Abstract
Background
The present study explored the expression of coiled-coil domain-containing 34 (CCDC34) in cervical cancer (CC) and its prognostic value.
Material/Methods
GEPIA and Oncomine cancer databases were mined to predict the CCDC34 differential expression level between a CC group and a normal group. Immunohistochemistry was performed to examine the CCDC34 expression in 67 CC and corresponding adjacent tissues. CD31 and vascular endothelial growth factor (VEGF) were stained to reflect tumor angiogenesis in 67 CC tissues. Kaplan-Meier univariate and Cox multivariate survival analysis were done to evaluate the correlation between CCDC34 expression and prognosis of CC patients.
Results
Both GEPIA and Oncomine cancer databases mining results revealed that CCDC34 was more highly expressed in the CC group than in the normal group (all P<0.05). Our immunochemical staining data showed that CCDC34 expression was dramatically higher in CC than in adjacent normal tissues (71.6 vs. 20.9%; P<0.001). High expression of CCDC34 was strongly associated with histological grade (P=0.022), lymph node metastasis (P=0.044), and FIGO stage (P=0.002). Furthermore, patients with CCDC34-positive expression had much more MVD than those with CCDC34-negative expression (P<0.001). Kaplan-Meier survival analysis showed that CCDC34-positive expression was associated with worse overall survival (OS) (P=0.004) and disease-free survival (DFS) (P=0.005). Additionally, Cox multivariate analysis revealed that CCDC34 was an independent unfavorable prognostic parameter of DFS and OS (P=0.040 and 0.039, respectively).
Conclusions
High expression of CCDC34 is an independent unfavorable prognostic parameter for OS and DFS of CC patients, which was strongly associated with tumor angiogenesis.
MeSH Keywords: Angiogenesis Inducing Agents, Prognosis, rho-Associated Kinases, Uterine Cervical Neoplasms
Background
Cervical cancer (CC) is the second leading cause of death among women worldwide [1]. Although there has been notable progress in CC treatment, including surgical techniques, chemotherapy, and radiotherapy, in the past 2 decades, there are still some early cases that have invasion and metastasis, which directly affects the prognosis of CC [2]. At present, the underlying molecular mechanism of CC pathogenesis is still unclear. Thus, to improve the survival of CC patients, it is important to find new potential prognostic biomarkers and therapeutic targets.
The coiled-coil domain-containing 34 (CCDC34), also named NY-REN-41, includes 373 amino acids and locates in 11p14.1 chromosome. CCDC34 was initially found in a patient with hamartoma of the retinal pigment epithelium and retina [3]. Recently, Gong et al. [4] found that CCDC34 was overexpressed in bladder cancer and promoted its cell proliferation and migration. However, the expression level and prognostic value of CCDC34 in human cervical cancer remain largely unclear.
In this study, GEPIA and Oncomine cancer databases were mined to predict the CCDC34 differential expression level between a CC group and a normal group. Then, immunohistochemical staining was performed to detect the expression levels of CCDC34, VEGF, and microvessel density (MVD) in CC. Moreover, CCDC34 expression level in CC and its correlations with patients’ clinicopathological characteristics and survival prognosis were investigated.
Material and Methods
Bioinformatics mining
GEPIA (http://gepia.cancer-pku.cn/) and Oncomine (https://www.oncomine.org/resource/login.html) cancer databases were mined to predict the CCDC34 differential expression level between a CC group and a normal group.
Patients and corresponding clinical characteristics
We retrospectively included 67 patients diagnosed with cervical cancer after radical surgery in our hospital from August 2009 to December 2011. Surgical staging was based on the FIGO system. All patients’ clinicopathological factors are listed in Table 1. This study was approved by the Ethics Committee of the Tumor Hospital of Ganzhou Review Board (No. 20140702). Informed consent was signed by each patient.
Table 1.
CCDC34 expression status in relation to selected clinicopathologic features in 67 cervical cancer patients.
| Characteristics | n | CCDC34 | χ2 | P | |
|---|---|---|---|---|---|
| − | + | ||||
| 19 | 48 | ||||
| Age | |||||
| <40 | 32 | 10 | 22 | 0.252 | 0.616 |
| ≥40 | 35 | 9 | 26 | ||
| Tumor length (cm) | |||||
| <4 | 30 | 7 | 23 | 0.675 | 0.411 |
| ≥4 | 37 | 12 | 25 | ||
| Histology | |||||
| SCC | 52 | 14 | 38 | 0.026 | 0.873 |
| Adenocarcinoma | 15 | 5 | 10 | ||
| Histological grade | |||||
| G1 | 31 | 13 | 18 | 5.235 | 0.022 |
| G2–3 | 36 | 6 | 30 | ||
| Cervical infiltration depth | |||||
| <2/3 | 24 | 9 | 15 | 1.538 | 0.215 |
| ≥2/3 | 43 | 10 | 33 | ||
| Lymphovascular permeation | |||||
| Yes | 29 | 7 | 22 | 0.448 | 0.503 |
| No | 38 | 12 | 26 | ||
| Lymph node metastasis | |||||
| Yes | 23 | 3 | 20 | 4.043 | 0.044 |
| No | 44 | 16 | 28 | ||
| Recurrence | |||||
| Yes | 26 | 5 | 21 | 1.742 | 0.187 |
| No | 41 | 14 | 27 | ||
| FIGO stage | |||||
| I | 33 | 15 | 18 | 9.356 | 0.002 |
| II | 34 | 4 | 30 | ||
Immunohistochemistry and results evaluation
Immunohistochemistry was performed to examine the expression levels of CCDC34 (ab122396, Abcam, Inc.), VEGF (ab2349, Abcam, Inc.) and CD31 (ab28364, Abcam, Inc.) according to the manufacturer’s instructions. The working concentration of each antibody was at a dilution of 1: 100. Immunohistochemical scores were calculated based on a previously reported method [5]: “+” score for ≥10% of tumor cells positive; “−” score when less than 10% of tumor cells or no visible staining was detected.
MVD counts
MVD counts were calculated by vascular endothelial cells with CD31-positive staining. We used low-magnification (×40) microscopy to identify regions with the maximum number of CD31-positive-staining microvessels. Then, we used high magnification (×100) to count the number of microvessels. Each section was independently observed and calculated by 2 pathologists.
Statistical analysis
SPSS 20.0 software was used to perform the statistical analysis. Data in GEPIA and Oncomine databases were analyzed by the independent-samples t test to compare the differential expression levels of CCDC34 mRNA between the cervical cancer group and the normal group. Pearson χ2 test or Fisher test was employed to evaluate the correlation between CCDC34 expression and clinicopathological factors. Kaplan-Meier univariate and Cox multivariate survival analyses were performed to assess the CCDC34 prognostic value in CC patients. A P value less than 0.05 was considered as a significantly statistical difference.
Results
High expression of CCDC34 and its relationship with CC patients’ clinicopathological factors
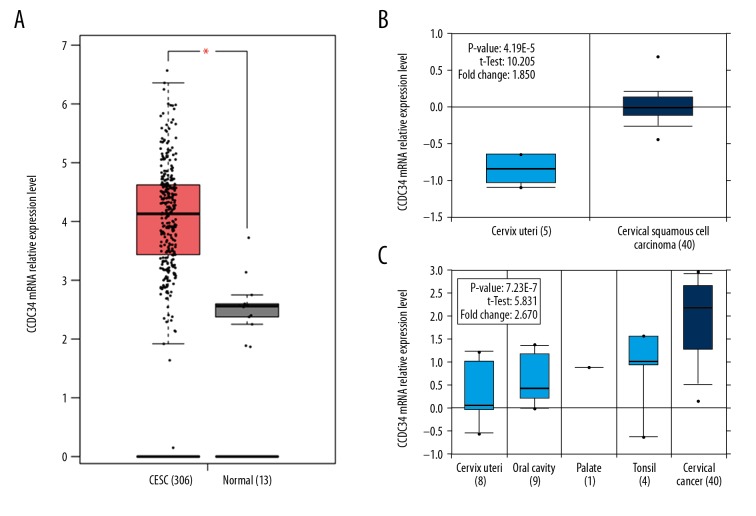
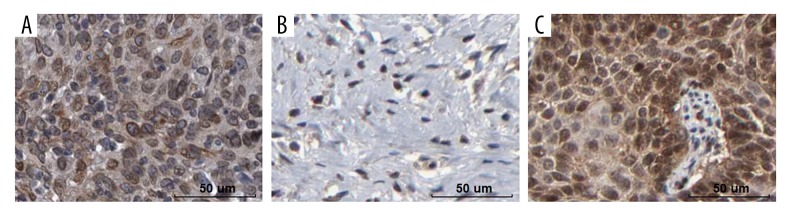
Both GEPIA and Oncomine cancer databases mining results revealed that CCDC34 was more highly expressed in the CC group than that in the normal group (all P<0.05, Figure 1). Then, immunohistochemistry was used in 67 cervical cancer and corresponding adjacent tissues to examine the clinicopathological and prognostic significance of CCDC34 in cervical cancer. The positive staining of CCDC34 was primarily located in the cytoplasm and membrane (Figure 2A). The CCDC34-positive staining rate was 71.6% (48/67) in cervical cancer tissues, while in adjacent non-cancerous tissues, only 14 of 67 (20.9%) corresponding paracarcinomatous showed CCDC34-positive staining (Figure 2B). A statistically significant difference was found between these 2 groups of tissues in the positive rate of CCDC34 (P<0.001, Figure 3). The relationship between CCDC34 expression levels and CC patients’ clinicopathological parameters are listed in Table 1. High expression of CCDC34 was significantly associated with histological grade (P=0.022), lymph node metastasis (P=0.044), and FIGO stage (P=0.002).
Figure 1.
Bioinformatics mining results. (A) GEPIA mining results (Based on TCGA data), * P<0.05. (B, C) Oncomine mining results based on GEO data, and the GSE number of each dataset was GSE7410 and GSE6791, respectively.
Figure 2.
Immunochemical staining of CCDC34 and VEGF. High (A) and low (B) expression of CCDC34 protein in cervical cancer tissues. (C) Expression of VEGF protein in cervical cancer tissues. Bar=50 um.
Figure 3.
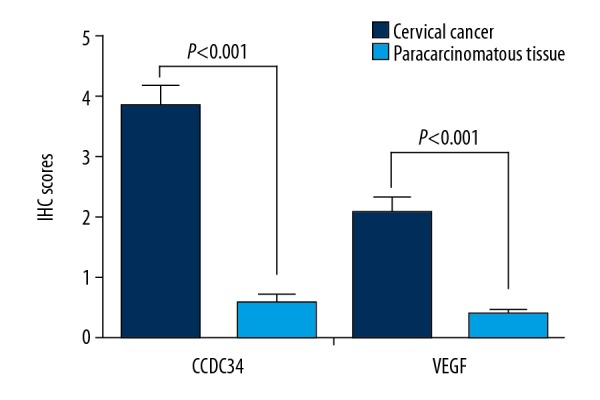
IHC scores of CCDC34 and VEGF expressions in cervical cancer and matched paracarcinomatous tissues.
Correlation between CCDC34 and VEGF expression in CC patients
VEGF-positive staining was mainly located in the cytoplasm (Figure 2C) and the VEGF-positive staining rate was 59.7% (40/67). The CCDC34-positive and VEGF-positive rate were both 55.2% (37/67), and the consistency of both CCDC34 and VEGF expression was 74.6% (50/67). Furthermore, Spearman’s test revealed a strong relationship between CCDC34 and VEGF expression in CC tissues (r=0.563, P=0.000; Table 2).
Table 2.
Correlation between CCDC34 and VEGF expression (cases).
| Staining indicator | CCDC34 | r | P value | |
|---|---|---|---|---|
| + | − | |||
| VEGF | ||||
| + | 37 | 3 | 0.563 | 0.000 |
| − | 11 | 16 | ||
Association between CCDC34 and MVD in CC
As shown in Figure 4, CC patients with CCDC34-positive expression had much more MVD than those with CCDC34-negative expression (P=0.000).
Figure 4.
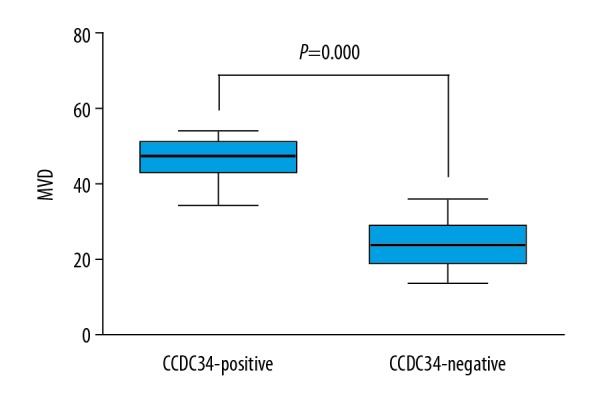
Intratumoral microvessel density (MVD) in relation to CCDC34 protein immunoreactivity. Cervical cancer patients with CCDC34-positive expression showed significantly higher intratumoral MVD than those with CCDC34-negative expression.
Correlation between CCDC34 and CC patients’ prognosis
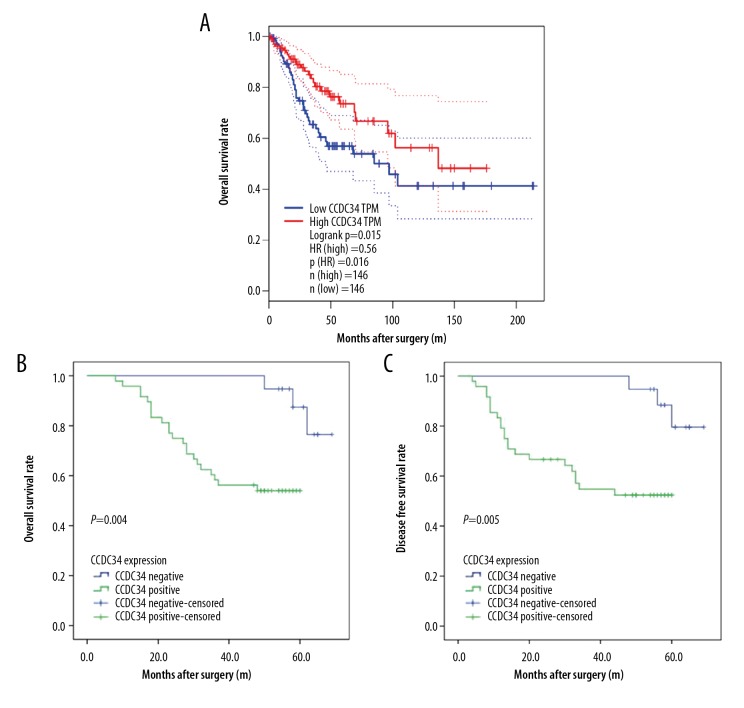
Based on TCGA database, patients with high expression of CCDC34 had worse OS than those with low expression of CCDC34 (P=0.015, Figure 5A). Compared to those with CCDC34-negative expression, CC patients with CCDC34-positive expression had shorter OS and DFS times (P=0.004, Figure 5B and P=0.005, Figure 5C, respectively).
Figure 5.
Kaplan-Meier analysis of overall survival (OS) and disease-free survival (DFS) curves of patients with cervical cancer based on CCDC34 expression levels. (A) OS curve of patients with cervical cancer based on TCGA database. (B) OS curve of patients with cervical cancer based on CCDC34 expression. (C). DFS curve of patients with cervical cancer based on CCDC34 expression.
CCDC34 can serve as a predictive parameter for the prognosis of CC patients
Kaplan-Meier univariate survival analysis showed that CCDC34 expression, recurrence, and FIGO stage had significant prognostic influence on OS and DFS (Table 3). Cox multivariate survival analysis revealed that CCDC34 was an independent prognostic factor for OS (P=0.039) and DFS (P=0.040) (Table 4).
Table 3.
Univariate analysis of factors associated with OS and DFS.
| Characteristics | DFS | OS | ||
|---|---|---|---|---|
| 95% CI | P | 95% CI | P | |
| CCDC34 | ||||
| Negative | 63.443–69.113 | 63.800–69.066 | ||
| Positive | 33.618–46.424 | 0.005 | 38.468–49.075 | 0.004 |
| Age | ||||
| <40 | 40.246–57.905 | 44.853–59.670 | ||
| ≥40 | 42.217–57.184 | 0.051 | 45.369–58.231 | 0.638 |
| Tumor length(cm) | ||||
| <4 | 38.817–55.453 | 42.416–56.706 | ||
| ≥4 | 43.864–59.824 | 0.606 | 47.735–61.180 | 0.575 |
| Histology | ||||
| SCC | 42.973–55.765 | 46.528–57.265 | ||
| Adenocarcinoma | 35.342–58.614 | 0.320 | 39.611–59.900 | 0.293 |
| Histological grade | ||||
| G1 | 43.619–58.282 | 46.997–59.257 | ||
| G2–3 | 40.429–57.501 | 0.835 | 44.663–59.294 | 0.801 |
| Cervical infiltration depth | ||||
| <2/3 | 47.721–62.705 | 49.522–62.853 | ||
| ≥2/3 | 38.829–54.533 | 0.118 | 43.487–56.755 | 0.128 |
| Lymphovascular permeation | ||||
| Yes | 35.604–53.187 | 39.757–55.143 | ||
| No | 46.073–61.170 | 0.196 | 49.869–62.297 | 0.198 |
| Lymph node metastasis | ||||
| Yes | 31.353–51.163 | 36.717–53.496 | ||
| No | 47.855–61.621 | 0.050 | 50.653–62.486 | 0.047 |
| Recurrence | ||||
| Yes | 30.894–50.851 | 36.434–53.160 | ||
| No | 49.095–62.285 | 0.045 | 51.663–63.093 | 0.043 |
| FIGO stage | ||||
| I | 30.681–47.422 | 36.617–50.266 | ||
| II | 53.245–64.636 | 0.030 | 54.065–64.858 | 0.046 |
Table 4.
Multivariate analysis of factors associated with OS and DFS.
| Factors | DFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| CCDC34 (negative vs. positive) | 4.798 | 1.072–21.478 | 0.040 | 5.617 | 1.094–28.838 | 0.039 |
| Age (<40 vs. ≥40) | 0.416 | 0.162–1.064 | 0.067 | 0.451 | 0.176–1.153 | 0.096 |
| Tumor length (<4 vs. ≥4 cm) | 0.726 | 0.262–2.006 | 0.536 | 0.746 | 0.276–2.016 | 0.563 |
| Histology (SCC vs. adenocarcinoma) | 0.440 | 0.162–1.200 | 0.109 | 0.448 | 0.167–1.199 | 0.110 |
| Histological grade (G1 vs. G2+G3) | 0.613 | 0.260–1.443 | 0.262 | 0.661 | 0.282–1.549 | 0.340 |
| Cervical infiltration depth (<2/3 vs. ≥2/3) | 3.116 | 0.903–10.760 | 0.072 | 2.922 | 0.856–9.979 | 0.087 |
| lymphovascular permeation (no vs. yes) | 1.745 | 0.760–4.003 | 0.189 | 1.593 | 0.702–3.616 | 0.265 |
| Lymph node metastasis (no vs. yes) | 1.045 | 0.419–2.609 | 0.925 | 1.041 | 0.418–2.592 | 0.932 |
| Recurrence (no vs. yes) | 3.879 | 1.343–11.204 | 0.012 | 3.807 | 1.343–10.795 | 0.012 |
| FIGO stage (I vs. II) | 1.865 | 0.710–4.898 | 0.206 | 1.688 | 0.629–4.529 | 0.299 |
Discussion
The coiled-coil domain-containing (CCDC) proteins exhibit diverse functions related to their highly versatile folding motif [6]. Previous studies have shown genetic or epigenetic alterations in several CCDC genes in human cancers, including CCDC34 in bladder cancer [4], colorectal cancer [7], pancreatic adenocarcinoma [8], and esophageal squamous cell carcinoma [9]. However, to date, there has been no study focusing on the relationship between CCDC34 and cervical cancer. In this study, our results proved that CCDC34 was overexpressed in cervical cancer compared to corresponding adjacent normal tissues. Further findings demonstrated that high expression of CCDC34 was significantly associated with histological grade, lymph node metastasis, and FIGO stage. These data suggest a key role of CCDC34 in progression and development of cervical cancer.
Accumulating evidence demonstrates that high expression of VEGF is closely related to aggressive behavior and worse prognosis of cancer [10]. In addition, high VEGF expression levels and MVD are strongly associated with unfavorable prognosis of CC patients [11–14]. In the present study, we similarly observed a significant positive correlation between CCDC34 and VEGF expression in CC tissues. Compared to those with CCDC34-negative expression, patients with CCDC34-positive expression had much more MVD. These findings suggest that CCDC34 participates in tumor angiogenesis of CC, possibly in coordination with VEGF. Similarly, Gong et al. [4] demonstrated that knockdown of CCDC34 retards bladder cancer proliferation and migration by downregulating the PI3K/AKT pathway. The AKT pathway has a pivotal regulatory role in multiple cellular survival pathways, primarily in tumorigenesis and angiogenesis, by regulating VEGF expression [15]. This suggests that CCDC34 facilitates VEGF expression by regulating the PI3K/AKT signaling pathway. Hu et al. [9] found that CCDC34 was highly expressed in ESCC, and high expression of CCDC34 was associated with poor prognosis of ESCC patients, the potential mechanism of which is closely related to the angiogenesis of ESCC; these results are consistent with our own. More in-depth studies are required to elucidate the role of CCDC34 in CC angiogenesis.
We also explored the relationship between CCDC34 expression and prognosis of CC patients. Kaplan-Meier univariate survival analysis demonstrated that patients with CCDC34-positive expression had shorter OS and DFS than those with CCDC34-negative expression. In addition, Cox multivariate survival analysis revealed that CCDC34 was an independent unfavorable predictor for OS and DFS of CC patients. The bioinformatics mining results were consistent with our experimental data.
The present study has certain limitations. First, small cervical cancer tissue samples were collected retrospectively, which might to some extent lead to biased statistical results. Second, only immunohistochemical staining, a semi-quantitative method, was used in our validation experiment, and some other quantitative methods like Western blot or qRT-PCR analysis are needed. Third, the exact biological function of CCDC34 in cervical cancer and its detailed molecular regulation mechanisms were not assessed in this study. We plan to address these deficiencies in our future experiments.
Conclusions
Our results proved that high expression of CCDC34 was closely associated with CC angiogenesis and is as an independent poor prognostic parameter in CC.
Footnotes
Source of support: This study was supported by the Jiangxi Provincial Department of Science and Technology (No. 20142BAB205053)
Conflicts of interest
None.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hu X, Schwarz JK, Lewis JS, Jr, et al. A microRNA expression signature for cervical cancer prognosis. Cancer Res. 2010;70:1441–48. doi: 10.1158/0008-5472.CAN-09-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kutsche K, Glauner E, Knauf S, et al. Cloning and characterization of the breakpoint regions of a chromosome 11;18 translocation in a patient with hamartoma of the retinal pigment epithelium. Cytogenet Cell Genet. 2000;91:141–47. doi: 10.1159/000056835. [DOI] [PubMed] [Google Scholar]
- 4.Gong Y, Qiu W, Ning X, et al. CCDC34 is up-regulated in bladder cancer and regulates bladder cancer cell proliferation, apoptosis and migration. Oncotarget. 2015;6:25856–67. doi: 10.18632/oncotarget.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudo Y, Ogawa I, Kitajima S, et al. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 2006;66:6928–35. doi: 10.1158/0008-5472.CAN-05-4540. [DOI] [PubMed] [Google Scholar]
- 6.Tamaki H, Sanda M, Katsumata O, et al. Pilt is a coiled-coil domain-containing protein that localizes at the trans-Golgi complex and regulates its structure. FEBS Lett. 2012;586:3064–70. doi: 10.1016/j.febslet.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 7.Geng W, Liang W, Fan Y, et al. Overexpression of CCDC34 in colorectal cancer and its involvement in tumor growth, apoptosis and invasion. Mol Med Rep. 2018;17:465–73. doi: 10.3892/mmr.2017.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi W, Shao F, Huang Q. Expression of coiled-coil domain containing 34 (CCDC34) and its prognostic significance in pancreatic adenocarcinoma. Med Sci Monit. 2017;23:6012–18. doi: 10.12659/MSM.907951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu DD, Li PC, He YF, et al. Overexpression of coiled-coil domain-containing protein 34 (CCDC34) and its correlation with angiogenesis in esophageal squamous cell carcinoma. Med Sci Monit. 2018;24:698–705. doi: 10.12659/MSM.908335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaya M, Wada T, Akatsuka T, et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res. 2000;6:572–77. [PubMed] [Google Scholar]
- 11.Cheng WF, Chen CA, Lee CN, et al. Vascular endothelial growth factor and prognosis of cervical carcinoma. Obstet Gynecol. 2000;96:721–26. doi: 10.1016/s0029-7844(00)01025-5. [DOI] [PubMed] [Google Scholar]
- 12.Bremer GL, Tiebosch AT, van der Putten HW, et al. Tumor angiogenesis: An independent prognostic parameter in cervical cancer. Am J Obstet Gynecol. 1996;174:126–31. doi: 10.1016/s0002-9378(96)70384-8. [DOI] [PubMed] [Google Scholar]
- 13.Dinh TV, Hannigan EV, Smith ER, et al. Tumor angiogenesis as a predictor of recurrence in stage Ib squamous cell carcinoma of the cervix. Obstet Gynecol. 1996;87:751–54. doi: 10.1016/0029-7844(96)00039-7. [DOI] [PubMed] [Google Scholar]
- 14.Wiggins DL, Granai CO, Steinhoff MM, et al. Tumor angiogenesis as a prognostic factor in cervical carcinoma. Gynecol Oncol. 1995;56:353–56. doi: 10.1006/gyno.1995.1062. [DOI] [PubMed] [Google Scholar]
- 15.Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]