Abstract
FK506-binding protein 51 (FKBP51) is a member of the immunophilin family, with relevant roles in multiple signaling pathways, tumorigenesis and chemoresistance. However, the function of FKBP51 in papillary thyroid carcinoma (PTC) remains largely unknown. In the present study, increased FKBP51 expression was detected in PTC tissues as compared with adjacent normal tissues, and the expression level was associated with clinical tumor, node and metastasis stage. Using FKBP51-overexpressing K1 cells and FKBP51-knockdown TPC-1 cells, both human PTC cell lines, it was identified that FKBP51 promoted the migration and invasion of PTC, without affecting cell proliferation. Further investigation revealed that FKBP51 activated the NF-κB pathway and epithelial-to-mesenchymal transition (EMT) genes, and EMT was suppressed when NF-κB was inhibited. It was also assessed whether FKBP51 promoted the formation of cytoskeleton to promote migration and invasion of PTC using a tubulin tracker; however, no evidence of such an effect was observed. These results suggested that FKBP51 promotes migration and invasion through NF-κB-dependent EMT.
Keywords: migration, invasion, epithelial-to-mesenchymal
Introduction
FK506-binding proteins (FKBPs) belong to the family of immunophilins, which may bind to immunosuppressant drugs (1). It contains a 3-unit tetratricopeptide repeat structural motif connected to a domain that mediates enzymatic function and peptidyl prolyl isomerase activity. FKBP51 interacts with glucocorticoid, androgen, estrogen, progestin and mineralocorticoid receptors (2–7) through a number of components of the molecular chaperone machinery, including heat shock protein 90. FKBP51 has been identified to be upregulated or downregulated in multiple types of cancer and to be associated with cell motility and invasion, for instance, Leach et al (8) identified that FKBP51 is increased in prostate cancer cells compared with normal prostate epithelial cells and improves the ability of stromal androgen receptor to predict prostate cancer-specific mortality. Hou et al (9) reported that low levels of FKBP51 promote pancreatic tumor growth. FKBP51 may promote cancer, including melanoma, through the nuclear factor (NF)-κB pathway (10,11) and suppress cancer through the phosphatidylinositol-4,5-bisphosphate 3 kinase/protein kinase B (AKT) signaling pathway (12,13). It has been demonstrated that the activation of NF-κB contributes to growth (14) and aggressiveness of papillary thyroid carcinoma, and lymph node metastases in papillary thyroid carcinoma (PTC) are significantly correlated with NF-κB levels (15). Recently, studies have demonstrated that FKBP51 serves an important role in epithelial-mesenchymal transition (EMT) (16–18). EMT refers to the biological process by which epithelial cells are transformed into mesenchymal phenotype cells by certain procedures. In this procedure, expression of epithelial cell characteristic proteins, including adhesion molecule E-cadherin, decreased and cytokeratin cytoskeleton changed into vimentin skeleton (19). In colorectal carcinoma, Rotoli D was determined to have increased microvessel density and tumor-associated macrophages in connective tissue surrounding FKBP51 positive lesions (16).
The morbidity rate of PTC is increasing worldwide (20,21). Surgery is the primary treatment for PTC. The prevalence of PTC is nearly 3 times higher in females than in males (22). This sex difference suggests that growth and progression of PTC may be influenced by female sex hormones, and it has been demonstrated that estrogen and progesterone receptors are more strongly expressed in thyroid hyperplasia diseases than in normal thyroid tissue (23).
As FKBP51 regulates the activity of sex-hormone receptors and correlates with a number of types of cancer, and PTC may be a sex hormone-associated cancer, in the present study, the function of FKBP51 in PTC was investigated.
Materials and methods
Materials
The human PTC cell lines K1 and TPC-1 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). RPMI-1640, Dulbecco's modified Eagle's medium and fetal bovine serum (FBS) were purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Anti-FKBP51 (ab46002) used in immunohistochemistry (IHC), anti-IκBα (ab32518), anti-N-cadherin (ab76011), anti-Vimentin (ab92547), anti-β-catenin (ab32572) and anti-MMP9 (ab76003) were purchased from Abcam (Cambridge, UK). Anti-p65 (sc-8008), anti-FKBP51 (sc-13983), anti-GAPDH (sc-47724), anti-α-tubulin (sc-53646), secondary antibody anti-mouse IgG-horseradish peroxidase (HRP; sc-516102) and anti-rabbit IgG-HRP (sc-2372) used in western blotting were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Anti-TGF-β1 (3709) and anti-histone 3 (12164) was purchased from Cell Signaling Technology (Danvers, MA, USA). Ammonium pyrrolidine dithiocarbamate (PDTC; T3147) was purchased from Target Molecule Corp. (Wellesley Hills, MA, USA).
IHC
IHC was used to detect expression of FKBP51. Thyroid tissue microarrays including 31 PTC tissues and 41 adjacent normal tissues were purchased from Shanxi Alena Biotechnology (Shanxi, China). Following staining with the Elivision™ plus kit (cat. no. 9902; Maxim Biotech, Inc., Rockville, MD, USA), according to the manufacturer's protocols, the specimens were incubated overnight with primary antibody (anti-FKBP51; 1:50 dilution) at 4°C, and washed with 0.1% Tween-20 PBS for 5 min 3 times. Subsequently, polymer enhancer (Elivision™ plus kit), which assists horse radish peroxidase-anti-rabbit polymer (Elivision™ plus kit) to combine with primary antibodies, was added and incubated for 20 min at room temperature, and then wash with 0.1% Tween-20 PBS for 5 min 3 times. Following this, the horse radish peroxidase-labeled anti-rabbit polymer (Elivision™ plus kit) was added and incubated at room temperature for 30 min, and then washed with 0.1% Tween-20 PBS for 5 min 3 times. Additionally, the specimens were stained with diaminobenzidine solution (DAB-0031/1031; Maxim Biotech, Inc.) for 5 min at room temperature, and distilled water was used to rinse the specimens and stop the coloration.
The expression of FKBP51 was scored by two professional pathologists as follows: 1+, 1–25% of positive tumor cells; 2+, 25–50%; 3+, 50–74%; and 4+, ≥75%. 0, negative staining; 1+, light brown; 2+, brown; 3+, dark brown. The total score was between 0–12 and was calculated as the positive percentage score multiplied by the intensity score. FKBP51 expression levels were ranked into four grades according to the total score: 0, -; 1–4, +; 5–8, ++; 9–12, +++.
The clinical stage of papillary thyroid carcinoma was guided by American Joint Committee on Cancer (24).
Lentivirus and plasmid transfection
The human FKBP51 gene sequence was retrieved from the NCBI gene bank (https://www.ncbi.nlm.nih.gov/nuccore/U71321.1) and restriction enzymes were used to cut appropriate sequences as the target gene and amplified through polymerase chain reaction [The target gene from chemical synthesis was denatured into a single strand at a high temperature ~95°C, and the single nucleotide was combined into the target gene from the 3′ end of the primer (FKBP51: Sense, 5′-AAAAGGCCAAGGAGCACAAC-3′ and antisense 5′-TTGAGGAGGGGCCGA194GTTC-3′) to synthesize a new complementary strand of DNA with a heat-resistant DNA polymerase (Takara Bio, Inc., Otsu, Japan). The full thermocycling conditions can be simplified into the following steps: Pre-denaturation (95°C for 30 sec), reaction cycles (95°C for 5 sec and 60°C for 30 sec), melting (95°C for 5 sec and 60°C for 1 min) and cooling (50°C for 30 sec). Subsequently, the FKBP51 cDNA was cloned into the GV303 lentiviral expression vector. All this process was conducted by Shanghai GeneChem (Shanghai GeneChem, Co., Ltd., Shanghai, China). Plasmids encoding human FKBP51 shRNA (SC-35380-SH) and scramble shRNA were purchased from Santa Cruz Biotechnology, Inc. K1 cells were seeded in a 96-well plate (5×103 cells/well) overnight. Then, the cells were infected with 1×106 units of the recombinant lentiviral vector carrying wild-type human FKBP51 (concentration, 2×108 U/ml) or randomized flanking sequences (concentration, 1×109 U/ml) as a control under the condition of the Polybrene (5 µg/ml) as the transfection reagent (Shanghai GeneChem, Co., Ltd.). After 8–12 h, the virus mixture was exchanged for fresh RPMI-1640 complete medium. Green fluorescent protein (GFP)-positive infected cells were detected by fluorescence microscopy (×10) at 72 h after transfection. TPC-1 cells were seeded in a 6-well plate (2×105 cells/well). The next day, shR-FKBP51 and shR-vec plasmids were added to the cells, and 5–7 h later, the mixture was changed into fresh medium containing 20% FBS. After 48 h, transfected cells were selected using puromycin (12 µg/ml). Subsequent experiments were performed following confirmation of the efficiency of infection (~72 h after infection).
Proliferation assay
FKBP51-overexpressing or knockdown cells and control cells were digested with trypsin and then seeded into 96-well plates (5×103 cells/well) and incubated at 37°C under 5% CO2. Following incubation for 24, 48 and 72 h, 10 µl of cell counting kit-8 and 90 µl of RPMI-1640 fresh medium were added into each well. Following incubation at 37°C in an atmosphere containing 5% CO2 for 1 h, the absorbance was measured at 450 nm using a SpectraMax M2 instrument. All experiments were performed in triplicate.
Migration and invasion assays
In total, 2×105 K1 cells or 3×104 TPC-1 cells in serum-free RPMI-1640 medium were added into Transwell upper chambers with 8-mm pore-size membranes, and complete medium was added to the lower chambers. To detect cell invasion ability, membranes were coated with Matrigel (diluted 1:6 in 1× PBS; BD Biosciences, Franklin Lakes, NJ, USA). The plate was incubated at 37°C in an atmosphere containing 5% CO2 for 24 h. Cells on the upper membrane surface were removed using cotton swabs and washed with 1× PBS, and the cells attached to the bottom surface of the membrane were stained using crystal violet at room temperature for 15 min. Images (×20) were captured using a Nikon fluorescence microscope (Nikon Corporation, Tokyo, Japan).
Western blotting
Cells were harvested and total protein was extracted using radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, Inc.). The protein concentration was determined using a bicinchoninic acid assay. A total of 30 µg of protein was separated via 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Following blocking for 1 h with 5% nonfat milk (Yili Dairy Corporation Group, Co., Ltd., Hohhot, China) diluted with PBS, the membranes were incubated with the following primary antibodies at 4°C overnight: Anti-FKBP51 (1:1,000), anti-p65 (1:250), anti-IκBα (1:1,000), anti-TGF-β1 (1:1,000), anti-N-cadherin (1:1,000), Vimentin (1:1,000), anti-β-catenin (1:1,000) and anti-MMP9 (1:1,000). Following washing three times with 1X TBS with Tween-20, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:1,000) for 1 h at room temperature and then washed three times with 1X TBS with Tween-20. The resulting signal was detected using Scion Image software version 4.03 (Scion Corporation, Frederick, MD, USA), and the quantity of protein was normalized to that of GAPDH (1:1,000), histone 3 (1:1,000), or α-tubulin (1:1,000) (for normalization to total protein, nuclear protein, or cytoplasmic protein, respectively).
Immunofluorescence microscopy
Cells on a slide were fixed with 3.7% formaldehyde for 10 min and washed with PBS containing 0.1% Triton X-100 twice. Tubulin-Tracker Red (Beyotime Institute of Biotechnology, Haimen, China) was diluted 1:250 in PBS containing 5% bovine serum albumin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and 0.1% Triton X-100, and the slides were incubated with this solution for 30 min at room temperature. The slides were then washed three times with PBS for 5 min each.
PDTC inhibition test
PDTC powder was dissolved in dimethyl sulfoxide (DMSO) as a storage solution (20 mM), and the working fluid concentration was 20 µM. In the Transwell test, 0.2 µl PDTC storage solution was added into 200 µl cell suspension in the upper chambers and 0.2 µl DMSO was added in the control group. Migrated and invaded cells were counted after 24 h. In the western blot assay, FKBP51-overexpressing K1 cells were seeded into 6-well plate (1×106 cells/well). The following day, 2 µl PDTC storage solution was added into 2 ml fresh RPMI-1640 medium in the plate and 2 µl DMSO was added in the control group. The protein of the two cell groups were extracted after culturing for 24 h at 37°C in an atmosphere containing 5% CO2. The migration/invasion and western blotting assays were similar to that aforementioned, with the difference being whether PDTC or DMSO was added.
Statistical analysis
All results were analyzed using SPSS software (version 20.0; IBM Corp., Armonk, NY, USA) and data are presented as the mean ± standard error of at least three independent experiments. The differences between two groups were evaluated using Student's t-test. Differences among multiple groups were assessed using one-way analysis of variance and the Student's Newman Keuls-q test was used for post-hoc analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
FKBP51 is highly expressed in PTC
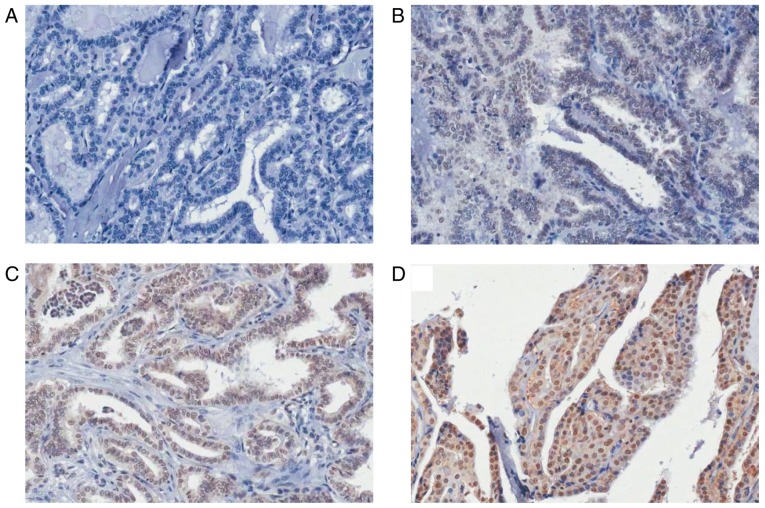
IHC analysis revealed higher FKBP51 expression in PTC than in the adjacent normal tissues. Furthermore, the FKBP51 expression level was associated with clinical tumor, node and metastasis (TNM) stage, with stronger FKBP51 expression indicating poorer TNM stage (Fig. 1; Table I).
Figure 1.
FK506-binding protein 51 expression level in papillary thyroid carcinoma tissues and adjacent normal tissues (magnification, ×200). Representative images of tissues rated (A) -, (B) +, (C) ++ and (D) +++.
Table I.
FKBP51 expression in different patients and tissues.
| Expression of FKBP51 | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Cases | − | + | ++ | +++ | P-value |
| Group | 0.015 | |||||
| PTC | 31 | 14 | 4 | 9 | 4 | |
| Normal | 41 | 23 | 15 | 3 | 0 | |
| Age | 0.140 | |||||
| <45 | 46 | 27 | 11 | 6 | 2 | |
| >45 | 26 | 11 | 7 | 5 | 3 | |
| TNM | 0.023 | |||||
| I | 19 | 11 | 4 | 3 | 1 | |
| II | 9 | 2 | 1 | 5 | 1 | |
| III | 2 | 1 | 0 | 1 | 0 | |
| IV | 1 | 0 | 0 | 0 | 1 | |
PTC, papillary thyroid carcinoma; FKBP51, FK506 binding protein 51.
FKBP51 promotes migration and invasion of PTC without affecting cell proliferation
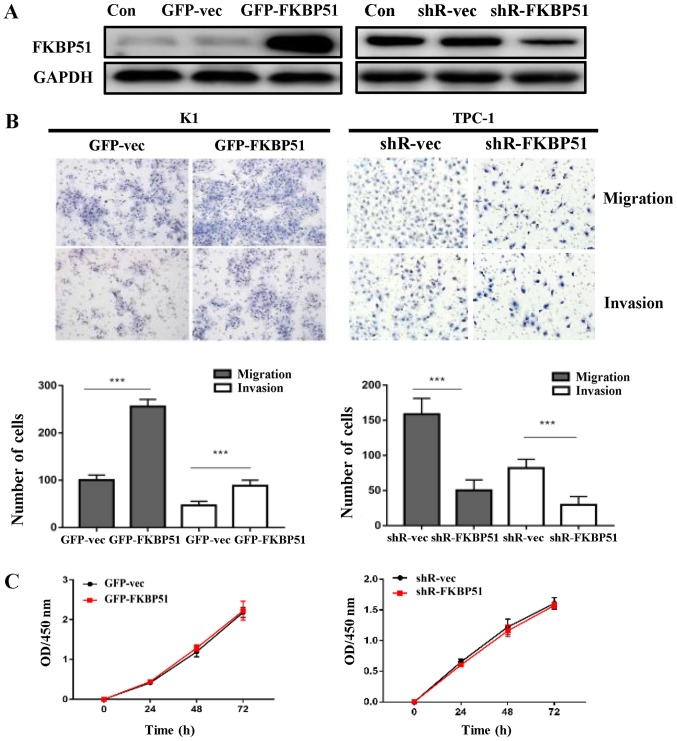
FKBP51-overexpressing and control K1 cells were successfully constructed, alongside FKBP51-knockdown and control TPC-1 cells (Fig. 2A). To determine the effect of FKBP51 on PTC cell migration and invasion, a transwell assay was performed. Fig. 2B presents that FKBP51 overexpression promoted cell migration and invasion as compared with the control group in K1 cells. The counts of migrated cells were 210±61.2 and 102.0±9.1 (P<0.05), and those of invaded cells were 75.3±11.5 and 48.8±6.2 (P<0.05), in overexpressing and control cells, respectively. FKBP51 knockdown inhibited cell migration and invasion in TPC-1 cells as compared with the control group. The counts of migrated cells were 158.5±11.4 and 50.25±7.4 (P<0.05), and those of invaded cells were 81.8±6.3 and 29.5±6.0 (P<0.05), respectively. There was no significant difference in proliferation between FKBP51-overexpressing and control K1 cells, and FKBP51-knockdown and control TPC-1 cells (Fig. 2C).
Figure 2.
FKBP51 promoted cell migration and invasion of papillary thyroid carcinoma cells without affecting proliferation. (A) FKBP51 overexpressing and control K1 cells and FKBP51 knockdown and control TPC-1 cells. (B) The cells migration and invasive capacities were determined using a transwell assay. The data represent the mean ± standard deviation of at least 3 independent experiments (***P<0.001). (C) Cell counting kit-8 analysis was used to determine cell proliferation difference between FKBP51-overexpressing and the control K1 cells as well as FKBP51-knockdown and the control TPC-1 cells. The result revealed no significance (P>0.05). FKBP51, FK506 binding protein 51; GFP, green fluorescent protein; vec, vector; Con, control.
FKBP51 regulates the NF-κB pathway
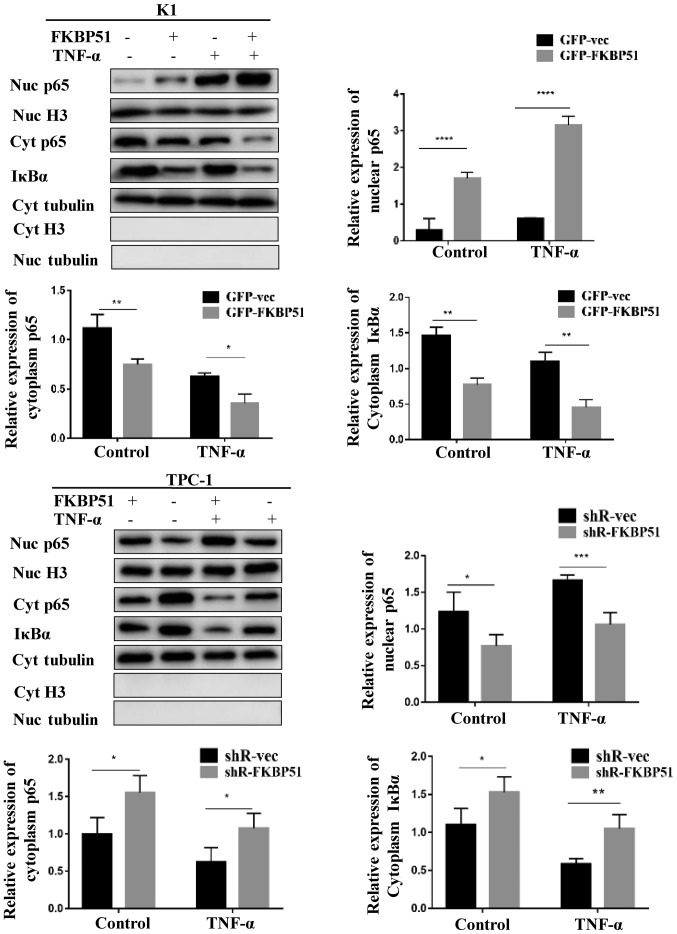
The molecular mechanism underlying the promoting effect of FKBP51 on migration and invasion was investigated. The results demonstrated that FKBP51 activated the NF-κB pathway. The activity of the NF-κB pathway was investigated at the basic level and at the TNF-α-stimulated level. Western blotting results revealed that both at the basic and the stimulated level, cytoplasmic IκBα and cytoplasmic P65 were decreased and nuclear P65 was increased in FKBP51-overexpressing K1 cells, while cytoplasmic IκBα and P65 were increased and nuclear P65 decreased in FKBP51-knockdown TPC-1 cells as compared with the control groups (Fig. 3).
Figure 3.
FKBP51 regulated the NF-κB pathway of papillary thyroid carcinoma. Western blot analysis of nuclear P65, cytoplasm P65 and IκBα in FKBP51-overexpressing and control K1 cells, and FKBP51-knockdown and control TPC-1 cells are presented. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. FKBP51, FK506 binding protein 51; GFP, green fluorescent protein; vec, vector; TNF, tumor necrosis factor; Nuc, nuclear; Cyt, cytoplasmic.
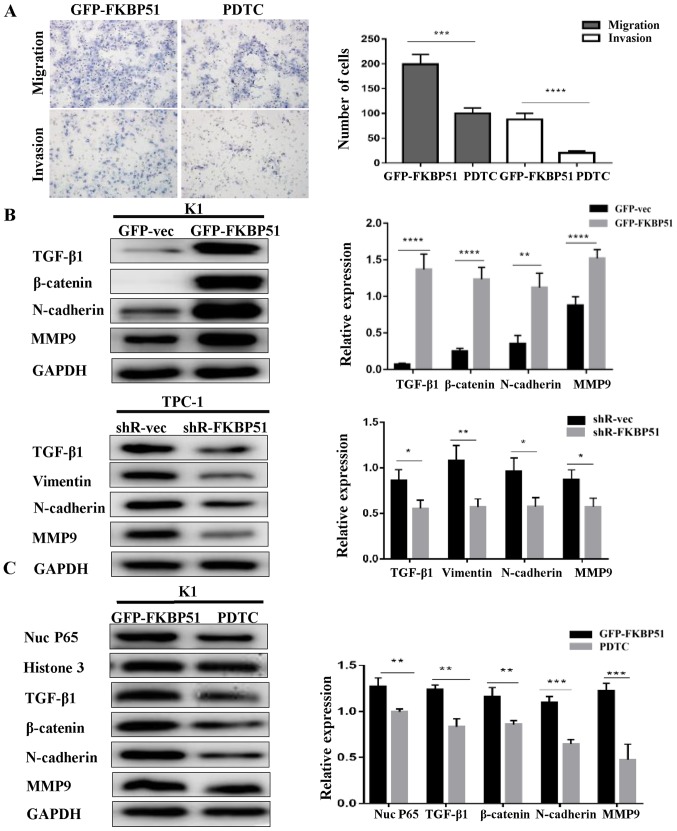
Further, the NF-κB pathway inhibitor PDTC (20 µM) suppressed migration and invasion of GFP-FKBP51 K1 (Fig. 4A). This confirmed that FKBP51 inhibited PTC cell migration and invasion via the NF-κB pathway.
Figure 4.
FKBP51 activated EMT through the NF-κB pathway. (A) GFP-FKBP51 K1 cells were treated with NF-κB pathway inhibitor PDTC (20 µM) for 24 h and the cells' migration and invasive capacities were determined using the transwell assay. (B) EMT-associated proteins TGF-β1, β-catenin, N-cadherin, MMP9 of K1 cells and TGF-β1, Vimentin, N-cadherin, MMP9 of TPC-1 cells were detected by western blotting. (C) FKBP51-overexpressing K1 cells were treated with NF-κB inhibitor PDTC (20 mM) for 24 h and expression of TGF-β1, β-catenin, N-cadherin and MMP9 was compared with control group was detected. *P<0.05; **P<0.01; ***P<0.001. FKBP51, FK506 binding protein 51; GFP, green fluorescent protein; vec, vector; MMP, matrix metalloproteinase; EMT, epithelial mesenchymal transition.
FKBP51 activates NF-κB-dependent EMT
EMT-associated protein expression was altered with FKBP51 expression level. TGF-β1, β-catenin, N-cadherin, and MMP9 were increased in FKBP51-overexpressing K1 cells, while TGF-β1, Vimentin, N-cadherin and MMP9 were decreased in FKBP51-knockdown TPC-1 cells, as presented in Fig. 4B. Subsequently, it was assessed whether EMT is regulated by the NF-κB pathway. FKBP51-overexpressing K1 cells were treated with PDTC (20 µM) for 24 h. TGF-β1, N-cadherin, β-catenin and MMP9 were decreased as compared with control group by this treatment (Fig. 4C).
FKBP51 does not alter the formation of tubulin
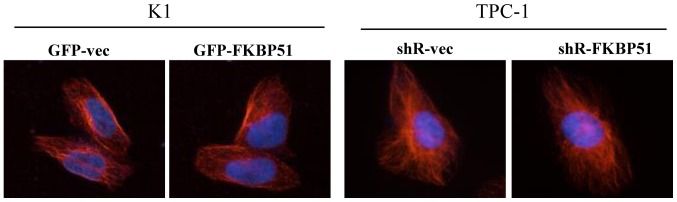
Further, it was assessed whether FKBP51 promotes migration and invasion of PTC cells through promoting the formation of tubulin cytoskeleton, using immunofluorescence staining. No difference in fluorescent signal was observed between FKBP51-overexpressing and control K1 cells, and FKBP51-knockdown and control TPC-1 cells (Fig. 5).
Figure 5.
FK506 binding protein 51 does not alter the formation of tubulin. Immunofluorescence was used to detect the tubulin formation, and relative fluorescence intensity and distribution of tubulin revealed no significant difference (P>0.05). GFP, green fluorescent protein.
Discussion
PTC accounted for 60–70% of thyroid cancer between 1992–2002 in Liaoning, China, mostly in adults aged between 20 and 50 years (25). There are numerous factors affecting PTC, including environmental factors and genetic mutations (26). However, gender is universally recognized as one of the risk factors since the occurrence is significantly higher in females than males (27). Therefore, most patient samples from the tissue microarray were female patients. PTC generally has small size and slow progression, but early metastasis may be identified (28). The underlying molecular mechanism and associated molecular markers of metastasis are not clear. Upregulation and downregulation of FKBP51 is observed in a number of human tumors. It is notable that FKBP51 is associated with the development of a variety of hormone-associated tumors, and previous studies have demonstrated that FKBP51 is overexpressed in androgen-dependent prostate cancer (8,10), and decreased in estrogen- and progesterone-associated breast cancer (29). In the present study, the expression of FKBP51 in PTC and its clinical significance were analyzed. It was identified that FKBP51 is expressed in both PTC and adjacent tissues, however its expression was significantly higher in cancer than in adjacent normal tissues. Furthermore, the FKBP51 expression level was associated with clinical TNM stage. Thus, it is hypothesized that FKBP51 may be associated with the proliferation and metastasis of thyroid cancer.
Accumulating data have demonstrated that FKBP51 serves important functions in tumor cell growth, apoptosis, and sensitivity to radiotherapy and chemotherapy (30,31). Studies have demonstrated that FKBP51 may serve a positive function in tumor progression by activating the NF-κB (32–34) pathway, or a negative function by promoting the Akt pathway through the dephosphorylation of Akt Ser473 to inhibit cell proliferation (9,35). The mechanism of FKBP51 in promoting cancer cell migration and invasion is not clear. Romano et al (17) and D'Angelillo et al (18) identified that FKBP51 may promote the migration and invasion of melanoma by activating EMT-associated genes. TGF-β1 as the key inducer of EMT not only promotes tumor cell metastasis but also mediates liver cirrhosis (36) and renal tubulointerstitial fibrosis (37). Further, FKBP51 reportedly not only promotes the activation of EMT genes but also induces certain melanoma stem cell genes (38). Srivastava et al (39) identified that FKBP51 promotes the proliferation and migration of melanoma by mediating interleukin IL-8. Certain researchers have demonstrated that FKBP51 promotes tubulin cytoskeleton formation through the ras homolog family member A pathway to promote cell invasion (40). In the present study, K1 and TPC-1 cell lines were selected, which are commonly used in study [the K1 cell line is a GLAG-66 derivative (41)] to overexpress and knockdown FKBP51 respectively. The results demonstrated that FKBP51 did not affect cell proliferation, but significantly promoted the migration and invasion of PTC cells. To clarify the underlying mechanism of the latter observation, NF-κB pathway- and EMT-associated proteins were evaluated. The results revealed that both the basic and the TNF-α-simulated activity of the NF-κB pathway were increased in FKBP51-overexpressing than in control cells. Similarly, EMT-associated proteins were altered in FKBP51-overexpressing K1 and FKBP51-knockdown TPC-1 cells. Subsequently, the present study aimed to evaluate whether there is an association between EMT and the NF-κB pathway. When the NF-κB inhibitor PDTC was added to FKBP51-overexpressing K1 cells for 24 h, TGF-β1, N-cadherin, and β-catenin and MMP9 expression were decreased. These results revealed that FKBP51 promotes migration and invasion through NF-κB pathway-dependent EMT. This result is consistent with previous findings, for example Ying et al (42) demonstrated that induced EMT was accompanied by nuclear translocation of NF-κB in lung cancer. Lv et al (43) revealed that twist1 regulates EMT via the NF-κB pathway in PTC. The present study also assessed whether FKBP51 is able to promote tubulin formation by using immunofluorescence, however no evidence of such an effect was identified. It was also assessed whether FKBP51 can promote tubulin formation by using immunofluorescence, however, once again no evidence of such an effect was observed.
To conclude, the present study demonstrates that FKBP51 promotes migration and invasion of PTC through the NF-κB pathway and activation of EMT-associated genes, indicating its diagnostic and therapeutic value.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 31370897), and all the results come from the joint efforts of all authors.
Availability of data and materials
The datasets generated/analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YG, RZ, EE, HY, SL, LS and YZ designed the study. YG, JD and MW performed the experiments. YG wrote the manuscript and analyzed the data.
Ethics approval and consent to participate
All patients provided written informed consent prior to their inclusion. The present study was approved by the Ethical Committee of Shandong Provincial Hospital.
Patient consent for publication
All patients provided written informed consent for the publication of their data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Dornan J, Taylor P, Walkinshaw MD. Structures of immunophilins and their ligand complexes. Curr Top Med Chem. 2003;3:1392. doi: 10.2174/1568026033451899. [DOI] [PubMed] [Google Scholar]
- 2.Cheung-Flynn J, Roberts PJ, Riggs DL, Smith DF. C-terminal sequences outside the tetratricopeptide repeat domain of FKBP51 and FKBP52 cause differential binding to Hsp90. J Biol Chem. 2003;278:17388–17394. doi: 10.1074/jbc.M300955200. [DOI] [PubMed] [Google Scholar]
- 3.Cheung J, Smith DF. Molecular chaperone interactions with steroid receptors: An update. Mol Endocrinol. 2000;14:939–946. doi: 10.1210/mend.14.7.0489. [DOI] [PubMed] [Google Scholar]
- 4.Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. Embo J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai PK, Maeda Y, Nakao K, Wakim NG, Duhring JL, Faber LE. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986;25:5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- 6.Gallo LI, Ghini AA, Pilipuk Piwien G, Galigniana MD. Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry. 2007;46:14044–14057. doi: 10.1021/bi701372c. [DOI] [PubMed] [Google Scholar]
- 7.Brucker SY, Eisenbeis S, König J, Lamy M, Salker MS, Zeng N, Seeger H, Henes M, Schöller D, Schönfisch B, et al. Decidualization is impaired in endometrial stromal cells from uterine rudiments in Mayer-Rokitansky-Küster-Hauser syndrome. Cell Physiol Biochem. 2017;41:1083–1097. doi: 10.1159/000464116. [DOI] [PubMed] [Google Scholar]
- 8.Leach DA, Trotta AP, Need EF, Risbridger GP, Taylor RA, Buchanan G. The prognostic value of stromal FK506-binding protein 1 and androgen receptor in prostate cancer outcome. Prostate. 2017;77:185–195. doi: 10.1002/pros.23259. [DOI] [PubMed] [Google Scholar]
- 9.Hou J, Wang L. FKBP5 as a selection biomarker for gemcitabine and Akt inhibitors in treatment of pancreatic cancer. PLoS One. 2012;7:e36252. doi: 10.1371/journal.pone.0036252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni L, Yang CS, Gioeli D, Frierson H, Toft DO, Paschal BM. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol. 2010;30:1243–1253. doi: 10.1128/MCB.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang W, Cazacu S, Xiang C, Zenklusen JC, Fine HA, Berens M, Armstrong B, Brodie C, Mikkelsen T. FK506 binding protein mediates glioma cell growth and sensitivity to rapamycin treatment by regulating NF-kappaB signaling pathway. Neoplasia. 2008;10:235–243. doi: 10.1593/neo.07929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solassol J, Mange A, Maudelonde T. FKBP family proteins as promising new biomarkers for cancer. Curr Opin Pharmacol. 2011;11:320–325. doi: 10.1016/j.coph.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Storer CL, Dickey CA, Galigniana MD, Rein T, Cox MB. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol Metab. 2011;22:481–490. doi: 10.1016/j.tem.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pyo JS, Kang G, Kim DH, Chae SW, Park C, Kim K, Do Si, Lee HJ, Kim JH, Sohn JH. Activation of nuclear factor-κB contributes to growth and aggressiveness of papillary thyroid carcinoma. Pathol Res Pract. 2013;209:228–232. doi: 10.1016/j.prp.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Wei L, Hui M, Sun D, Li W, Wang D, Zhang G, Tan J. The relationship between BRAFV600E, NF-κB and TgAb expression in papillary thyroid carcinoma. Pathol Res Pract. 2017;213:183–188. doi: 10.1016/j.prp.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Rotoli D, Morales M, Del Carmen Maeso M, Del Pino García M, Morales A, Ávila J, Martín-Vasallo P. Expression and localization of the immunophilin FKBP51 in colorectal carcinomas and primary metastases, and alterations following oxaliplatin-based chemotherapy. Oncol Lett. 2016;12:1315–1322. doi: 10.3892/ol.2016.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romano S, D'Angelillo A, D'Arrigo P, Staibano S, Greco A, Brunetti A, Scalvenzi M, Bisogni R, Scala I, Romano MF. FKBP51 increases the tumour-promoter potential of TGF-beta. Clin Transl Med. 2014;3:1. doi: 10.1186/2001-1326-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Angelillo A, Staibano S, Russo M, Romano MF, Romano S. Molecular aspects of FKBP51 that enable melanoma dissemination. Curr Mol Pharmacol. 2015;9:141–147. doi: 10.2174/1874467208666150519115242. [DOI] [PubMed] [Google Scholar]
- 19.Song IH, Kim KR, Lim S, Kim SH, Sung CO. Expression and prognostic significance of epithelial-mesenchymal transition-related markers and phenotype in serous ovarian cancer. Pathol Res Pract. 2018 doi: 10.1016/j.prp.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Ceresini G, Corcione L, Michiara M, Sgargi P, Teresi G, Gilli A, Usberti E, Silini E, Ceda GP. Thyroid cancer incidence by histological type and related variants in a mildly iodine-deficient area of Northern Italy, 1998 to 2009. Cancer. 2012;118:5473–5480. doi: 10.1002/cncr.27591. [DOI] [PubMed] [Google Scholar]
- 21.Jonklaas J, Murthy S, Liu D, Klubo-Gwiezdzinska J, Krishnan J, Burman KD, Boyle L, Carrol N, Felger E, Loh YP. Novel biomarker SYT12 may contribute to predicting papillary thyroid cancer outcomes. Future Sci OA. 2017;4:FSO249. doi: 10.4155/fsoa-2017-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer. 2014;21:T273–T283. doi: 10.1530/ERC-14-0053. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Chen G, Meng XY, Liu ZH, Dong S. Serum levels of sex hormones and expression of their receptors in thyroid tissue in female patients with various types of thyroid neoplasms. Pathol Res Pract. 2014;210:830–835. doi: 10.1016/j.prp.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 25.Haixia Guan, Zhongyan Shan, Xiaoyi Mi, et al. 11 years of pathological analysis of thyroid cancer before and after common salt iodization. J China Med Univer. 2006;35:284–285. [Google Scholar]
- 26.Zhang Q, Liu SZ, Zhang Q, Guan YX, Chen QJ, Zhu QY. Meta-analyses of association between BRAF(V600E) mutation and clinicopathological features of papillary thyroid carcinoma. Cell Physiol Biochem. 2016;38:763–776. doi: 10.1159/000443032. [DOI] [PubMed] [Google Scholar]
- 27.Chen GG, Vlantis AC, Zeng Q, van Hasselt CA. Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Curr Cancer Drug Targets. 2008;8:367–377. doi: 10.2174/156800908785133150. [DOI] [PubMed] [Google Scholar]
- 28.Choi MH, Moon JY, Cho SH, Chung BC, Lee EJ. Metabolic alteration of urinary steroids in pre- and post-menopausal women, and men with papillary thyroid carcinoma. BMC Cancer. 2011;11:342. doi: 10.1186/1471-2407-11-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bihan S, Marsaud V, Mercier-Bodard C, Baulieu EE, Mader S, White JH, Renoir JM. Calcium/calmodulin kinase inhibitors and immunosuppressant macrolides rapamycin and FK506 inhibit progestin-and glucocorticosteroid receptor-mediated transcription in human breast cancer T47D cells. Mol Endocrinol. 1998;12:986–1001. doi: 10.1210/mend.12.7.0128. [DOI] [PubMed] [Google Scholar]
- 30.Rotoli D, Morales M, Ávila J, Maeso MDC, García MDP, Mobasheri A, Martín-Vasallo P. Commitment of scaffold proteins in the onco-biology of human colorectal cancer and liver metastases after oxaliplatin-based chemotherapy. Int J Mol Sci. 2017;18:E891. doi: 10.3390/ijms18040891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo K, Li Y, Yin Y, Li L, Wu C, Chen Y, Nowsheen S, Hu Q, Zhang L, Lou Z, Yuan J. USP49 negatively regulates tumorigenesis and chemoresistance through FKBP51-AKT signaling. EMBO J. 2017;36:1434–1446. doi: 10.15252/embj.201695669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loercher A, Lee TL, Ricker JL, Howard A, Geoghegen J, Chen Z, Sunwoo JB, Sitcheran R, Chuang EY, Mitchell JB, et al. Nuclear factor-kappaB is an important modulator of the altered gene expression profile and malignant phenotype in squamous cell carcinoma. Cancer Res. 2004;64:6511–6523. doi: 10.1158/0008-5472.CAN-04-0852. [DOI] [PubMed] [Google Scholar]
- 33.Romano S, Xiao Y, Nakaya M, D'Angelillo A, Chang M, Jin J, Hausch F, Masullo M, Feng X, Romano MF, Sun SC. FKBP51 employs both scaffold and isomerase functions to promote NF-κB activation in melanoma. Nucleic Acids Res. 2015;43:6983–6993. doi: 10.1093/nar/gkv615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li P, Zhang X, Wang L, Du L, Yang Y, Liu T, Li C, Wang C. lncRNA HOTAIR contributes to 5FU resistance through suppressing miR-218 and activating NF-κB/TS signaling in colorectal cancer. Mol Ther Nucleic Acids. 2017;8:356–369. doi: 10.1016/j.omtn.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stechschulte LA, Hinds TD, Jr, Ghanem SS, Shou W, Najjar SM, Sanchez ER. FKBP51 reciprocally regulates GRα and PPARγ activation via the Akt-p38 pathway. Mol Endocrinol. 2014;28:1254–1264. doi: 10.1210/me.2014-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JY, An HJ, Kim WH, Gwon MG, Gu H, Park YY, Park KK. Anti-fibrotic effects of synthetic oligodeoxynucleotide for TGF-β1 and smad in an animal model of liver cirrhosis. Mol Ther Nucleic Acids. 2017;8:250–263. doi: 10.1016/j.omtn.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu J, Zhu Q, Li PL, Wang W, Yi F, Li N. Stem cell conditioned culture media attenuated albumin-induced epithelial-mesenchymal transition in renal tubular cells. Cell Physiol Biochem. 2015;35:1719–1728. doi: 10.1159/000373984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romano S, Staibano S, Greco A, Brunetti A, Nappo G, Ilardi G, Martinelli R, Sorrentino A, Di Pace A, Mascolo M, et al. FK506 binding protein 51 positively regulates melanoma stemness and metastatic potential. Cell Death Dis. 2013;4:e578. doi: 10.1038/cddis.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava SK, Bhardwaj A, Arora S, Tyagi N, Singh AP, Carter JE, Scammell JG, Fodstad Ø, Singh S. Interleukin-8 is a key mediator of FKBP51-induced melanoma growth, angiogenesis and metastasis. Br J Cancer. 2015;112:1772–1781. doi: 10.1038/bjc.2015.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takaoka M, Ito S, Miki Y, Nakanishi A. FKBP51 regulates cell motility and invasion via RhoA signaling. Cancer Sci. 2017;108:380–389. doi: 10.1111/cas.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro FR, Meireles AM, Rocha AS, Teixeira MR. Conventional and molecular cytogenetics of human non-medullary thyroid carcinoma: Characterization of eight cell line models and review of the literature on clinical samples. BMC Cancer. 2008;8:371. doi: 10.1186/1471-2407-8-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ying Y, Qingwu L, Mingming X, Zhenju S, Chaoyang T, Zhengang T. Emodin: One main ingredient of shufeng jiedu capsule reverses chemoresistance of lung cancer cells through inhibition of EMT. Cell Physiol Biochem. 2017;42:1063–1072. doi: 10.1159/000478754. [DOI] [PubMed] [Google Scholar]
- 43.Lv N, Shan Z, Gao Y, Guan H, Fan C, Wang H, Teng W. Twist1 regulates the epithelial-mesenchymal transition via the NF-κB pathway in papillary thyroid carcinoma. Endocrine. 2016;51:469–477. doi: 10.1007/s12020-015-0714-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated/analyzed during the present study are available from the corresponding author on reasonable request.