Abstract
The aim of the present study was to investigate the racial disparities in the presentation, treatment and survival time of patients with hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma (ICC) between Chinese and other racial groups from the Surveillance, Epidemiology, and End Results (SEER) database between January 1st 2004, and December 31st 2013. Key covariates, including clinical presentation, treatment and survival time, were recorded and compared, demonstrating the racial differences. Kaplan-Meier analysis and Cox regression models were performed to identify these disparities in survival time. A total of 30,954 patients were identified in the SEER database. Among these, 27,767 (89.7%) had HCC and 3,187 (10.3%) had ICC. In the HCC cohort, Chinese patients had the highest survival time. Compared with the mortality risk of Chinese patients, the mortality risk of Other Asian, non-Hispanic white, Hispanic and African-American patients increased by 16.8, 35.1, 28.3 and 33.3%, respectively. Compared with other groups, Chinese patients were more likely to present with localized stage, and without vascular invasion, adjacent invasion and metastasis. In the ICC cohort, the Chinese group had improved survival time, compared with the other groups following univariate analysis, although no significant differences were observed between Chinese and Other Asian and Hispanic patients following adjusting for contributing factors. Furthermore, there was no significant differences in the presentation between the groups, which differed from the HCC analysis. In conclusion, race/ethnicity was a significant independent prognostic factor in the HCC cohort, whereas it was not significant in the ICC cohort. The synergistic effect of contributing factors, including demographic, socioeconomic, biological and treatment differences, caused the racial disparity observed in primary liver cancer survival time.
Keywords: primary liver cancer, racial disparities, survival analysis, SEER database, population
Introduction
In 2012, it was reported that primary liver cancer (PLC) is one of the most common malignancy types and is a leading cause of mortality globally (1,2). Previously, a series of advanced surgical techniques and pharmacotherapy, including liver transplantation, loco-regional therapy and sorafenib, have been implemented in medical practice to improve the survival time of patients with liver cancer (3–6). However, in the United States between 1975 and 2012, the incidence and mortality of this disease continued to increase and was disproportionally distributed in the population (7).
Racial disparities in hepatocellular carcinoma (HCC) survival time have been confirmed in a number of studies (8–11). Numerous studies, between 2006 and 2012, have demonstrated that Asian patients have the highest survival time, while African-American patients have the lowest survival time (9,11–13). Furthermore, it has been reported that between 1988 and 2012, Asian patients, who were treated in the United States, have an increased probability of being treated via liver resection, compared with other racial groups (11,12). There are numerous contributing factors, including etiology, tumor stage and social economic status, affecting the racial disparities observed in PLC survival time (9,12,14). The mechanisms underlying factors affecting HCC survival time and their relative importance in disease progression remain undetermined.
The Asian population was reported as the fastest growing and most heterogeneous ethnic group in the USA between 1992 and 2009 (15). There are numerous differences in the etiology, genetic characteristics, cancer susceptibility and living habits of the Asian subgroups (16,17). In 2012, it was reported that the Chinese population is one of the largest Asian subgroups and account for a large proportion of patients with HCC in the United States (1,18). Furthermore, previous studies have demonstrated that Chinese patients with colorectal and esophageal cancer have improved survival time, compared with patients of other racial groups (19,20). However, there are limited studies examining differences in HCC outcomes between Chinese patients and patients of other racial groups. Stewart et al (11) reported that compared with Caucasians, Chinese patients had reduced HCC cause-specific mortality [hazard ratio (HR)=0.81; 95% confidence interval (CI), 0.77–0.86], although a detailed analysis with respect to clinical presentation, treatment and survival time was not provided. Intrahepatic cholangiocarcinoma (ICC) is the second most common PLC type and has been increasing in incidence and mortality over the last 10 to 20 years (21,22); however, limited studies investigating the racial disparities in ICC outcomes have been performed (21,23).
Therefore, in the present study, racial disparities in HCC and ICC survival time between Chinese patients and patients of other racial groups were retrospectively analyzed with the Surveillance, Epidemiology, and End Results (SEER) database between January 1st 2004, and December 31st 2013 (24). Furthermore, the racial disparities between Asian subgroups were analyzed.
Patients and methods
Database
Data from the SEER database, the largest public cancer database and sponsored by the National Cancer Institute, were retrospectively analyzed. The SEER program collects demographics, including age, sex and race/ethnicity, as well as clinical characteristics, including cancer stage, histology, primary therapy and survival time, in the USA from 1973. It is a population-based database that includes ~28% of the USA population and multiple racial groups. The exact database used for the present analysis was the Incidence-SEER 18 Registries Research Data + Hurricane Katrina Impacted Louisiana Cases, November 2015 Sub (1973–2013 varying) (25) using the SEER*Stat 8.3.2 software (National Cancer Institute, Bethesda, MD, USA). Permission was received for the use of these data (SEER ID: 13724-Nov 2015).
Patient selection
Patients who were histologically diagnosed as HCC or ICC between January 1st 2004, and December 31st 2013 were included in the present study. Patients were excluded if they were <18 years of age, had a history of a prior malignancy, the cause of mortality was unknown (UNK), the follow-up period was <1 month, or any key covariate data were incomplete or UNK, including diagnosis confirmation methods, age, marital status, histological stage, tumor size, residence and race/ethnicity.
Covariates
Key covariates with respect to baseline characteristics, including year of diagnosis, age, sex, race/ethnicity, residence, marital status, household income, educational level, poverty, pathology, histological grade, tumor size, vascular and adjacent invasion, metastasis, cancer stage, serum α-fetoprotein (AFP) level, fibrosis score and primary therapy were extracted from the database. Race/ethnicity was classified as non-Hispanic White (NHW), Hispanic, African-American or Chinese, as determined in the SEER database. Asian subgroups except Chinese were merged into ‘Other Asian’ for the overall analysis and subsequently categorized as Korean, Japanese, Vietnamese, Philippines Filipino or Other Minority for subgroup analysis. Treatment was categorized as radiation, resection, transplantation, tumor destructive therapy (TDT), none or UNK. TDT included radio-frequency ablation, laser therapy, photodynamic therapy, electrocautery and cryosurgery, as described in the SEER database (26). Data were extracted from the dataset with the reference of the SEER Coding and Staging Manual and Collaborative Staging System Manual (26).
Statistical analysis
Baseline demographic and clinical characteristics data were summarized with descriptive statistics in Tables I and II. Continuous variables were presented as mean ± standard deviation. Comparison of baseline characteristics between races/ethnicities was calculated by one-way analysis of variance, followed by Bonferroni's multiple comparison test, and the χ2 test.
Table I.
Baseline demographic and clinical characteristics of patients with hepatocellular carcinoma from the SEER database.
| Variables | Total | Chinese | NHW | African-American | Other Asian | Hispanic | P-value |
|---|---|---|---|---|---|---|---|
| Total (%) | 27,767 (100) | 1,241 (4.47) | 13,867 (49.94) | 3,645 (13.13) | 3,437 (12.38) | 5,577 (20.08) | |
| Age, years | <0.001a | ||||||
| Mean ± SD | 62±11 | 64±13 | 63±11 | 60±10 | 64±12 | 61±11 | |
| Median (IQR) | 61 (54–69) | 64 (55–73) | 61 (55–69) | 59 (54–64) | 64 (55–72) | 60 (53–68) | |
| P-valuec | Ref | 0.008 | <0.001 | 1.000 | 0.008 | ||
| Sex | <0.001b | ||||||
| Female | 6,274 (22.59) | 307 (24.74) | 2,854 (20.58) | 815 (22.36) | 944 (27.47) | 1,354 (24.28) | |
| Male | 21,493 (77.41) | 934 (75.26) | 11,013 (79.42) | 2,830 (77.64) | 2,493 (72.53) | 4,223 (75.72) | |
| Marital status, n (%) | <0.001b | ||||||
| Married | 15,364 (55.33) | 985 (79.37) | 7,582 (54.68) | 1,336 (36.56) | 2,462 (71.63) | 2,999 (53.77) | |
| Single | 5,806 (20.91) | 96 (7.74) | 2,724 (19.64) | 1,334 (36.51) | 369 (10.74) | 1,283 (23.00) | |
| Divorced | 4,131 (14.88) | 54 (4.35) | 2,312 (16.67) | 690 (18.83) | 249 (7.24) | 826 (14.81) | |
| Widowed | 2,466 (8.88) | 106 (8.54) | 1,249 (9.01) | 285 (7.80) | 357 (10.39) | 469 (8.41) | |
| Residence, n (%) | <0.001b | ||||||
| Rural | 2,176 (7.84) | 5 (0.41) | 1,721 (12.41) | 188 (5.16) | 67 (1.95) | 195 (3.50) | |
| Urban | 25,591 (92.16) | 1,236 (99.59) | 12,146 (87.59) | 3,457 (94.84) | 3,370 (98.05) | 5,382 (96.50) | |
| Household income (USD) | <0.001a | ||||||
| Mean ± SD | 47,512±10,980 | 53,820±10,109 | 46,872±11,042 | 44,837±9,999 | 52,439±10,920 | 46,412±10,239 | |
| P-valuec | Ref | <0.001 | <0.001 | 0.001 | <0.001 | ||
| Educationd (%) | <0.001a | ||||||
| Mean ± SD | 20.79±7.34 | 19.97±6.26 | 19.8±7.54 | 21.09±6.65 | 20.06±6.70 | 23.68±7.18 | |
| P-valuec | Ref | 1.000 | <0.001 | 1.000 | <0.001 | ||
| Povertye (%) | <0.001a | ||||||
| Mean ± SD | 12.86±5.17 | 11.83±4.05 | 12.15±5.29 | 14.35±5.46 | 11.92±4.31 | 14.48±4.79 | |
| P-valuec | Ref | 0.318 | <0.001 | 1.000 | <0.001 | ||
| Grade, n (%) | <0.001b | ||||||
| I | 3,855 (13.88) | 146 (11.76) | 2,029 (14.63) | 491 (13.47) | 378 (10.99) | 811 (14.54) | |
| II | 5,170 (18.62) | 249 (20.06) | 2,672 (19.27) | 675 (18.52) | 698 (20.31) | 876 (15.71) | |
| III | 2,198 (7.92) | 142 (11.44) | 1,051 (7.58) | 295 (8.09) | 347 (10.10) | 363 (6.51) | |
| IV | 202 (0.73) | 3 (0.24) | 110 (0.80) | 24 (0.66) | 38 (1.11) | 27 (0.48) | |
| UNK | 16,342 (58.85) | 701 (56.49) | 8,005 (57.72) | 2,160 (59.26) | 1,976 (57.49) | 3,500 (62.76) | |
| SEER stage (26), n (%) | <0.001b | ||||||
| Localized | 16,435 (59.19) | 794 (63.98) | 8,202 (59.15) | 2,025 (55.56) | 1,985 (57.75) | 3,429 (61.48) | |
| Regional | 8,312 (29.93) | 336 (27.07) | 4,186 (30.19) | 1,151 (31.58) | 1,070 (31.13) | 1,569 (28.13) | |
| Distant | 3,020 (10.88) | 111 (8.94) | 1,479 (10.66) | 469 (12.87) | 382 (11.11) | 579 (10.38) | |
| Tumor size diameter (cm), n (%) | <0.001b | ||||||
| 0–3 | 8,859 (31.90) | 369 (29.73) | 4,717 (34.02) | 1,034 (28.37) | 950 (27.64) | 1,789 (32.08) | |
| 3–5 | 7,294 (26.27) | 314 (25.30) | 3,665 (26.43) | 922 (25.29) | 842 (24.50) | 1,551 (27.81) | |
| >5 | 11,614 (41.83) | 558 (44.96) | 5,485 (39.55) | 1,689 (46.34) | 1,645 (47.86) | 2,237 (40.11) | |
| Vascular invasion, n (%) | <0.001b | ||||||
| No | 12,269 (44.19) | 593 (47.78) | 6,128 (44.19) | 1,489 (40.85) | 1,486 (43.24) | 2,573 (46.14) | |
| Yes | 10,363 (37.32) | 414 (33.36) | 5,344 (38.54) | 1,363 (37.39) | 1,239 (36.05) | 2,003 (35.92) | |
| UNK | 5,135 (18.49) | 234 (18.86) | 2,395 (17.27) | 793 (21.76) | 712 (20.71) | 1,001 (17.94) | |
| Adjacent invasion, n (%) | <0.001b | ||||||
| No | 18,347 (66.07) | 860 (69.30) | 9,222 (66.46) | 2,281 (62.58) | 2,190 (63.72) | 3,794 (68.03) | |
| Yes | 6,881 (24.78) | 282 (22.72) | 3,349 (24.15) | 996 (27.33) | 906 (26.36) | 1,348 (24.17) | |
| UNK | 2,539 (9.14) | 99 (7.98) | 1,296 (9.35) | 368 (10.10) | 341 (9.92) | 435 (7.80) | |
| Regional lymph nodes, n (%) | <0.001b | ||||||
| No | 24,358 (87.72) | 1,127 (90.81) | 12,078 (87.10) | 3,174 (87.08) | 3,072 (89.38) | 4,907 (87.99) | |
| Yes | 1,710 (6.16) | 34 (2.74) | 985 (7.103) | 268 (7.35) | 152 (4.42) | 271 (4.86) | |
| UNK | 1,699 (6.12) | 80 (6.45) | 804 (5.797) | 203 (5.57) | 213 (6.20) | 399 (7.15) | |
| Metastasis, n (%) | 0.001b | ||||||
| No | 24,060 (86.65) | 1,107 (89.20) | 12,051 (86.90) | 3,103 (85.13) | 2,961 (86.15) | 4,838 (86.75) | |
| Yes | 3,129 (11.27) | 111 (8.94) | 1,547 (11.16) | 474 (13.00) | 390 (11.35) | 607 (10.88) | |
| UNK | 578 (2.08) | 23 (1.85) | 269 (1.94) | 68 (1.87) | 86 (2.50) | 132 (2.37) | |
| AFP, n (%) | <0.001b | ||||||
| Negative | 5,889 (21.21) | 297 (23.93) | 3,239 (23.36) | 492 (13.50) | 640 (18.62) | 1,221 (21.89) | |
| Borderline | 71 (0.26) | 3 (0.24) | 47 (0.34) | 8 (0.22) | 4 (0.12) | 9 (0.16) | |
| Positive | 16,752 (60.33) | 780 (62.85) | 7,819 (56.39) | 2,547 (69.88) | 2,218 (64.53) | 3,388 (60.75) | |
| UNK | 5,055 (18.21) | 161 (12.97) | 2,762 (19.91) | 598 (16.41) | 575 (16.73) | 959 (17.20) | |
| Fibrosis score, n (%) | <0.001b | ||||||
| No to moderate | 1,573 (5.66) | 167 (13.46) | 739 (5.33) | 205 (5.62) | 251 (7.30) | 211 (3.78) | |
| Severe to cirrhosis | 6,600 (23.77) | 266 (21.43) | 3,526 (25.43) | 755 (20.71) | 708 (20.60) | 1,345 (24.11) | |
| UNK | 19,594 (70.57) | 808 (65.11) | 9,602 (69.24) | 2,685 (73.67) | 2,478 (72.10) | 4,021 (72.10) | |
| Treatment, n (%) | <0.001b | ||||||
| None/UNK | 16,963 (61.09) | 628 (50.60) | 8,143 (58.72) | 2,380 (65.29) | 1,997 (58.10) | 3,815 (68.41) | |
| Radiation | 1,513 (5.45) | 32 (2.58) | 878 (6.33) | 228 (6.26) | 150 (4.36) | 225 (4.03) | |
| Resection | 3,337 (12.02) | 309 (24.90) | 1,568 (11.3) | 419 (11.50) | 632 (18.39) | 409 (7.33) | |
| Transplant | 2,321 (8.36) | 75 (6.04) | 1,449 (10.45) | 192 (5.27) | 184 (5.35) | 421 (7.55) | |
| TDT | 3,633 (13.08) | 197 (15.87) | 1,829 (13.19) | 426 (11.69) | 474 (13.79) | 707 (12.68) |
One-way analysis of variance
Person χ2 test
Bonferroni's multiple comparison test (multiple comparisons were performed between the Chinese group and other groups)
Education indicated the percentage of adults aged ≥25 years who had <12 years of education
Poverty indicated the percentage of individuals living below the poverty line. NHW, non-Hispanic white; SD, standard deviation; IQR, interquartile range; SEER, Surveillance, Epidemiology, and End Results; UNK, unknown; AFP, α fetoprotein; TDT, tumor destructive therapy; Ref, reference cohort.
Table II.
Baseline demographic and clinical characteristics of patients with intrahepatic cholangiocarcinoma in the SEER database.
| Variables | Total | Chinese | NHW | African-American | Other Asian | Hispanic | P-value |
|---|---|---|---|---|---|---|---|
| Total (%) | 3,187 | 113 (3.55) | 2,093 (65.67) | 264 (8.28) | 280 (8.79) | 437 (13.71) | |
| Age, years | <0.001a | ||||||
| Mean ± SD | 64±12 | 65±12 | 65±12 | 61±13 | 64±12 | 63±12 | |
| Median (IQR) | 64 (55–72) | 65 (57–73) | 65 (56–73) | 61 (52–69) | 65 (55–73) | 63 (54–71) | |
| P-valuec | Ref | 1.000 | 0.062 | 1.000 | 0.664 | ||
| Sex, n (%) | 0.069b | ||||||
| Female | 1,611 (50.55) | 59 (52.21) | 1,026 (49.02) | 150 (56.81) | 139 (49.64) | 237 (54.23) | |
| Male | 1,576 (49.45) | 54 (47.79) | 1,067 (50.98) | 114 (43.19) | 141 (50.36) | 200 (45.77) | |
| Marital status, n (%) | <0.001b | ||||||
| Married | 1,962 (61.56) | 85 (75.22) | 1,316 (62.88) | 108 (40.91) | 198 (70.71) | 255 (58.35) | |
| Single | 409 (12.83) | 12 (10.62) | 221 (10.56) | 71 (26.89) | 27 (9.64) | 78 (17.85) | |
| Divorced | 364 (11.42) | 3 (2.65) | 247 (11.80) | 49 (18.56) | 18 (6.43) | 47 (10.76) | |
| Widowed | 452 (14.18) | 13 (11.51) | 309 (14.76) | 36 (13.64) | 37 (13.22) | 57 (13.04) | |
| Residence, n (%) | <0.001b | ||||||
| Rural | 345 (10.83) | 1 (0.88) | 300 (14.33) | 21 (7.95) | 7 (2.50) | 16 (3.66) | |
| Urban | 2,842 (89.17) | 112 (99.12) | 1,793 (85.67) | 243 (92.05) | 273 (97.50) | 421 (96.34) | |
| Household income (USD) | <0.001a | ||||||
| Mean ± SD | 47,560±11,341 | 52,581±10,994 | 47,334±11,678 | 45,261±10,385 | 51,178±9,921 | 46,413±10,327 | |
| P-valuec | Ref | <0.001 | <0.001 | 1.000 | <0.001 | ||
| Educationd (%) | <0.001a | ||||||
| Mean ± SD | 20.26±7.73 | 21.18±6.85 | 19.35±7.77 | 21.21±7.05 | 19.40±6.79 | 24.33±7.33 | |
| P-valuec | Ref | 0.121 | 1.000 | 0.338 | 0.001 | ||
| Povertye (%) | <0.001a | ||||||
| Mean ± SD | 12.31±5.42 | 12.60±4.89 | 11.66±5.48 | 13.79±5.31 | 11.86±4.34 | 14.71±5.09 | |
| P-valuec | Ref | 0.670 | 0.467 | 1.000 | 0.002 | ||
| Grade, n (%) | 0.572b | ||||||
| I | 178 (5.59) | 8 (7.08) | 106 (5.06) | 21 (7.95) | 17 (6.07) | 26 (5.95) | |
| II | 832 (26.11) | 26 (23.01) | 561 (26.80) | 72 (27.27) | 71 (25.36) | 102 (23.34) | |
| III | 734 (23.03) | 25 (22.12) | 470 (22.46) | 55 (20.83) | 65 (23.21) | 119 (27.23) | |
| IV | 18 (0.56) | 1 (0.88) | 14 (0.67) | 0 (0.00) | 2 (0.71) | 1 (0.23) | |
| UNK | 1,425 (44.71) | 53 (46.89) | 942 (45.01) | 116 (43.94) | 125 (44.64) | 189 (43.25) | |
| SEER stage (26), n (%) | 0.084b | ||||||
| Localized | 1,103 (34.61) | 38 (33.63) | 720 (34.40) | 102 (38.64) | 97 (34.64) | 146 (33.41) | |
| Regional | 1,133 (35.55) | 41 (36.28) | 781 (37.31) | 82 (31.06) | 86 (30.71) | 143 (32.72) | |
| Distant | 951 (29.84) | 34 (30.09) | 592 (28.28) | 80 (30.30) | 97 (34.64) | 148 (33.87) | |
| Tumor size (cm), n (%) | 0.163b | ||||||
| 0–3 | 472 (14.81) | 20 (17.70) | 315 (15.05) | 33 (12.50) | 48 (17.14) | 56 (12.81) | |
| 3–5 | 636 (19.96) | 31 (27.43) | 420 (20.07) | 54 (20.45) | 54 (19.28) | 77 (17.62) | |
| >5 | 2,079 (65.23) | 62 (54.87) | 1,358 (64.88) | 177 (67.05) | 178 (63.57) | 304 (69.57) | |
| Vascular invasion, n (%) | 0.899b | ||||||
| No | 1,520 (47.69) | 48 (42.48) | 995 (47.55) | 122 (46.21) | 138 (49.29) | 217 (49.66) | |
| Yes | 720 (22.59) | 26 (23.01) | 477 (22.79) | 63 (23.86) | 64 (22.86) | 90 (20.59) | |
| UNK | 947 (29.71) | 39 (34.51) | 621 (29.67) | 79 (29.92) | 78 (27.85) | 130 (29.75) | |
| Adjacent invasion, n (%) | 0.001b | ||||||
| No | 2,324 (72.92) | 74 (65.49) | 1,538 (73.48) | 188 (71.21) | 210 (75.00) | 314 (71.85) | |
| Yes | 826 (25.92) | 35 (30.97) | 541 (25.85) | 67 (25.38) | 68 (24.29) | 115 (26.33) | |
| UNK | 37 (1.16) | 4 (3.54) | 14 (0.67) | 9 (3.41) | 2 (0.71) | 8 (1.83) | |
| Regional iymph nodes, n (%) | 0.264b | ||||||
| No | 2,091 (65.61) | 81 (71.68) | 1,357 (64.84) | 178 (67.42) | 190 (67.86) | 285 (65.22) | |
| Yes | 799 (25.07) | 21 (18.58) | 550 (26.28) | 65 (24.62) | 63 (22.50) | 100 (22.88) | |
| UNK | 297 (9.32) | 11 (9.74) | 186 (8.88) | 21 (7.95) | 27 (9.64) | 52 (11.90) | |
| Metastasis, n (%) | 0.054b | ||||||
| No | 2,102 (65.96) | 72 (63.72) | 1,420 (67.85) | 176 (66.67) | 172 (61.43) | 262 (59.95) | |
| Yes | 1,014 (31.91) | 38 (33.63) | 628 (30.00) | 84 (31.82) | 103 (36.79) | 161 (36.84) | |
| UNK | 71 (2.23) | 3 (2.65) | 45 (2.15) | 4 (1.51) | 5 (1.78) | 14 (3.21) | |
| AFP | <0.001b | ||||||
| Negative | 1,151 (36.12) | 46 (40.71) | 752 (35.93) | 89 (33.71) | 124 (44.29) | 140 (32.04) | |
| Borderline | 4 (0.13) | 0 (0.00) | 0 (0.00) | 1 (0.38) | 1 (0.36) | 2 (0.46) | |
| Positive | 549 (17.22) | 14 (12.39) | 331 (15.81) | 68 (25.76) | 42 (15.00) | 94 (21.51) | |
| UNK | 1,483 (46.53) | 53 (46.90) | 1,010 (48.26) | 106 (40.15) | 113 (40.35) | 201 (46.59) | |
| Fibrosis score | 0.001b | ||||||
| No to moderate | 239 (7.50) | 18 (15.93) | 150 (7.17) | 19 (7.20) | 32 (11.43) | 20 (4.58) | |
| Severe to cirrhosis | 148 (4.64) | 4 (3.54) | 103 (4.92) | 14 (5.30) | 8 (2.86) | 19 (4.35) | |
| UNK | 2,800 (87.86) | 91 (80.53) | 1,840 (87.91) | 231 (87.50) | 240 (85.71) | 398 (91.07) | |
| Treatment | 0.001b | ||||||
| None/UNK | 1,851 (58.08) | 56 (49.56) | 1,156 (55.23) | 163 (61.74) | 177 (63.21) | 299 (68.42) | |
| Radiation | 379 (11.89) | 20 (17.70) | 264 (12.61) | 29 (10.98) | 23 (8.21) | 43 (9.84) | |
| Resection | 831 (26.07) | 32 (28.32) | 583 (27.85) | 60 (22.73) | 70 (25.00) | 86 (19.68) | |
| Transplant | 30 (0.94) | 1 (0.88) | 23 (1.10) | 2 (0.76) | 1 (0.36) | 3 (0.69) | |
| TDT | 96 (3.01) | 4 (3.54) | 67 (3.21) | 10 (3.79) | 9 (3.22) | 6 (1.37) |
One-way analysis of variane
Pearson χ2 test
Bonferroni's multiple comparison test based on mean value (multiple comparisons were performed between the Chinese group and other groups)
Education indicated the percentage of adults aged ≥25 years who had <12 years of education
Poverty indicated the percentage of individuals living below the poverty line. NHW, non-Hispanic white; SD, standard deviation; IQR, interquartile range; SEER, Surveillance, Epidemiology, and End Results; UNK, unknown; AFP, α fetoprotein; TDT, tumor destructive therapy; Ref, reference cohort.
The primary endpoints of the present study were the overall survival (OS) time and cause-specific survival (CSS) time. Survival time was calculated from the date of diagnosis to the date of mortality. In the OS analysis, any cause of mortality was treated as an event. In the CSS analysis, an event was defined as mortality attributed to PLC. Survival time was estimated and compared using Kaplan-Meier analysis and the log-rank test.
Multivariate analysis was performed to evaluate the prognostic power of variables for survival time. Variables were divided into three categories: i) Demographic factors, including age, sex, residence, race, marital status, household income and poverty; ii) tumor biological and clinical factors, including pathology, histological grade, tumor size, vascular invasion, regional lymph nodes, distant metastasis, SEER stage, AFP level and fibrosis score; and iii) treatment-associated factors, including radiation, resection, transplantation, TDT, and none or UNK therapy. For the multivariate Cox model, for the process for selecting a category of variables in SPSS software, race/ethnicity was entered as block 1, the remaining demographic variables were entered as block 2, tumor biological and clinical variables were entered as block 3, and treatment-associated factors were entered as block 4. Variables in blocks 2 and 3 were entered in a forward stepwise method using the Likelihood Ratio test to determine their impact on survival time (9). The HR changes for each group following the stepwise adjustment are depicted in forest plots.
There are a number of missing values for categorical variables in the present study. Due to no significant differences being determined from the analysis of all cases and cases of known value, the missing values were separated into the subcategory ‘UNK’ and presented in Tables I and II.
P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed by using SPSS Statistics software, version 22 for Windows (IBM Corp., Armonk, NY, USA) and GraphPad Prism software, version 7 for Windows (GraphPad Software Inc., La Jolla, CA, USA).
Results
Baseline characteristics
According to the inclusion and exclusion criteria of the present study, 30,954 patients were identified in the SEER database from 2004 to 2013. There were 27,767 (89.7%) patients diagnosed with HCC and 3,187 (10.3%) diagnosed with ICC. Tables I and II show the baseline demographic and clinical characteristics of the patients with HCC and ICC in the present study.
In the HCC group (Table I), 1,241 (4.47%) patients were Chinese, 13,867 (49.94%) were NHW, 3,654 (13.13%) were African-American, 3,437 (12.38%) were Other Asian and 5,577 (20.08%) were Hispanic. The mean age of Asian patients (including Chinese) was increased, compared with the other groups. African-American patients had the lowest mean age, compared with the other groups. The sex ratio between the groups was not identical, with the NHW group having the highest proportion of males. Additionally, the Chinese group had the highest proportion of married patients and the lowest proportion of divorced patients. Of the Chinese patients, approximately 99.59% were living in rural areas. The household income of the Chinese group was significantly increased, compared with the other groups. Furthermore, the Chinese patients had a notably increased education level and a reduced poverty level. The proportion of localized stage, no vascular invasion, no adjacent invasion, no regional lymph node metastasis and no distant metastasis was increased in the Chinese group, compared with the other groups. Chinese patients with HCC had a significantly increased probability of exhibiting no to moderate fibrosis, compared with the other groups. Additionally, Chinese patients had an increased probability of receiving surgical resection and had the lowest proportion of no or UNK treatment, compared with the other groups.
In the ICC group (Table II), there were 113 (3.55%) Chinese, 2,093 (65.67%) NHW, 264 (8.28%) African-American, 280 (8.79%) Other Asian and 437 (13.71%) Hispanic patients. The mean age of the Chinese and other groups was similar, although African-American patients had the lowest mean age. The sex ratio was similar in each group at approximately 1:1, which was different from the HCC group. The proportion of each characteristic for marital status, residence, household income, education level and poverty level in each group was similar to the HCC distribution; however, there was no significant difference in differentiation grade, stage, tumor size, vascular invasion and metastasis among each group, which was different from the HCC data. Finally, Chinese patients had the highest proportion of surgical resection and the lowest proportion of no or UNK treatment.
Effect of race on PLC survival time
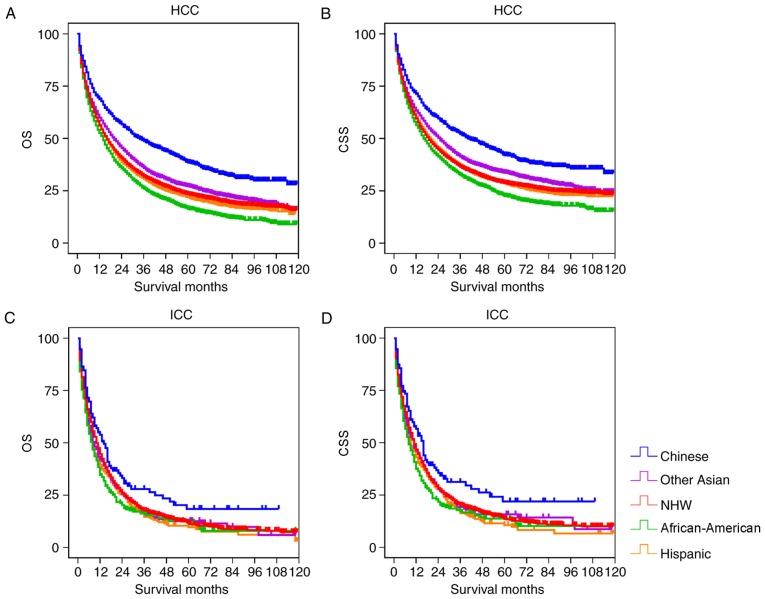
At the time of analysis, 20,767 patients had succumbed, with 17,413 succumbing due to cancer. The study cohort was divided into the HCC and ICC groups for survival analysis. Fig. 1 depicts the OS and CSS rates of different races in the two groups. In the HCC cohort, the Chinese patients had significantly increased OS and CSS, compared with the other groups (P<0.001; Table III). The OS and CSS of Other Asian patients were significantly increased, compared with the NHW, Hispanic and African-American groups (P<0.001), but was reduced, compared with the Chinese group. African-American patients had the lowest OS and CSS compared with other examined groups. In the ICC cohort, the Chinese group had significantly increased OS and CSS, compared with the other groups (P<0.05), but there was no significant differences between the other races (Fig. 1; Table IV).
Figure 1.
The OS and CSS of patients with primary liver cancer according to race/ethnicity. (A) The OS of the HCC cohort. (B) The CSS of the HCC cohort. (C) The OS of the ICC cohort. (D) The CSS of the ICC cohort. OS, overall survival; CSS, cause-specific survival; NHW, non-Hispanic white; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.
Table III.
Univariate analysis of prognostic factors for OS and CSS of patients with hepatocellular carcinoma with the log-rank test.
| OS | CSS | ||||||
|---|---|---|---|---|---|---|---|
| Race/ethnicity | Total | Median (months) | Log-ranka | P-value | Median (months) | Log-ranka | P-value |
| Overall | 27,767 | 17 | 22 | ||||
| Chinese | 1,241 | 34 | 46 | ||||
| NHW | 13,867 | 16 | 114.253 | <0.001 | 21 | 74.836 | .000 |
| African-American | 3,645 | 14 | 200.141 | <0.001 | 18 | 140.375 | .000 |
| Other Asian | 3,437 | 20 | 55.665 | <0.001 | 27 | 33.551 | .000 |
| Hispanic | 5,577 | 16 | 122.820 | <0.001 | 21 | 78.138 | .000 |
Survival was compared with the Chinese group. OS, overall survival; CSS, cause-specific survival; NHW, non-Hispanic white.
Table IV.
Univariate analysis of prognostic factors for overall and cause-specific survival of intrahepatic cholangiocarcinoma by the log-rank test.
| OS | CSS | ||||||
|---|---|---|---|---|---|---|---|
| Race/ethnicity | Total | Median (months) | Log-ranka | P-value | Median (months) | Log-ranka | P-value |
| Overall | 3,187 | 10 | 11 | ||||
| Chinese | 113 | 14 | 16 | ||||
| NHW | 2,093 | 10 | 4.895 | 0.027 | 11 | 5.343 | 0.021 |
| African-American | 264 | 8 | 9.490 | 0.002 | 8 | 10.069 | 0.002 |
| Other Asian | 280 | 9 | 5.017 | 0.025 | 11 | 4.331 | 0.037 |
| Hispanic | 437 | 8 | 8.345 | 0.004 | 9 | 8.667 | 0.003 |
Survival was compared with the Chinese group. OS, overall survival; CSS, cause-specific survival; NHW, non-Hispanic white.
Multivariate analysis of PLC CSS
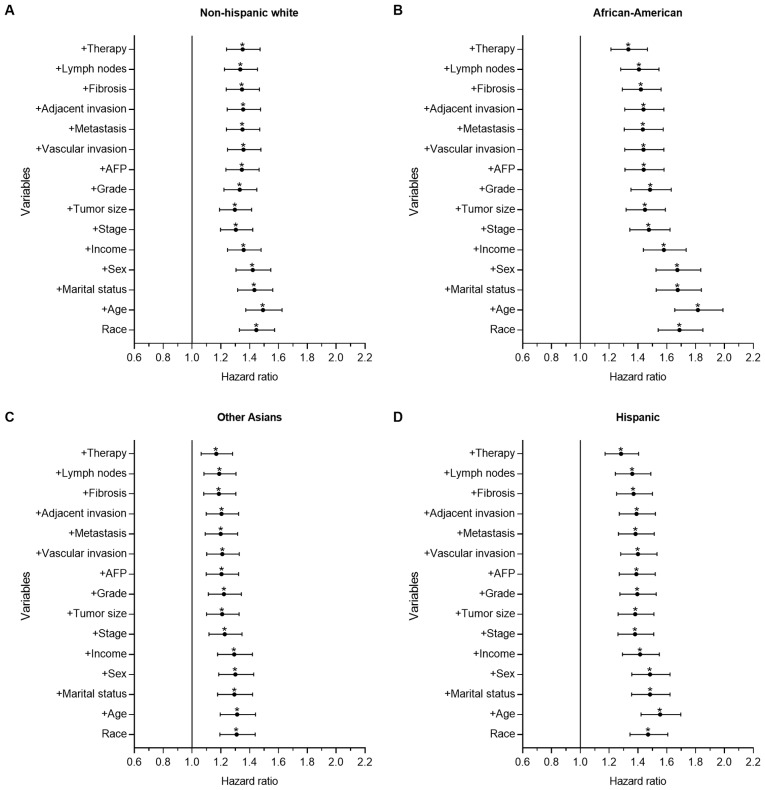
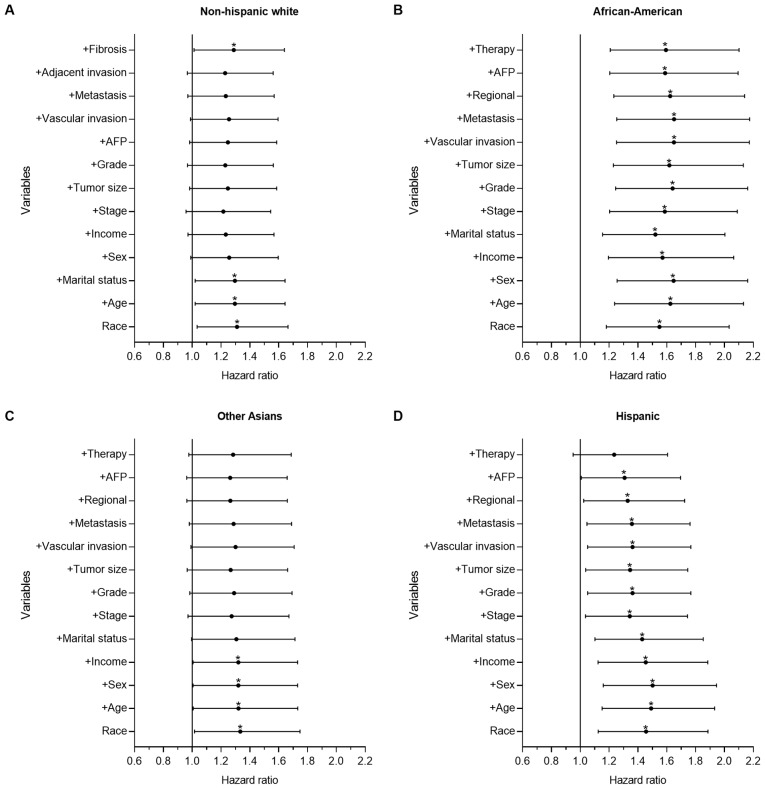
To further determine the prognostic power of a number of demographic-, socioeconomic-, tumor- and treatment-associated factors on racial disparities observed in survival time, forward stepwise multivariate analysis was performed to identify the HR changes between the Chinese group and the other groups. Figs. 2 and 3 show forest plots displaying the results from the multivariate Cox regression model of CSS for all groups in the present study (the Chinese group was set as the reference).
Figure 2.
Forest plot displaying the estimated HR and 95% CI of ethnicity for the CSS of hepatocellular carcinoma from the multivariate Cox models of all groups. The Chinese group was set as the reference. The first HR in the bottom is the crude effect of race/ethnicity followed by HRs following adjusting for contributing variables in a forward stepwise method, in order to determine their impact on CSS. Chinese patients had significantly improved survival time, which was demonstrated with univariate and multivariate analysis in each group. HRs of (A) NHW, (B) African-American, (C) Other Asian and (D) Hispanic patients. *P<0.05. HR, hazard ratio; CI, confidence interval; CSS, cause-specific survival time; NHW, non-Hispanic white; AFP, α fetoprotein.
Figure 3.
Forest plot displaying the estimated HR and 95% CI of ethnicity for the CSS of intrahepatic cholangiocarcinoma from the multivariate Cox models of all groups. The Chinese group was set as the reference. The first HR in the bottom is the crude effect of race/ethnicity followed by HRs following adjusting for contributing variables in a forward stepwise method, in order to determine their impact on CSS. (A) HR of NHW patients, where treatment was the only key factor. (B) HR of African-American patients, where Chinese patients had significantly improved survival time, which was demonstrated with univariate and multivariate analysis. (C) HR of Other Asian patients, where marital status, tumor biological and clinical variables were the key factors. (D) HR of Hispanic patients, where treatment was the only key factor. *P<0.05. HR, hazard ratio; CI, confidence interval; CSS, cause-specific survival time; NHW, non-Hispanic white; AFP, α fetoprotein.
Fig. 2 depicts the results for the HCC group. There were significant differences between the Chinese group and the other groups, as demonstrated with univariate and multivariate analysis. Compared with the Chinese group, the mortality risk of the Other Asian (P<0.001; HR=1.310; 95% CI, 1.193–1.438), NHW (P<0.001; HR=1.446; 95% CI, 1.329–1.573), Hispanic (P<0.001; HR=1.470; 95% CI, 1.345–1.607) and African-American groups (P<0.001; HR=1.688; 95% CI, 1.540–1.850) increased by 31.0, 44.6, 47.0 and 68.8%, respectively. Following adjusting for all other contributing factors, the mortality risk of the Other Asian (P=0.001; HR=1.168; 95% CI. 1.063–1.282), NHW (P<0.001; HR=1.351; 95% CI, 1.240–1.472), Hispanic (P<0.001; HR=1.283; 95% CI, 1.172–1.404) and African-American groups (P<0.001; HR=1.333; 95% CI, 1.213–1.465) increased by 16.8, 35.1, 28.3 and 33.3%, respectively. In the HCC cohort, age, tumor size, stage and treatment served important roles affecting the observed racial disparities in survival time.
Fig. 3 shows the results of the ICC group. There were significant differences in the univariate analysis between the Chinese and the other groups. In the multivariate analysis, there was no significant difference between the Chinese group and the Other Asian (P=0.073; HR=1.284; 95% CI, 0.977–1.687) and Hispanic groups (P=0.113; HR=1.235; 95% CI, 0.951–1.605), although significant differences remained between the Chinese group and the African-American (P=0.001; HR=1.594; 95% CI, 1.208–2.101) and NHW groups (P=0.039; HR=1.288; 95% CI, 1.013–1.639). Compared with the Chinese group, the mortality risk of the NHW and African-American groups increased by 28.8 and 59.4%, respectively. Treatment served an important role in racial survival disparities between the Chinese and the other groups.
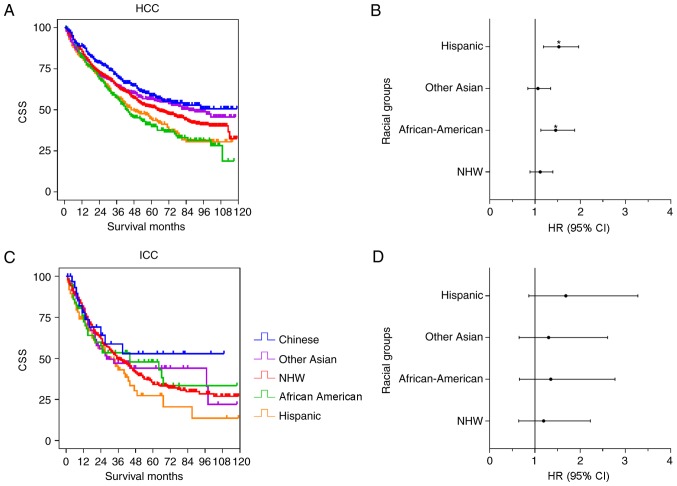
Racial disparities of CSS in patients that received surgical resection
Surgery was the optimal method for curative therapy. The racial disparities among patients who received surgical resection was further analyzed. Fig. 4 shows the results from the univariate and multivariate analysis of the HCC and ICC cohorts. In the HCC cohort (Fig. 4A and B), Chinese, Other Asian and NHW patients demonstrated improved survival time, compared with the Hispanic and African-American patients. There were significant differences between the Chinese group and the NHW (P=0.018), Hispanic (P<0.001) and African-American (P<0.001) groups, although no significant difference was observed between the Chinese group and Other Asian group (P=0.149). Following adjustment for contributing factors, there was no significant difference between the Chinese group and the Other Asian (P=0.605; HR=1.064; 95% CI, 0.841–1.345) and NHW groups (P=0.350; HR=1.113; 95% CI, 0.889–1.393), whereas significant differences were observed between the Chinese group and the Hispanic (P=0.001; HR=1.525; 95% CI, 1.186–1.962) and African-American (P=0.004; HR=1.455; 95% CI, 1.128–1.876) groups (Table V). Compared with the Chinese group, the mortality risk of the Hispanic and African American groups increased by 45.5 and 52.5%, respectively. Fig. 4C and D shows the ICC data distribution, and there were no significant differences between the Chinese group and the other groups in the univariate and multivariate analysis (Table V).
Figure 4.
Univariate and multivariate analysis of CSS for patients with HCC and ICC following surgery. The results of the univariate and multivariate analysis are presented as a Kaplan-Meier curve and forest plotting, respectively. Forest plotting depicted the HR and 95% CI of each racial group, compared with the Chinese group, as a reference. (A) The Kaplan-Meier curve for patients with HCC. (B) Forest plotting of CSS for patients with HCC. (C) The Kaplan-Meier curve for patients with ICC. (D) Forest plotting of CSS for patients with ICC. *P<0.05. CSS, cause-specific survival time; NHW, non-Hispanic white; HR, hazard ratio; CI, confidence interval; ICC, intrahepatic cholangiocarcinoma; HCC, hepatocellular carcinoma.
Table V.
Multivariate analysis of survival time in HCC and ICC with liver resection.
| HCC | ICC | |||||
|---|---|---|---|---|---|---|
| Race/ethnicity | P-value | HR | 95% CI | P-value | HR | 95% CI |
| Chinese | 1 | 1 | ||||
| NHW | 0.350 | 1.113 | 0.889–1.393 | 0.577 | 1.194 | 0.641–2.226 |
| African-American | 0.004 | 1.455 | 1.128–1.876 | 0.409 | 1.352 | 0.660–2.770 |
| Other Asian | 0.605 | 1.064 | 0.841–1.345 | 0.457 | 1.302 | 0.650–2.608 |
| Hispanic | 0.001 | 1.525 | 1.186–1.962 | 0.125 | 1.683 | 0.865–3.276 |
HR, hazard ratio; 95% CI, 95% confidence interval; NHW, non-Hispanic white; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.
Racial disparities in CSS among Asian subgroups
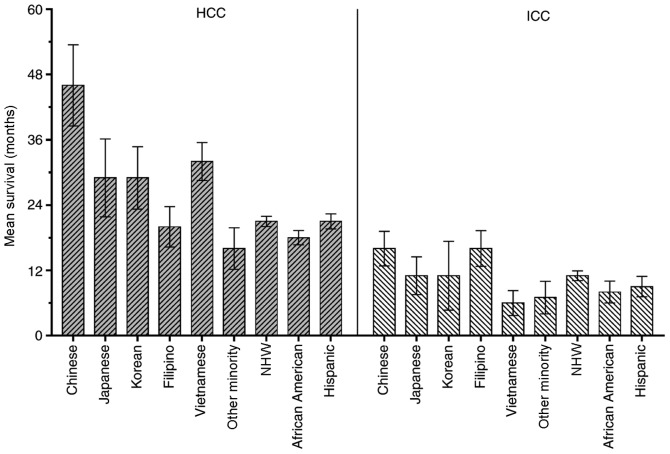
The racial disparities for survival time among Asian subgroups in the present study cohort was further analyzed. In the HCC group, there were 1,241 (26.5%) Chinese, 434 (9.3%) Japanese, 589 (12.6%) Korean, 759 (16.2%) Filipino, 1,193 (25.5%) Vietnamese and 462 (9.9%) ‘Other Minority’, including Indian, Pakistani, Kampuchean and Thai patients. In the ICC group, there were 113 (28.8%) Chinese, 36 (9.2%) Japanese, 53 (13.5%) Korean, 96 (24.4%) Filipino, 37 (9.4%) Vietnamese and 58 (14.8%) Other Minority patients. Fig. 5 shows the mean CSS of the different ethnic groups in the present study. Primarily, the mean CSS of the HCC group was improved, compared with the ICC group.
Figure 5.
Comparison of the mean survival time for patients with HCC and ICC in Asian and other racial groups. ICC, intrahepatic cholangiocarcinoma; HCC, hepatocellular carcinoma; NHW, non-Hispanic white.
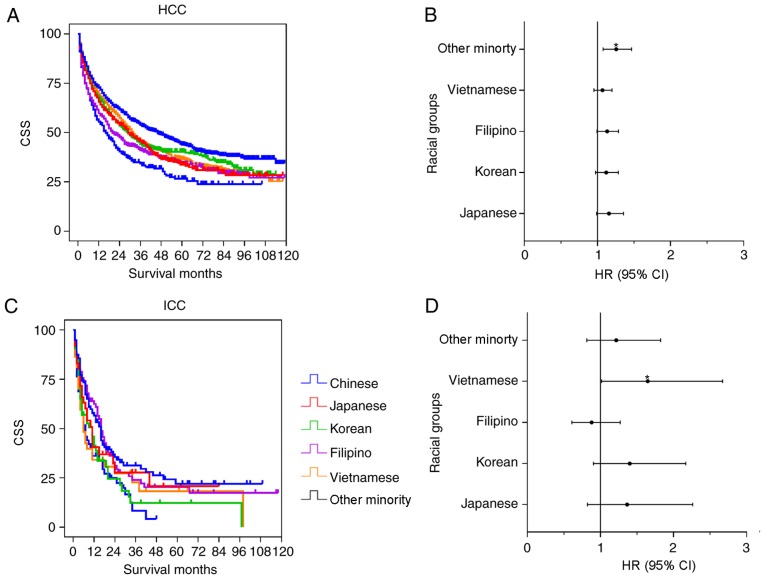
Fig. 6 shows the results of the univariate and multivariate analysis in the Asian subgroups. The Chinese group had significantly improved survival time, compared with the Vietnamese (P=0.002), Japanese (P=0.001), Korean (P=0.009), Filipino (P<0.001) and Other Minority groups (P<0.001) in the HCC univariate analysis. However, no significant differences were observed among the Chinese group and the other subgroups except for the Other Minority group (P=0.004; HR=1.257; 95% CI, 1.077–1.466) in the multivariate analysis (Table VI). For the ICC cohort, the univariate analysis indicated that the survival time of Chinese patients was significantly increased, compared with the Korean (P=0.029) and Other Minority (P=0.004) groups, whereas there were no significant differences between the Chinese group and the Japanese (P=0.377), Filipino (P=0.767) and Vietnamese (P=0.062) groups. In the multivariate analysis, there was a significant difference between the Chinese group and the Vietnamese group (P=0.043; HR=1.647; 95% CI, 1.016–2.670). No significant differences between the Chinese group and the other subgroups were determined (Table VI).
Figure 6.
Univariate and multivariate analysis of CSS for patients from Asian subgroups with HCC and ICC. The results of the univariate and multivariate analysis were presented as a Kaplan-Meier curve and forest plots, respectively. The forest plots depicted the HR and 95% CI of each racial group, compared with the Chinese group. (A) The Kaplan-Meier curve for patients with HCC. (B) Forest plotting of CSS for patients with HCC. (C) The Kaplan-Meier curve for patients with ICC. (D) Forest plotting of CSS for patients with ICC. *P<0.05. CSS, cause-specific survival; HR, hazard ratio; CI, confidence interval; ICC, intrahepatic cholangiocarcinoma; HCC, hepatocellular carcinoma.
Table VI.
Multivariate analysis of survival time among Asian subgroups.
| HCC | ICC | |||||
|---|---|---|---|---|---|---|
| Race/ethnicity | P-value | HR | 95% CI | P-value | HR | 95% CI |
| Chinese | ||||||
| Japanese | 0.070 | 1.158 | 0.988–1.356 | 0.229 | 1.364 | 0.822–2.263 |
| Korean | 0.119 | 1.118 | 0.972–1.286 | 0.130 | 1.401 | 0.905–2.167 |
| Filipino | 0.061 | 1.132 | 0.994–1.288 | 0.495 | 0.879 | 0.608–1.272 |
| Vietnamese | 0.263 | 1.068 | 0.951–1.200 | 0.043 | 1.647 | 1.016–2.670 |
| Other minority | 0.004 | 1.257 | 1.077–1.466 | 0.340 | 1.217 | 0.813–1.823 |
HR, hazard ratio; 95% CI, 95% confidence interval; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.
Discussion
PLC is one of the cancer types increasing in incidence and mortality in the USA over the past three decades (1,2,7). Race/ethnicity has been confirmed as one of the independent risk factors affecting liver cancer survival time and thus has attracted the attention of numerous population-based studies (14,19,27).
Primarily, for PLC, Asian patients have an increased survival rate, compared with other groups, and African-American patients have the lowest survival rate (4,27); however, the way this racial disparity is caused and the factors that contribute the most important roles remains controversial. A number of reports demonstrated that Chinese patients have improved survival outcomes for colorectal and esophageal cancer types (19,20), but whether this phenomenon occurs for PLC is UNK. Due to the high prevalence of PLC among the Asian population (1,28), it was necessary to investigate prognostic factors in Chinese and other Asian patients.
In the present study, the racial disparities in PLC were retrospectively analyzed between a Chinese group and other racial groups for the clinical presentation, treatment and survival time from a 10-year cohort in the SEER database. It was demonstrated that the Chinese group had improved survival time, compared with the other groups in the HCC cohort, following univariate and multivariate analysis, and analyzed the significance of the contributing factors generating the racial disparities. The racial disparities in patients with ICC was also investigated and it was demonstrated that the Chinese group also had improved survival time, compared with the other groups, following univariate analysis, but following multivariate analysis this survival time benefit was not observed. It was confirmed that a synergistic effect of contributing factors, including demographic, socioeconomic, tumor biology and treatment, caused the racial survival disparity for PLC types.
The association between tumor biological factors and survival time has been widely investigated (29–31). HCC and ICC are the two most common types of PLC. Primarily, tumor size, invasion, differentiation and stage are the dominant contributing factors for prognosis in HCC and ICC (29,30). However, due to the differences in etiology and carcinogenesis, there are significant differences in the tumor biological factors characteristics of HCC and ICC (21).
Vascular invasion has been confirmed as a key factor for tumor stage and survival time. Lee et al (32) reported that vascular invasion was associated with a younger age, aggressive tumor behavior, poor liver function reserve and poor performance in daily activity, which negatively impacts HCC survival time. Patients with HCC vascular invasion are frequently diagnosed with an advanced stage in the Barcelona Clinic for Liver Cancer staging system (6). In the present study, Chinese patients had a reduced percentage of vascular invasion and adjacent HCC invasion, which may partially explain why Chinese patients had improved survival time. It was also determined that Chinese patients in the HCC group had an increased probability to exhibit poor differentiation and increased tumor size, which are associated with poor survival time (29); however, there was no significant difference in ICC vascular invasion among the Chinese and the other groups, although Chinese patients demonstrated an increased proportion of adjacent invasion. These results were consistent with the survival analysis that indicated no significant differences among these groups following adjusting for contributing factors.
Demographic and socioeconomic factors also contribute to cancer survival (17,33). Aizer et al (34) reported that marriage was an important socioeconomic factor affecting cancer survival time. He et al (35) demonstrated that for PLC, married patients had the highest survival time, and widowed patients had the lowest. In the present study, the Chinese group had the highest proportion of being married, compared with the other ethnic groups in the HCC and ICC cohorts; therefore, marriage may have a positive effect on cancer prognosis. Married patients have an increased probability to receive timely detection and are more compliant with proper therapy recommendations, including surgery, chemotherapy and medications. Additionally, their spouses provide encouragement and sufficient social and economic support for medical intervention and curative therapy. Marriage also has a positive impact on cardiovascular and endocrine function, cortisol level and immune function, which may improve the effects of cancer treatment and management (34).
Treatment was another dominant factor affecting survival time (3,36). In the present study, Chinese patients had the highest proportion of resection and the lowest proportion of no or UNK treatment for the HCC and ICC cohorts. Surgical resection has been recognized as the optimal curative therapy method for early-stage liver tumor types. The racial disparities among the Chinese patients who received surgical resections and patients of other races who received surgical resection were further analyzed. The results indicated that Chinese patients had improved survival time, compared with the other groups, in the HCC cohort following univariate analysis; however, following multivariate analysis, Chinese patients only had improved survival time when compared with African-American and Hispanic patients. Additionally, there was no significant difference between the Chinese group and the NHW and Other Asian groups. Furthermore, there were no significant differences between the Chinese group and the other groups in the ICC cohort. Factors associated with racial disparities affecting liver cancer survival time following surgical resection are complex (3,37,38). For example, the majority of Asian patients with HCC had a hepatitis B virus (HBV) infection (5). Following virus screening and antiviral therapy, Chinese patients and other patients with HBV-associated HCC frequently have an improved liver function, daily activity and exhibit less comorbidity, reducing the difficulty of surgical resection and management (37,39); however, NHW, Hispanic and African-American patients frequently exhibit hepatitis C virus, non-alcohol fatty liver disease and alcohol abuse, which are frequently accompanied by cirrhosis, diabetes and obesity, resulting in disease management difficulties. Additionally, race was significantly associated with treatment selection. Hoehn et al (37) reported that Asian and NHW patients with HCC had an increased probability of receiving curative therapy, including surgical resection and transplantation, whereas African-American patients had a reduced probability of receiving curative therapy. Stewart et al (11) reported that Chinese, Korean and Japanese patients with HCC had an increased probability of receiving surgical treatment, compared with other Asian populations (11). Selecting an appropriate treatment is complex. Factors, including the disease itself, socioeconomic status, insurance status and social support, serve an important role in treatment selection; however, in the present study, these data were not available. Furthermore, although surgical resection was the only curative therapy method for liver tumor types, patients with ICC still had a poor prognosis, due to its low early detection rate and higher rate of mortality (22). No other studies reporting racial disparities in the survival time of patients with ICC have been reported previously. It is necessary to further investigate the risk and prognostic factors in the ICC cohort and their effect on different races.
Previous studies on racial disparities in liver cancer usually grouped Asian populations into one category and demonstrated that Asian patients had improved survival time, compared with other groups (12,37); however, the term ‘Asian’ encompasses numerous subgroups with different genetic characteristics, geographic origins and living habits, each of which has a different etiology, susceptibility and clinical presentation (16). It was necessary to investigate the racial disparities in survival time between Asian subgroups, which assisted in providing treatment strategies with increased precision for ethnic groups with different characteristics; therefore, the racial disparities among Asian subgroups were further analyzed. It was demonstrated that the Chinese group had improved survival time, compared with other Asian subgroups, following univariate analysis for the HCC and ICC cohorts, but the difference was not significant following adjusting for contributing factors. This result requires further confirmation, due to the small size of the Other Minority group in the present study.
There were a number of limitations to the present study. Firstly, this was a retrospective study, and a number of the contributing factors associated with prognosis were not included, due to the limited data in the SEER database. Therefore, the effects of etiology, comorbidity and chemotherapy on PLC survival time could not be evaluated, which introduces information bias and reduces the validity of multifactorial analysis. Secondly, not all patients in the present study had full data for each covariate, due to the characteristics of the SEER database. The SEER database is the only public database that provides large samples for analysis, which allowed beneficial information to be obtained. Finally, the underlying mechanisms at the genetic and carcinogenic level could not be obtained from the SEER database. Nevertheless, to the best of our knowledge, the present study is the first to analyze racial disparities between Chinese patients and patients of other racial groups for PLC, and it provided beneficial information for clinical practice and future oncological studies.
In conclusion, the racial disparities in clinical presentation, treatment and survival time of PLC between a Chinese group and other racial groups was analyzed. It was demonstrated that Chinese patients had improved survival time, compared with other patients in the HCC cohort. Furthermore, the prognostic power of demographic, socioeconomic, tumor biological and treatment-associated factors on PLC survival time was investigated. Further study is required with a focus on the large-scale analysis of genetic characteristics from different ethnic groups, in order to investigate the underlying mechanisms. Additionally, personalized therapy based on demographic and socioeconomic status is beneficial for improving the survival time of patients with PLC.
Acknowledgements
The authors would like to thank the National Cancer Institute and the Surveillance, Epidemiology and End Results Program for the large-scale population-based cancer data, and Dr Baibing Mi, Department of Epidemiology, Xi'an Jiaotong University for statistical analysis support.
Glossary
Abbreviations
- PLC
primary liver cancer
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- ICC
intrahepatic cholangiocarcinoma
- AFP
α fetoprotein
- NHW
non-Hispanic white
- HR
hazard ratio
- CI
confidence interval
- SEER
Surveillance, Epidemiology, and End Results
- UNK
unknown
- TDT
tumor destructive therapy
- OS
overall survival
- CSS
cause-specific survival
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81727802).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FR, XC, LH, RW and YL designed the study. FR, JZ, ZG, WL, WG and LH acquired and analyzed the data. FR, JZ, ZG, HZ, ZX and LH interpreted the data. FR, JZ, ZG, XC and LH drafted the manuscript. LH, RW and YL revised the manuscript critically. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The SEER database does not include any human or demographic identifying information, and the data used for analysis were de-identified. Therefore, ethics approval and formal informed consent to participate was not required.
Patient consent for publication
All identifying information is removed in the present study; therefore, formal consent for publication was not required.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Zak Y, Rhoads KF, Visser BC. Predictors of surgical intervention for hepatocellular carcinoma: Race, socioeconomic status, and hospital type. Arch Surg. 2011;146:778–784. doi: 10.1001/archsurg.2011.37. [DOI] [PubMed] [Google Scholar]
- 4.Artinyan A, Mailey B, Sanchez-Luege N, Khalili J, Sun CL, Bhatia S, Wagman LD, Nissen N, Colquhoun SD, Kim J. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367–1377. doi: 10.1002/cncr.24817. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Q, Li N, Zeng X, Han Q, Li F, Yang C, Lv Y, Zhou Z, Liu Z. Hepatocellular carcinoma in a large medical center of China over a 10-year period: Evolving therapeutic option and improving survival. Oncotarget. 2015;6:4440–4450. doi: 10.18632/oncotarget.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forner A, Reig ME, Rodriguez De Lope C, Bruix J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 7.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, et al. Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen MH, Garcia RT, Simpson PW, Wright TL, Keeffe EB. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410–417. doi: 10.1053/jhep.2002.34744. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Hansen BE, Peppelenbosch MP, De Man RA, Pan Q, Sprengers D. Factors associated with ethnical disparity in overall survival for patients with hepatocellular carcinoma. Oncotarget. 2017;8:15193–15204. doi: 10.18632/oncotarget.14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Serag HB, Kramer J, Duan Z, Kanwal F. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am J Gastroenterol. 2014;109:1427–1435. doi: 10.1038/ajg.2014.214. [DOI] [PubMed] [Google Scholar]
- 11.Stewart SL, Kwong SL, Bowlus CL, Nguyen TT, Maxwell AE, Bastani R, Chak EW, Chen MS., Jr Racial/ethnic disparities in hepatocellular carcinoma treatment and survival in California, 1988-2012. World J Gastroenterol. 2016;22:8584–8595. doi: 10.3748/wjg.v22.i38.8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alawadi ZM, Phatak UR, Kao LS, Ko TC, Wray CJ. Race not rural residency is predictive of surgical treatment for hepatocellular carcinoma: Analysis of the texas cancer registry. J Surg Oncol. 2016;113:84–88. doi: 10.1002/jso.24101. [DOI] [PubMed] [Google Scholar]
- 13.Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: Tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98:1934–1939. [PMC free article] [PubMed] [Google Scholar]
- 14.Welzel TM, Graubard BI, Quraishi S, Zeuzem S, Davila JA, El-Serag HB, McGlynn KA. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314–1321. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong LL, Hernandez B, Kwee S, Albright CL, Okimoto G, Tsai N. Healthcare disparities in Asians and Pacific Islanders with hepatocellular cancer. Am J Surg. 2012;203:726–732. doi: 10.1016/j.amjsurg.2011.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawai H, Nishida N, Mbarek H, Matsuda K, Mawatari Y, Yamaoka M, Hige S, Kang JH, Abe K, Mochida S, et al. No association for Chinese HBV-related hepatocellular carcinoma susceptibility SNP in other East Asian populations. BMC Med Genet. 2012;13:47. doi: 10.1186/1471-2350-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, Greten TF, McGlynn KA. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757–1765. doi: 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Qiu M, Xu R, Sandra Dobs A. Comparison of survival and clinicopathologic features in colorectal cancer among African American, Caucasian and Chinese patients treated in the United States: Results from the surveillance epidemiology and end results (SEER) database. Oncotarget. 2015;6:33935–33943. doi: 10.18632/oncotarget.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin MQ, Li YP, Wu SG, Sun JY, Lin HX, Zhang SY, He ZY. Differences in esophageal cancer characteristics and survival between Chinese and Caucasian patients in the SEER database. Onco Targets Ther. 2016;9:6435–6444. doi: 10.2147/OTT.S112038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esnaola NF, Meyer JE, Karachristos A, Maranki JL, Camp ER, Denlinger CS. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122:1349–1369. doi: 10.1002/cncr.29692. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016;379:198–205. doi: 10.1016/j.canlet.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Antwi SO, Mousa OY, Patel T. Racial, ethnic, and age disparities in incidence and survival of intrahepatic cholangiocarcinoma in the united states; 1995-2014. Ann Hepatol. 2018;17:604–614. doi: 10.5604/01.3001.0010.8663. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute. https://seer.cancer.gov/about/overview.html Overview of the SEER Program.
- 25.National Cancer Institute. https://seer.cancer.gov/data-software/documentation/seerstat/nov. SEER*Stat Databases. 2015 Nov; Submission. 2015/
- 26.National Cancer Institute. https://seer.cancer.gov/tools/codingmanuals/index.html SEER Program Coding and Staging Manual. 2018
- 27.Davila JA, El-Serag HB. Racial differences in survival of hepatocellular carcinoma in the United States: A population-based study. Clin Gastroenterol Hepatol. 2006;4:104–110. doi: 10.1016/S1542-3565(05)00745-7. [DOI] [PubMed] [Google Scholar]
- 28.Chen JG, Zhang SW. Liver cancer epidemic in China: Past, present and future. Semin Cancer Biol. 2011;21:59–69. doi: 10.1016/j.semcancer.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 30.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 31.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 32.Lee YH, Hsu CY, Huang YH, Hsia CY, Chiou YY, Su CW, Lin HC, Huo TI. Vascular invasion in hepatocellular carcinoma: Prevalence, determinants and prognostic impact. J Clin Gastroenterol. 2014;48:734–741. doi: 10.1097/MCG.0b013e3182a8a254. [DOI] [PubMed] [Google Scholar]
- 33.Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, Setiawan VW, El-Khoueiry A. Impact of sex on the survival of patients with hepatocellular carcinoma: A surveillance, epidemiology and end results analysis. Cancer. 2014;120:3707–3716. doi: 10.1002/cncr.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He XK, Lin ZH, Qian Y, Xia D, Jin P, Sun LM. Marital status and survival in patients with primary liver cancer. Oncotarget. 2016;8:64954–64963. doi: 10.18632/oncotarget.11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teo EK, Han SH, Terrault N, Luketic V, Jensen D, Keeffe EB, Lok AS HBV-OLT Study Group, corp-author. Liver transplantation in patients with hepatitis B virus infection: Outcome in Asian versus white patients. Hepatology. 2001;34:126–132. doi: 10.1053/jhep.2001.25271. [DOI] [PubMed] [Google Scholar]
- 37.Hoehn RS, Hanseman DJ, Wima K, Ertel AE, Paquette IM, Abbott DE, Shah SA. Does race affect management and survival in hepatocellular carcinoma in the United States? Surg. 2015;158:1244–1251. doi: 10.1016/j.surg.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Couto CA, Gelape CL, Calmet F, Martin P, Levy C. Effect of ethnicity on liver transplant for hepatocellular carcinoma. Exp Clin Transplant. 2013;11:339–345. doi: 10.6002/ect.2013.0008. [DOI] [PubMed] [Google Scholar]
- 39.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145:1158–1163. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.