Plazomicin is an FDA-approved aminoglycoside for the treatment of complicated urinary tract infections. In this open-label study, 24 adults with normal renal function or mild, moderate, or severe renal impairment (n = 6 per group) received a single 7.5-mg/kg of body weight dose of plazomicin as a 30-min intravenous infusion.
KEYWORDS: aminoglycosides, antimicrobial agents, pharmacokinetics, renal impairment
ABSTRACT
Plazomicin is an FDA-approved aminoglycoside for the treatment of complicated urinary tract infections. In this open-label study, 24 adults with normal renal function or mild, moderate, or severe renal impairment (n = 6 per group) received a single 7.5-mg/kg of body weight dose of plazomicin as a 30-min intravenous infusion. Total clearance declined with renal impairment, resulting in 1.98-fold and 4.42-fold higher plazomicin exposures, as measured by the area under the concentration-time curve from 0 h to infinity, in subjects with moderate and severe impairment, respectively, than in subjects with normal renal function. (This study has been registered at ClinicalTrials.gov under identifier NCT01462136.)
INTRODUCTION
Gram-negative bacteria have become increasingly resistant to multiple antibiotics (1–3), and treatment options for infections due to multidrug-resistant Enterobacteriaceae are limited (4, 5). Plazomicin is an aminoglycoside that was engineered to overcome aminoglycoside-modifying enzymes, the most common mechanism of aminoglycoside resistance in Enterobacteriaceae. In vitro, plazomicin has bactericidal activity against multidrug-resistant Enterobacteriaceae, including strains that produce extended-spectrum β-lactamases (6), carbapenemases (7, 8), and most aminoglycoside-modifying enzymes (9, 10). Plazomicin is approved by the U.S. Food and Drug Administration for the treatment of complicated urinary tract infections, including pyelonephritis (11).
Similar to other aminoglycosides, plazomicin displays linear dose-proportional pharmacokinetics (PK) (12, 13), has low plasma protein binding (∼20%), and does not undergo metabolism. As plazomicin is eliminated primarily via urinary excretion of the parent drug (14), we evaluated the impact of renal function on the PK of plazomicin.
This open-label phase 1 study (ClinicalTrials registration no. NCT01462136) was conducted at three U.S. centers from 2011 to 2012. The protocol was approved by the institutional review board at each site. All subjects provided written informed consent. The study included a screening period of ≤21 days, treatment on day 1, collection of PK samples on days 1 to 5, and follow-up evaluation on day 14. Adults with normal renal function or preexisting renal impairment age 18 to 75 years with a body mass index (BMI) of 19 to 32 kg/m2 and body weight of ≥40 kg were eligible for enrollment. Subjects requiring hemodialysis or peritoneal dialysis were excluded. Eligible subjects were stratified based on the mean of two predose creatinine clearance (CLCR) values, as estimated by the Cockcroft and Gault equation (15), and were enrolled concurrently into the mild (CLCR, 60 to 89 ml/min), moderate (CLCR, 30 to 59 ml/min), or severe (CLCR, 15 to 29 ml/min) renal impairment group (n = 6 per group). Subjects in the normal renal function group (CLCR, ≥90 ml/min) were enrolled and treated only after dosing had been completed in the renal impairment groups. Staggered enrollment was employed to approximately match the demographic characteristics of subjects in the normal renal function group with those of subjects in the renal impairment groups.
A single 7.5-mg/kg of body weight dose of plazomicin was administered via a 30-min intravenous infusion. The single 7.5-mg/kg dose was selected to avoid high plazomicin exposures in subjects with severe renal impairment. Blood samples were collected before dosing and at 36 and 45 min and 1, 1.5, 3, 6, 10, 16, 24, 36, 48, 72, and 96 h after the start of infusion. Noncompartmental PK analysis was conducted using Phoenix WinNonlin v.6.1 (Pharsight Corp., Mountain View, CA, USA) (details are described in the supplemental material).
Twenty-four subjects were enrolled and completed the study. The mean age was 63.9 years (range, 50 to 75 years). While the renal function groups were well matched for age and sex, values for mean body weight and BMI were slightly higher in the normal renal function group than in the impaired renal function groups (Table 1).
TABLE 1.
Subject demographic and baseline characteristicsa
| Characteristic | Normal renal function (n = 6) |
Mild renal impairment (n = 6) |
Moderate renal impairment (n = 6) |
Severe renal impairment (n = 6) |
Total (N = 24) |
|---|---|---|---|---|---|
| Age (yr) | 61.8 ± 8.08 | 62.7 ± 7.55 | 65.7 ± 9.27 | 65.3 ± 7.28 | 63.9 ± 7.73 |
| Female (no. [%]) | 3 (50.0) | 2 (33.3) | 3 (50.0) | 4 (66.7) | 12 (50.0) |
| White (no. [%]) | 5 (83.3) | 5 (83.3) | 5 (83.3) | 4 (66.7) | 19 (79.2) |
| Wt (mean ± SD) (kg) | 83.1 ± 14.7 | 72.4 ± 8.5 | 77.7 ± 11.1 | 74.1 ± 21.5 | 76.8 ± 14.4 |
| BMI (mean ± SD) (kg/m2) | 28.1 ± 3.93 | 25.7 ± 2.41 | 27.4 ± 2.40 | 27.4 ± 3.89 | 27.2 ± 3.15 |
| CLCR (mean ± SD) (ml/min) | 115 ± 18.5 | 75.9 ± 5.02 | 49.5 ± 8.05 | 21.2 ± 6.95 | 65.5 ± 36.9 |
BMI, body mass index; CLCR, creatinine clearance; Wt, weight.
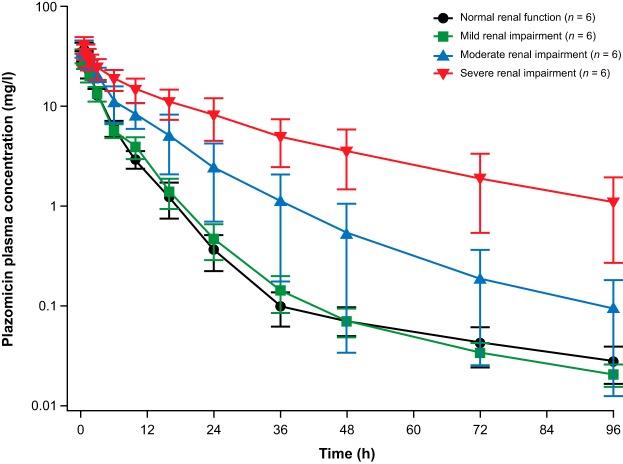
Plazomicin plasma drug concentrations declined in a multiphasic manner over time in each renal function group (Fig. 1). Subjects with mild renal impairment had plazomicin plasma concentration-time profiles that were comparable to those in subjects with normal renal function. Mean plazomicin plasma concentrations were higher in subjects with moderate and severe renal impairment than in subjects with normal renal function.
FIG 1.
Semilog plot of the mean (SD) plazomicin plasma concentration-time profile by renal function group.
Following a single dose of plazomicin, maximum concentration of drug in plasma (Cmax) values did not appear to be impacted by renal impairment (P > 0.05; Table 2). Plasma exposures based on the area under the plasma concentration-time curve from time zero to infinity (AUC0–∞) were similar in subjects with mild renal impairment and normal renal function, but they were 1.98-fold and 4.42-fold higher in subjects with moderate and severe impairment, respectively, than in subjects with normal renal function (Table 3). The volume of distribution at steady state (Vss) was approximately 20% to 30% lower in subjects with renal impairment than in subjects with normal renal function. Total clearance (CLT) values were not notably different in subjects with mild renal impairment versus those with normal function but were substantially lower with moderate and severe impairment than with normal renal function (Table 2).
TABLE 2.
Plazomicin plasma pharmacokinetic parameters by renal function groupa
| PK parameter | Normal renal function (n = 6) |
Mild renal impairment (n = 6) |
Moderate renal impairment (n = 6) |
Severe renal impairment (n = 6) |
|---|---|---|---|---|
| Cmax (mg/liter) | 37.9 ± 5.01 | 32.8 ± 4.30 | 39.2 ± 6.43 | 41.4 ± 7.83 |
| AUC0–∞ (mg · h/liter) | 136 ± 17.2 | 138 ± 23.7 | 281 ± 96.0 | 647 ± 259 |
| Vss (liters) | 36.0 ± 7.76 | 28.5 ± 2.17 | 25.8 ± 6.96 | 25.1 ± 7.89 |
| CLT (liters/h) | 4.64 ± 1.17 | 3.98 ± 0.481 | 2.25 ± 0.685 | 0.96 ± 0.379 |
All values are reported as mean ± SD. PK, pharmacokinetics; Cmax, maximum concentration of drug in plasma; AUC0–∞, area under the concentration-time curve from 0 h to infinity; Vss, volume of distribution at steady state; CLT, total clearance.
TABLE 3.
Pairwise comparisons of plazomicin plasma pharmacokinetic parameters by renal function groupa
| PK parameter | Pairwise group comparison geometric mean ratio (90% CI), P value |
||
|---|---|---|---|
| Mild renal impairment/normal renal function |
Moderate renal impairment/normal renal function |
Severe renal impairment/normal renal function |
|
| Cmax (mg/liter) | 0.87 (0.753–0.997), 0.093 | 1.03 (0.883–1.21), 0.719 | 1.08 (0.899–1.30), 0.460 |
| AUC0–∞ (mg · h/liter) | 1.01 (0.849–1.19), 0.942 | 1.98 (1.51–2.60), 0.0023 | 4.42 (3.04–6.44), 0.0003 |
PK, pharmacokinetic; CI, confidence interval; Cmax, maximum concentration of drug in plasma; AUC0–∞, area under the concentration-time curve from 0 h to infinity.
A single 7.5-mg/kg dose of plazomicin was well tolerated across renal function groups. Three adverse events were reported, none of which was considered by the investigator to be related to plazomicin. The results of the safety analyses are provided in the supplemental material.
Our results show that similar to other aminoglycosides (16, 17), the PK of plazomicin is impacted by renal function. Total clearance declined with renal impairment, resulting in significantly increased plazomicin exposures with moderate and severe impairment. Given the magnitude of increases in AUC0–∞, adjustments to plazomicin dosing are indicated for patients with moderate or severe renal impairment to attain a range of exposures similar to those of patients with normal renal function (11).
Similar to other plazomicin PK studies and to other aminoglycosides, the Vss for plazomicin approximated the extracellular fluid volume of 15 to 25 liters (12, 13, 18–20). Lower mean Vss values observed with renal impairment than with normal function may in part be related to the slightly higher body weights in subjects with normal renal function than in those with renal impairment.
A strength of this study is that PK was evaluated in subjects with a broad range of baseline CLCR, including three subjects with CLCR of <20 ml/min. An important limitation is the exclusion of subjects with end-stage renal disease requiring dialysis, precluding evaluation of the impact of renal replacement therapy on plazomicin PK. In conclusion, while dosage regimen adjustments of plazomicin do not appear to be warranted for patients with mild renal impairment, dosage adjustments are recommended in patients with moderate or severe renal impairment.
Data availability.
Individual participant data that underlie the results reported in this article, after deidentification, will be made available (unless prohibited by applicable law) to researchers for noncommercial purposes beginning 6 months following article publication; proposals should be directed to datarequests@achaogen.com.
Supplementary Material
ACKNOWLEDGMENTS
We thank INC Research (Raleigh, NC, USA), Almac Group (Souderton, PA, USA), ACM Global Laboratory (Rochester, NY, USA), Alturas Analytics, Inc. (Moscow, ID, USA), the Institute for Clinical Pharmacodynamics (ICPD, Schenectady, NY, USA), Jamie P. Dwyer (Vanderbilt University Medical Center, Nashville, TN, USA), Lynn E. Connolly, Tony Baca, Robert Cass, Todd Clobes, and Jen Yang.
Editorial support was provided by Jean Turner and Kate Bradford of PAREXEL and funded by Achaogen, Inc.
This project was funded in whole or in part with federal funds from the Biomedical Advanced Research and Development Authority (BARDA), Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary, Department of Health and Human Services, under contract no. HHSO100201000046C.
A.S.K., J.A.G., and J.D.S. are employees or former employees of and stockholders in Achaogen, Inc. S.V.W. is a former employee of the Institute for Clinical Pharmacodynamics and received research funding from Achaogen, Inc. V.R. is a paid contractor with Achaogen, Inc.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01128-18.
REFERENCES
- 1.Center for Disease Dynamics Economics & Policy (CDDEP). 2015. The state of the world’s antibiotics 2015. Center for Disease Dynamics Economics & Policy, Washington, DC: https://cddep.org/wp-content/uploads/2017/06/swa_edits_9.16.pdf. [Google Scholar]
- 2.U.S. Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. U.S. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. [Google Scholar]
- 3.European Centre for Disease Prevention and Control. 2016. Surveillance of antimicrobial resistance in Europe 2016: annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). European Centre for Disease Prevention and Control, Stockholm, Sweden: https://ecdc.europa.eu/sites/portal/files/documents/AMR-surveillance-Europe-2016.pdf. [Google Scholar]
- 4.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. 2015. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery and development of new antibiotics. World Health Organization, Geneva, Switzerland: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1. [Google Scholar]
- 6.López-Diaz MD, Culebras E, Rodriguez-Avial I, Rios E, Vinuela-Prieto JM, Picazo JJ, Rodriguez-Avial C. 2017. Plazomicin activity against 346 extended-spectrum-β-lactamase/AmpC-producing Escherichia coli urinary isolates in relation to aminoglycoside-modifying enzymes. Antimicrob Agents Chemother 61:e02454-16. doi: 10.1128/AAC.02454-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galani I, Souli M, Daikos GL, Chrysouli Z, Poulakou G, Psichogiou M, Panagea T, Argyropoulou A, Stefanou I, Plakias G, Giamarellou H, Petrikkos G. 2012. Activity of plazomicin (ACHN-490) against MDR clinical isolates of Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. from Athens, Greece. J Chemother 24:191–194. doi: 10.1179/1973947812Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Kashikar A, Bush K. 2017. In vitro activity of plazomicin against β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE). J Antimicrob Chemother 72:2792–2795. doi: 10.1093/jac/dkx261. [DOI] [PubMed] [Google Scholar]
- 9.Cox G, Ejim L, Stogios PJ, Koteva K, Bordeleau E, Evdokimova E, Sieron AO, Savchenko A, Serio AW, Krause KM, Wright GD. 2018. Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis 4:980–987. doi: 10.1021/acsinfecdis.8b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castanheira M, Davis AP, Mendes RE, Serio AW, Krause KM, Flamm RK. 2018. In vitro activity of plazomicin against Gram-negative and Gram-positive isolates collected from United States hospitals and comparative activity of aminoglycosides against carbapenem-resistant Enterobacteriaceae and isolates carrying carbapenemase genes. Antimicrob Agents Chemother 62:e00313-18. doi: 10.1128/AAC.00313-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achaogen, Inc. 2018. Zemdri (plazomicin) injection, for intravenous use. Achaogen, Inc, South San Francisco, CA: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210303Orig1s000lbl.pdf. [Google Scholar]
- 12.Cass RT, Brooks CD, Havrilla NA, Tack KJ, Borin MT, Young D, Bruss JB. 2011. Pharmacokinetics and safety of single and multiple doses of ACHN-490 injection administered intravenously in healthy subjects. Antimicrob Agents Chemother 55:5874–5880. doi: 10.1128/AAC.00624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seroogy J, Choi T, Gall J, Van Wart S. 2017. Pharmacokinetics (PK) of plazomicin in healthy adults, poster 206. ASM Microbe, 1 to 5 June 2017, New Orleans, LA. [DOI] [PubMed] [Google Scholar]
- 14.Choi T, Seroogy J, Sanghvi M, Dhuria S. 2018. Mass balance, metabolism, and excretion of [14C]-plazomicin in healthy human subjects, poster 1400. IDWeek, 3 to 7 October 2018, San Francisco, CA. [Google Scholar]
- 15.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Nayak-Rao S. 2010. Aminoglycoside use in renal failure. Indian J Nephrol 20:121–124. doi: 10.4103/0971-4065.70839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhanel GG, Lawson CD, Zelenitsky S, Findlay B, Schweizer F, Adam H, Walkty A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2012. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev Anti Infect Ther 10:459–473. doi: 10.1586/eri.12.25. [DOI] [PubMed] [Google Scholar]
- 18.Pai MP, Nafziger AN, Bertino JS Jr. 2011. Simplified estimation of aminoglycoside pharmacokinetics in underweight and obese adult patients. Antimicrob Agents Chemother 55:4006–4011. doi: 10.1128/AAC.00174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkpatrick CM, Duffull SB, Begg EJ. 1999. Pharmacokinetics of gentamicin in 957 patients with varying renal function dosed once daily. Br J Clin Pharmacol 47:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dager WE. 1994. Aminoglycoside pharmacokinetics: volume of distribution in specific adult patient subgroups. Ann Pharmacother 28:944–951. doi: 10.1177/106002809402800719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results reported in this article, after deidentification, will be made available (unless prohibited by applicable law) to researchers for noncommercial purposes beginning 6 months following article publication; proposals should be directed to datarequests@achaogen.com.