High doses of rifampin may help patients with tuberculous meningitis (TBM) to survive. Pharmacokinetic pharmacodynamic evaluations suggested that rifampin doses higher than 13 mg/kg given intravenously or 20 mg/kg given orally (as previously studied) are warranted to maximize treatment response.
KEYWORDS: Indonesia, Mycobacterium tuberculosis, RCT, meningeal, pharmacokinetics, rifampin, survival, tolerability
ABSTRACT
High doses of rifampin may help patients with tuberculous meningitis (TBM) to survive. Pharmacokinetic pharmacodynamic evaluations suggested that rifampin doses higher than 13 mg/kg given intravenously or 20 mg/kg given orally (as previously studied) are warranted to maximize treatment response. In a double-blind, randomized, placebo-controlled phase II trial, we assigned 60 adult TBM patients in Bandung, Indonesia, to standard 450 mg, 900 mg, or 1,350 mg (10, 20, and 30 mg/kg) oral rifampin combined with other TB drugs for 30 days. The endpoints included pharmacokinetic measures, adverse events, and survival. A double and triple dose of oral rifampin led to 3- and 5-fold higher geometric mean total exposures in plasma in the critical early days (2 ± 1) of treatment (area under the concentration-time curve from 0 to 24 h [AUC0–24], 53.5 mg · h/liter versus 170.6 mg · h/liter and 293.5 mg · h/liter, respectively; P < 0.001), with proportional increases in cerebrospinal fluid (CSF) concentrations and without an increase in the incidence of grade 3 or 4 adverse events. The 6-month mortality was 7/20 (35%), 9/20 (45%), and 3/20 (15%) in the 10-, 20-, and 30-mg/kg groups, respectively (P = 0.12). A tripling of the standard dose caused a large increase in rifampin exposure in plasma and CSF and was safe. The survival benefit with this dose should now be evaluated in a larger phase III clinical trial. (This study has been registered at ClinicalTrials.gov under identifier NCT02169882.)
INTRODUCTION
In 2016, the WHO published data on 10.4 million new tuberculosis (TB) cases and 1.3 million deaths caused by this disease worldwide, making it the leading single infectious disease killer (1). In turn, tuberculous meningitis (TBM) is the most devastating form of TB. It occurs in 1% to 6% of patients with TB (2, 3), leading to death or neurological disability in more than 30% of affected patients (2, 4, 5).
The antimicrobial treatment for TBM is according to the model for pulmonary TB, with intensive and continuation phases of treatment. It adheres to the same first-line TB drugs and dosing guidelines (6), although it is known that some first-line TB drugs, including rifampin, achieve suboptimal concentrations beyond the blood-brain and blood-cerebrospinal fluid (CSF) barriers. Rifampin is a crucial TB drug, evidenced by the high mortality rate in TBM patients with resistance to rifampin (7, 8). As it takes a long time to develop new drugs to treat TB and TBM, it is important to make the best possible use of existing drugs. We performed a series of studies to evaluate higher doses of rifampin in Indonesian patients with TBM.
A first open-label, randomized phase II clinical trial showed that a 33% higher dose of rifampin administered intravenously (i.v.) (13 mg/kg) for 2 weeks led to a 3-fold higher exposure to rifampin in plasma and CSF during the first critical days of treatment and a strong reduction in mortality at 6 months after the treatment started (adjusted hazard ratio [HR], 0.42; 95% confidence interval [CI], 0.20 to 0.91) (9). Although we found a clear relationship between concentration and effect and derived threshold values for lower mortality, our data suggested that the highest desirable exposures had not been reached (10).
Intravenous rifampin is not widely available in low-to-middle-income countries, is expensive, and must be administered by health care workers, which is impractical over a longer period of time or unfeasible after discharge from the hospital. We conducted a second open-label pharmacokinetic study; doses of 17 or 20 mg/kg of oral rifampin resulted in average total exposures in plasma (area under the concentration-time curve from 0 to 24 h [AUC0–24]) that were approximately similar to the values obtained with 13 mg/kg rifampin i.v., but the average peak plasma concentrations (Cmax) were lower with large interindividual variabilities (11).
Both our first and second clinical trials called for the evaluation of doses of rifampin higher than 13 mg/kg intravenously and 17 to 20 mg/kg orally. The current study evaluated the pharmacokinetics, safety/tolerability, and efficacy of higher (up to 30 mg/kg) doses of oral rifampin as a TBM treatment.
(Presented in part at the 10th International Workshop on Pharmacology of Tuberculosis Drugs, Atlanta, GA, 15 October 2017 [12].)
RESULTS
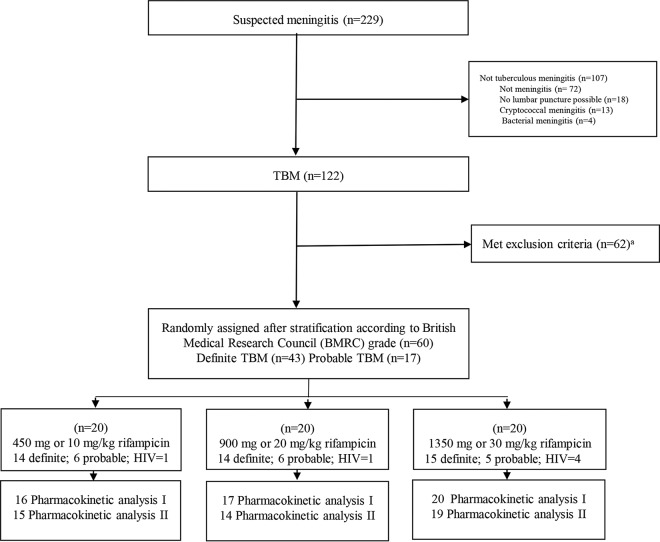
Of the 229 patients with suspected TBM during the study period, 122 patients were diagnosed with TBM and 107 were excluded as a result of alternative diagnoses. The 60 TBM patients who fulfilled all eligibility criteria were randomly assigned to each study arm (Fig. 1). The baseline characteristics (Table 1) show that the patients were equally balanced across the groups, except for fewer male patients in the 20-mg/kg group (P = 0.343) and a higher percentage of HIV-positive patients (P = 0.261) and those with lower CSF protein concentrations (P = 0.057) in the 30-mg/kg group. Overall, most of the patients (90%) had British Medical Research Council (BMRC) grade 2 TBM, 53% were male, the median age was 29.5 years, and 10% of patients had HIV infections.
FIG 1.
Study design.
TABLE 1.
Patients’ characteristics
| Characteristica | Treatment arm |
P valueb
|
||
|---|---|---|---|---|
| 450 mg (10 mg/kg; n = 20) |
900 mg (20 mg/kg; n = 20) |
1,350 mg (30 mg/kg; n = 20) |
||
| Male sex (n [%]) | 12 (60) | 8 (40) | 12 (60) | 0.343 |
| Age (yr) (median [IQR]) | 28 (22.3–45.8) | 29 (21.3–38.3) | 33 (24.3–37.3) | 0.976 |
| Body wt (kg) (median [IQR]) | 45 (40–47.3) | 45 (38.5–50.5) | 48.4 (41.6–54.7) | 0.491 |
| BMI (median [IQR]) | 17.6 (15.4–19) | 18.2 (16–20.4) | 19.1 (16.2–20.2) | 0.396 |
| Temp (°C) (median [IQR]) | 37.2 (36.5–38.2) | 38 (37.2–38.2) | 37.6 (37–38) | 0.162 |
| Chief complaint (n [%]) | ||||
| Lowered consciousness | 17 (85) | 16 (80) | 18 (90) | 0.594 |
| Headache | 19 (33.3) | 19 (33.3) | 19 (33.3) | 0.558 |
| Neck stiffness | 20 (100) | 19 (95) | 15 (75) | 0.401 |
| Cranial nerve palsy | 15 (75) | 15 (75) | 17 (85) | 0.614 |
| Motoric abnormality | 11 (55) | 12 (60) | 13 (65) | 0.642 |
| TBM grade (n [%]) | 0.663 | |||
| 1 | 0 (0.0) | 1 (5.0) | 0 (0) | |
| 2 | 18 (90.0) | 18 (90.0) | 18 (90) | |
| 3 | 2 (10.0) | 1 (5.0) | 2 (10) | |
| HIV positive (n [%]) | 1 (5.0) | 1 (5.0) | 4 (20) | 0.261 |
| GCS (median [IQR]) | 13 (12–14) | 12.5 (11.3–13.8) | 13 (11.3–13.8) | 0.877 |
| Chest X ray, TB (n [%]) | 13 (86.7) | 12 (85.7) | 12 (85.7) | 0.752 |
| CSF baselines (median [IQR]) | ||||
| Leukocytes (cells/µl) | 188 (60.5–413.8) | 245 (98.8–698.8) | 240 (128.3–482.5) | 0.528 |
| PMN (cells/µl) | 30 (18–46) | 29 (13.3–67) | 41 (21.3–65.3) | 0.625 |
| MN (cells/µl) | 70 (54–82) | 71 (33–86.8) | 59 (34.8–78.8) | 0.580 |
| Protein (mg/dl) | 257 (172.7–372.3) | 216.5 (158.8–428) | 169 (116.5–230.8) | 0.057 |
| CSF/blood glucose ratio baseline (median [IQR]) | 0.17 (0.09–0.26) | 0.2 (0.12–0.21) | 0.2 (0.1–0.3) | 0.303 |
| Bacteriologically confirmed TBM (n [%]) | 14 (70) | 14 (70) | 15 (75) | 0.921 |
| Culture positive | 13 (65) | 13 (65) | 14 (70) | 0.928 |
| ZN staining positive | 8 (40) | 4 (20) | 6 (30) | 0.386 |
| Gene Xpert positive | 9 (45) | 8 (40) | 10 (50) | 0.582 |
| Drug administration through NGT on PK1 (n [%]) | 10 (58.8) | 12 (60.0) | 13 (65) | 0.917 |
| Drug administration through NGT on PK2 (n [%]) | 1 (6.7) | 3 (21.4) | 5 (26.3) | 0.33 |
| Dose (median [IQR]) | ||||
| Isoniazid (mg/kg) | 6.7 (6.4–7.5) | 6.7 (6–7.9) | 6.2 (5.5–7.2) | 0.308 |
| Pyrazinamide (mg/kg) | 33.7 (33.3–37.5) | 33.3 (31.6–39.5) | 31.06 (27.4–36) | 0.203 |
| Ethambutol (mg/kg) | 16.8 (16.7–20.1) | 16.7 (15–21.1) | 15.5 (14–18.5) | 0.244 |
| Rifampin (mg/kg) | 10 (9.5–11.3) | 20 (17.8–23.4) | 28 (24.7–32.4) | <0.001 |
TB, tuberculosis; IQR, interquartile range; BMI, body mass index; TBM, tuberculous meningitis; GCS, Glasgow coma scale; CSF, cerebrospinal fluid; ZN, Ziehl-Neelsen; NGT, nasogastric tube; PK, pharmacokinetic(s); PMN, polymorphonuclear cells; MN, mononuclear cells.
Kruskal-Wallis tests for comparisons of continuous variables, χ2 tests for comparisons of the proportions in categorical variables.
An initial pharmacokinetic (PK1) assessment was performed for 53 patients, and Fifty-three patients had, a second PK (PK2) evaluation was performed for 48 patients. PK assessments were not performed for patients who died or were withdrawn from the study. The study drugs were administered via a nasogastric tube (NGT) for 33 patients (62%) at the first PK assessment and for nine patients (19%) at the second assessment. In some patients, the eventual plasma AUC0–24 and Cmax values could not be assessed reliably at sampling times of 1, 2, 4, 8, and 12 h after the dose (Table 2), and in some patients, concomitant CSF was not sampled during the two PK assessments.
TABLE 2.
Pharmacokinetic data for rifampin
| Parametera | Treatment arm (mean or medianf [range]) |
P valueb | ||
|---|---|---|---|---|
| 450 mg (10 mg/kg) | 900 mg (20 mg/kg) | 1,350 mg (30 mg/kg) | ||
| First PK assessment | ||||
| Treatment day | 1 (1–3) | 1 (1–3) | 1 (1–3) | |
| AUC0–24 (mg · h/liter) | 53.5 (13.4–116.5) | 170.6 (26.4–377.1) | 293.5 (190.1–533.3) | <0.001 |
| T1/2 (h) | 4.9 (2.1–20) | 6.3 (3.7–10.6) | 8.8 (3.7–25.6) | 0.022 |
| Cmax (mg/liter) | 7.2 (2.2–14.1) | 18.1 (2–43.6) | 25.5 (11.9–55.5) | <0.001 |
| VF (liter) | 59.2 (26.0–217.1) | 47.9 (22.4–516.3) | 58.1 (24.8–146.3) | 0.644 |
| CLF (liter/h) | 8.4 (3.9–33.6) | 5.3 (2.4–34.1) | 4.6 (2.5–7.1) | 0.020 |
| Tmax (h) | 3.6 (1–9) | 3.9 (0.9–12) | 3.9 (1–12) | 0.882c |
| CSF3–9 (mg/liter) | 0.28 (0.13–0.48) | 0.59 (0.13–1.7) | 0.74 (0.24–1.20) | <0.001 |
| Time to LP (h:min)d | 04:02 (03:02–05:45) | 04:19 (03:02–06:13) | 03:57 (03:15–05:29) | |
| AUC0–24 of >116 mg · h/liter (no./total [%]) | 1/11 (9.1) | 13/15 (86.7) | 17/17 (100) | <0.001e |
| Cmax of >22 mg/liter (no./total [%]) | 0/14 | 7/18 (39) | 14/19 (74) | <0.001e |
| Second PK assessment | ||||
| Treatment day | 10 (9–11) | 10 (9–11) | 10 (9–11) | |
| AUC0–24 (mg · h/liter) | 39.23 (17.41–66.53) | 144.15 (86.25–241.67) | 187.89 (41.61–392.14) | <0.001 |
| T1/2 (h) | 2.09 (1.27–7.75) | 3.12 (2.02–6.10) | 3.27 (1.65–7.39) | 0.008 |
| Cmax (mg/liter) | 7.55 (4.45–14.3) | 21.89 (13.28–32) | 25.14 (9.9–54.78) | <0.001 |
| VF (liter) | 34.57 (16.30–80.66) | 28.21 (17.20–66.77) | 34.47 (19.27–77.37) | 0.464 |
| CLF (liter/h) | 11.47 (6.72–25.85) | 6.24 (3.72–10.44) | 7.19 (3.44–32.44) | 0.004 |
| Tmax (h) | 2.72 (1–4) | 2.92 (1–8) | 3.59 (1–12) | 0.768c |
| CSF3–9 (mg/liter) | 0.23 (0.13–0.63) | 0.39 (0.13–0.82) | 0.51 (0.13–1.18) | 0.008 |
| Time to LP (h:min)d | 03:41 (03:02–06:00) | 03:51(03:28–05:52) | 04:02 (03:17–05:57) | |
AUC0–24, area under the concentration-time curve from 0 to 24 h; Cmax, maximum plasma concentration; Tmax, time to Cmax; CSF, cerebrospinal fluid; CSF3–9, concentration in CSF between 3 and 9 h after drug administration; VF, apparent volume of distribution; CLF, apparent total clearance; T1/2, half-life; LP, lumbar puncture. First PK was at day 2 ± 1, second PK was at day 10 ± 1. AUC0–24 values were estimated in 43 patients both in PK1 and PK2. The rest could not be estimated because the elimination rate constant could not be assessed reliably with sampling at 1, 2, 4, 8, and 12 h after dosing, preventing extrapolation of exposures beyond the last measurable concentration. Cmax could not be assessed in 7 patients.
One-way analysis of variance on log-transformed PK data, with Tukey’s honestly significant difference post hoc test, unless otherwise indicated.
Kruskal-Wallis test.
CSF samples had to be taken between 3 and 9 h after the dose and were mostly taken around 4 h after the dose.
Threshold values for reduced mortality based on previous work (10).
Geometric means are used for the pharmacokinetic parameters, except for values related to time, which are medians.
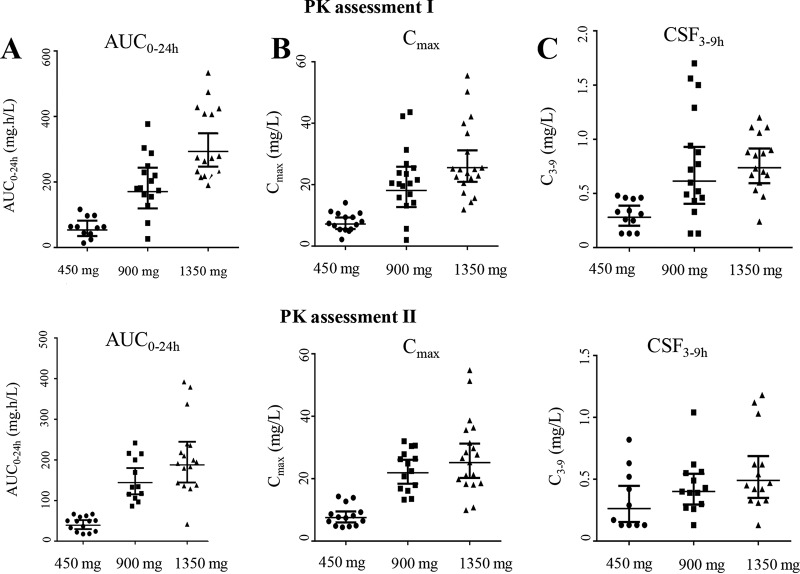
A higher dose of oral rifampin resulted in higher geometric mean AUC0–24 values, i.e., 3-fold higher for the 20-mg/kg group and 5-fold higher for the 30-mg/kg group, whereas Cmax and CSF concentrations increased proportionally with the increasing rifampin doses, both in PK1 and PK2 (Table 2).
Large interindividual variability was observed in plasma rifampin AUC0–24 and Cmax values and in CSF concentrations. For example, the differences between the minimum and maximum plasma AUC0–24 values at the first PK assessment were 9-fold for the 10-mg/kg group, 14-fold for the 20-mg/kg group, and 3-fold for the 30-mg/kg group (Table 2, Fig. 2). In the multivariate analysis, the dose administered was the only predictor of plasma AUC0–24, Cmax, and concentrations in CSF, whereas sex, age, and BMRC grade were not significant predictors (data not shown). The numbers of patients administered the drug via an NGT were not significantly different between the three study groups and did not predict AUC0–24, Cmax, or CSF concentrations. The few HIV-infected patients in the study (n = 6) (Table 1) were not using antiretroviral treatment at the start of the study, but four patients began treatments 2 months after TBM treatment was started (13). This excludes any possible effect of antiretroviral drugs on the pharmacokinetic endpoints or on the interpatient pharmacokinetic variability in the study. Plasma AUC0–24, Cmaxs, and CSF concentrations were all highly and positively correlated with each other. For example, the correlation coefficient (Spearman’s ρ) between plasma AUC0–24 and CSF concentrations was 0.7 (P < 0.01).
FIG 2.
Distribution of rifampin. Area under the concentration-time curve from 0 to 24 h after the dose (AUC0–24) (A), maximum concentration in plasma (Cmax) (B), and concentration in CSF at 3 to 9 h after the dose (C), at the first (day 2 ± 1 of treatment, top) and second (day 10 ± 1of treatment, bottom) pharmacokinetic (PK) assessments. The x axes show the three arms in the study using standard 450 mg, 900 mg, or 1,350 mg (10, 20, and 30 mg/kg) oral rifampin combined with other TB drugs. Data points represent values from individual patients. Horizontal lines represent geometric means with 95% confidence intervals.
A comparison of the AUC0–24 values at the first and second PK assessments showed a significant decrease of 33% at the second assessment (P = 0.014, all groups combined), with decreases of 27%, 16%, and 36% in the 10-, 20-, and 30-mg/kg groups, respectively (P = 0.21, 0.003, and 0.004, respectively, paired t test on log-transformed AUC0–24 values). Similarly, CSF concentrations decreased from day 2 to day 10 of the treatment, but Cmaxs did not (Table 2).
The adverse events during the 30 days after the start of study drugs were equally distributed among the groups (Table 3). The majority of the adverse events were mild. Hepatotoxicity was the most common grade 3 adverse event. All patients with grade 3 adverse events continued treatment, and those with grade 3 hepatotoxicity had normal transaminases after a median of 20 days (interquartile range [IQR], 7.5 to 52 days) of continued treatment without an interruption or dose change of rifampin. One patient in the 30-mg/kg group had grade 4 hepatotoxicity at day 13 of treatment, as indicated by hyperbilirubinemia of >10 times the upper limit of normal (ULN) and transaminases 3 times the ULN. All TB drugs were interrupted, the blinding to the dose of rifampin was removed, and the higher rifampin dose was replaced by a standard dose. The blood total bilirubin decreased to 2 times the ULN, and transaminase levels normalized by day 26 of treatment. Rifampin AUC0–24 and Cmaxs in those with and without grade 3 or 4 adverse events were not significantly different (P = 0.325 and 0.772, respectively).
TABLE 3.
Safety and tolerability
| Category | Treatment arm (n [%]) |
P value | |||
|---|---|---|---|---|---|
| All | 450 mg (10 mg/kg; n = 20) | 900 mg (20 mg/kg; n = 20) | 1,350 mg (30 mg/kg; n = 20) | ||
| All AEa | |||||
| Grade I–II AE | 51 (85) | 17 (85) | 16 (80) | 18 (90) | 0.676 |
| Grade III–IV AE | 15 (25) | 3 (15) | 8 (40) | 4 (20) | 0.503 |
| Specific adverse effects | |||||
| Purpura | |||||
| Grade I–II | 1 (1.7) | 0 | 0 | 1 (5) | 0.608 |
| Thrombocytopenia | |||||
| Grade I–II | 8 (13.3) | 2 (10) | 4 (20) | 2 (10) | 0.686 |
| Leukopenia | |||||
| Grade I–II | 3 (5) | 0 | 1 (5) | 2 (10) | 0.593 |
| Anemia | |||||
| Grade I–II | 20 (33.3) | 11 (55) | 6 (30) | 3 (15) | 0.107 |
| Grade III | 1 (1.7) | 0 | 1 (5) | 0 | |
| Hepatotoxicity | |||||
| Grade I–II | 26 (43) | 9 (45) | 7 (35) | 10 (50) | 0.824 |
| Grade III–IV | 12 (20) | 3 (15) | 5 (25) | 4 (20) | |
| Nausea | |||||
| Grade I–II | 27 (45) | 9 (45) | 8 (40) | 10 (50) | 0.444 |
| Vomitus | |||||
| Grade I–II | 21 (35) | 8 (40) | 6 (30) | 7 (35) | 0.359 |
| Abdominal discomfort | |||||
| Grade I–II | 15 (25) | 4(20) | 6 (30) | 5 (25) | 0.189 |
| Diarrhea | |||||
| Grade I–II | 8 (13.3) | 1 (5) | 3(15) | 4 (20) | 0.602 |
| Pruritus | |||||
| Grade I–II | 27 (43.3) | 9 (45) | 6 (30) | 12 (60) | 0.442 |
| Grade III | 1 (1.7) | 0 | 1 (5) | 0 | |
| Rash | |||||
| Grade I–II | 21 (35) | 5 (25) | 5 (25) | 11 (55) | 0.410 |
| Grade III | 1(1.7) | 0 | 1 (5) | 0 | |
AE, adverse events; classification was based on the U.S. National Institutes of Health CTCAE version 4.03.
The administration of higher doses of rifampin increased the percentage of patients who achieved plasma AUC0–24 and Cmax threshold values for decreased mortality, as derived from our first clinical trial (9) (Table 2). Two of the sixty patients were withdrawn from the study on day 2 on the basis of the clinician’s consideration. One patient was withdrawn because of a sudden and rapid deterioration (30-mg/kg group), as it was considered unethical to perform multiple blood or CSF samplings in this patient. The other patient was withdrawn after rifampin resistance was identified by Gene Xpert (20-mg/kg group), which enabled the investigator to withdraw the patient according to the study protocol; resistance to rifampin was not an exclusion criterion (see Text S1 in the supplemental material), considering the turnaround time of even fast tests for rifampin resistance in a resource-poor setting. Only an “intention-to-treat” analysis was deemed useful for this study, as most deaths occurred before the patients could complete the full “per protocol” intervention for 1 month. This means that the two withdrawn patients were considered survivors in our explorative analysis of efficacy.
The 6-month mortality was 32% (95% CI, 19.5% to 48.1%), and the majority (12/19 [63%]) of deaths occurred in the first 2 weeks after admission. The causes of death were suspected respiratory failure (n = 7), septic shock (n = 4), brain herniation (n = 3), sudden cardiac event (n = 2), pulmonary embolism (n = 2), and head injury (n = 1). The 6-month mortality was nonsignificantly lower in the 30-mg/kg group both among all patients (P = 0.12) and among the cases with definite TBM (P = 0.07) (Table 4). The exclusion of the one patient with resistance to rifampin in the analyses caused a small change in the mortality rates (Table 4). A Cox regression analysis showed that the HR for the administration of the triple dose versus the standard dose was 0.44 with a 95% CI of 0.1 to 1.9 (P = 0.27) in all TBM patients, whereas it was 0.23 (95% CI, 0.03 to 2.05; P = 0.19) among patients with definite TBM (both adjusted for HIV status and Glasgow coma scale [GCS] score) (see also Fig. S1).
TABLE 4.
Patients’ cumulative mortality per time point
| Mortality assessment (day) |
All TBM patients |
Bacteriologically confirmed (definite) TBMa |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment arm (n [%]) |
P value | Treatment arm (n [%]) |
P value | ||||||
| All (n = 60) | 450 mg (10 mg/kg; n = 20 |
900 mg (20 mg/kg; n = 20)b |
1,350 mg (30 mg/kg; n = 20) |
450 mg (10 mg/kg; n = 14) |
900 mg (20 mg/kg; n = 14)b |
1,350 mg (30 mg/kg; n = 15) |
|||
| At discharge | 13 (22) | 5 (25) | 5 (25) | 3 (15) | 3 (21) | 4 (29) | 1 (7) | ||
| 30 | 14 (23) | 5 (25) | 6 (30) | 3 (15) | 3 (21) | 4 (29) | 1 (7) | ||
| 45 | 15 (25) | 5 (25) | 7 (35) | 3 (15) | 3 (21) | 5 (36) | 1 (7) | ||
| 60 | 15 (25) | 5 (25) | 7 (35) | 3 (15) | 3 (21) | 5 (36) | 1 (7) | ||
| 180 | 19 (32) | 7 (35) | 9 (45) | 3 (15) | 0.116b | 5 (36) | 6 (43) | 1 (7) | 0.069b |
TBM was classified as definite (microbiologically proven) if either CSF microscopy for acid-fast bacilli, Mycobacterium tuberculosis culture, or PCR results were positive.
One patient included with bacteriologically confirmed TBM was withdrawn from the study due to resistance to rifampin and was included as a survivor in the intention to treat analysis (see Results). If this patient is excluded from the analysis, the mortality at 180 days would be 9/19 (47%) among all TBM patients and 6/13 (46%) among bacteriologically confirmed patients in the 900 mg (20 mg/kg) arm; P = 0.091 for all patients and P = 0.054 for patients with bacteriologically confirmed TBM.
New neurological events at 3, 7, 30, 60, and 180 days, as well as functional outcome measured by the modified Rankin scale (MRS) and Glasgow outcome scale (GOS), were equally distributed among all three groups of patients (data not shown).
DISCUSSION
This study revealed that tripling the standard dose of oral rifampin for 1 month strongly increased the exposure to this pivotal TB drug in plasma and CSF, did not increase the incidence of grade 3 and 4 adverse events, improved the attainment of previously determined exposure threshold values for lower mortality, and showed a trend for a lower 6-month mortality rate among patients with microbiologically proven (definite) TBM.
The standard dose of rifampin resulted in low average exposures in CSF, around the MICs of 0.2 to 0.4 mg/liter of this drug for Mycobacterium tuberculosis (14, 15). These results are in agreement with data from the literature showing that individual or mean rifampin CSF concentrations >1 mg/liter are rare (15–18). Due to its protein binding in plasma (19), only 15% to 20% of rifampin is available for transport to other tissues, and penetration to the brain is even more limited.
Fortunately, our intervention with higher doses of rifampin was effective from a pharmacokinetic point of view. A tripling of the rifampin dose provided a more than proportional increase in AUC0–24 and a proportional increase in Cmax and CSF concentrations, which were all correlated with each other. A possible explanation for the nonlinear pharmacokinetics is the saturation of hepatic extraction or of the excretion of the drug in bile upon increasing the dose (20, 21). The resulting geometric mean AUC0–24s in the 20-mg/kg and 30-mg/kg groups in the first critical days of treatment were higher than that of 600 mg i.v. rifampin in our previous examination (11) (170.6 and 293.5 versus 145.7 h · mg/liter, respectively); the average Cmax in the 20-mg/kg group was still lower, but the 30-mg/kg group had a similar Cmax to that for 600 mg i.v. rifampin (25.5 versus 24.7 mg/liter).
Our data also showed large interindividual variability in rifampin exposure, which is in agreement with the literature and may be enhanced by pharmacokinetic changes in critically ill patients (22, 23). Of note, the lowest observed AUC0–24, Cmax, and CSF concentrations increased with the higher dose (Table 2, Fig. 2); these lowest exposures may be associated with more treatment failures and mortality.
After the first critical days of treatment, we observed decreased exposures to rifampin after ten days of treatment, which is explained by the autoinducing enzyme properties of rifampin (20).
The adverse events after the treatments were started were equally distributed among the three rifampin dose groups. The highest 30-mg/kg daily dose of rifampin was not associated with an increase in the incidence of severe (grade 3 or 4) adverse events, although it should be noted that the number of patients in this phase II study was relatively small. Hepatotoxicity, as reflected by alanine aminotransferase (ALT) and aspartate transaminase (AST) increases, was the most common grade 3 adverse event, and these increases all resolved without any interruption of the study drugs. Thus, on the basis of our data, grade 3 and 4 adverse events, specifically, ALT and AST increases, were not dependent on rifampin dose or exposure. Indeed, hepatotoxicity to rifampin appears to be a hypersensitivity reaction, which is more common with intermittent administration of larger doses (24, 25). Studies of higher doses of rifampin in pulmonary TB also suggest that hepatotoxicity to rifampin is idiosyncratic and not dose related (26, 27).
As to the efficacy, the tripling of the dose of oral rifampin for a period of 30 days led to an increase in the attainment of plasma AUC0–24 and Cmax thresholds for decreased mortality and highlighted a trend in survival benefit (P = 0.07) among those with microbiologically proven (definite) TBM. Although it is important to note this clear trend, our study was not designed and powered to detect a difference in mortality. For example, the apparent small differences between the performances of the 10- and 20-mg/kg doses (Table 4 and Fig. S1) were not statistically significant.
The overall aim of this trial was to find and substantiate the dose of rifampin to be studied in a larger (phase III) follow-up trial. We propose this dose to be 30 mg/kg given orally, as this yielded the highest exposure in plasma and CSF, was tolerable in severely ill TBM patients, and showed a trend for reduced mortality among patients with definite TBM. A study in Africa with a similar 35-mg/kg oral rifampin dose was associated with an increase in bactericidal activity (12, 26), and the same dose achieved a decrease in time to culture conversion in patients with pulmonary TB (27). Ideally, a follow-up trial with rifampin at a 30-mg/kg dose should be performed among TBM patients from various continents.
The strengths of our study are the double-blinding that we applied and the high proportion of bacteriologically confirmed TBM cases (71%). Our study also has limitations. First, our study mainly included patients with TBM grade (BMRC grade) 2 and few patients with the more severe grade 3 TBM. It cannot be excluded that pharmacokinetics, the safety/tolerability, and the efficacy of high dose rifampin are different in the more severe grade 3 TBM population. Second, the sample size was limited, inherent to the study’s phase II characteristics and objectives, and the study was not powered to compare the efficacy of the three doses of oral rifampin. As a result, the apparent mortality benefits of high-dose oral rifampin should be interpreted with caution. Third, we could only take single CSF samples for pharmacokinetic purposes; thus, no pharmacokinetic curves could be generated for CSF.
In summary, the tripling of the dose of oral rifampin to 30 mg/kg resulted in an increased exposure to rifampin, both in plasma and CSF, which was not associated with an increase in the incidence of grade 3 and 4 adverse events. The higher 30-mg/kg rifampin dose resulted in an increase in pharmacokinetic target attainment. In terms of efficacy, further investigation in a larger population is needed to confirm our findings.
MATERIALS AND METHODS
Patients.
All patients over 14 years old (adults) with clinically suspected meningitis who presented themselves at Hasan Sadikin Hospital, Bandung, Indonesia (the referral hospital for West Java), between December 2014 and November 2016 had an initial screening that included standard CSF tests, blood measurements, and chest radiography.
TBM was classified as definite (microbiologically proven) if either CSF microscopy for acid-fast bacilli, M. tuberculosis culture, or PCR results were positive. On the basis of prior evaluations of CSF characteristics of definite and clinically suspected cases in the Bandung cohort, the patients were classified as having probable TBM if they had a CSF/blood glucose ratio of <0.5 combined with a CSF cell count of ≥5 cells/µl (4).
Patients with clinical suspicion of TBM were eligible for the study if they fulfilled the inclusion and exclusion criteria listed in Text S1 in the supplemental material. An HIV test was performed for every patient. Microbiological examinations were performed for cryptococci in HIV-infected patients (India ink microscopy and cryptococcal antigen testing), M. tuberculosis (Ziehl-Neelsen microscopy, culture, and Gene Xpert) (28), and bacterial pathogens (Gram staining). The neurological status of patients was classified according to a modified BMRC grading system as 1 (GCS of 15 with no focal neurological signs), 2 (GCS of 11 to 14 or 15 with focal neurological signs), or 3 (GCS < 10).
Written informed consent to participate in the trial was obtained from all patients or from their relatives if the patient could not provide informed consent. In the latter case, patients who regained the capacity to consider participation were consulted, and the study was continued after obtaining informed consent.
Study design.
The overall aim of this study was to find and substantiate the dose of rifampin to be studied in a larger follow-up trial on the basis of pharmacokinetic, safety/tolerability, and efficacy considerations. The primary objective was to describe the pharmacokinetics of higher doses of rifampin in TBM patients. The secondary objectives were to evaluate the safety and tolerability and to explore the efficacy of treatment regimens with higher doses of rifampin.
The study was a double-blinded, randomized, placebo-controlled phase II trial with three parallel arms. Eligible patients were assigned a standard (450 mg, ∼10 mg/kg, one active and two placebo tablets), double (900 mg, ∼20 mg/kg, two active and one placebo tablet), or triple (1,350 mg, ∼30 mg/kg, three active tablets) dose of rifampin for 30 days, in addition to other TB drugs, according to Indonesian national guidelines. Randomization occurred in variable block sizes and was stratified by BMRC grade. Adjunctive dexamethasone was administered intravenously, using the dosing and tapering schedule as applied in a previous study in Vietnam (29), but with a change in mode of administration of the same doses, i.e., intravenous administration during hospitalization and oral administration after discharge. After 30 doses of the standard or high-dose rifampin regimen, all patients continued taking the standard TB regimen up to 2 months, with subsequent daily doses of rifampin (450 mg) and isoniazid (300 mg) for a minimum of 4 months. During hospitalization in the first 4 weeks of treatment, the adherence to the treatment was secured by facility-based directly observed treatment (DOT). After hospitalization, the adherence was monitored through community-based DOT, frequently through close family members, pill counts, and the patients’ medication intake diary.
Compared to our prior clinical trials (9, 11), the current trial was performed in a blind manner rather than open, and we administered higher doses of rifampin for a longer period of 1 month instead of 2 weeks. The longer duration was evaluated considering (i) that mortality due to TBM is less, but still occurring, beyond the first weeks of treatment, (ii) that the longer duration of high-dose rifampin was likely to be safe and tolerable and without unduly harm, on the basis of our prior two studies in TBM patients and an ongoing study among African patients with pulmonary TB (26), and (iii) that such a longer intervention was feasible in clinical practice when using oral rather than intravenous administration of rifampin. The intermediate dose of rifampin (900 mg or 20 mg/kg daily) was included because our prior study with this oral dose (11) did not have efficacy as an endpoint, was not performed in a blind manner, and evaluated the shorter (2 weeks) course of the intervention.
Rifampin 450 mg (active) tablets and matching placebo tablets were manufactured at PT Kimia Farma, Bandung, Indonesia. The study drugs and other standard TB treatments were administered to patients with empty stomachs. For unconscious patients who could not swallow, the drugs were crushed and delivered through a nasogastric tube.
The study was approved by the ethical review board of the Medical Faculty of Universitas Padjadjaran, Bandung, Indonesia. External monitoring assessed compliance with the study’s protocol and the data safety monitoring board (DSMB) performed an interim analysis after half of the subjects were included. This trial is registered with ClinicalTrials.gov as trial number NCT02169882.
Bioanalysis and PK assessments.
PK sampling was performed twice, at day 2 (± 1) during the first critical days of treatment (PK1) and then at day 10 (± 1) of treatment (PK2). On each sampling day, serial blood sampling was performed just before and at 1, 2, 4, 8, and 12 h after dosing. CSF samples for PK assessment were collected on both PK sampling days, between 3 and 9 h after dosing. Patients had an overnight fast before PK sampling and remained fasted until 2 h after the administration of the study drugs. Blood samples were centrifuged at 3,000 rpm for 15 min, and plasma and CSF samples were stored at −80°C within 30 min of collection. The analyses of the rifampin concentrations in plasma and CSF were performed as described previously (11). The PK parameters for rifampin in plasma were assessed using standard noncompartmental methods in Phoenix WinNonlin v.6.3 (Certara USA Inc., Princeton, NJ) as described previously (30).
Follow-up.
Patients were followed up until 6 months after their treatment started. All patients were hospitalized for a minimum of 10 days, enabling real-time surveillance of any TBM-related and drug-related adverse events during that period. Liver transaminases and full blood counts were monitored on days 3, 7, 10, 14, 30, 45, and 60. Any further investigations, e.g., bilirubin tests, were performed if indicated.
The adverse events in these severely ill patients were defined as those possibly/probably related to their treatment (hematological, gastrointestinal, and skin disorders). All other adverse events (e.g., new neurological events and respiratory failure) were not incorporated in the assessment of safety and tolerability.
The classification and grading of adverse events were based on the common terminology criteria for adverse events (CTCAE version 4.03). Treatment was stopped in patients who had grade 4 adverse events.
Efficacy was assessed by mortality and clinical and neurological responses on days 3, 7, 30, 60, and 180. New neurological events were defined as the occurrence of any of the following: cranial nerve palsy, motor deficits, or seizures. The modified Rankin scale (MRS) and Glasgow outcome scale (GOS) were used to evaluate the clinical and neurological outcomes.
After discharge, survival was monitored by phone calls or home visits for patients who could not be reached via phone. Verbal autopsies were performed via phone calls and no postmortem autopsy was done.
Sample size and statistical analysis.
On the basis of the results from a previous clinical trial, the number of participants needed per study arm to estimate the total exposure to rifampin (AUC0–24) at a dose of 20 mg/kg, using 95% confidence and a margin of error of 20%, was 18 at day 2 ± 1 and 16 at day 10 ± 1 (11). With a consideration of dropouts, 20 patients were needed for each study arm. This number of participants was also shown to be sufficient to demonstrate a significant difference in AUC0–24 achieved after 10, 20, and 30 mg/kg of rifampin.
Patient characteristics, rifampin PK parameters, and adverse events were presented descriptively for all patients and by study group. A multiple linear regression analysis was performed to find predictors of PK parameters. The number of patients with plasma AUC0–24 and Cmax values above the threshold values for lower mortality of 116 h · mg/liter and 22 mg/liter, respectively, at day 2 ± 1 (as determined previously) (10) was assessed for each group. The data for safety/tolerability and survival were analyzed by the intention-to-treat principle. The mortality data are presented as proportions in each group at various time points, and the proportions were compared using the chi-square test. A Cox regression analysis assessed the effect of rifampin dose on 6-month mortality in all patients and for culture-confirmed TBM patients only. IBM SPSS Statistics (version 22.0) for Windows was used for the statistical analyses; GraphPad Prism version 5.03 and statistical software R version 3.4.2 (31) were used to generate graphs. P values of less than 0.05 were considered significant in all analyses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ayi Djembarsari and Siti Aminah Soepalarto, Hasan Sadikin Hospital, for accommodating the research, Feby Purnama, Sofia Immaculata, Sri Margi, Shehika Shulda, and Rani Trisnawati for monitoring the patients and data recording, Atu Purnama Dewi for rifampin bioanalysis, Jessi Annisa for microbiological analysis, and Lika Apriani as the random list holder.
All the authors worked collectively to develop the protocols and methods described in this report. R.R. was the principal investigator. A.R.G. and S.D. were responsible for the clinical data and the follow-up. V.Y., A.C., and L.T.B. performed the pharmacokinetic data analysis under the supervision of R.R. and R.A., S.D. and R.A. performed the statistical analyses with support from K.W., S.D. performed the literature search and wrote the first draft of the report, and all other authors provided contributions and suggestions.
This work was supported by Peer Health (National Academy of Sciences [NAS]-United States Agency for International Development [USAID]), the Ministry of Research, Technology and Higher Education, Indonesia (PKSLN grant to T.H.A., R.R., and S.D.), the Direktorat Jenderal Pendidikan Tinggi (BPPLN fellowship to S.D.), and Radboud University Medical Center, the Netherlands (fellowship to S.D.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01014-18.
REFERENCES
- 1.World Health Organization. 2017. Global tuberculosis report 2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Wilkinson RJ, Rohlwink U, Misra UK, van Crevel R, Mai NTH, Dooley KE, Caws M, Figaji A, Savic R, Solomons R, Thwaites GE, Consortium TMIR. 2017. Tuberculous meningitis. Nat Rev Neurol 13:581–598. doi: 10.1038/nrneurol.2017.120. [DOI] [PubMed] [Google Scholar]
- 3.Gomes T, Reis-Santos B, Bertolde A, Johnson JL, Riley LW, Maciel EL. 2014. Epidemiology of extrapulmonary tuberculosis in Brazil: a hierarchical model. BMC Infect Dis 14:9. doi: 10.1186/1471-2334-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Laarhoven A, Dian S, Ruesen C, Hayati E, Damen MSMA, Annisa J, Chaidir L, Ruslami R, Achmad TH, Netea MG, Alisjahbana B, Rizal Ganiem A, van Crevel R. 2017. Clinical parameters, routine inflammatory markers, and LTA4H genotype as predictors of mortality among 608 patients with tuberculous meningitis in Indonesia. J Infect Dis 215:1029–1039. doi: 10.1093/infdis/jix051. [DOI] [PubMed] [Google Scholar]
- 5.Heemskerk AD, Bang ND, Mai NT, Chau TT, Phu NH, Loc PP, Chau NV, Hien TT, Dung NH, Lan NT, Lan NH, Lan NN, Phong LT, Vien NN, Hien NQ, Yen NT, Ha DT, Day JN, Caws M, Merson L, Thinh TT, Wolbers M, Thwaites GE, Farrar JJ. 2016. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med 374:124–134. doi: 10.1056/NEJMoa1507062. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2017. Treatment of tuberculosis guidelines for treatment of drug-susceptible tuberculosis and patient care. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Thwaites GE, Lan NT, Dung NH, Quy HT, Oanh DT, Thoa NT, Hien NQ, Thuc NT, Hai NN, Bang ND, Lan NN, Duc NH, Tuan VN, Hiep CH, Chau TT, Mai PP, Dung NT, Stepniewska K, White NJ, Hien TT, Farrar JJ. 2005. Effect of antituberculosis drug resistance on response to treatment and outcome in adults with tuberculous meningitis. J Infect Dis 192:79–88. doi: 10.1086/430616. [DOI] [PubMed] [Google Scholar]
- 8.Cecchini D, Ambrosioni J, Brezzo C, Corti M, Rybko A, Perez M, Poggi S, Ambroggi M. 2007. Tuberculous meningitis in HIV-infected patients: drug susceptibility and clinical outcome. AIDS 21:373–374. doi: 10.1097/QAD.0b013e328012b84d. [DOI] [PubMed] [Google Scholar]
- 9.Ruslami R, Ganiem AR, Dian S, Apriani L, Achmad TH, van der Ven AJ, Borm G, Aarnoutse RE, van Crevel R. 2013. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis 13:27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 10.te Brake L, Dian S, Ganiem AR, Ruesen C, Burger D, Donders R, Ruslami R, van Crevel R, Aarnoutse R. 2015. Pharmacokinetic/pharmacodynamic analysis of an intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis. Int J Antimicrob Agents 45:496–503. doi: 10.1016/j.ijantimicag.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Yunivita V, Dian S, Ganiem AR, Hayati E, Hanggono Achmad T, Purnama Dewi A, Teulen M, Meijerhof-Jager P, van Crevel R, Aarnoutse R, Ruslami R. 2016. Pharmacokinetics and safety/tolerability of higher oral and intravenous doses of rifampicin in adult tuberculous meningitis patients. Int J Antimicrob Agents 48:415–421. doi: 10.1016/j.ijantimicag.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Svensson RJ, Svensson EM, Aarnoutse RE, Diacon AH, Dawson R, Gillespie SH, Boeree MJ, Simonsson USH. 15 October 2017. Prediction of increase in time-to-positivity after higher doses of rifampicin based on PKPD modelling. 10th International Workshop on Pharmacology of Tuberculosis Drugs, Atlanta, GA. [Google Scholar]
- 13.Torok ME, Yen NT, Chau TT, Mai NT, Phu NH, Mai PP, Dung NT, Chau NV, Bang ND, Tien NA, Minh NH, Hien NQ, Thai PV, Dong DT, Anh do TT, Thoa NT, Hai NN, Lan NN, Lan NT, Quy HT, Dung NH, Hien TT, Chinh NT, Simmons CP, de Jong M, Wolbers M, Farrar JJ. 2011. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin Infect Dis 52:1374–1383. doi: 10.1093/cid/cir230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rastogi N, Labrousse V, Goh KS. 1996. In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr Microbiol 33:167–175. doi: 10.1007/s002849900095. [DOI] [PubMed] [Google Scholar]
- 15.Donald PR. 2010. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb) 90:279–292. doi: 10.1016/j.tube.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Furesz S, Scotti R, Pallanza R, Mapelli E. 1967. Rifampicin: a new rifamycin. 3. Absorption, distribution, and elimination in man. Arzneimittelforschung 17:534–537. [PubMed] [Google Scholar]
- 17.Ellard GA, Humphries MJ, Allen BW. 1993. Cerebrospinal fluid drug concentrations and the treatment of tuberculous meningitis. Am Rev Respir Dis 148:650–655. doi: 10.1164/ajrccm/148.3.650. [DOI] [PubMed] [Google Scholar]
- 18.D’Oliveira JJ. 1972. Cerebrospinal fluid concentrations of rifampin in meningeal tuberculosis. Am Rev Respir Dis 106:432–437. doi: 10.1164/arrd.1972.106.3.432. [DOI] [PubMed] [Google Scholar]
- 19.te Brake LH, Ruslami R, Later-Nijland H, Mooren F, Teulen M, Apriani L, Koenderink JB, Russel FG, Burger DM, Alisjahbana B, Wieringa F, van Crevel R, Aarnoutse RE. 2015. Exposure to total and protein-unbound rifampin is not affected by malnutrition in Indonesian tuberculosis patients. Antimicrob Agents Chemother 59:3233–3239. doi: 10.1128/AAC.03485-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acocella G. 1978. Clinical pharmacokinetics of rifampicin. Clin Pharmacokinet 3:108–127. doi: 10.2165/00003088-197803020-00002. [DOI] [PubMed] [Google Scholar]
- 21.Chirehwa MT, Rustomjee R, Mthiyane T, Onyebujoh P, Smith P, McIlleron H, Denti P. 2016. Model-Based Evaluation of Higher Doses of Rifampin Using a Semimechanistic Model Incorporating Autoinduction and Saturation of Hepatic Extraction. Antimicrob Agents Chemother 60:487–494. doi: 10.1128/AAC.01830-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felton TW, Hope WW, Roberts JA. 2014. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn Microbiol Infect Dis 79:441–447. doi: 10.1016/j.diagmicrobio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Blot SI, Pea F, Lipman J. 2014. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 77:3–11. doi: 10.1016/j.addr.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Burman WJ, Gallicano K, Peloquin C. 2001. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet 40:327–341. doi: 10.2165/00003088-200140050-00002. [DOI] [PubMed] [Google Scholar]
- 25.Grosset J, Leventis S. 1983. Adverse effects of rifampin. Rev Infect Dis 5:S440–S450. doi: 10.1093/clinids/5.Supplement_3.S440. [DOI] [PubMed] [Google Scholar]
- 26.Boeree MJ, Diacon AH, Dawson R, Narunsky K, du Bois J, Venter A, Phillips PP, Gillespie SH, McHugh TD, Hoelscher M, Heinrich N, Rehal S, van Soolingen D, van Ingen J, Magis-Escurra C, Burger D, Plemper van Balen G, Aarnoutse RE, Consortium P. 2015. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med 191:1058–1065. doi: 10.1164/rccm.201407-1264OC. [DOI] [PubMed] [Google Scholar]
- 27.Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, Kibiki GS, Churchyard G, Sanne I, Ntinginya NE, Minja LT, Hunt RD, Charalambous S, Hanekom M, Semvua HH, Mpagama SG, Manyama C, Mtafya B, Reither K, Wallis RS, Venter A, Narunsky K, Mekota A, Henne S, Colbers A, van Balen GP, Gillespie SH, Phillips PPJ, Hoelscher M, PanACEA consortium . 2017. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 17:39–49. doi: 10.1016/S1473-3099(16)30274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaidir L, Annisa J, Dian S, Parwati I, Alisjahbana A, Purnama F, van der Zanden A, Ganiem AR, van Crevel R. 2018. Microbiological diagnosis of adult tuberculous meningitis in a ten-year cohort in Indonesia. Diagn Microbiol Infect Dis 91:42–46. doi: 10.1016/j.diagmicrobio.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, Nguyen QH, Nguyen TT, Nguyen NH, Nguyen TN, Nguyen NL, Nguyen HD, Vu NT, Cao HH, Tran TH, Pham PM, Nguyen TD, Stepniewska K, White NJ, Tran TH, Farrar JJ. 2004. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 351:1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 30.Ruslami R, Nijland HM, Alisjahbana B, Parwati I, van Crevel R, Aarnoutse RE. 2007. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob Agents Chemother 51:2546–2551. doi: 10.1128/AAC.01550-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.