The oral drug miltefosine (MIL) was introduced in the Indian subcontinent in the year 2002 for the treatment of visceral leishmaniasis (VL). However, recent reports on its declining efficacy and increasing relapse rates pose a serious concern.
KEYWORDS: Leishmania donovani, lipase precursor, miltefosine, overexpression, parasite persistence
ABSTRACT
The oral drug miltefosine (MIL) was introduced in the Indian subcontinent in the year 2002 for the treatment of visceral leishmaniasis (VL). However, recent reports on its declining efficacy and increasing relapse rates pose a serious concern. An understanding of the factors contributing to MIL tolerance in Leishmania parasites is critical. In the present study, we assessed the role of the lipase precursor-like protein (Lip) in conferring tolerance to miltefosine by episomally overexpressing Lip in Leishmania donovani (LdLip++). We observed a significant increase (∼3-fold) in the MIL 50% inhibitory concentration (IC50) at both the promastigote (3.90 ± 0.68 µM; P < 0.05) and intracellular amastigote (9.10 ± 0.60 µM; P < 0.05) stages compared to the wild-type counterpart (LdNeo) (MIL IC50s of 1.49 ± 0.20 µM at the promastigote stage and 3.95 ± 0.45 µM at the amastigote stage). LdLip++ parasites exhibited significantly (P < 0.05) increased infectivity to host macrophages and increased metacyclogenesis and tolerance to MIL-induced oxidative stress. The susceptibility of LdLip++ to other antileishmanial drugs (sodium antimony gluconate and amphotericin B) remained unchanged. In comparison to LdNeo, the LdLip++ parasites elicited high host interleukin-10 (IL-10) cytokine expression levels (1.6-fold; P < 0.05) with reduced expression of the cytokine tumor necrosis factor alpha (TNF-α) (1.5-fold; P < 0.05), leading to a significantly (P < 0.01) increased ratio of IL-10/TNF-α. The above-described findings suggest a role of lipase precursor-like protein in conferring tolerance to the oral antileishmanial drug MIL in L. donovani parasites.
INTRODUCTION
Miltefosine (MIL), an alkylphosphocholine, was registered as the first oral drug to treat visceral leishmaniasis (VL) in the Indian subcontinent in the year 2002 (1). Although it exhibited an initial cure rate of more than 94%, reports of its declining efficacy (2) and increasing rates of relapse in the Indian subcontinent are of great concern (3). The long half-life of the drug (150 to 200 h) may cause parasites to develop resistance in the field, as evident by the quick in vitro induction of resistance against MIL in Leishmania donovani (4). A recent study reported the existence of field isolates of VL in India that were resistant to MIL (5).
Leishmania parasites are rich in ether-lipid complexes that are found mainly in the glycosylphosphatidylinositol-anchored glycolipids and glycoproteins present on the surface of the parasites (6). MIL induces apoptosis-like cell death in Leishmania, and inhibition of apoptotic cell death has been documented in MIL-resistant parasites (7–9). MIL plays a role in impairment of acidocalcisome function, activation of the sphingosine-dependent plasma membrane Ca2+ channel, and inhibition of cytochrome c oxidase in L. donovani (10, 11). Leishmania undergoes metabolic reconfiguration during oxidative stress to resist reactive oxygen species (ROS) (12). Fatty acids are involved in the biosynthesis of sphingolipids and ether-lipids and also serve as an important bioenergetic fuel for Leishmania via the beta oxidation pathway (13). Defective inward translocation of drugs due to mutations in the putative MIL transporter LdMT and its accessory protein LdRoS3 has been well explained in experimental resistant parasites (14).
A study of differential gene expression between MIL-resistant and MIL-sensitive L. donovani parasites revealed upregulated expression of genes associated with lipid metabolism, viz., lipase (LinJ.31.2540) and the lipase precursor (LinJ.31.0870), indicating the involvement of lipase-mediated free fatty acid metabolism in MIL-resistant Leishmania (15). Lipases are the building blocks for the synthesis of complex parasite lipids, important for membrane remodeling, and help in the acquisition of the host’s resources for energy metabolism in a variety of parasitic organisms (16). MIL resistance in Leishmania affects lipid biochemical pathways, like fatty acid elongation, fatty acid desaturase, and C-24-alkylation of sterols. A comparative study of MIL-sensitive and -resistant Leishmania parasites revealed lower contents of unsaturated phospholipid alkyl chains in the MIL-resistant parasite plasma membrane, suggesting a lower fluidity of the MIL-resistant parasite membrane (17).
Here, we investigated the role of the lipase precursor (Lip) molecule in imparting increased tolerance to MIL in L. donovani parasites. We episomally expressed Lip in a wild-type L. donovani isolate and assessed the parasites overexpressing Lip (LdLip++) for in vitro drug susceptibility, infectivity to macrophages, metacyclogenesis, tolerance to MIL-induced oxidative stress, and accumulation of MIL in the parasites. Since host immune responses are critical for jeopardizing the chemotherapeutic efficacy of antileishmanials (18), we also assessed modulation of the expression of proinflammatory and anti-inflammatory cytokines in macrophages infected with LdLip++ parasites.
RESULTS
Comparative sequence analysis of the lipase precursor from MIL-sensitive and MIL-resistant L. donovani parasites.
The DNA sequence for the lipase precursor was determined for both sensitive and resistant L. donovani parasites. The amino acid sequence was identical and showed 99% similarity with the lipase precursor from Leishmania infantum (Fig. 1). A synonymous mutation was seen at 46th position, where valine was replaced by alanine.
FIG 1.
Clustal W amino acid sequence alignment of the lipase precursor in both wild-type L. donovani parasites (LdK133 and LdAG83) and L. donovani parasites made experimentally resistant to MIL (LdM30). There is a synonymous mutation at the 46th position in L. donovani in comparison with L. infantum.
LdLip++ parasites exhibit enhanced enzymatic activity of lipase precursor protein.
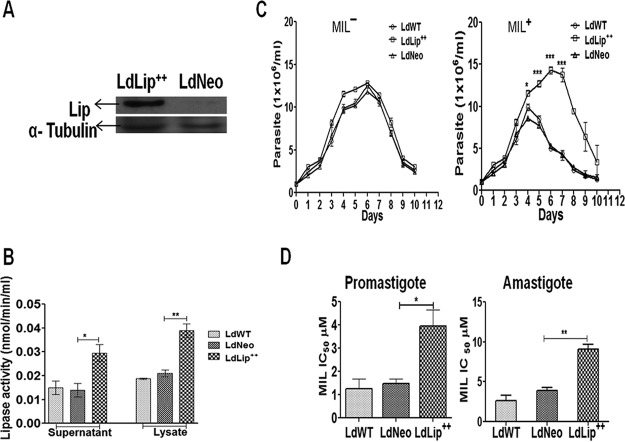
Western blot results using whole-cell lysates showed that the chimeric gene construct was translated into the ∼26-kDa LdLip++::HA (hemagglutinin) protein in LdLip++ parasites (Fig. 2A). We measured the enzymatic activity of the lipase precursor-like protein in culture supernatants and cell lysates of LdLip++, LdNeo, and wild-type L. donovani (LdWT) parasites. The activities in culture supernatants and cell lysates of LdLip++ parasites (0.029 mU/ml and 0.039 mU/ml) were significantly (∼2-fold) higher than those of LdNeo (0.014 mU/ml and 0.021 mU/ml) or LdWT (0.015 mU/ml and 0.012 mU/ml) parasites (Fig. 2B).
FIG 2.
Western blot analysis, lipase activity, growth kinetics, and in vitro MIL susceptibility of LdLip++ parasites. (A) Whole-cell lysates from LdLip++ and LdNeo parasites probed with HRP-conjugated mouse anti-HA monoclonal antibody. The arrow denotes the 26-kDa LdLip++::HA chimeric protein. (B) Lipase activity in culture supernatants and whole-cell lysates of L. donovani transfectants. Lipase activity was measured fluorometrically by using BioVision fluorometric assay kit III as described in Materials and Methods. Asterisks show levels of significance (*, P < 0.05; **, P < 0.01). (C) Growth kinetics of wild-type and transfected L. donovani parasites without MIL (MIL−) or with MIL (MIL+) pressure. Asterisks show significance (*, P < 0.05; ***, P < 0.001). (D) In vitro MIL susceptibility (IC50) of transfected parasites in comparison with the wild type at the promastigote level following a standard resazurin assay and at the intracellular amastigote level using mouse peritoneal macrophages as described in Materials and Methods. Values represent means ± SD of data from three independent experiments. Asterisks show levels of significance (*, P < 0.05; **, P < 0.01).
LdLip++ parasites show enhanced proliferation in the presence of miltefosine.
The growth of transfected LdLip++ and LdNeo parasites were monitored in comparison with LdWT parasites under MIL pressure (3 µM) at the promastigote level for a period of 10 days. In the presence of MIL, the transfected LdLip++ parasites showed significantly increased proliferation compared to LdNeo or LdWT parasites at day 4 (P < 0.05) and at days 5 to 7 (P < 0.001), while in the absence of MIL, the growth of LdLip++ parasites was comparable to that of LdNeo or LdWT parasites (Fig. 2C).
LdLip++ parasites display a reduction in susceptibility to miltefosine.
In vitro, the MIL susceptibility of LdLip++ parasites was significantly reduced (up to 3-fold) compared to LdNeo, at both the promastigote (P < 0.05) and intracellular amastigote (P < 0.01) stages (Fig. 2D). The susceptibility of LdLip++ parasites to other antileishmanial drugs tested (sodium antimony gluconate [SAG] and amphotericin B [AmB]) was not significantly different in comparison with LdWT or LdNeo parasites (Table 1).
TABLE 1.
In vitro drug susceptibility of transfected parasites overexpressing the lipase precursor (LdLip++) to antileishmanial drugsa
| Isolate | Mean MIL IC50 (µM) ± SD |
Mean AmB IC50 (µM) ± SD |
Mean SAG IC50 (µg/ml) ± SD at amastigote stage |
||
|---|---|---|---|---|---|
| Promastigote | Amastigote | Promastigote | Amastigote | ||
| LdWT | 1.26 ± 0.40 | 2.66 ± 0.67 | 0.78 ± 0.13 | 0.44 ± 0.03 | 4.83 ± 0.42 |
| LdNeo | 1.49 ± 0.20 | 3.90 ± 0.45 | 0.76 ± 0.16 | 0.42 ± 0.16 | 5.33 ± 0.57 |
| LdLip++ | 3.90 ± 0.68 | 9.10 ± 0.60 | 0.89 ± 0.11 | 0.49 ± 0.20 | 6.22 ± 0.46 |
MIL, miltefosine; AmB, amphotericin B; SAG, sodium antimony gluconate; IC50, 50% inhibitory concentration; LdWT, wild-type L. donovani parasites; LdNeo, mock-transfected (vector only) L. donovani parasites; LdLip++, transfected L. donovani parasites episomally expressing the lipase precursor protein.
LdLip++ parasites do not exhibit alterations in miltefosine uptake.
Liquid chromatography-mass spectrometry (LCMS) analysis revealed that levels of MIL uptake (nanograms per 108 promastigotes) were comparable in both LdLip++ (163.0 ± 2.8 ng/108 promastigotes) and LdWT (158.0 ± 1.4 ng/108 promastigotes) parasites. Overexpression of the lipase precursor protein therefore did not affect MIL uptake by L. donovani parasites (Fig. 3A).
FIG 3.
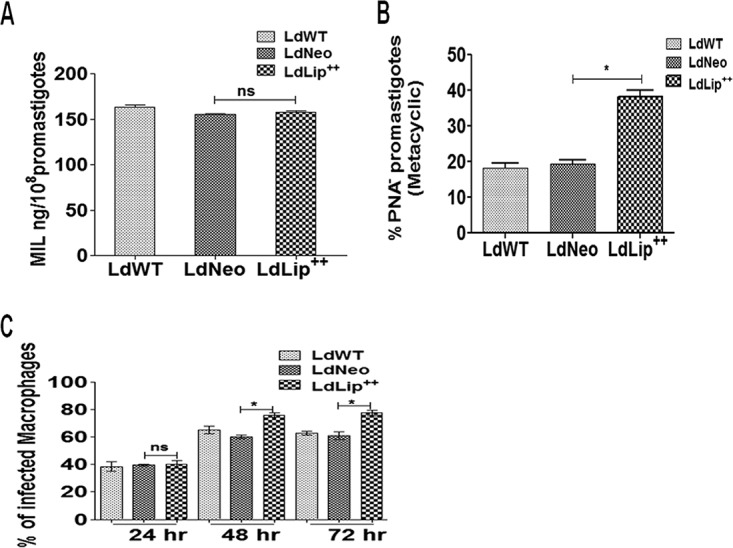
MIL uptake, percent metacyclogenesis, and infectivity to macrophages of transfected parasites with respect to wild-type parasites. (A) MIL uptake, estimated using LCMS, in 1 × 108 promastigotes. Data represent means ± SD of results from two independent experiments, each in triplicate. (B) Percent metacyclogenesis of the promastigote population estimated based on negative selection with peanut agglutinin (%PNA− promastigote). Values represent means ± SD of data from two independent experiments. Asterisks indicate significance (*, P < 0.05). (C) Mouse peritoneal macrophages infected with wild-type or transfected parasites at a 1:10 (cell/parasite) ratio. Percent infectivity was determined at 24 h, 48 h, and 72 h postinfection by counting the number of infected cells out of 100 macrophages at a ×1,000 magnification after staining with Diff-Quik. Data represent means ± SD of results from three independent experiments, each in duplicate. Asterisks indicate significance (*, P < 0.05). ns, not significant.
Metacyclogenesis is enhanced in LdLip++ parasites.
We observed a significantly increased percentage of the metacyclic LdLip++ promastigote population (38.20% ± 1.82%; P < 0.05) compared to LdNeo parasites (19.40% ± 1.10%), based on negative selection with peanut agglutinin (PNA) (Fig. 3B).
LdLip++ parasites display increased infectivity to host macrophages.
LdLip++ parasites showed a significantly increased percent infectivity in comparison to their wild-type counterparts at both 48 h (LdLip++ = 76.0% ± 1.4%; LdNeo = 65.0% ± 2.8% [P < 0.05]) and 72 h (LdLip++ = 77.5% ± 2.1%; LdNeo = 63.0% ± 1.4% [P < 0.05]) postinfection (Fig. 3C).
LdLip++ parasites show increased tolerance to reactive oxygen species.
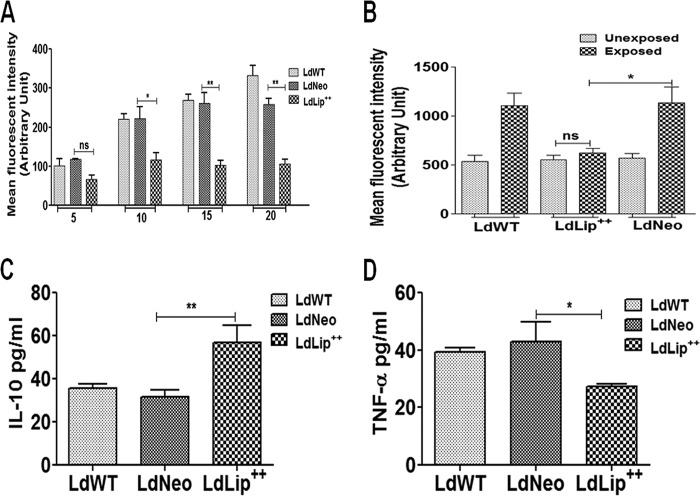
The level of ROS increased with increasing MIL concentrations in LdNeo and LdWT parasites, while it remained unaltered in LdLip++ parasites. The ROS level was significantly lower in LdLip++ promastigotes at higher MIL concentrations (10 to 20 µM) than in LdNeo parasites (Fig. 4A). Similarly, we evaluated the levels of ROS without and with MIL exposure in macrophages infected with LdLip++, LdNeo, or LdWT parasites. Results showed comparable levels of ROS in macrophages infected with LdLip++ parasites without (mean fluorescence intensity [MFI], 551.5) and with (MFI, 622.0) MIL exposure (20 µM). After MIL exposure, the level of ROS in macrophages infected with LdLip++ was significantly lower than the level of ROS (MFI, 1,137.0) in macrophages infected with LdNeo parasites (Fig. 4B).
FIG 4.
MIL-induced oxidative stress (ROS level) and cytokine expression in culture supernatants of infected macrophages. (A) Dose-dependent accumulation of ROS in LdWT, LdNeo, and LdLip++ promastigotes, measured fluorometrically at 495-nm excitation and 535-nm emission wavelengths using the cell-permeable probe H2DCFDA (40 nM). Data represent means ± SD of results from three independent experiments, each performed in duplicate. Asterisks indicate significance (*, P < 0.05; **, P < 0.01). (B) Accumulation of ROS in macrophages infected with LdWT, LdNeo, or LdLip++ parasites before and after MIL exposure (20 μM), assayed fluorometrically at 495-nm excitation and 535-nm emission wavelengths using the cell-permeable probe H2DCFDA (30 μM). Data represent means ± SD of results from three independent experiments, each in triplicate. Asterisks indicate significance (*, P < 0.05). (C and D) Expression of IL-10 and TNF-α in host macrophages infected with LdWT, LdNeo, or LdLip++ parasites. Data represent means ± SD from two separate assays, each in triplicate. Asterisks indicate significance (*, P < 0.05; **, P < 0.01).
Cytokine profile of host macrophages infected with LdLip++.
Host macrophages prestimulated with lipopolysaccharide (LPS) (1 µg/ml) and infected with LdLip++ parasites showed significantly elevated expression levels of interleukin-10 (IL-10) (1.6-fold; P < 0.05) and reduced expression levels of tumor necrosis factor alpha (TNF-α) (1.5-fold; P < 0.05) compared with macrophages infected with LdNeo (Fig. 4C and D). The IL-10/TNF-α ratio in LdLip++ parasites (2.04) was elevated by 3.9-fold compared with that in LdNeo parasites (0.52), which may contribute to the increased persistence of LdLip++ parasites within host macrophages. IL-4, IL-12, and gamma interferon (IFN-γ) could not be detected, while expression levels of IL-2 were comparable in the two.
DISCUSSION
Proposed targets of MIL in Leishmania include disturbed ether-lipid metabolism, glycosylphosphatidylinositol anchor biosynthesis, and signal transduction as well as decreased activity of acyl transferase, an enzyme involved in lipid remodeling (6). Lipase and the lipase precursor involved in free fatty acid metabolism provide an alternate source of energy generation during stress (15). Therefore, we chose to investigate the role of the lipase precursor, which showed consistently high expression levels in all L. donovani isolates from cases that relapsed after MIL treatment (19). Lip encodes a putative enzyme lipase precursor that is supposedly involved in free fatty acid metabolism via the beta oxidation pathway and helps parasites to evade drug-induced oxidative stress by providing extra energy. We generated transfected L. donovani parasites, designated LdLip++, exhibiting 2-fold-higher lipase activity and investigated LdLip++ parasites for various parameters, including (i) in vitro susceptibility to MIL, SAG, and AmB; (ii) infectivity to macrophages; (iii) metacyclogenesis; (iv) MIL uptake; (v) tolerance to oxidative stress; and (vi) cytokine expression in LdLip++ parasite-infected host macrophages. Episomal expression of a gene provides an essential tool to study the phenotypic changes in parasites and to assess their fitness toward antileishmanial drugs (20). Leishmania parasites overexpressing histone H2A, HSP83, and P299 genes exhibited reduced susceptibility to antimony and MIL (20–22). In the present study, we observed that parasites overexpressing Lip (LdLip++) showed a 3-fold reduction in MIL susceptibility compared to wild-type parasites; however, the susceptibility of LdLip++ to SAG and AmB remained unaltered. Possibly, lipase overexpression perturbed the membrane dynamics, leading to a reduced interaction of MIL with the ether-lipid complex, which might be responsible for reduced MIL susceptibility in LdLip++ parasites. We observed higher MIL 50% inhibitory concentration (IC50) values at the amastigote stage than at the promastigote stage. Higher or similar MIL IC50 values at the amastigote stage compared to those at the promastigote stage have been observed in previous studies (23–25). A strong correlation (r = 0.70; P = 0.0018) between MIL susceptibilities of amastigotes and promastigotes was observed (24). Additionally, we have used mouse peritoneal macrophages (PEMs) for in vitro assays, which would have contributed to higher MIL IC50s of the amastigotes, as the in vitro activity of miltefosine is host cell dependent, being the lowest in PEMs (26).
LdLip++ parasites exhibited enhanced proliferation in the presence of MIL compared with wild-type and mock-transfected parasites. Based on previous findings implicating Lip in increased virulence (16, 27), we assessed metacyclogenesis and infectivity to host macrophages of LdLip++ parasites. We observed that metacyclogenesis and macrophage infectivity of LdLip++ parasites were significantly increased. Lipase-mediated hydrolysis of triacylglycerol leads to products that compromise host immunity, thus allowing the pathogen to survive within host cell (27). Increased infectivity and increased metacyclogenesis have been associated with MIL tolerance and documented in L. donovani from relapse cases after MIL treatment in the Indian subcontinent (19, 28).
The investigation for MIL-induced oxidative stress tolerance in LdLip++ parasites revealed that ROS production was significantly reduced in LdLip++ promastigotes at higher MIL concentrations. Subsequent to MIL exposure in macrophages infected with LdLip++ parasites, the ROS level was substantially decreased, which reiterates the findings with the MIL-tolerant phenotype in our previous study (19). Likewise, the intracellular ROS level was lower in a mutant strain of Escherichia coli efficient in utilizing fatty acids as a carbon source than in wild-type E. coli (29).
Previous studies have demonstrated that increasing resistance to MIL in L. donovani might be associated with underdosing or underexposure of the drug to the parasites (30, 31). Differential expression of the aminophospholipid LdMT and its accessory protein LdRoS3 as well as point mutations in these genes have been linked with poor accumulation of MIL within parasites (32). Such altered expression or point mutations in LdMT-LdRoS3 could not be detected in a set of clinical isolates of L. donovani from relapsed cases of VL (33). The parasite isolate studied here did not exhibit any point mutation in the LdMT-LdRoS3 genes, and increased MIL tolerance in LdLip++ parasites could not be associated with reduced MIL accumulation (15).
The expression of the anti-inflammatory cytokine IL-10 is responsible for pathogen persistence (34). MIL has an immunostimulatory role that helps in parasite elimination from the host cell (35). Infected macrophages show low levels of proinflammatory cytokines (TNF-α, IL-12, and IFN-γ) that are significantly increased upon MIL treatment (35). IL-10 expression shows a positive correlation with parasite burden in VL (36) and plays an important role in parasite persistence and inhibition of host leishmanicidal activity (37, 38). Studies have shown that an increased ratio of IL-10/TNF-α correlates positively with Plasmodium vivax parasitemia (39–42). In experimental models of leishmaniasis, TNF-α plays a critical role in controlling parasite growth (43). However, in splenic aspirate cultures of Leishmania, neutralization of TNF-α does not affect parasite replication, although it inhibits IFN-γ production, while IL-10 levels remain unaltered (44). LdLip++ parasites induced increases in IL-10 and reductions in TNF-α levels in host macrophages, leading to marked increases in the IL-10/TNF-α ratios. It has been reported that overexpression of lipase increases virulence by downregulating the Th1 immune response in Mycobacterium tuberculosis-infected mice (27). The data suggest that the lipase precursor molecule favors parasite persistence within host macrophages, as evident by increased proliferation and infectivity during MIL stress compared to control parasites.
In conclusion, the present findings suggest a role of the lipase precursor molecule in increasing tolerance to MIL in L. donovani by (i) promoting the utilization of host free fatty acids as an alternate source of energy generation; (ii) membrane remodeling that reduces the drug interaction on the parasite surface, resulting in a lower level of accumulation of ROS; and (iii) increasing parasite infectivity and proliferation within host macrophages by promoting IL-10 expression in host cells. This molecule can be a potential target to counter the parasite’s defense mechanism that operates during MIL exposure.
MATERIALS AND METHODS
Parasite cultures.
The L. donovani isolate (K133 [MHOM/IN/2000/K133]; termed LdWT here) was derived from bone marrow aspirates of a VL patient admitted to Safdarjung Hospital, New Delhi, India, under the guidelines of the ethics committee. L. donovani promastigotes were propagated in M199 medium (Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (Gibco, USA), 100 IU/ml penicillin G, and 100 µg/ml streptomycin at 25°C. Wild-type parasites were made experimentally resistant by stepwise exposure to increasing drug concentrations (MIL up to 30 µg/ml) as described previously (15).
Generation of Leishmania parasites with episomal expression of lipase precursor-like protein (LdLip++).
DNA encoding the L. donovani lipase precursor-like protein was amplified with gene primers containing the SpeI site and an HA tag for subcloning into the Leishmania expression plasmid pKSNEO using forward primer 5ʹ-ACTAGTATGCGTCGCGTTCAGTCATGGACGTG-3ʹ and reverse primer 5ʹ-ACTAGTCTACGCGTAGTCCGGCACGTCGTACGGGTAAGCCCCACGAAGAACATTGCGAG-3ʹ (the SpeI site is underlined, and the HA tag is in boldface type).
The construct (20 µg) was transfected into a wild-type L. donovani isolate (K133) by electroporation using a Gene pulser XCell instrument in 2-mm-gap cuvettes at 450 V and 500 mF to produce K133Lip++ (LdLip++) parasites. The positive transfectants were selected by using G418 (50 µg/ml) as described previously (45). Parasites transfected with the empty vector pKSNEO (LdNeo) were used as a control.
Western blotting.
Preparation of the parasite lysate and Western blot analysis were performed according to methods described previously (46). Lysates (100 µg) from LdLip++ and LdNeo parasites were transferred onto a nitrocellulose membrane following separation by SDS-PAGE. The membrane was probed with horseradish peroxidase (HRP)-conjugated monoclonal anti-HA antibody (1:4,000 dilution) produced in mouse (Sigma-Aldrich, USA). The blot was developed by using Western blot detection enhanced chemiluminescence (ECL) detection reagent (GE Healthcare, UK). The image was scanned with ChemiDoc (Bio-Rad, USA) and analyzed by using Image Lab 5.1 software (46).
Estimation of enzymatic activity of lipase precursor-like protein.
The enzymatic activity of the lipase precursor-like protein was assessed fluorometrically, as described previously (16), in culture supernatants and whole-cell lysates from LdLip++, LdNeo, and LdWT promastigotes using lipase activity fluorometric assay kit III (BioVision, USA), according to the manufacturer’s instructions.
Briefly, 2 × 107 promastigotes (day 5) were centrifuged at 1,800 × g for 10 min, the supernatant was collected, and the cells were lysed in 400 µl of chilled assay buffer. Both the supernatant and cell lysate were analyzed by measuring florescence at excitation/emission (Ex/Em) wavelengths of 529/600 nm at two different time points (T1 and T2) of incubation.
A standard curve was generated in the range of 0 to 100 pmol, using different volumes (0 to 10 µl) of a methylresorufin solution (0.1mM), and the volume was adjusted to 100 µl/well with lipase assay buffer. Florescence was measured at Ex/Em wavelengths of 529/600 nm using a cytofluorimeter (Infinite M200; Tecan, Switzerland).
Activity was calculated by using the formula lipase activity (nmol/min/ml = mU/ml) = [B/(T2 – T1) × V] × sample dilution factor, where B is the amount of methylresorufin from the standard curve and V represents the pretreated sample volume (in milliliters).
First and second readings (R1 and R2) were taken at 30 min (T1) and 60 min (T2), respectively.
Growth kinetics of LdLip++ parasites.
A total of 1 × 106 LdWT, LdNeo, or LdLip++ promastigotes were inoculated in complete M199 medium without or with MIL (3 µM). Growth of LdLip++, LdNeo, or LdWT parasites was monitored for a period of 10 days by counting parasite numbers using a Neubauer chamber under a microscope at a ×200 magnification.
In vitro drug susceptibility of LdLip++ promastigotes.
In vitro susceptibilities to MIL and AmB were assessed by using a resazurin-based fluorometric assay as described previously (23). Briefly, late-log-phase LdLip++, LdNeo, and LdWT promastigotes were plated into a 96-well culture plate (105 promastigotes/well) and exposed to increasing concentrations of either MIL (0.4 µM to 390 µM) or AmB (0.027 µM to 2.157 µM) for 72 h. Plates were further incubated for 24 h after the addition of 50 μl of resazurin (0.0125%). Viability of cells was measured fluorometrically (excitation wavelength, 550 nm; emission wavelength, 590 nm). Calculation of the half-maximal inhibitory concentration (IC50) was done by sigmoidal regression analysis using Microcal Origin 6.0 software. All experiments were performed twice in quadruplicates.
In vitro drug susceptibility of LdLip++ intracellular amastigotes.
In vitro susceptibility to MIL, AmB, and sodium antimony gluconate (SAG) was assessed by using an intracellular amastigote model described previously (47). Briefly, primary peritoneal macrophages derived from 6-week-old female BALB/c mice were infected with 6-day-old LdLip++, LdNeo, or LdWT promastigotes at a ratio of 10:1 (parasites/macrophages) in 200 µl complete RPMI 1640 medium in 8-well chambered slides and incubated for 16 h at 37°C in 5% CO2. Infected macrophages were washed and incubated further for 48 h, with increasing concentrations of either MIL (1, 5, 10, 20, and 30 µM), AmB (0.027, 0.054, 0.108, 0.539, and 2.0 µM), or SAG (1, 5, 10, 20, 30, and 40 µg/ml). Macrophages were stained with Diff-Quik solutions and subsequently examined for intracellular amastigotes. Calculation of the IC50 was done by using Microcal Origin 6.0 software.
Infectivity to macrophages.
The percent infectivity of LdLip++ parasites to macrophages was investigated by using a mouse macrophage-amastigote model as described previously (19, 48). The percent infectivity of LdLip++ parasites was calculated by counting the number of infected macrophages.
Metacyclogenesis in L. donovani.
Metacyclogenesis of LdLip++ promastigotes was assessed according to methods described previously (19, 49). The percent metacyclic population was calculated by counting the promastigotes that did not agglutinate with peanut agglutinin (PNA) out of the total promastigote cell density.
Oxidative stress tolerance.
MIL-induced oxidative stress tolerance in LdLip++ parasites was assessed fluorometrically by using the cell-permeable probe 2′,7′-dichlorodihydrofluorescin diacetate (H2DCFDA; Molecular Probes, USA) as described previously (19, 47, 50). Fluorimetric measurements were expressed as mean fluorescence intensity (MFI) units, which represent the levels of ROS.
MIL accumulation in LdLip++ parasites.
Accumulation of MIL was investigated in log-phase promastigote cultures according to standard procedures (19, 51). Briefly, promastigotes (108 cells/ml), after treatment with 100 µM MIL for 90 min, were centrifuged at 1,500 × g for 10 min. The cell pellet was digested in 2 N HNO3 by overnight incubation. The supernatant was collected by centrifugation at 2,000 × g for 15 min and later analyzed for MIL content by using liquid chromatography-mass spectrometry (LCMS).
Multiplex enzyme-linked immunosorbent assay (ELISA) for cytokine estimation.
Estimation of cytokine levels was done according to methods described previously, with modifications (52). Cytokine levels in culture supernatants of mouse peritoneal macrophages prestimulated with 1 µg/ml of lipopolysaccharide (LPS) from Escherichia coli and then infected with LdLip++, LdWT, or LdNeo parasites for 48 h were determined by using a Bio-PlexPro mouse cytokine kit (Bio-Rad, USA) according to the manufacturer’s protocol. Briefly, a 50-µl cell supernatant sample was incubated with antibody-coupled beads. Immune complexes were washed and incubated with biotinylated detection antibody, followed by streptavidin-phycoerythrin treatment prior to assessing cytokine concentrations. Manufacturer-provided standards were used to prepare the standard curve for each cytokine. Proinflammatory (IL-2, IL-12, TNF-α, and IFN-γ) and anti-inflammatory (IL-4 and IL-10) cytokines were analyzed by using a multiplex array reader from the Luminex instrumentation system (Bio-Plex workstation; Bio-Rad Laboratories).
Statistical analysis.
Statistical analysis of the data was carried out by using Graph Pad Prism 5 software (San Diego, CA, USA). Results are represented as means ± standard deviations (SD). The P value was calculated by performing Student’s t test. P values of <0.05 were considered significant.
Ethics statement.
The study was approved by the Institutional Animal Ethics Committee (IAEC-3/2010) of the National Institute of Pathology, New Delhi, India. Guidelines for animal care and handling protocols recommended by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) were followed.
ACKNOWLEDGMENTS
This work was supported by intramural funding and research grant number 63/4/2007-BMS by the Indian Council of Medical Research. P.S. is a recipient of a J. C. Bose national fellowship (DST, India).
We report no conflict of interest.
REFERENCES
- 1.Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, Junge K, Bryceson A, Berman J. 2002. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med 347:1739–1746. doi: 10.1056/NEJMoa021556. [DOI] [PubMed] [Google Scholar]
- 2.Sundar S, Singh A, Rai M, Prajapati VK, Singh AK, Ostyn B, Boelaert M, Dujardin JC, Chakravarty J. 2012. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis 55:543–550. doi: 10.1093/cid/cis474. [DOI] [PubMed] [Google Scholar]
- 3.Pandey BD, Pandey K, Kaneko O, Yanagi T, Hirayama K. 2009. Relapse of visceral leishmaniasis after miltefosine treatment in a Nepalese patient. Am J Trop Med Hyg 80:580–582. doi: 10.4269/ajtmh.2009.80.580. [DOI] [PubMed] [Google Scholar]
- 4.Seifert K, Matu S, Perez-Victoria FJ, Castanys S, Gamarro F, Croft SL. 2003. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int J Antimicrob Agents 22:380–387. doi: 10.1016/S0924-8579(03)00125-0. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava S, Mishra J, Gupta AK, Singh A, Shankar P, Singh S. 2017. Laboratory confirmed miltefosine resistant cases of visceral leishmaniasis from India. Parasit Vectors 10:49. doi: 10.1186/s13071-017-1969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lux H, Heise N, Klenner T, Hart D, Opperdoes FR. 2000. Ether-lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether-lipid analogues in Leishmania. Mol Biochem Parasitol 111:1–14. doi: 10.1016/S0166-6851(00)00278-4. [DOI] [PubMed] [Google Scholar]
- 7.Paris C, Loiseau PM, Bories C, Breard J. 2004. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother 48:852–859. doi: 10.1128/AAC.48.3.852-859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das M, Saudagar P, Sundar S, Dubey VK. 2013. Miltefosine-unresponsive Leishmania donovani has a greater ability than miltefosine-responsive L. donovani to resist reactive oxygen species. FEBS J 280:4807–4815. doi: 10.1111/febs.12449. [DOI] [PubMed] [Google Scholar]
- 9.Mishra J, Singh S. 2013. Miltefosine resistance in Leishmania donovani involves suppression of oxidative stress-induced programmed cell death. Exp Parasitol 135:397–406. doi: 10.1016/j.exppara.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Pinto-Martinez AK, Rodriguez-Duran J, Serrano-Martin X, Hernandez-Rodriguez V, Benaim G. 2018. Mechanism of action of miltefosine on Leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane Ca2+ channel. Antimicrob Agents Chemother 62:e01614-17. doi: 10.1128/AAC.01614-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luque-Ortega JR, Rivas L. 2007. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob Agents Chemother 51:1327–1332. doi: 10.1128/AAC.01415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh AK, Sardar AH, Mandal A, Saini S, Abhishek K, Kumar A, Purkait B, Singh R, Das S, Mukhopadhyay R, Roy S, Das P. 2015. Metabolic reconfiguration of the central glucose metabolism: a crucial strategy of Leishmania donovani for its survival during oxidative stress. FASEB J 29:2081–2098. doi: 10.1096/fj.14-258624. [DOI] [PubMed] [Google Scholar]
- 13.Canuto GA, Castilho-Martins EA, Tavares MF, Rivas L, Barbas C, Lopez GA. 2014. Multi-analytical platform metabolomic approach to study miltefosine mechanism of action and resistance in Leishmania. Anal Bioanal Chem 406:3459–3476. doi: 10.1007/s00216-014-7772-1. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Victoria FJ, Castanys S, Gamarro F. 2003. Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob Agents Chemother 47:2397–2403. doi: 10.1128/AAC.47.8.2397-2403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulshrestha A, Sharma V, Singh R, Salotra P. 2014. Comparative transcript expression analysis of miltefosine-sensitive and miltefosine-resistant Leishmania donovani. Parasitol Res 113:1171–1184. doi: 10.1007/s00436-014-3755-6. [DOI] [PubMed] [Google Scholar]
- 16.Shakarian AM, McGugan GC, Joshi MB, Stromberg M, Bowers L, Ganim C, Barowski J, Dwyer DM. 2010. Identification, characterization, and expression of a unique secretory lipase from the human pathogen Leishmania donovani. Mol Cell Biochem 341:17–31. doi: 10.1007/s11010-010-0433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakotomanga M, Saint-Pierre-Chazalet M, Loiseau PM. 2005. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug-membrane interactions. Antimicrob Agents Chemother 49:2677–2686. doi: 10.1128/AAC.49.7.2677-2686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdan C. 2008. Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell Microbiol 10:1221–1234. doi: 10.1111/j.1462-5822.2008.01146.x. [DOI] [PubMed] [Google Scholar]
- 19.Deep DK, Singh R, Bhandari V, Verma A, Sharma V, Wajid S, Sundar S, Ramesh V, Dujardin JC, Salotra P. 2017. Increased miltefosine tolerance in clinical isolates of Leishmania donovani is associated with reduced drug accumulation, increased infectivity and resistance to oxidative stress. PLoS Negl Trop Dis 11:e0005641. doi: 10.1371/journal.pntd.0005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh R, Kumar D, Duncan RC, Nakhasi HL, Salotra P. 2010. Overexpression of histone H2A modulates drug susceptibility in Leishmania parasites. Int J Antimicrob Agents 36:50–57. doi: 10.1016/j.ijantimicag.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Vergnes B, Gourbal B, Girard I, Sundar S, Drummelsmith J, Ouellette M. 2007. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol Cell Proteomics 6:88–101. doi: 10.1074/mcp.M600319-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury K, Zander D, Kube M, Reinhardt R, Clos J. 2008. Identification of a Leishmania infantum gene mediating resistance to miltefosine and SbIII. Int J Parasitol 38:1411–1423. doi: 10.1016/j.ijpara.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Kulshrestha A, Bhandari V, Mukhopadhyay R, Ramesh V, Sundar S, Maes L, Dujardin JC, Roy S, Salotra P. 2013. Validation of a simple resazurin-based promastigote assay for the routine monitoring of miltefosine susceptibility in clinical isolates of Leishmania donovani. Parasitol Res 112:825–828. doi: 10.1007/s00436-012-3212-3. [DOI] [PubMed] [Google Scholar]
- 24.Kumar D, Kulshrestha A, Singh R, Salotra P. 2009. In vitro susceptibility of field isolates of Leishmania donovani to miltefosine and amphotericin B: correlation with sodium antimony gluconate susceptibility and implications for treatment in areas of endemicity. Antimicrob Agents Chemother 53:835–838. doi: 10.1128/AAC.01233-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escobar P, Matu S, Marques C, Croft SL. 2002. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop 81:151–157. doi: 10.1016/S0001-706X(01)00197-8. [DOI] [PubMed] [Google Scholar]
- 26.Seifert K, Escobar P, Croft SL. 2010. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J Antimicrob Chemother 65:508–511. doi: 10.1093/jac/dkp500. [DOI] [PubMed] [Google Scholar]
- 27.Singh VK, Srivastava M, Dasgupta A, Singh MP, Srivastava R, Srivastava BS. 2014. Increased virulence of Mycobacterium tuberculosis H37Rv overexpressing LipY in a murine model. Tuberculosis (Edinb) 94:252–261. doi: 10.1016/j.tube.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Rai K, Cuypers B, Bhattarai NR, Uranw S, Berg M, Ostyn B, Dujardin JC, Rijal S, Vanaerschot M. 2013. Relapse after treatment with miltefosine for visceral leishmaniasis is associated with increased infectivity of the infecting Leishmania donovani strain. mBio 4:e00611-13. doi: 10.1128/mBio.00611-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidetaka D, Hoshino Y, Kentaro K, Usud Y. 2014. Reduction of hydrogen peroxide stress derived from fatty acid beta-oxidation improves fatty acid utilization in Escherichia coli. Appl Microbiol Biotechnol 98:629–639. doi: 10.1007/s00253-013-5327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorlo TP, Rijal S, Ostyn B, de Vries PJ, Singh R, Bhattarai N, Uranw S, Dujardin JC, Boelaert M, Beijnen JH, Huitema AD. 2014. Failure of miltefosine in visceral leishmaniasis is associated with low drug exposure. J Infect Dis 210:146–153. doi: 10.1093/infdis/jiu039. [DOI] [PubMed] [Google Scholar]
- 31.Ostyn B, Hasker E, Dorlo TP, Rijal S, Sundar S, Dujardin JC, Boelaert M. 2014. Failure of miltefosine treatment for visceral leishmaniasis in children and men in South-East Asia. PLoS One 9:e100220. doi: 10.1371/journal.pone.0100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Victoria FJ, Gamarro F, Ouellette M, Castanys S. 2003. Functional cloning of the miltefosine transporter. A novel P-type phospholipid translocase from Leishmania involved in drug resistance. J Biol Chem 278:49965–49971. doi: 10.1074/jbc.M308352200. [DOI] [PubMed] [Google Scholar]
- 33.Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, Ramesh V, Sundar S, Schonian G, Dujardin JC, Salotra P. 2012. Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl Trop Dis 6:e1657. doi: 10.1371/journal.pntd.0001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanley AC, Engwerda CR. 2007. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol 85:138–147. doi: 10.1038/sj.icb7100011. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee AK, Gupta G, Adhikari A, Majumder S, Mahapatra SK, Majumdar SB, Majumdar S. 2012. Miltefosine triggers a strong proinflammatory cytokine response during visceral leishmaniasis: role of TLR4 and TLR9. Int Immunopharmacol 12:565–572. doi: 10.1016/j.intimp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Verma S, Kumar R, Katara GK, Singh LC, Negi NS, Ramesh V, Salotra P. 2010. Quantification of parasite load in clinical samples of leishmaniasis patients: IL-10 level correlates with parasite load in visceral leishmaniasis. PLoS One 5:e10107. doi: 10.1371/journal.pone.0010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nylen S, Sacks D. 2007. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol 28:378–384. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee B, Mukhopadhyay R, Bannerjee B, Chowdhury S, Mukherjee S, Naskar K, Allam US, Chakravortty D, Sundar S, Dujardin JC, Roy S. 2013. Antimony-resistant but not antimony-sensitive Leishmania donovani up-regulates host IL-10 to overexpress multidrug-resistant protein 1. Proc Natl Acad Sci U S A 110:E575–E582. doi: 10.1073/pnas.1213839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeyrek FY, Kurcer MA, Zeyrek D, Simsek Z. 2006. Parasite density and serum cytokine levels in Plasmodium vivax malaria in Turkey. Parasite Immunol 28:201–207. doi: 10.1111/j.1365-3024.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 40.Jain V, Singh PP, Silawat N, Patel R, Saxena A, Bharti PK, Shukla M, Biswas S, Singh N. 2010. A preliminary study on pro- and anti-inflammatory cytokine profiles in Plasmodium vivax malaria patients from central zone of India. Acta Trop 113:263–268. doi: 10.1016/j.actatropica.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Medina TS, Costa SP, Oliveira MD, Ventura AM, Souza JM, Gomes TF, Vallinoto AC, Povoa MM, Silva JS, Cunha MG. 2011. Increased interleukin-10 and interferon-gamma levels in Plasmodium vivax malaria suggest a reciprocal regulation which is not altered by IL-10 gene promoter polymorphism. Malar J 10:264. doi: 10.1186/1475-2875-10-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goncalves RM, Scopel KK, Bastos MS, Ferreira MU. 2012. Cytokine balance in human malaria: does Plasmodium vivax elicit more inflammatory responses than Plasmodium falciparum? PLoS One 7:e44394. doi: 10.1371/journal.pone.0044394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engwerda CR, Ato M, Stager S, Alexander CE, Stanley AC, Kaye PM. 2004. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in the control of Leishmania donovani infection. Am J Pathol 165:2123–2133. doi: 10.1016/S0002-9440(10)63262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh N, Kumar R, Engwerda C, Sacks D, Nylene S, Sundar S. 2016. Tumor necrosis factor alpha neutralization has no direct effect on parasite burden, but causes impaired IFN-γ production by spleen cells from human visceral leishmaniasis patients. Cytokine 85:184–190. doi: 10.1016/j.cyto.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papadopoulou B, Roy G, Ouellette M. 1992. A novel antifolate resistance gene on the amplified H circle of Leishmania. EMBO J 11:3601–3608. doi: 10.1002/j.1460-2075.1992.tb05444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma V, Sharma P, Selvapandiyan A, Salotra P. 2016. Leishmania donovani-specific Ub-related modifier-1: an early endosome-associated ubiquitin-like conjugation in Leishmania donovani. Mol Microbiol 99:597–610. doi: 10.1111/mmi.13253. [DOI] [PubMed] [Google Scholar]
- 47.Mookerjee BJ, Mookerjee A, Sen P, Bhaumik S, Banerjee S, Naskar K, Choudhuri SK, Saha B, Raha S, Roy S. 2006. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob Agents Chemother 50:1788–1797. doi: 10.1128/AAC.50.5.1788-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukhopadhyay R, Mukherjee S, Mukherjee B, Naskar K, Mondal D, Decuypere S, Ostyn B, Prajapati VK, Sundar S, Dujardin JC, Roy S. 2011. Characterisation of antimony-resistant Leishmania donovani isolates: biochemical and biophysical studies and interaction with host cells. Int J Parasitol 41:1311–1321. doi: 10.1016/j.ijpara.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Sacks DL, Perkins PV. 1985. Development of infective stage Leishmania promastigotes within phlebotomine sand flies. Am J Trop Med Hyg 34:456–459. doi: 10.4269/ajtmh.1985.34.456. [DOI] [PubMed] [Google Scholar]
- 50.Kim S, Cheon HS, Kim SY, Juhnn YS, Kim YY. 2013. Cadmium induces neuronal cell death through reactive oxygen species activated by GADD153. BMC Cell Biol 14:4. doi: 10.1186/1471-2121-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dorlo TP, Hillebrand MJ, Rosing H, Eggelte TA, de Vries PJ, Beijnen JH. 2008. Development and validation of a quantitative assay for the measurement of miltefosine in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 865:55–62. doi: 10.1016/j.jchromb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Singh UP, Singh S, Taub DD, Lillard JW Jr.. 2003. Inhibition of IFN-γ-inducible protein-10 abrogates colitis in IL-10−/− mice. J Immunol 171:1401–1406. doi: 10.4049/jimmunol.171.3.1401. [DOI] [PubMed] [Google Scholar]