Isavuconazole may be useful in treating and preventing fungal infections in solid-organ transplant (SOT) recipients due to its safety profile and activity against Aspergillus and some Mucorales. Isavuconazole has favorable pharmacokinetics based on clinical trials in various patient populations, but data are limited in SOT recipients.
KEYWORDS: isavuconazole, organ transplant, pharmacokinetics
ABSTRACT
Isavuconazole may be useful in treating and preventing fungal infections in solid-organ transplant (SOT) recipients due to its safety profile and activity against Aspergillus and some Mucorales. Isavuconazole has favorable pharmacokinetics based on clinical trials in various patient populations, but data are limited in SOT recipients. We evaluated the steady-state pharmacokinetics of isavuconazole in 26 SOT recipients receiving the drug intravenously for prophylaxis. There was moderate interpatient variability in isavuconazole pharmacokinetic parameters (coefficients of variation of 51% for the area under the plasma concentration-versus-time curve [AUC] and 59% for the trough plasma concentration [Ctrough]). AUC and steady-state Ctrough were significantly lower in women, patients with a body mass index of ≥18.5 kg/m2, and those receiving hemodialysis. Trough plasma concentrations were highly correlated with AUCs (R2 = 0.94) and can serve as a suitable measure of isavuconazole exposure in patients. In conclusion, moderate interpatient variability in isavuconazole exposure, the identification of factors associated with lower exposure, the recognition that Ctrough is a surrogate marker for AUC, and the availability of a simple analytical method suggest that therapeutic drug monitoring (TDM) may be useful for guiding treatment in at least some SOT recipients. Future studies are needed to correlate isavuconazole exposure with patients’ clinical outcomes and to determine the clinical role of TDM.
INTRODUCTION
Invasive fungal infections (IFIs) are major causes of morbidity and mortality among solid-organ transplant (SOT) recipients (1–5). Broad-spectrum triazole antifungal agents have improved the outcomes of SOT recipients by offering safe and effective alternatives to the more toxic agent amphotericin B for treating IFIs, in particular invasive mold infections like aspergillosis and mucormycosis. These agents are also employed for prophylaxis by many SOT programs (6). Isavuconazole (ISA) is a triazole agent that was recently approved by the Food and Drug Administration and the European Medicines Agency, which offers potential pharmacokinetic (PK) advantages over other triazoles like voriconazole or posaconazole (POS). ISA is administered as a water-soluble prodrug (isavuconazonium sulfate) that is rapidly converted in vivo by plasma esterases to the active ISA and an inactive cleavage product. Unlike intravenous (i.v.) voriconazole and posaconazole, isavuconazonium sulfate is highly water soluble; thus, the intravenous formulation does not require solubilization by cyclodextrin, which might cause nephrotoxicity. Other potential PK advantages of ISA include linear and dose-proportional pharmacokinetics and excellent bioavailability (98%) (7). ISA is a substrate and a moderate inhibitor of CYP3A4 (8, 9). ISA PK has low clearance (CL), a large volume of distribution (V), and a long half-life, which allow it to be administered once a day (10, 11). Like posaconazole, ISA provides broader-spectrum coverage than voriconazole by having activity against at least some species of Mucorales (7).
ISA PK has been studied in healthy volunteers (10, 11), subjects with hepatic (12) and renal (13) impairments, and patients with acute myeloid leukemia and neutropenia (14). ISA PK has not been investigated in SOT recipients. The primary aims of this prospective study were to evaluate steady-state PK of i.v. ISA used for prophylaxis in SOT recipients and identify factors that impact ISA PK.
RESULTS
Patient demographics and clinical characteristics.
Two hundred thirty-one samples from 26 patients were assayed (Table 1). Twenty-three patients had 9 samples tested. In 3 patients, samples at 24 h (plasma concentration at 24 h [C24]) were unavailable, and steady-state trough plasma concentration (Ctrough) (plasma concentration at time zero [C0]) data were used for analysis. The median time from SOT to collection of the first sample was 7.5 days, and the median time from the ISA loading dose to sample collection was 6 days. Eighty-eight percent (23/26) of patients were enrolled within 2 months of transplantation; the remaining patients were enrolled at 7 months (n = 2) and 46 months (n = 1) posttransplantation. The median time from the loading dose to sample collection was 6 days. During the course of ISA prophylaxis, toxicity was not observed in any patient, nor was ISA prematurely discontinued. None of the patients developed breakthrough IFI.
TABLE 1.
Patient demographic and clinical characteristicsa
| Demographic or clinical characteristic | Value for all patients (n = 26) |
|---|---|
| Median age (yr) (range) | 50 (21–73) |
| % (no.) men | 62 (16) |
| % (no.) of patients of white race | 100 (26) |
| % (no.) of patients not Hispanic or Latino | 100 (26) |
| Median wt (kg) (range) | 63.4 (36–108) |
| Median BMI (kg/m2) (range) | 24.0 (16.6–33.2) |
| % (no.) of patients with cachexia (BMI, <18.5) | 19 (5) |
| % (no.) of patients with normal wt (BMI, 18.5–24.9) | 38 (10) |
| % (no.) of overweight patients (BMI, 25.0–29.9) | 27 (7) |
| % (no.) of obese patients (BMI, ≥30) | 15 (4) |
| % (no.) of patients with type of transplant | |
| Lung | 81 (21) |
| Liver | 8 (2) |
| Heart | 8 (2) |
| Pancreas/kidney | 4 (1) |
| % (no.) of patients with underlying disease | |
| Cystic fibrosis | 27 (7) |
| % (no.) of patients with requirement for renal replacement therapy | |
| Patients on HD | 19 (5) |
| Patients on CRRT | 23 (6) |
| Mean concn ± SD (range) for laboratory test | |
| AST (IU/liter) | 24 (0.8–173) |
| ALT (IU/liter) | 19 (10–248) |
| ALP (IU/liter) | 70 (22–491) |
| Bilirubin (mg/dl) | 0.7 (0.3–6.5) |
| Albumin (g/dl) | 2.85 (2–4.3) |
| Creatinine (µmol/liter) | 146 (53–345) |
| CrCl (ml/min/1.73 m2)b | 52 (19–115) |
All data were collected at the time of enrollment, prior to ISA administration. BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; CrCl, creatinine clearance; HD, hemodialysis; CRRT, continuous renal replacement therapy.
CrCl = {[140 – age (years)] × weight (kilograms) × 0.85 for females × 1.73}/[0.818 × serum creatinine (micromoles per milliliter) × BSA], where BSA (square meters) equals [height (meters) × weight (kilograms)/36]1/2.
PK analyses.
The mean (± standard deviation [SD]) steady-state plasma concentration-time profiles of ISA after i.v. administration are presented in Fig. 1. The plasma ISA concentration reached a maximum 1 h after the start of infusion and declined subsequently in a biphasic manner. Due to its long half-life (approximately 66 h in our study), ISA PK was characterized by a relative flat plasma concentration profile between 6 h and 24 h after dosing. Ctrough was quantifiable (greater than limit of detection) in all the patients, with a mean level of 2.3 ± 1.4 µg/ml and a median level of 1.9 µg/ml (range, 0.3 to 6.6 µg/ml). Ctrough values were >1 µg/ml, >2 µg/ml, and >3 µg/ml in 88% (23/26), 42% (11/26), and 12% (3/26) of patients, respectively. Twelve percent (3/26) of patients had levels of <1 µg/ml. There was an excellent correlation between Ctrough and C24 (R2 = 0.95; P < 0.0001) and between Ctrough and the area under the plasma concentration-versus-time curve from time zero to 24 h (AUC0–24 h) (R2 = 0.94; P < 0.0001) (Fig. 2). The Ctrough/C24 ratio was 1.02, and the average difference between Ctrough and C24 (C24 − Ctrough/Ctrough) was 2.7%, suggesting that steady state was reached in our patients at the time of study.
FIG 1.
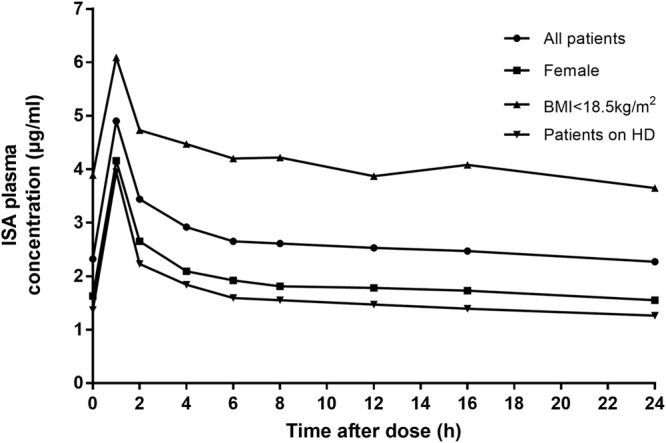
Isavuconazole time-concentration profiles in 26 SOT recipients. Data are presented as means (circles). Time zero is immediately prior to the administration of a dose of intravenous isavuconazole.
FIG 2.
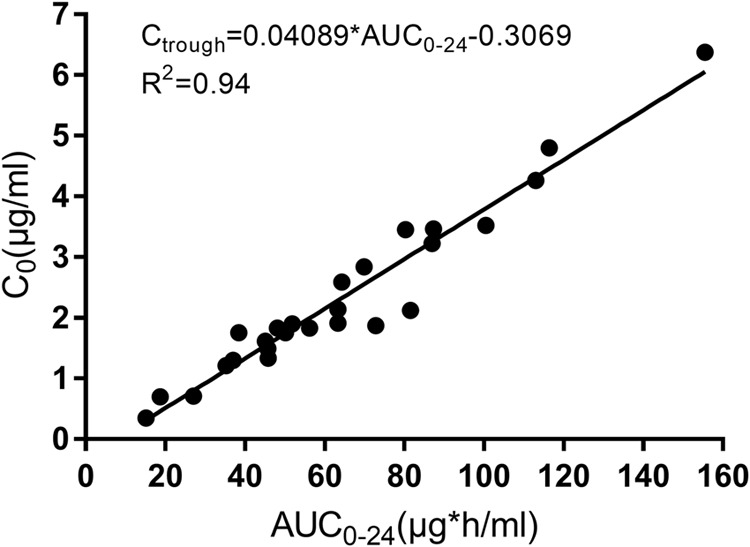
Correlation between Ctrough and AUC0–24 h.
PK parameters of i.v. ISA are summarized in Table 2. At steady state, there were moderate interpatient variabilities in AUC (coefficient of variation [CV] of 51%) and trough levels (CV of 59%) but high interpatient variability in the volume of distribution at steady state (Vss) (CV of 135%).
TABLE 2.
Intravenous isavuconazole plasma pharmacokinetic parametersa
| Parameter | Value for group |
|||||||
|---|---|---|---|---|---|---|---|---|
| All patients |
Female patients |
Patients with BMI of <18.5 kg/m2 |
Patients on HD |
|||||
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | Mean ± SD | CV (%) | Mean ± SD | CV (%) | |
| AUC0–24 h (μg · h/ml) | 64.2 ± 32.4 | 51 | 45.8 ± 23.7 | 52 | 99.7 ± 40.9 | 41 | 38.7 ± 29.6 | 76 |
| Cssmax (μg/ml) | 4.9 ± 1.8 | 36 | 4.3 ± 1.7 | 41 | 6.1 ± 1.4 | 23 | 3.9 ± 2.1 | 54 |
| Cssmin/trough (μg/ml) | 2.3 ± 1.4 | 59 | 1.6 ± 1.0 | 59 | 3.9 ± 1.9 | 48 | 1.4 ± 1.3 | 92 |
| Cav (μg/ml) | 2.7 ± 1.4 | 50 | 1.9 ± 1.0 | 52 | 4.2 ± 1.7 | 41 | 1.6 ± 1.2 | 76 |
| CL (liters/h) | 4.1 ± 2.7 | 66 | 5.8 ± 3.6 | 62 | 2.4 ± 1.2 | 51 | 7.6 ± 4.4 | 59 |
| Vss (liters) | 338 ± 458 | 135 | 530 ± 708 | 134 | 170 ± 55 | 33 | 295 ± 85 | 29 |
CV, coefficient of variation; AUC0–24 h, area under plasma concentration-versus-time curve from time zero to 24 h after dosing; Cssmax, maximum plasma concentration at steady state; Cssmin/trough, trough concentration at steady state; Cssav, average concentration at steady state; CL, clearance at steady state; Vss, volume of distribution at steady state.
Factors associated with AUC0–24 h and Ctrough.
Patients’ sex, body mass index (BMI), and receipt of hemodialysis (HD) were associated with AUC0–24 h (Table 3). The median AUC0–24 h values were significantly higher for men than for women (66.6 versus 42.1 μg · h/ml; P = 0.02), for patients with a BMI of <18.5 kg/m2 (cachectic) than for patients with a BMI of ≥18.5 kg/m2 (100.5 versus 51.8 μg · h/ml; P = 0.024), and for patients who were not receiving renal replacement therapy than for those receiving HD (64.3 versus 27.1 μg · h/ml; P = 0.04). There was no difference in AUC0–24 h among patients receiving continuous renal replacement therapy (CRRT) (59.8 μg · h/ml) and patients not receiving renal replacement therapy (P = 0.73). It is evident from Fig. 1 that the mean ISA time-concentration profiles in plasma were highest for patients with a BMI of <18.5 kg/m2 and lowest for women and patients on HD.
TABLE 3.
Factors associated with AUC0–24 h and Ctrougha
| Factor | Linear regression P value |
|
|---|---|---|
| AUC0–24 h | Ctrough | |
| Age | 0.69 | 0.77 |
| Sex | 0.02 | 0.03 |
| Cystic fibrosis | 0.44 | 0.60 |
| Liver transplant | 0.30 | 0.76 |
| Time from transplant | 0.72 | 0.66 |
| BMI | 0.049 | 0.07 |
| BMI of <18.5 kg/m2 | 0.004 | 0.002 |
| HD versus none | 0.048 | 0.08 |
HD, hemodialysis; BMI, body mass index; Ctrough, trough concentration at steady state; AUC0–24 h, area under plasma concentration-versus-time curve from time zero to 24 h after dosing.
Sex and BMI were also significantly associated with Ctrough (Table 3). In a subset analysis of women with a BMI of <18.5 kg/m2, the median Ctrough was significantly higher among those who were not receiving renal replacement therapy than among those receiving HD (1.9 µg/ml versus 0.7 µg/ml; P = 0.02). HD was also associated with Ctrough, although this did not reach statistical significance (P = 0.08) (Table 3). There was no difference in Ctrough between patients receiving CRRT and those not receiving renal replacement therapy (P = 0.73).
DISCUSSION
To our knowledge, this is the first PK study of ISA administered intravenously for antifungal prophylaxis in SOT recipients. Several findings are particularly noteworthy. First, there was moderate interpatient variability in ISA AUC0–24 h (CV of 51%), which was comparable to those described in previous ISA PK studies (15–17). Second, the plasma trough concentration was highly correlated with the ISA AUC0–24 h (R2 = 0.94; P < 0.0001). Finally, women, patients with a BMI of ≥18.5 kg/m2, and those receiving HD had lower ISA AUC0–24 h and Ctrough than other patients. Taken together, the interpatient variability in drug exposure, the identification of factors associated with lower ISA concentrations, and the demonstration that Ctrough is an accurate proxy for AUC0–24 h suggest a future role for therapeutic drug monitoring (TDM) in guiding the usage of ISA in at least some SOT recipients.
The interpatient variabilities in ISA AUC0–24 h and Ctrough (CV of 51% and 59%, respectively) were similar to the variability for AUC previously reported in ISA clinical trials or in clinical samples (CV of 68%), where ISA was given either i.v. or orally (15–17). The range of ISA exposure in our patients was striking, with 10-fold and 18-fold differences in low and high values for AUC0–24 h (15.16 to 155.54 μg · h/ml) and Ctrough (0.35 to 6.37 μg/ml), respectively. Female sex, a BMI of ≥18.5 kg/m2, and HD are biologically plausible risk factors for reduced AUC0–24 h and Ctrough. Sixty-six percent of ISA is metabolized by hepatic CYP3A (8, 9), and women have greater hepatic CYP3A activity than men (18). Cachectic patients (BMI of <18.5 kg/m2) have decreased plasma and liver volumes and reduced levels of CYP3A4 and other cytochromes, resulting in attenuated ISA metabolism (19). Drug clearance (CL) might also be reduced in cachectic patients (19–21). Indeed, CL among our patients with a BMI of <18.5 kg/m2 was lower than that in patients with a BMI of ≥18.5 kg/m2 (2.0 liters/h versus 3.9 liters/h; P = 0.02). In contrast, ISA CL was significantly higher among patients on HD than patients not receiving renal replacement therapy (median of 7.4 liters/h versus 3.1 liters/h; P = 0.04). A previous study showed that less than 1% of the administered ISA was recovered in dialysate fluid (13), a finding that was consistent with the highly albumin-bound nature of ISA. Indeed, protein binding of ISA is over 99%, and the concentration of free drug in plasma is less than 1%. Accordingly, the serum albumin level was lower among HD patients (2.5 g/dl versus 3.2 g/dl; P = 0.04), and clearance of ISA is likely increased due to an increased unbound fraction in patients on HD (22). We did not find any differences between ISA AUC0–24 h and CL among patients receiving CRRT (59.8 μg · h/ml and 2.8 liters/h, respectively) and those who were not receiving HD or CRRT (64.3 μg · h/ml and 3.1 liters/h, respectively; P = 0.6 for both). Serum albumin levels for the former and latter groups were not significantly different (2.85 g/dl and 3.2 g/dl, respectively; P = 0.6), again suggesting the critical role of plasma protein binding of ISA on its clearance from the body.
In clinical practice, itraconazole, voriconazole, and posaconazole exposure is monitored through measurements of trough concentrations, which serve as surrogate markers of AUC0–τ. At present, the need for ISA TDM is unclear. The European Conference on Infections in Leukemia (ECIL-6) recommends ISA TDM for breakthrough infections or infections unresponsive to treatment, for treatment of pathogens with reduced susceptibility, or in the setting of drug interactions but acknowledges that there is limited evidence to support these recommendations (23–25). The AUC/MIC ratio is the PK-pharmacodynamic (PD) parameter that best correlates with the activity of azole agents, but corresponding targets for ISA treatment effectiveness in humans or for optimal prophylaxis are not defined; if human AUC/MIC targets can be identified in future studies, our findings indicate that it should be feasible to assign ISA Ctrough values to be targeted during TDM.
We acknowledge that the relatively small size of this study limits the generalizability of our findings and our ability to identify additional factors that might impact ISA PK. Future studies are needed to confirm the effects of BMI and hemodialysis on ISA PK and to identify additional factors that might impact ISA PK. As one example, we did not find a significant difference in AUC0–24 h or Ctrough among 7 patients with cystic fibrosis (CF) and 19 patients without CF. In general, patients with CF have increased V and fast clearance (26), and low voriconazole and posaconazole exposures have been documented in this population (27, 28). It is also important to recognize that all of our patients were non-Hispanic whites, and all received i.v. ISA. Clearly, larger studies in diverse SOT populations are needed.
In conclusion, we have shown that i.v. ISA drug exposure in SOT recipients may be particularly impacted in women, cachectic SOT recipients, and those receiving HD. Future studies are needed to correlate i.v. ISA exposure with patients’ clinical outcomes and to determine if there is a role for TDM in clinical practice. In addition, follow-up population PK modeling and assessments of probabilities of PK-PD target attainment are warranted to expand upon the findings here.
MATERIALS AND METHODS
Subjects.
Patients were eligible for study participation if they were older than 18 years of age, underwent SOT, and were initiated on i.v. ISA prophylaxis between March 2016 and September 2017. The study was approved by the Institutional Review Board at the University of Pittsburgh and conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization guidelines for good clinical practice. Written informed consent was obtained from all subjects.
Study design and sample collection.
All patients received standard i.v. ISA dosing (372 mg of isavuconazonium sulfate, which corresponds to 200 mg of the active component of ISA, every 8 h for 6 doses, followed by 372 mg daily). Per our institution protocol, the durations of ISA prophylaxis (either i.v. or by mouth) were 3 to 4 months for lung and 30 days for other solid-organ transplants. All patients received tacrolimus as part of their immunosuppression regimen. We routinely performed tacrolimus therapeutic drug monitoring until ISA reached steady state rather than altering the tacrolimus dosage. We previously showed that this approach did not lead to tacrolimus toxicity (29).
The i.v. formulation was infused over 1 h. After a minimum of 7 doses, serial blood samples were collected in heparinized tubes from each patient just prior to (0 h) and 1, 2, 4, 6, 8, 12, 16, and 24 h following administration of ISA. Blood samples were centrifuged at 1,500 × g for 10 min and frozen at −80°C until they were analyzed for ISA concentrations.
Determination of ISA plasma concentration.
ISA plasma concentrations were determined by a validated ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) assay technique. Plasma sample (100 μl) was treated with acetonitrile, with posaconazole (POS) as the internal standard. The standard calibration curves were linear over a concentration range of 0.200 to 9 µg/ml (data not shown). The lower limit of quantification (LLOQ) was 50 ng/ml. A weighing factor of 1/Y was used to construct the equations for the standard curve. The intra- and interday relative standard deviations (RSDs) were ≤7.7% and ≤3.0%, respectively. The mean rates of extraction recovery and ion suppression relative recovery were 94.3% and 100.3%, respectively. Quality control (QC) samples in duplicate at three concentrations (500, 2,500, and 7,500 ng/ml) were incorporated into each run. The results of the QC samples provided the basis for accepting or rejecting the run. At low concentrations, QC samples had to be within ±20% of their nominal values; medium- and high-concentration QC samples also had to be within ±15% of their respective nominal values.
ISA PK analyses.
PK analyses were performed by using Phoenix WinNonlin 6.4 (Certara, Princeton, NJ). ISA plasma concentrations were used to determine PK parameters using a noncompartmental model. Uniform weighting was used for noncompartmental analysis. The maximum plasma concentration at steady state (Cmax), the time to Cmax (Tmax), and the minimum plasma concentration at steady state (Cmin) were obtained from each patient’s plasma concentration-time profile. Ctrough was defined as concentration at time zero. The area under the plasma concentration-versus-time curve from time zero to 24 h (AUC0–24 h) was determined using the trapezoidal method. The average concentration at steady state (Cav) was calculated as AUC0–24 h/24. ISA clearance (CL) was calculated as daily dose/AUC0–24 h. Mean residence time (MRT) was calculated as [AUMC0–24 h + 24(AUC0–INF − AUC0–24 h)]/AUC0–24 h − TI/2, where TI represents the infusion duration and AUMC is the area under the first moment of the concentration-time curve. The volume of distribution at steady state (Vss) was calculated as (dose/AUC) × MRT.
Statistical analyses.
Statistical analyses were conducted by using STATA (StataCorp., College Station, TX). Univariate linear regression was performed to identify covariates that might affect AUC0–24 h. PK variables are presented as means (±SD). For subgroup comparisons, data are presented as medians, and statistical differences between groups were computed by using the Mann-Whitney nonparametric test. A P value of <0.05 was considered significant.
REFERENCES
- 1.Gavalda J, Meije Y, Fortun J, Roilides E, Saliba F, Lortholary O, Munoz P, Grossi P, Cuenca-Estrella M, ESCMID Study Group for Infections in Compromised Hosts . 2014. Invasive fungal infections in solid organ transplant recipients. Clin Microbiol Infect 20(Suppl 7):27–48. doi: 10.1111/1469-0691.12660. [DOI] [PubMed] [Google Scholar]
- 2.Kauffman CA, Freifeld AG, Andes DR, Baddley JW, Herwaldt L, Walker RC, Alexander BD, Anaissie EJ, Benedict K, Ito JI, Knapp KM, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Schuster MG, Chiller TM, Pappas PG. 2014. Endemic fungal infections in solid organ and hematopoietic cell transplant recipients enrolled in the Transplant-Associated Infection Surveillance Network (TRANSNET). Transpl Infect Dis 16:213–224. doi: 10.1111/tid.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Ling Z, Li L, Ruan B. 2011. Invasive fungal infections in liver transplantation. Int J Infect Dis 15:e298–e304. doi: 10.1016/j.ijid.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Neofytos D, Fishman JA, Horn D, Anaissie E, Chang CH, Olyaei A, Pfaller M, Steinbach WJ, Webster KM, Marr KA. 2010. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis 12:220–229. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 5.Sole A, Salavert M. 2008. Fungal infections after lung transplantation. Transplant Rev 22:89–104. doi: 10.1016/j.trre.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Patel TS, Eschenauer GA, Stuckey LJ, Carver PL. 2016. Antifungal prophylaxis in lung transplant recipients. Transplantation 100:1815–1826. doi: 10.1097/TP.0000000000001050. [DOI] [PubMed] [Google Scholar]
- 7.Natesan SK, Chandrasekar PH. 2016. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: current evidence, safety, efficacy, and clinical recommendations. Infect Drug Resist 9:291–300. doi: 10.2147/IDR.S102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend R, Dietz A, Hale C, Akhtar S, Kowalski D, Lademacher C, Lasseter K, Pearlman H, Rammelsberg D, Schmitt-Hoffmann A, Yamazaki T, Desai A. 2017. Pharmacokinetic evaluation of CYP3A4-mediated drug-drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin Pharmacol Drug Dev 6:44–53. doi: 10.1002/cpdd.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groll AH, Desai A, Han D, Howieson C, Kato K, Akhtar S, Kowalski D, Lademacher C, Lewis W, Pearlman H, Mandarino D, Yamazaki T, Townsend R. 2017. Pharmacokinetic assessment of drug-drug interactions of isavuconazole with the immunosuppressants cyclosporine, mycophenolic acid, prednisolone, sirolimus, and tacrolimus in healthy adults. Clin Pharmacol Drug Dev 6:76–85. doi: 10.1002/cpdd.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt-Hoffmann A, Roos B, Maares J, Heep M, Spickerman J, Weidekamm E, Brown T, Roehrle M. 2006. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:286–293. doi: 10.1128/AAC.50.1.286-293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt-Hoffmann A, Roos B, Heep M, Schleimer M, Weidekamm E, Brown T, Roehrle M, Beglinger C. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:279–285. doi: 10.1128/AAC.50.1.279-285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt-Hoffmann A, Roos B, Spickermann J, Heep M, Peterfai E, Edwards DJ, Stoeckel K. 2009. Effect of mild and moderate liver disease on the pharmacokinetics of isavuconazole after intravenous and oral administration of a single dose of the prodrug BAL8557. Antimicrob Agents Chemother 53:4885–4890. doi: 10.1128/AAC.00319-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend RW, Akhtar S, Alcorn H, Berg JK, Kowalski DL, Mujais S, Desai AV. 2017. Phase I trial to investigate the effect of renal impairment on isavuconazole pharmacokinetics. Eur J Clin Pharmacol 73:669–678. doi: 10.1007/s00228-017-2213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornely OA, Bohme A, Schmitt-Hoffmann A, Ullmann AJ. 2015. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother 59:2078–2085. doi: 10.1128/AAC.04569-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai A, Kovanda L, Kowalski D, Lu Q, Townsend R, Bonate PL. 2016. Population pharmacokinetics of isavuconazole from phase 1 and phase 3 (SECURE) trials in adults and target attainment in patients with invasive infections due to Aspergillus and other filamentous fungi. Antimicrob Agents Chemother 60:5483–5491. doi: 10.1128/AAC.02819-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovanda LL, Desai AV, Lu Q, Townsend RW, Akhtar S, Bonate P, Hope WW. 2016. Isavuconazole population pharmacokinetic analysis using nonparametric estimation in patients with invasive fungal disease (results from the VITAL study). Antimicrob Agents Chemother 60:4568–4576. doi: 10.1128/AAC.00514-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andes D, Kovanda L, Desai A, Kitt T, Zhao M, Walsh TJ. 2018. Isavuconazole concentration in real-world practice: consistency with results from clinical trials. Antimicrob Agents Chemother 62:e00585-18. doi: 10.1128/AAC.00585-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu ZY, Zhao YS. 2010. Sex-dependent differences in cytochrome p450 3a activity as assessed by midazolam disposition in humans: a meta-analysis. Drug Metab Dispos 38:817–823. doi: 10.1124/dmd.109.031328. [DOI] [PubMed] [Google Scholar]
- 19.Cvan Trobec K, Kerec Kos M, Trontelj J, Grabnar I, Tschirner A, Palus S, Anker SD, Springer J, Lainscak M. 2015. Influence of cancer cachexia on drug liver metabolism and renal elimination in rats. J Cachexia Sarcopenia Muscle 6:45–52. doi: 10.1002/jcsm.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naito T, Tashiro M, Ishida T, Ohnishi K, Kawakami J. 2013. Cancer cachexia raises the plasma concentration of oxymorphone through the reduction of cyp3a but not cyp2d6 in oxycodone-treated patients. J Clin Pharmacol 53:812–818. doi: 10.1002/jcph.112. [DOI] [PubMed] [Google Scholar]
- 21.Naito T, Tashiro M, Yamamoto K, Ohnishi K, Kagawa Y, Kawakami J. 2012. Impact of cachexia on pharmacokinetic disposition of and clinical responses to oxycodone in cancer patients. Eur J Clin Pharmacol 68:1411–1418. doi: 10.1007/s00228-012-1266-x. [DOI] [PubMed] [Google Scholar]
- 22.Pea F, Viale P, Pavan F, Furlanut M. 2007. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet 46:997–1038. doi: 10.2165/00003088-200746120-00003. [DOI] [PubMed] [Google Scholar]
- 23.Stott KE, Hope WW. 2017. Therapeutic drug monitoring for invasive mould infections and disease: pharmacokinetic and pharmacodynamic considerations. J Antimicrob Chemother 72(Suppl 1):i12–i18. doi: 10.1093/jac/dkx029. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen R, Andersen SR, Astvad KMT, Arendrup MC. 2017. Implementation of isavuconazole in a fluorescence-based high-performance liquid chromatography kit allowing simultaneous detection of all four currently licensed mold-active triazoles. mSphere 2:e00098-17. doi: 10.1128/mSphere.00098-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis R, Brüggemann R, Padoin C, Maertens J, Marchetti O, Groll A, Johnson E, Arendrup M. 2015. Triazole antifungal therapeutic drug monitoring. Abstr ECIL-6, Sophia Antipolis, France, 11 to 12 September 2015. [Google Scholar]
- 26.Touw DJ. 1998. Clinical pharmacokinetics of antimicrobial drugs in cystic fibrosis. Pharm World Sci 20:149–160. doi: 10.1023/A:1008634911114. [DOI] [PubMed] [Google Scholar]
- 27.Berge M, Guillemain R, Boussaud V, Pham MH, Chevalier P, Batisse A, Amrein C, Dannaoui E, Loriot MA, Lillo-Le Louet A, Billaud EM. 2009. Voriconazole pharmacokinetic variability in cystic fibrosis lung transplant patients. Transpl Infect Dis 11:211–219. doi: 10.1111/j.1399-3062.2009.00384.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Nguyen MH, Clancy CJ, Joshi R, Zhao W, Ensor C, Venkataramanan R, Shields RK. 2016. Pharmacokinetics of posaconazole suspension in lung transplant patients with and without cystic fibrosis. Antimicrob Agents Chemother 60:3558–3562. doi: 10.1128/AAC.00424-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivosecchi RM, Clancy CJ, Shields RK, Ensor CR, Shullo MA, Falcione BA, Venkataramanan R, Nguyen MH. 2017. Effects of isavuconazole on the plasma concentrations of tacrolimus among solid-organ transplant patients. Antimicrob Agents Chemother 61:e00970-17. doi: 10.1128/AAC.00970-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


