level [16]. This may contribute to an increased occurrence of complicated gout [17]. In addition, people with gout are exposed to hyperuricaemia and subclinical MSU crystal deposition long before the initial clinical presentation and diagnosis of the disease. By the time people present clinically (usually with an acute attack of gout) and are diagnosed, substantial irreversible joint damage may have already occurred [18–21]. Therefore it is possible that initiating ULT after the occurrence of symptomatic arthritis may be insufficient to reduce the risk of TJR. To the best of our knowledge, no previous study has examined the risk of TJR of the knee or hip in patients with gout and the possible effect of current ULT practice on the risk of TJR.
We therefore used two population-based health databases, the Taiwan National Health Insurance (NHI) database and the UK Clinical Practice Research Datalink (CPRD) to test the hypotheses that people with gout are at increased risk of joint damage and subsequent TJR and that the current practice of ULT usage may retard the progression of joint damage in people with gout. We used a cohort study of patients with incident gout and matched people without gout identified from the general population of Taiwan and the UK to compare the risk of TJR prior to and following diagnosis. In addition, we used a nested case–control study within the incident gout cohort to examine the effect of the prior use of ULT on the risk of TJR.
Methods
Sources of data
This study was approved by the institutional review boards of Chang Gung Memorial Hospital and data holders of the Taiwan NHI database and the UK CPRD. The NHI database contains clinical and demographic data of the entire population of Taiwan generated from the routine clinical practices of primary, secondary and specialist care since 1996. The database covers ∼29 million people, both alive and deceased as of 2016. The data collected include demographic information, medical diagnoses, prescriptions, surgical and diagnostic procedures, referrals, vaccinations, Chinese herbal medicine, pharmacy, dental care and maternity care. The centralized health insurance scheme ensures a coverage rate of 99.5% and uniform data recording. Diagnoses and procedures are recorded using the International Classification of Diseases, 9th revision (ICD-9) diagnosis and procedure codes. The NHI database has been used for a wide variety of clinical and epidemiological research, including the assessment of disease burden, pharmaco-epidemiology, genetic epidemiology and planning for health policy [13, 14, 16, 22, 23]. Multiple studies have validated the NHI database and, in general, the accuracy of the recorded diagnoses in this database is high [24–29].
The CPRD is an anonymized longitudinal database from UK general practices containing individual-level medical records. It was initiated in 1987 and includes ∼14 million individuals in the UK. This database records comprehensive information on demographics, lifestyle factors, medical diagnoses, results of investigations and examinations, operations, consultations, referrals and prescribed medications. The diagnostic coding system in the CPRD is the Read code. The database has been well validated for many diagnoses [30–33]. Patient consent was not required per ethical approval by the institutional review board because all data obtained from the Taiwan NHI database and the UK CPRD were anonymized by the data holder.
Study design
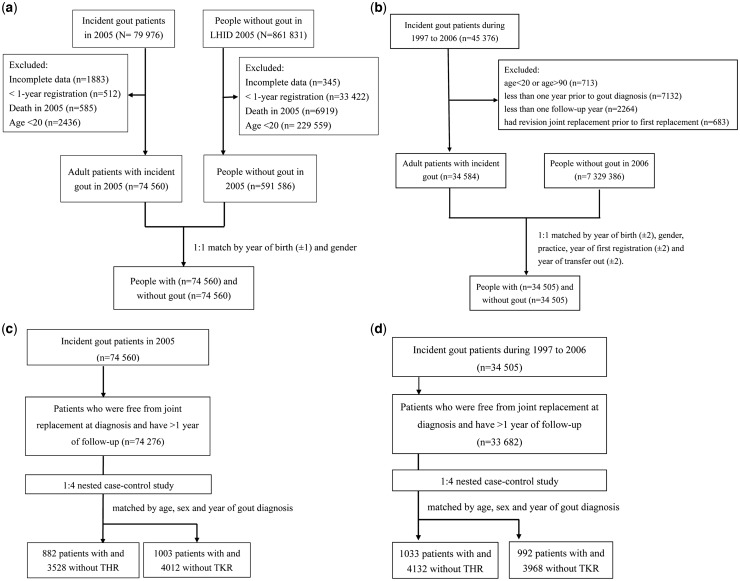
We conducted this matched cohort study to examine the risk of TJR, including THR and TKR in patients with incident gout and people without any record of a diagnosis of gout or prescription for ULT. We identified all incident gout patients diagnosed in 2005 in the general population of Taiwan from the main NHI database and identified people without gout using a systematically random sample of one million people from the general population in 2005 (Fig. 1A). This calendar year was chosen because we wanted to have appropriate periods to examine the TJR risk both prior to (1996–2005) and after the diagnosis of gout (2005–13). The patients with incident gout had to have at least one record of a diagnosis of gout (ICD-9 code: 274.x) and had prescriptions for ULT after the diagnosis of gout in 2005, no evidence of gout and no prescriptions for ULT prior to the date of diagnosis (index date) and at least 1 year of continuous registration in the NHI programme prior to the index date. The validity of the gout diagnosis has been performed in a previous study that showed a sensitivity of 76% and specificity of 1.00 [26]. For the systematically random sample, we identified at random one subject who had no record of gout and no prescriptions for ULT per gout patient. People without gout were matched individually in a 1:1 ratio to the patients with incident gout by year of birth (±1 year) and gender. The same index date was assigned to each of the matched unexposed subjects. As with the patients with incident gout, the unexposed subjects had at least 1 year of active participation in the NHI programme prior to the index date. They were followed up and censored at the earliest date of the first recorded TJR, death, deregistration from the NHI or the end date of the study (31 December 2013). For those who had both THR and TKR, their first record of TKR or THR was used for TJR. The identification of a TJR was based on hospital records for the surgical procedure.
Fig. 1.
Flow chart of the study population
(A) Cohort study based on the Taiwan NHI database. (B) Cohort study based on the UK CPRD. (C) Nested case–control study based on the Taiwan NHI database. (D) Nested case–control study based on the UK CPRD.
For the UK, we identified patients with a new diagnosis of gout from 1997 to 2006 who had at least a 1 year continuous registration prior to the index date (Fig. 1B). To ensure the data quality recorded by each general practitioner (GP), only those registered with up-to-standard practices were included. The Read codes used to identify gout and ULT are listed in supplementary Table S1, available at Rheumatology online. This code list was developed by our research team as described in our previous publications [13, 16, 34]. In addition, the validity of a gout diagnosis in the CPRD has been performed previously. When compared with a retrospective chart review, the recorded diagnosis of gout in the CPRD is accurate in 90% of cases [33]. People without gout included participants from up-to-standard practices between 1997 and 2006 who had at least 1 year of continuous registration and no record of gout or any ULT use prior to the index date. We matched the patients with gout at a ratio of 1:1 to unexposed subjects by year of birth (±2 years), gender, general practice, year of first continuous registration (±2 years) and year of transfer out (±2 years) at the same CPRD practice. The index date was defined as the initial date of diagnosis of gout in the cases, with the same date applied to their matched unexposed subjects.
To examine the effect of ULT on TJR in the patients with gout, we carried out a nested case–control study using data from the incident gout cohorts for both the Taiwan and UK populations. Incident cases of TJR were identified during the follow-up and matched at a 1:4 ratio to people without TJR by age, sex and year of gout diagnosis. The index date of each TJR (first date of hospitalization for TJR for the Taiwan cohort and the date of the first GP record of TJR in the UK population) was allocated to the matched unexposed subjects who had not undergone joint replacement (Fig. 1C for the Taiwan population and Fig. 1D for the UK population). The reason for matching the year of gout diagnosis and the index date was to ensure that those with and without TJR had the same duration of ULT exposure. Separate analyses were conducted for THR and TKR.
Outcomes
The main outcome was TJR. In the Taiwan population, ascertainment of TJR was based on inpatient records. We used ICD-9 procedure codes to ascertain primary TKR (815.1–815.3) and THR (815.4–815.5). In the UK population, the ascertainment of TJR was based on GP records. The Read codes used to identify TJR are shown in supplementary Table S1, available at Rheumatology online.
ULT
In Taiwan, both xanthine oxidase inhibitors (allopurinol and febuxostat) and uricosuric agents (benzbromarone, sulfinpyrazone and probenecid) are approved and reimbursed by the NHI. Therefore ULT was further classified into xanthine oxidase inhibitors and uricosuric agents. However, in general practice in the UK, allopurinol is the only widely available ULT. Therefore, in the UK analysis, we only analysed the use of allopurinol.
The cumulative defined daily dose (cDDD; the average maintenance dose per day for a drug used for its primary indication in adults) was used to define the prescribed amount of ULT from the time of first diagnosis of gout to the index date of TJR [35]. Since we have transformed cumulative dose into the same units (DDDs), cumulative doses of different ULTs can be summed. We categorized exposure to ULT into four groups (<28, 28–90, 91–180 and >180 cDDD) to evaluate the dose–response effect between ULT use and TJR.
Confounding variables
We included confounding covariates that were likely to be associated with the risk of TJR. These included age at diagnosis of gout, gender, socio-economic status, Charlson co-morbidity index [36], other co-morbidities relevant to TJR (RA, OA, diabetes and hip fracture) and medications (proton pump inhibitors, NSAIDs, vitamin D, bisphosphonate, glucocorticoids, insulin, other hypoglycaemic agents, anti-hypertensives, nitrates, lipid-lowering agents and anticonvulsants). In the UK population, we also adjusted for BMI, smoking status and alcohol consumption.
Statistical analysis
The prevalence of TJR at the time of diagnosis of gout was estimated in both the Taiwan and UK cohorts. Multivariate conditional logistic regression was performed to estimate odds ratios (ORs) and 95% CIs for TJR. The Kaplan–Meier method was used to compute the cumulative incidence of TJR after a gout diagnosis in the patients with incident gout and matched unexposed subjects. Unadjusted and adjusted hazard ratios (HRs) and 95% CIs with adjustments for confounding variables were calculated for the risk of TJR using a Cox proportional hazards model. The results of the nested case–control study for the effect of ULT on TJR are presented as OR (95% CI) estimated by conditional logistic regression. We conducted a sensitivity analysis by excluding people with OA, RA or hip fractures prior to or at the diagnosis of gout. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Prevalence of TJR at the initial diagnosis of gout compared with people without gout
As shown in Fig. 1A and B, we identified 74 560 patients with gout and 74 560 matched subjects in the Taiwan cohort and 34 505 patients with gout and 34 505 matched unexposed subjects in the UK cohort. Table 1 shows the characteristics of the patients with gout and their matched unexposed subjects at diagnosis. The mean age at diagnosis of gout in the UK cohort [61.4 (s.d. 14.8) years] was significantly older than that in the Taiwan cohort [48.8 (s.d. 16.4) years; P < 0.001]. The Taiwan cohort had a longer observation period before a diagnosis of gout, however, the median follow-up period was similar (8.5 vs 8.8 years). In both cohorts the patients with incident gout tended to have more co-morbidities with a significantly higher Charlson co-morbidity index. Co-morbidities related to TJR that were more common in the patients with gout included diabetes and OA, and this was consistent in both cohorts.
Table 1.
Demographic and co-morbid characteristics of the Taiwan and UK cohorts
| Characteristics | Taiwan cohort | UK cohort | ||||
|---|---|---|---|---|---|---|
| Gout cases (n = 74 560) | People without gout (n = 74 560) | P-value | Gout cases (n = 34 505) | People without gout (n = 34 505) | P-value | |
| Age, mean (s.d.), years | 48.8 (16.4) | 48.8 (16.4) | NA | 61.4 (14.8) | 61.1 (14.8) | NA |
| Male, n (%) | 54 583 (73.21) | 54 583 (73.21) | NA | 25 410 (73.64) | 25 410 (73.64) | NA |
| Observation years, median (IQR) | ||||||
| Prior to index date | 10.3 (10.0–10.5) | 10.3 (10.0–10.5) | 0.277 | 5.7 (3.1–9.3) | 5.6 (2.9–9.3) | <0.001 |
| After index date | 8.5 (8.2–8.7) | 8.5 (8.2–8.7) | <0.001 | 8.8 (4.9–11.6) | 8.8 (4.8–11.7) | <0.001 |
| CCI scores, n (%) | ||||||
| Mean (s.d.) | 0.28 (0.74) | 0.22 (0.71) | <0.001 | 0.32 (0.75) | 0.19 (0.57) | <0.001 |
| 0 | 60 774 (81.51) | 63 913 (85.72) | <0.001 | 27 038 (78.36) | 29 717 (86.12) | <0.001 |
| 1 | 9283 (12.45) | 7036 (9.44) | 4939 (14.31) | 3425 (9.92) | ||
| ≥2 | 4503 (6.04) | 3611 (4.84) | 2528 (7.33) | 1363 (3.95) | ||
| Co-morbidities, n (%) | ||||||
| RA | 99 (0.13) | 157 (0.21) | <0.001 | 108 (0.31) | 139 (0.40) | 0.048 |
| Diabetes mellitus | 5297 (7.10) | 4504 (6.04) | <0.001 | 2295 (6.65) | 1792 (5.19) | <0.001 |
| OA | 5219 (7.00) | 3134 (4.20) | <0.001 | 1957 (5.67) | 1319 (3.82) | <0.001 |
| Hip fracture | 147 (0.20) | 150 (0.20) | 0.862 | 11 (0.03) | 15 (0.04) | 0.432 |
| Co-medications, n (%) | ||||||
| Proton pump inhibitor | 2523 (3.38) | 1976 (2.65) | <0.001 | NA | ||
| NSAIDs | 54 801 (73.50) | 42 524 (57.03) | <0.001 | 26 055 (75.5) | 8498 (24.6) | <0.001 |
| Vitamin D | 63 (0.08) | 50 (0.07) | 0.221 | 709 (2.1) | 587 (1.7) | <0.001 |
| Bisphosphonate | 155 (0.21) | 187 (0.25) | 0.083 | 720 (2.1) | 713 (2.1) | 0.852 |
| Glucocorticoids | 15 257 (20.46) | 10 592 (14.21) | <0.001 | NA | ||
| Insulin | 1097 (1.47) | 847 (1.14) | <0.001 | 429 (1.2) | 427 (1.2) | 0.945 |
| Other hypoglycaemic agents | 5299 (7.11) | 4243 (5.69) | <0.001 | 1596 (4.6) | 1350 (3.9) | <0.001 |
| Anti-hypertensives | 25 401 (34.07) | 15 383 (20.63) | <0.001 | 14 963 (43.4) | 7674 (22.2) | <0.001 |
| Nitrates | 3371 (4.52) | 2159 (2.90) | <0.001 | 4196 (12.2) | 2266 (6.6) | <0.001 |
| Lipid-lowering agents | 5222 (7.00) | 3178 (4.26) | <0.001 | 670 (1.9) | 272 (0.8) | <0.001 |
| Anticonvulsants | 2845 (3.82) | 2218 (2.97) | <0.001 | 691 (2.0) | 706 (2.0) | 0.685 |
| Place of residence, n (%) | ||||||
| Urban | 40 328 (54.09) | 43 004 (57.68) | <0.001 | NA | ||
| Suburban | 23 858 (32.00) | 23 600 (31.65) | ||||
| Rural | 8194 (10.99) | 6338 (8.50) | ||||
| Unknown | 2180 (2.92) | 1618 (2.17) | ||||
| Income level, n (%) | ||||||
| Quintile 1 | 15 499 (20.79) | 15 627 (20.96) | <0.001 | NA | ||
| Quintile 2 | 5698 (7.64) | 5599 (7.51) | ||||
| Quintile 3 | 24 768 (33.22) | 22 386 (30.02) | ||||
| Quintile 4 | 14 783 (19.83) | 15 086 (20.23) | ||||
| Quintile 5 | 13 812 (18.52) | 15 862 (21.27) | ||||
| Occupationb, n (%) | ||||||
| 1 | 13 746 (18.44) | 14 094 (18.90) | <0.001 | NA | ||
| 2 | 2625 (3.52) | 3116 (4.18) | ||||
| 3 | 22 401 (30.04) | 24 029 (32.23) | ||||
| 4 | 25 176 (33.77) | 22 504 (30.18) | ||||
| 5 | 10 612 (14.23) | 10 817 (14.51) | ||||
| BMI, n (%), kg/m2 | ||||||
| 18.5–24.9 | NA | 6856 (19.9) | 10 224 (29.6) | <0.001 | ||
| <18.5 | 223 (0.6) | 512 (1.5) | ||||
| 25.0–29.9 | 13 739 (39.8) | 11 126 (32.2) | ||||
| ≥30 | 11 094 (32.2) | 5278 (15.3) | ||||
| Unknown | 2593 (7.5) | 7365 (21.3) | ||||
| Smoking, n (%) | ||||||
| Non-smoker | NA | 11 088 (32.1) | 9064 (26.3) | <0.001 | ||
| Current smoker | 9172 (26.6) | 6514 (18.9) | ||||
| Ex-smoker | 10 869 (31.5) | 11 117 (32.2) | ||||
| Unknown | 3376 (9.8) | 7810 (22.6) | ||||
| Alcohol consumption, n (%), U/week | ||||||
| Never/ex-drinker | NA | 3368 (9.8) | 3616 (10.5) | <0.001 | ||
| Current 1–9 | 13 963 (40.5) | 13 750 (39.8) | ||||
| Current ≥10 | 11 553 (33.5) | 6931 (20.1) | ||||
| Unknown | 5621 (16.3) | 10 208 (29.6) | ||||
CCI: Charlson co-morbidity index; NA: not available.
At the index date, the prevalence of TJR was significantly higher in the patients with incident gout than in people without gout (1.16 vs 0.82% in the Taiwan cohort and 2.61 vs 1.76% in the UK cohort). In the unadjusted analysis, gout was associated with a statistically significant increased risk of TJR in both Taiwan [OR 1.44 (95% CI 1.29, 1.60)] and the UK [OR 1.50 (95% CI 1.35, 1.67)] (supplementary Table S2, available at Rheumatology online). After adjusting for covariates, the association between gout and TJR at diagnosis was still statistically significant [adjusted OR in Taiwan 1.21 (95% CI 1.09, 1.35); adjusted OR in the UK 1.21 (95% CI 1.07, 1.37)].
Incidence of TJR after a diagnosis of gout compared with the people without gout
Overall there were 1898 incident cases of TJR in the Taiwan cohort and 1906 cases in the UK cohort. The incidence of TJR was higher in the gout group than in unexposed subjects in both the Taiwan (3.23 vs 1.91/1000 person-years) and UK (6.87 vs 4.61/1000 person-years) cohorts. Fig. 2 shows the cumulative incidence of THR and TKR in both the Taiwan and UK cohorts. Crude risk estimates for THR, TKR and TJR were significantly higher in the gout group compared with people without gout. The unadjusted HR for TJR was 1.70 (95% CI 1.58, 1.83) in the Taiwan cohort and 1.49 (95% CI 1.38, 1.60) in the UK cohort. After adjusting for covariates, the HR was 1.56 (95% CI 1.45, 1.68) for TJR in the Taiwan cohort and 1.14 (95% CI 1.05, 1.22) in the UK cohort. The HRs and 95% CIs for THR and TJR are presented in Table 2.
Fig. 2.
The cumulative incidence of THR and TKR in patients with gout and without gout
(A) THR in Taiwan. (B) TKR in Taiwan. (C) THR in the UK. (D) TKR in the UK.
Table 2.
Risk of TJR after diagnosis in the patients with gout compared with their matched unexposed subjects
| Gout group | People without gout | Risk estimates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk estimates | Events | Total person-years | Incidence (95% CI)a | Events | Total person-years | Incidence (95% CI) a | Absolute risk difference (95% CI)a | Unadjusted HR (95% CI)b | Adjusted HR (95% CI)b |
| Taiwan cohort | |||||||||
| THR | 944 | 594 021 | 1.59 (1.49, 1.69) | 647 | 600 227 | 1.08 (0.99, 1.16) | 0.51 (0.38, 0.64) | 1.47 (1.33, 1.63)* | 1.37 (1.25, 1.52)* |
| TKR | 1041 | 593 516 | 1.75 (1.65, 1.86) | 533 | 600 962 | 0.89 (0.81, 0.96) | 0.86 (0.74, 1.00) | 1.98 (1.78, 2.20)* | 1.77 (1.59, 1.97)* |
| TJR | 1898 | 587 296 | 3.23 (3.09, 3.38) | 1137 | 596 641 | 1.91 (1.79, 2.02) | 1.33 (1.14, 1.51) | 1.70 (1.58, 1.83)* | 1.56 (1.45, 1.68)* |
| UK cohort | |||||||||
| THR | 1058 | 284 787 | 3.72 (3.49, 3.94) | 715 | 284 816 | 2.51 (2.33, 2.69) | 1.21 (0.92, 1.49) | 1.45 (1.32, 1.60)* | 1.19 (1.08, 1.32)* |
| TKR | 1021 | 285 650 | 3.57 (3.36, 3.79) | 665 | 285 353 | 2.33 (2.15, 2.51) | 1.24 (0.96, 1.53) | 1.53 (1.39, 1.69)* | 1.09 (0.98, 1.20) |
| TJR | 1906 | 277 586 | 6.87 (6.56, 7.17) | 1290 | 280 010 | 4.61 (4.36, 4.86) | 2.26 (1.86, 2.66) | 1.49 (1.38, 1.60)* | 1.14 (1.05, 1.22)* |
Unit: cases per 1000 person-years.
HRs and 95% CIs estimated by Cox proportional hazards analysis and adjusted for socio-economic status, Charlson co-morbidity index, co-morbidities and co-medications in the Taiwan cohort and Charlson co-morbidity index, BMI, smoking status, alcohol consumption, co-morbidities and co-medications in the UK cohort.
P < 0.05.
Association between ULT and risk of TJR in the patients with gout
Table 3 shows the associations between cDDDs of ULT and TJR in both cohorts. Compared with the patients with gout with a cDDD <28 for xanthine oxidase inhibitors, cDDDs of 28–90, 91–180 and >180 were associated with adjusted ORs for TJR of 1.06 (95% CI 0.83, 1.34), 1.02 (0.70, 1.50) and 1.25 (0.83, 1.88) in the Taiwan cohort and 1.09 (0.83, 1.42), 0.93 (0.68, 1.27) and 1.08 (0.94, 1.24) in the UK cohort, but these estimates did not reach statistical significance. We conducted further analyses of uricosuric agents in Taiwan only, as they were not available in the UK. Similarly, no association was found between the use of uricosuric agents and the risk of TJR.
Table 3.
Nested case–control study to assess the association between ULT use and TJR among patients with gout
| Use of uric acid drugs | Taiwan cohort | UK cohort | ||||
|---|---|---|---|---|---|---|
| THR, adjusted OR (95% CI) | TKR, adjusted OR (95% CI) | TJR, adjusted OR (95% CI) | THR, adjusted OR (95% CI) | TKR, adjusted OR (95% CI) | TJR, adjusted OR (95% CI) | |
| Xanthine oxidase inhibitors | ||||||
| <28 cDDD | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 28–≤90 cDDD | 1.03 (0.74, 1.44) | 1.06 (0.75, 1.50) | 1.06 (0.83, 1.34) | 1.13 (0.82, 1.56) | 0.93 (0.62, 1.40) | 1.09 (0.83, 1.42) |
| 91–≤180 cDDD | 0.92 (0.52, 1.62) | 0.91 (0.54, 1.52) | 1.02 (0.70, 1.50) | 1.36 (0.92, 2.02) | 0.69 (0.43, 1.11) | 0.93 (0.68, 1.27) |
| >180 cDDD | 1.27 (0.74, 2.20) | 0.75 (0.43, 1.33) | 1.25 (0.83, 1.88) | 1.02 (0.85, 1.22) | 1.01 (0.83, 1.22) | 1.08 (0.94, 1.24) |
| P for trend | 0.57 | 0.42 | 0.31 | 0.59 | 0.88 | 0.34 |
| Uricosuric agentsa | ||||||
| <28 cDDD | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | NA | NA | NA |
| 28–≤90 cDDD | 0.91 (0.73, 1.13) | 0.97 (0.78, 1.20) | 0.89 (0.77, 1.04) | NA | NA | NA |
| 91–≤180 cDDD | 0.98 (0.73, 1.30) | 0.95 (0.70, 1.27) | 1.00 (0.81, 1.23) | NA | NA | NA |
| >180 cDDD | 0.96 (0.73, 1.27) | 1.16 (0.89, 1.52) | 1.04 (0.86, 1.26) | NA | NA | NA |
| P for trend | 0.67 | 0.48 | 0.93 | NA | NA | NA |
| Urate-lowering therapy | ||||||
| <28 cDDD | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | NA | NA | NA |
| 28–≤90 cDDD | 0.89 (0.72, 1.10) | 0.98 (0.79, 1.20) | 0.89 (0.77, 1.03) | NA | NA | NA |
| 91–≤180 cDDD | 0.99 (0.76, 1.30) | 1.03 (0.79, 1.35) | 1.03 (0.85, 1.24) | NA | NA | NA |
| >180 cDDD | 1.04 (0.81, 1.33) | 1.09 (0.85, 1.40) | 1.12 (0.94, 1.34) | NA | NA | NA |
| P for trend | 0.91 | 0.53 | 0.36 | NA | NA | NA |
ORs and 95% CIs estimated by conditional logistic regression analysis and adjusted for socio-economic status, Charlson co-morbidity index, co-morbidities and co-medications in the Taiwan cohort and Charlson co-morbidity index, BMI, smoking status, alcohol consumption, co-morbidities and co-medications in the UK cohort.
*P < 0.05.
Sensitivity analysis
We conducted a sensitivity analysis by excluding people with TJR, RA, OA and hip fractures prior to the initial diagnosis of gout. Comparisons of the risk of TJR at and after a diagnosis of gout in both cohorts are shown in supplementary Table S3, available at Rheumatology online. Similar to the primary analysis, in general the patients with gout had a higher risk of TJR at or after the initial diagnosis compared with their matched unexposed subjects. The results of nested case–control studies examining the impact of ULT on the risk of TJR were shown in supplementary Table S4, available at Rheumatology online. Similar to the primary analysis, ULT did not alter the risk of TJR in the patients with gout in both countries.
Discussion
In this population-based study using data from the general populations of Taiwan and the UK, we found that the risk of TJR at both the hip and the knee was significantly higher in patients with gout than in people without gout. However, among the patients with gout, a higher dose of current ULT was not associated with any reduction in the risk of TJR after considering multiple factors including socio-economic status, lifestyle and co-morbidities. These results were consistent in both the Taiwan and UK cohorts. To the best of our knowledge, this is the first study to examine the risk of both THR and TKR considering a temporal relationship with an initial diagnosis of gout and the impact of ULT on the risk of TJR in patients with gout.
This study utilizes two nationwide databases that enable an indirect comparison of TJR risks in gout patients. First, the average age of gout diagnosis is much younger in Taiwan (49 years) than in the UK (61 years) and both the crude TJR prevalence at diagnosis and the incidence afterward in Taiwanese gout patients is approximately half that of the UK. The UK cohort therefore had a higher ‘background’ TJR risk despite a numerically lower relative risk. Previous studies have repeatedly documented a high incidence of gout in the young population in Taiwan [37]. The reason is unclear, but Taiwanese aboriginals, who are genetically close to Pacific Islanders, are one of the populations with the highest gout incidence in the world [14]. Second, due to the limitation of covariate availability, BMI was not adjusted for in the Taiwan cohort. In the UK cohort, 32.2% of gout patients had a BMI >30 kg/m2, which is much higher than in people without gout. It is evident that adjustment in the UK results in a greater attenuation of HR estimates, particularly for the TKR risk. Therefore obesity at least partly contributes to the risk of TJR. In general, Taiwanese tend to have a smaller body size and less obesity. A nationwide nutritional survey found the average adult BMI was 23.8 kg/m2 [38], but whether BMI affects TJR risk in Taiwan needs further study.
Although gout has long been considered to be a minor disease with negligible adverse long-term outcomes [39], contemporary evidence supports an increased risk of multiple co-morbidities and mortality in people with gout [13]. MSU crystal deposition can cause mechanical and inflammatory damage to tissues within and around joints and clinically results in chronic usage-related pain, functional impairment and radiographic structural changes of OA in people with gout. Therefore gout is a potential risk factor for TJR, although there is currently only limited evidence regarding the risk of TJR in people with gout [40]. TJR is a major surgical procedure that is only undertaken for ‘failed joints’ that are unresponsive to other interventions. Therefore it can be considered a surrogate for irreversible and severe joint damage. Although the knee is a known target joint for gout, hip involvement has been considered to be uncommon in acute attacks of gout [41], even though silent MSU accumulation and consequent joint damage is possible. This study supports the significant effects of gout on both the knees and hips.
This study is based on nationwide databases in Taiwan and the UK and the results showed that gout was a risk factor for TJR, independent from OA and RA, the two major causes of TJR, and other accepted risk factors. The large difference between crude and adjusted risk measures suggests that covariates associated with gout are also significant risk factors for TJR. However, more gout patients had undergone TJR at the time of a diagnosis of gout, suggesting that factors present before the first attack of gout increased the risk of joint damage. Moreover, the risk seemed to continue after gout had been diagnosed. In this regard, hyperuricaemia occurs in people with gout long before an initial clinical presentation and diagnosis and continues in the absence of adequate treatment. A recent study found that serum urate levels were correlated with the degree of joint space narrowing in the knees of people without gout [42]. Moreover, synovial uric acid levels have been associated with the severity of OA in patients with and without gout [43]. In addition to hyperuricaemia, urate crystal deposition may also predate the clinical presentation by a long period of time, and occult joint damage from the mechanical and chronic inflammatory effects of microtophi may already be present at the time of the first clinical presentation [18–21, 44]. Therefore effective ULT could potentially retard the progression of joint damage and reduce the risk of TJR both by reducing urate levels and by dissolving existing crystals.
However, the current study found that the risk of both THR and TKR was not reduced by the use of ULT following a diagnosis of gout, and the results were consistent in both the Taiwan and UK cohorts. In addition, the ORs for TJR were not significant across a range of cumulative doses of ULT, which suggests that ULT at commonly used doses is not effective in alleviating progressive joint damage and reducing TJR. Our recent study using UK primary care data found that a diagnosis of gout was associated with OA, with an OR of 1.27, and that following the initial diagnosis of gout the HR for OA was 1.45 [13]. Therefore irreversible joint damage may occur before the initial diagnosis of gout. Preclinical asymptomatic MSU crystal deposition has been demonstrated in one-third of people with ‘asymptomatic’ hyperuricaemia [18–21], and extensive MSU crystal deposition can be seen on imaging such as dual-energy computerized tomography at the time of diagnosis of gout [44]. This prolonged preclinical asymptomatic phase of gout that may associate with joint damage is a relatively new concept, and a new staging system for gout proposed in 2014 by Dalbeth and Stamp [45] formally partitions the ‘asymptomatic’ spectrum into asymptomatic hyperuricaemia (stage A) and MSU deposition without signs or symptoms of gout (stage B). The results of this study reflect the possible existence of irreversible joint damage at the time of diagnosis of gout and support the clinical relevance of the new staging system. Therefore screening and treating asymptomatic disease, particularly for patients with stage B (i.e. subclinical gout), seems to be a rational approach, although in most countries ULT is not licensed to treat hyperuricaemia per se in the absence of gout or urolithiasis.
Currently there are no explicit evidence-based recommendations concerning when in the clinical course of gout patients should be considered for ULT. Current trends increasingly favour early intervention with ULT, even around the time of first diagnosis, to prevent further crystal deposition and long-term complications such as subcutaneous tophi and joint damage, rather than waiting until these have occurred [39]. This is indirectly supported by previous studies showing frequent attacks in untreated patients [46] and a high prevalence of associated co-morbidities such as chronic kidney disease [8, 47] and urolithiasis. Our previous study using UK CPRD data found that many co-morbidities, including OA, are more frequently observed in patients at the time of diagnosis of gout compared with matched people without gout, indicating that damage occurred earlier than clinically apparent gout [13]. The current study further demonstrates that such existing joint damage may already be irreversible at diagnosis. An alternative explanation for the lack of effect of ULT in reducing the risk of TJR is that many people prescribed ULT are not ‘treated to target’ by up-titration of ULT against serial serum urate levels until a target level below the saturation point for MSU crystal formation is reached. More studies are needed to ascertain the possible beneficial effects of ULT prescribed according to individualized ‘treat-to-target’ recommendations to prevent joint damage in asymptomatic people with hyperuricaemia.
Limitations
There are several limitations to this study. First, misclassification may exist since identification of patients with gout was by physician-based diagnosis without requirement to demonstrate urate crystal presence. In addition, the identification of joint replacement was based on procedure codes, and only those who received the respective procedures could be coded for reimbursement purposes. Currently both THR and TJR are subject to case payment schemes that require application prior to surgery. Therefore the case definition for joint replacement is robust in the Taiwan NHI database. In the UK CPRD, the recording of TJR has previously been validated [48]. Second, residual confounding may exist, for example, with respect to BMI and physical activity in Taiwan. Such information cannot be obtained from the NHI database. However, we included BMI, alcohol and smoking in the model using the UK cohort, and this yielded similar results. Third, we only observed ULT prescriptions rather than real patient consumption, and gout patients are recognized to have possibly the worst adherence to long-term medications of all patients with chronic illnesses [39]. Fourth, the definition of doses was based on cDDD, which is the accumulated ‘unit’ of daily assumed average dose of ULT. Therefore our data reflect the assumed average consumption of ULT as defined by the World Health Organization. This definition cannot account for possible differences in hypouricaemic efficacy. However, using this definition, it is feasible to compare drug use between different drugs or between different health care systems, such as in Taiwan and the UK. Fifth, the serum uric acid levels were not available in the Taiwan NHI database and were uncommonly and inconsistently recorded in the UK CPRD. Therefore we cannot adjust hyperuricaemia as a covariate.
Conclusions
More gout patients already had joint replacements at the time of diagnosis of gout than their matched unexposed subjects, and after diagnosis the patients with gout continued to have a higher risk of both THR and TJR after adjustment for OA. This suggests that chronic hyperuricaemia and asymptomatic MSU crystal deposition prior to the first clinical presentation of gout may result in joint damage. The current standard of ULT treatment after diagnosis of gout did not to reduce this risk, and either earlier interventions or more optimal treat-to-target use of ULT, or both, may be required.
Supplementary Material
Acknowledgements
The authors thank the Maintenance Project for the Center for Data Analytics and Statistics (CLRPG3D0043) of Chang Gung Memorial Hospital for statistical assistance. This study was supported by the University of Nottingham for methodology and infrastructure. This study is based in part on NHI Research Database data provided by the Administration of National Health Insurance, Ministry of Health and Welfare, Taiwan. The interpretation and conclusions contained herein do not represent the positions of the Administration of National Health Insurance or the National Health Research Institutes.
Funding: This work was funded by the National Science Council of Taiwan (project 105-2314-B-182A-135-MY2, 106-2314-B-182A-161-MY3) and Chang Gung Memorial Hospital (projects CORPG3E0143, CMRPG3F0833, CMRPG3F0843, CMRPD1F0253, CORPG3G0111, CORPG3G0161, CORPG3G0231, CORPG3G0191, CMRPG3F2141, CMRPG3G1401, CMRPG3E1962 and CMRPG3E1952).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Wortmann R. Crystal-induced Arthropathies: Gout, Pseudogout, and Apatite-associated Syndromes. Boca Raton, FL: CRC Press, 2006. [Google Scholar]
- 2. Clarson LE, Chandratre P, Hider SL. et al. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol 2015;22:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abbott RD, Brand FN, Kannel WB, Castelli WP.. Gout and coronary heart disease: the Framingham Study. J Clin Epidemiol 1988;41:237–42. [DOI] [PubMed] [Google Scholar]
- 4. Choi HK, Curhan G.. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007;116:894–900. [DOI] [PubMed] [Google Scholar]
- 5. Kuo CF, Yu KH, See LC. et al. Risk of myocardial infarction among patients with gout: a nationwide population-based study. Rheumatology 2013;52:111–7. [DOI] [PubMed] [Google Scholar]
- 6. Krishnan E, Baker JF, Furst DE, Schumacher HR.. Gout and the risk of acute myocardial infarction. Arthritis Rheum 2006;54:2688–96. [DOI] [PubMed] [Google Scholar]
- 7. De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK.. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Ann Rheum Dis 2010;69:1162–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu KH, Kuo CF, Luo SF. et al. Risk of end-stage renal disease associated with gout: a nationwide population study. Arthritis Res Ther 2012;14:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilcox WR, Khalaf AA.. Nucleation of monosodium urate crystals. Ann Rheum Dis 1975;34:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chhana A, Callon KE, Pool B. et al. The effects of monosodium urate monohydrate crystals on chondrocyte viability and function: implications for development of cartilage damage in gout. J Rheumatol 2013;40:2067–74. [DOI] [PubMed] [Google Scholar]
- 11. Chhana A, Callon KE, Pool B. et al. Monosodium urate monohydrate crystals inhibit osteoblast viability and function: implications for development of bone erosion in gout. Ann Rheum Dis 2011;70:1684–91. [DOI] [PubMed] [Google Scholar]
- 12. Roddy E, Zhang W, Doherty M.. Are joints affected by gout also affected by osteoarthritis? Ann Rheum Dis 2007;66:1374–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M.. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis 2016;75:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuo CF, Grainge MJ, Zhang W, Doherty M.. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 2015;11:649–62. [DOI] [PubMed] [Google Scholar]
- 15. Becker MA, Schumacher HR Jr, Wortmann RL. et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450–61. [DOI] [PubMed] [Google Scholar]
- 16. Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M.. Eligibility for and prescription of urate-lowering treatment in patients with incident gout in England. JAMA 2014;312:2684–6. [DOI] [PubMed] [Google Scholar]
- 17. Elfishawi MM, Zleik N, Kvrgic Z. et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol 2018;45:574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howard RG, Pillinger MH, Gyftopoulos S. et al. Reproducibility of musculoskeletal ultrasound for determining monosodium urate deposition: concordance between readers. Arthritis Care Res 2011;63:1456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Puig JG, de Miguel E, Castillo MC. et al. Asymptomatic hyperuricemia: impact of ultrasonography. Nucleosides Nucleotides Nucleic Acids 2008;27:592–5. [DOI] [PubMed] [Google Scholar]
- 20. Pineda C, Amezcua-Guerra LM, Solano C. et al. Joint and tendon subclinical involvement suggestive of gouty arthritis in asymptomatic hyperuricemia: an ultrasound controlled study. Arthritis Res Ther 2011;13:R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Miguel E, Puig JG, Castillo C. et al. Diagnosis of gout in patients with asymptomatic hyperuricaemia: a pilot ultrasound study. Ann Rheum Dis 2012;71:157–8. [DOI] [PubMed] [Google Scholar]
- 22. Kuo CF, Grainge MJ, See LC. et al. Epidemiology and management of gout in Taiwan: a nationwide population study. Arthritis Res Ther 2015;17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuo CF, Grainge MJ, See LC. et al. Familial aggregation of gout and relative genetic and environmental contributions: a nationwide population study in Taiwan. Ann Rheum Dis 2015;74:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng CL, Lee CH, Chen PS. et al. Validation of acute myocardial infarction cases in the National Health Insurance Research Database in Taiwan. J Epidemiol 2014;24:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng CL, Chien HC, Lee CH, Lin SJ, Yang YH.. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int J Cardiol 2015;201:96–101. [DOI] [PubMed] [Google Scholar]
- 26. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML.. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011;20:236–42. [DOI] [PubMed] [Google Scholar]
- 27. Chen CC, Chen LS, Yen MF, Chen HH, Liou HH.. Geographic variation in the age- and gender-specific prevalence and incidence of epilepsy: analysis of Taiwanese National Health Insurance-based data. Epilepsia 2012;53:283–90. [DOI] [PubMed] [Google Scholar]
- 28. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY.. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc 2005;104:157–63. [PubMed] [Google Scholar]
- 29. Kao WH, Hong JH, See LC. et al. Validity of cancer diagnosis in the National Health Insurance database compared with the linked National Cancer Registry in Taiwan. Pharmacoepidemiol Drug Saf 2017;doi:10.1002/pds.4267. [DOI] [PubMed] [Google Scholar]
- 30. Jick H, Jick SS, Derby LE.. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ 1991;302:766–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan NF, Harrison SE, Rose PW.. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract 2010;60:e128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ.. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meier CR, Jick H.. Omeprazole, other antiulcer drugs and newly diagnosed gout. Br J Clin Pharmacol 1997;44:175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M.. Effect of allopurinol on all-cause mortality in adults with incident gout: propensity score-matched landmark analysis. Rheumatology 2015;54:2145–50. [DOI] [PubMed] [Google Scholar]
- 35. Merlo J, Wessling A, Melander A.. Comparison of dose standard units for drug utilisation studies. Eur J Clin Pharmacol 1996;50:27–30. [DOI] [PubMed] [Google Scholar]
- 36. Charlson M, Szatrowski TP, Peterson J, Gold J.. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- 37. Yu KH, Luo SF.. Younger age of onset of gout in Taiwan. Rheumatology 2003;42:166–70. [DOI] [PubMed] [Google Scholar]
- 38. Chang HY, Pan WH, Yeh WT, Tsai KS.. Hyperuricemia and gout in Taiwan: results from the Nutritional and Health Survey in Taiwan (1993–96). J Rheumatol 2001;28:1640–6. [PubMed] [Google Scholar]
- 39. Doherty M, Jansen TL, Nuki G. et al. Gout: why is this curable disease so seldom cured? Ann Rheum Dis 2012;71:1765–70. [DOI] [PubMed] [Google Scholar]
- 40. Buck M, Delaney M.. Diagnosis and management of gout in total knee arthroplasty. Orthop Nurs 2014;33:37–40; quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 41. Huet T, Ottaviani S, Coustet B, Dieude P.. Hip gout arthritis revealed by sonography. J Ultrasound Med 2016;35:1828–9. [DOI] [PubMed] [Google Scholar]
- 42. Krasnokutsky S, Oshinsky C, Attur M. et al. Serum urate levels predict joint space narrowing in non-gout patients with medial knee osteoarthritis. Arthritis Rheumatol 2017;69:1213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Howard RG, Samuels J, Gyftopoulos S. et al. Presence of gout is associated with increased prevalence and severity of knee osteoarthritis among older men: results of a pilot study. J Clin Rheumatol 2015;21:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choi HK, Al-Arfaj AM, Eftekhari A. et al. Dual energy computed tomography in tophaceous gout. Ann Rheum Dis 2009;68:1609–12. [DOI] [PubMed] [Google Scholar]
- 45. Dalbeth N, Stamp L.. Hyperuricaemia and gout: time for a new staging system? Ann Rheum Dis 2014;73:1598–600. [DOI] [PubMed] [Google Scholar]
- 46. Yu TF, Gutman AB.. Efficacy of colchicine prophylaxis in gout. Prevention of recurrent gouty arthritis over a mean period of five years in 208 gouty subjects. Ann Intern Med 1961;55:179–92. [DOI] [PubMed] [Google Scholar]
- 47. Choi HK, Soriano LC, Zhang Y, Rodriguez LA.. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012;344:d8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Culliford DJ, Maskell J, Beard DJ. et al. Temporal trends in hip and knee replacement in the United Kingdom: 1991 to 2006. J Bone Joint Surg Br 2010;92:130–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.