Abstract
Background
Leukemia cells have strong proliferation and anti-apoptosis capabilities. The purpose of this study was to investigate the effect of silencing the leucine-rich alpha-2-glycoprotein1 (LRG1) gene, which was found to regulate tumor proliferation and apoptosis in acute myeloid leukemia (AML) cell lines.
Material/Methods
Plasmid interference technique was used to silence the LRG1 gene in the KASUMI-1 cell line. The cell counting kit-8 (CCK-8) assay was used to test the effect of transduction on cell viability. Cell cycle and apoptosis were detected by flow cytometry. Western blot and quantitative real-time polymerase chain reaction (RT-qPCR) were applied to detect the expression levels of proteins and mRNA, respectively.
Results
KASUMI-1 cells with the CD34+CD38− phenotype were sorted by flow cytometry. After transfection of the siLRG1 plasmid, the level of LRG1 expression was downregulated and cell viability was reduced. Silencing of LRG1 gene blocked KASUMI-1 cells in G0/G1 phase and promoted apoptosis. Further experiments found that LRG1 gene silencing significantly downregulated cell cycle-associated proteins and anti-apoptotic proteins, while upregulating pro-apoptotic proteins. Downregulation of LRG1 gene expression also inhibits signal transduction of the JAK-STAT pathway.
Conclusions
LRG1 gene silencing regulates the expression of cyclin and apoptosis-related proteins to reduce cell viability and promote apoptosis, probably through inhibition of the JAK-STAT pathway.
MeSH Keywords: Apoptosis; Cell Cycle; Leukemia, Myeloid, Acute
Background
Acute myeloid leukemia (AML) is a type of malignant clonal hematological disease in which hematopoietic stem and progenitor cells are derived [1]. The annual incidence rate is 2–4 per 100 000 population, and the median age of onset is 64–70 years, accounting for more than 80% of adult leukemia cases. In the past 40 years, AML treatment has made great progress, and the complete remission rate has reached 50–80% [1]. However, the toxic and adverse effects of chemotherapy drugs, the increase in drug resistance, and relapse are still problems [2]. Leukemia cells continuously proliferate and self-renew, and there are defects in apoptosis and differentiation [3]. Therefore, a therapeutic approach that seeks to increase the apoptosis of leukemic cells is of great significance to AML.
The leucine-rich alpha-2-glycoprotein1 (LRG1) gene-encoded LRG1 protein is a membrane-associated leucine-rich repeat (LRR) family member that is induced by pro-inflammatory cytokines [4]. Previous studies have shown that LRG1 is overexpressed in ulcerative colitis, immune responses, and neovascularization [5–7]. Recent studies have found that the LRG1 gene is also overexpressed in a variety of tumors, including colorectal cancer, hepatocellular carcinoma, and ovarian cancer [7–9]. Although a study has found that the expression level of LRG1 gene is increased in leukemia, there are few studies on the biological function of the LRG1 gene in leukemia and the regulation of downstream signaling pathways [10].
A previous study has found that the growth of cancer cells can be inhibited by silencing the LRG1 gene in colon cancer, and LRG1 gene silencing has been shown to inhibit tumor cell proliferation by affecting apoptosis at the protein and cyclin levels [11,12]. The JAK/STAT signaling pathway is a rapidly transmitting signal pathway that is a central pathway for activation of binding of various cytokines to receptors, and it participates in the processes of tumor proliferation, differentiation, and apoptosis [13]. Activation of the JAK/STAT signaling pathway is closely related to the occurrence and development of various tumors, and a variety of inhibitors of JAK/STAT signaling pathways have been discovered and shown to play an important role in the treatment of tumors [14]. Since the US Food and Drug Administration (FDA) approved Ruxotinib for myelofibrosis indications in 2012, many other similar preparations have also entered clinical trials [15]. This has contributed to the research interest in inhibition of the JAK/STAT signaling pathway in hematological neoplastic diseases [16].
To investigate the biological function of LRG1 in leukemia cells and its mechanism of action, we used plasmid interference gene silencing techniques to study cell viability and apoptosis after LRG1 gene silencing in the human AML cell line KASUMI-1. By examining the expression levels of the relevant apoptotic proteins, cyclins, and signal pathways, we explored the mechanism of action of LRG1 in the KASUMI-1 cell line and provide ideas for the treatment of AML.
Material and Methods
Cells culture
The human AML cell line KASUMI-1 was purchased from ATCC (USA). Cells were cultured in RPMI 1640 medium containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 incubator. Culture-related reagents were purchased from GIBCO Invitrogen (USA).
Flow cytometry detection and sorting of CD34+/CD38− cells
We collected 1.0×106 KASUMI-1 cells, washed them with PBS and resuspended them. Then, we added 20 μL mouse anti-human monoclonal antibodies CD34-PE (ab157304, Abcam, dilution: 1: 4000) and CD38-FITC (ab1173, Abcam, dilution: 1: 6000) and the sample was incubated at room temperature in the dark for 30 min, and then washed with PBS. A flow cytometer (Becton Dickerson, San Jose, CA, USA) was used to test and sort CD34+/CD38− cells, and FITC and PE fluorescent antibodies were used as isotype controls. The same method was applied to detect the sorted cells.
Construction and transfection of siLRG plasmid
siLRG was purchased from GenePharma (China). siRNA transfections were performed using Lipofectamine 2000 (Invitrogen, USA) as the siLRG1 group. Negative-siRNA as the NC group and untransfected group (control group) was also established. The transfection efficiencies were detected using quantitative real-time polymerase chain reaction (RT-qPCR) and Western blot, respectively.
Cell viability analysis
The Cell counting kit-8 (CCK-8) assay was used to detect cell viability at 6 h, 12 h, and 24 h after transfection. The kit was purchased from Tongren (Japan). We inoculated 100 mL of the transfected cells in a 96-well plate and pre-incubated it at 37°C in a 5% CO2 incubator, and then 10 mL of CCK-8 reagent was added and cultured at 37°C in 5% CO2 atmosphere for 4 h. The absorbance of each well at 450 nm was measured using a microplate reader (ELX 800, Bio-Teck, USA), and cell viability was calculated according to the standard curve.
Evaluation of cell cycle
The transfected cells were collected and washed with PBS at 0°C, and then fixed with 75% ethanol at −20°C for 12 h. After fixation, the cells were treated with 10 μL of RNase A (10 mg/mL, TaKaRa, Japan) for 30 min at 37°C. Then, the cells were stained with 50 μL of PI (250 μg/mL) and detected by flow cytometry. The flow cytometry results were processed using FlowJo V10 software (Becton, Dickinson & Company, USA).
Evaluation of cell apoptosis
Flow cytometry was used to detect cell apoptosis with kits purchased from BD Pharmingen (USA). We washed 1×106 cells with PBS at 4°C and resuspend them to a concentration of 4×105 cells/mL. We added 5 μL of Annexin V-FITC into 200 μL of cell solution, and then 10 μL propidium iodide (PI, 20 μg/mL) was added. The samples were incubated at room temperature in the dark for 10 min. Flow cytometry was applied to assess cell apoptosis. The wavelength of the light source was 488 nm.
RT-qPCR analysis
RT-qPCR was used to detect the expression levels of Cyclin D1, Proliferating Cell Nuclear Antigen (PCNA), Bax, Bcl-2, pro-Caspase-3, and cleaved-Caspase-3 genes. The cells were triturated and lysed using Trizol (TaKaRa, Japan) at 0°C for 5 min. RNAs were extracted by CCl3 (Aladdin, China) and dissolved in DEPC water (Sigma aliquots). RNA concentration was measured by using a UV spectrophotometer (NanoDrop One Microvolume UV-Vis spectrophotometer, ThermoFisher, USA). Reverse transcription assays were performed on RNA samples using a reverse transcription kit (TaKaRa, Japan) to synthesize cDNA. The reverse transcription reaction condition was 37°C for 15 min and the reverse transcriptase inactivation condition was 85°C for 15 s. RT-qPCR experiments were performed with the SYBR Prellix Ex TaqTM Real-Time PCR Kit (TaKaRa, Japan). PCR was performed by activating the DNA polymerase at 95°C for 5 min, followed by 40 cycles of two-step PCR (95°C for 10 s and 60°C for 30 s and a final extension at 75°C for 10 min and held at 4°C. DnaSe and RNase-free water were used as the templates of negative control experiments. All primers were obtained from Genewiz (Suzhou, Jiangsu China) and are listed in Table 1. GAPDH was considered as an internal control. The formula 2−ΔΔCT was used to analyze the gene expression.
Table 1.
The sequences of primers.
| Primer name | Sequence (5′-3′) | Product size (bp) |
|---|---|---|
| LRG1-Forward | GCAAUUAGAACGGCUACAUT T | 195 |
| LRG1-Reverse | AUGUAGCCGUUCUAAUUGCTT | |
| Cyclin D1-Forward | CTGGCCATGAACTACCTGGA | 245 |
| Cyclin D1-Reverse | GTCACACTTGATCACTCTGG | |
| PCNA-Forward | CACCTTAGCACTAGTATTCGAAGCAC | 137 |
| PCAN-Reverse | CACCCGACGGCATCTTTATTAC | |
| Bcl-2-Forward | CTGGTGGACAACATCGC | 317 |
| Bcl-2-Reverse | GGAGAAATCAAACAGAGGC | |
| Bax-Forward | TCCACCAAGAAGCTGAGCGAG | 345 |
| Bax-Reverse | TTCTTTGAGTTCGGTGGGGTC | |
| pro-Caspase-3-Forward | TCTGGAGCATCTGCTGTCTG | 317 |
| pro-Caspase-3-Reverse | TTGACGTCTGTGGTCCGTCC | |
| cleaved-Caspase-3-Forward | TCCGAATTCATGGAGAACACTGAAAACTC | 435 |
| cleaved-Caspase-3-Reverse | ATCGTCGACTCAAACATCACGCATCAATTCCAC | |
| GAPDH-Forward | CCATCTTCCAGGAGCGAGAT | 222 |
| GAPDH-Reverse | TGCTGATGATCTTGAGGCTG |
Western blot
Western blot analysis was applied to detect cell cycle-associated proteins (Cyclin D1 and PCNA), apoptosis-related proteins (Bax, Bcl-2, pro-Caspase-3 and cleaved-Caspase-3), and JAK-STAT pathway-related proteins (p-JAK2, JAK2, p-STAT3, STAT3). Cells were lysed with liquid nitrogen and blocked with RIPA (Abmole, USA), followed by 1% cleavage in PMSF and phosphatase inhibitors (Abmole, USA) and lysis for 30 min at 4°C. The supernatant was collected by centrifugation at 12 000 rpm and 4 min at 4°C for 15 min. A standard curve was drawn using the BCA method to determine the protein concentration. A 10% SDS-PAGE gel was prepared without RNase dH2O and used for electrophoresis. The PVDF membrane (Bio-Rad, USA) was transferred using a Trans-Blot Transfer Slot (Bio-Rad, USA) and blocked with 5% fat-free milk for 2 h at room temperature. The primary antibodies (anti-LRG1, Abcam, ab178698, dilution: 1: 800; anti-Cyclin D1, Abcam, ab134175, dilution: 1: 800; anti-PCNA, ab29, Abcam, dilution: 1: 700; anti-Bax, Abcam, ab32503, dilution: 1: 600; anti-Bcl-2, Abcam, ab32124, dilution: 1: 1000; anti-pro-Caspase-3, Abcam, ab32150, dilution: 1: 600; anti-cleaved-Caspase-3, Abcam, ab13585, dilution: 1: 800; anti-p-JAK2, Abcam, ab32101, dilution: 1: 600; anti-JAK2, Abcam, ab108596, dilution: 1: 700; anti-p-STAT3, Abcam, ab76315, dilution: 1: 900; anti-STAT3, Abcam, ab119352, dilution: 1: 800) were added according to the kit instructions, shaken at room temperature for 2 h, then incubated at 4°C for 12 h. The secondary antibodies (goat anti-mouse IgG, Abcam, ab6785, 1: 8000; rabbit anti-mouse IgG, Abcam, ab99697, dilution: 1: 9000; mouse anti-rabbit IgG, Invitrogen, BA1034, 1: 7000; donkey anti-rabbit IgG, R&D, NL004, 1: 5000; Rabbit Anti-Human IgG, Abcam, ab6759, dilution: 1: 10000) were added and incubated at room temperature for 1.5 h. Chemiluminescence detection was carried out using ECL reagent (Huiying, Shanghai, China).
Statistical analysis
All the experimental data are presented as mean ± standard deviation (SD). Statistical analysis was performed using SPSS 20 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison was carried out to evaluate differences between experimental groups. Statistical significant is expressed as P<0.05.
Results
Expression of surface molecules of CD34 and CD38 of KASUMI-1 cells
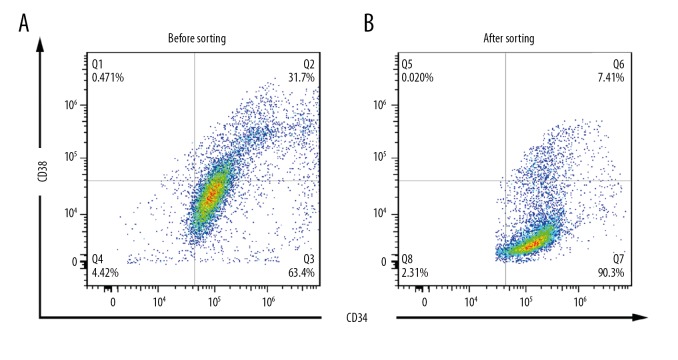
Hematopoietic stem cells and early-differentiated progenitor cells are defined as CD34+CD38−. The experimental results showed that the majority of KASUMI-1 cells were CD34+CD38−, but there were still other antigenic phenotype cells (Figure 1A). After sorting by flow cytometry, higher purity CD34+CD38− cells were obtained (Figure 1B). CD34+CD38− cells were considered to be hematopoietic stem cells and early-differentiated progenitor cells. The cells used in this experiment were all sorted KASUMI-1 cells with CD34+CD38−.
Figure 1.
Flow CD34+CD38− KASUMI-1 cells were sorted by flow cytometry. (A) Only 63.4% of KASUMI-1 cells were CD34+CD38− phenotype before sorting. (B) After selection, 90.3% of KASUMI-1 cells had CD34+CD38− phenotype.
Transfection of the siLRG1 plasmid reduced the expression level of LRG1
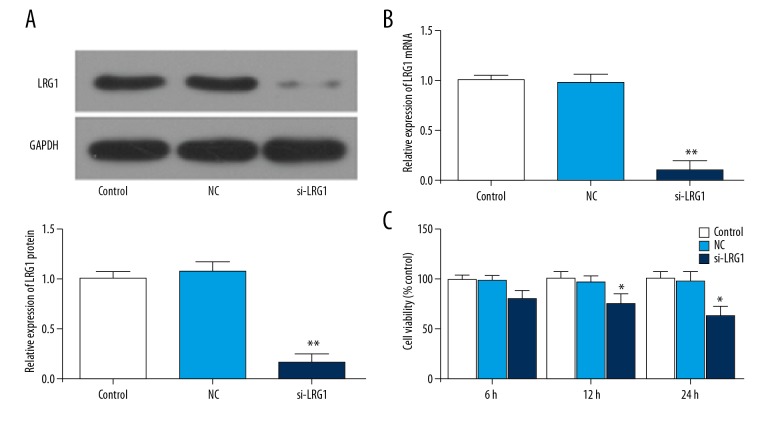
To study the transfection efficiency of siLRG1 plasmids, RT-qPCR and Western blot were applied to study transfection efficiency. The results showed that siLRG1 mRNA and protein expression levels decreased significantly after transfection (P<0.01) (Figure 2A, 2B). This shows that our plasmid construction and transfection were successful.
Figure 2.
Effects of siLRG1 plasmid on the expression of LRG1 and cell viability in KASUMI-1 cells. KASUMI-1 cells were transfected with PBS (control group), negative-siRNA plasmid (NC group), siLRG1 plasmid (siLRG1 group). (A) Western blot was used to assess the protein level of LRG1 in different gruops. (B) RT-qPCR was performed to test the mRNA level of LRG1 in different gruops. (C) The cell viability was analyzed by CCK-8 analysis. * P<0.05, ** P<0.01, versus control group.
Transfection of the siLRG1 plasmid reduced KASUMI-1 cell viability
Cell viability was tested by using the CCK-8 assay. After 12 h and 24 h of transfection, the viability of KASUMI-1 cells was decreased (P<0.05) (Figure 2C). Therefore, we speculated that silencing of LRG1 gene can inhibit the growth of KASUMI-1 cells.
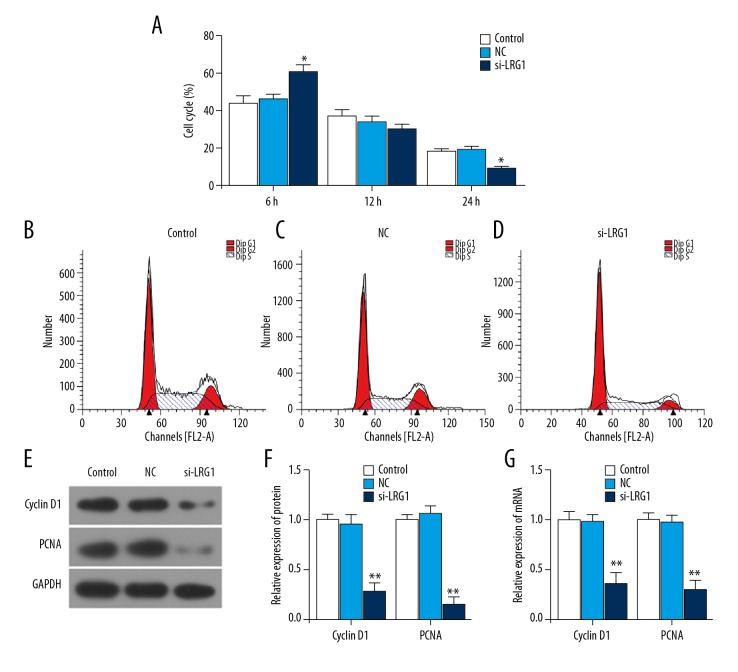
LRG1 gene silencing induced cell arrest at G0/G1 and downregulated the expression of Cyclin D1 and PCNA proteins
To investigate how LRG1 gene silencing affects cell viability, the cell cycle was detected by flow cytometry. Compared with the control group and the NC group, the number of cells in the G0/G1 phase was increased in the siLRG1 group, while the number of cells in G2/M phase was decreased (P<0.05) (Figure 3A–3D). This indicates that downregulation of LRG1 gene expression can induce cell arrest in the G0/G1 phase and reduce cell viability. To investigate the cause of cell blocking in G0/G1, Western blot analysis and RT-qPCR were applied to assess the expression of cell cycle-related proteins Cyclin D1 and PCNA. The results showed that LRG1 gene silencing significantly downregulated the expression of Cyclin D1 and PCNA genes and proteins (P<0.01) (Figure 3E–3G). This suggests that after silencing the LRG1 gene, KASUMI-1 cells were arrested in G0/G1 phase by downregulating the Cyclin D1 and PCNA expression levels.
Figure 3.
Effect of siLRG1 plasmid transfection on cell cycle. (A–D) Flow cytometry was applied to detect the cell cycle of each group after transfection. (E, F) Western blot was used to assess the protein level of Cyclin D1 and PCNA in different gruops. (G) RT-qPCR was used to detect mRNA of M Cyclin D1 and PCNA. * P<0.05, ** P<0.01, versus control group.
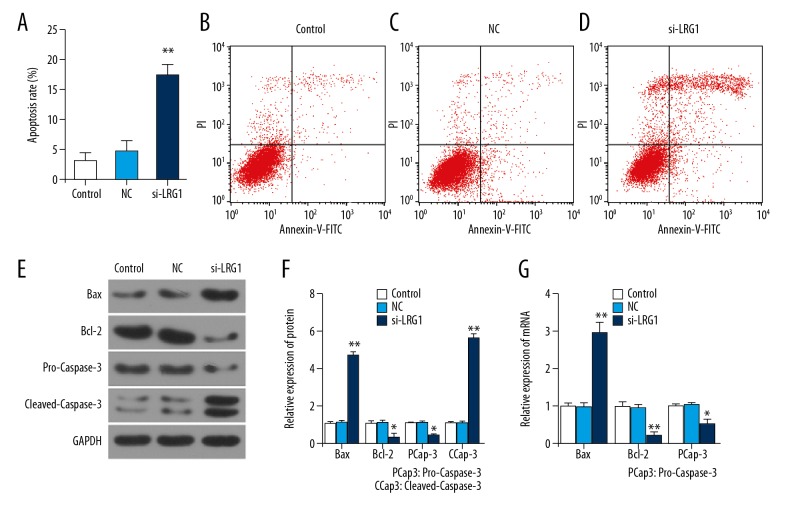
Silencing of LRG1 gene promoted apoptosis, promoted Bax and Cleaved-Caspase-3, and inhibited Bcl-2 and Pro-Caspase-3 expression
To fully explore how LRG1 downregulation affects cell viability, flow cytometry was used to detect apoptosis. There was no difference between the NC group and the control group. The proportion of apoptosis in the siLRG1 group was higher than in the NC group (P<0.01) (Figure 4A–4D). To further explore the causes of apoptosis, were detected Bax, Cleaved-Caspase-3, Bcl-2, and Pro-Caspase-3 mRNAs and proteins by Western blot analysis and RT-qPCR, respectively. The results showed that Bax and Cleaved-Caspase-3 in the siLRG1 group were higher than in the NC group, but Bcl-2 and Pro-Caspase-3 were lower (P<0.05) (Figure 4E–4G). This suggests that silencing of the LRG1 gene promoted KASUMI-1 cell apoptosis by upregulating pro-apoptotic proteins and downregulating anti-apoptotic proteins.
Figure 4.
Effect of siLRG1 plasmid transfection on cell apoptosis. (A–D) Flow cytometry was applied to detect the cell apoptosis of each group after transfection. (E, F) Western blot was used to assess the protein level of Bcl-2, Bax, pro-Caspase-3 and cleaved-Caspase-3 in different gruops. (G) RT-qPCR was used to detect mRNA of Bcl-2, Bax, pro-Caspase-3 and cleaved-Caspase-3. * P<0.05, ** P<0.01, versus control group.
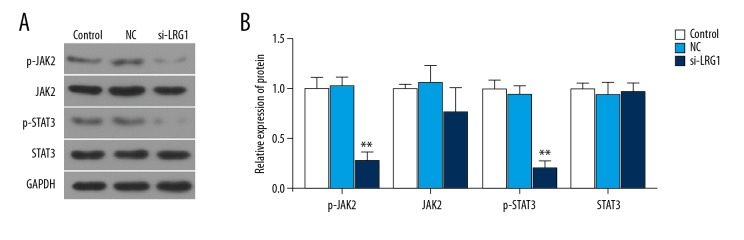
Downregulation of LRG1 inhibited JAK-STAT pathway
To further investigate downregulation of the LRG1 gene on molecular substrates affecting cyclins and apoptosis-related proteins, Western blot analysis was used to detect JAK-STAT pathway-related proteins. The results showed that the expression of JAK2 and STAT3 protein in the siLRG group cells was not changed among groups (P>0.05), but the phosphorylation levels of JAK2 and STAT3 were significantly downregulated when LRG1 was silenced (P<0.05) (Figure 5A, 5B). This result shows that downregulation of the LRG1 gene inhibits signal transduction of the JAK-STAT pathway.
Figure 5.
Effect of siLRG1 plasmid transfection on JAK-STAT pathway. (A, B) Western blot was used to assess the protein level of JAK-2, p-JAK-2, STAT3 and p-STAT3 in different gruops. * P<0.05, ** P<0.01, versus control group.
Discussion
LRG was first isolated from human serum by Haupt and Baundner in 1977 and the amino acid sequence was determined in 1985 [17]. LRRs often play an important role in cell adhesion, survival, and signal transduction, as well as in gene repair, recombination, transcription, and other processes [18]. Studies have suggested that LRG-1 can promote angiogenesis, especially abnormal blood vessel growth [7]. Previous studies have suggested that overexpression of LRG1 can increase the ability of tumor cells to invade and metastasize, and that the downregulation of LRG1 expression levels may occur via regulation of extracellular matrix and epithelial-mesenchymal transition [19]. We also found that LRG1 has a relationship with cell proliferation. To improve the stringency of the experiment and the credibility of the results, we used flow cytometry to define the KASUMI-1 leukemia cells of CD34+CD39−. For leukemic cell lines, the proliferation or apoptosis response should be studied. The results of this study showed that the cell viability of KASUMI-1 cells decreased when LRG1 was silenced. This suggests that LRG gene silencing has the effect of inhibiting KASUMI-1 cell viability.
Although overexpression of LRG1 has been demonstrated in a variety of tumor cells, its molecular mechanism is still unclear. To further investigate how LRG silencing downregulates cell viability, we found through flow cytometry that downregulation of LRG1 gene expression results in cell cycle arrest in G0/G1 phase and induction of apoptosis. This shows that silencing the LRG1 gene causes KASUMI-1 cells to stay in the G0/G1 phase and promote apoptosis. Subsequent experiments also confirmed a significant downregulation of Cyclin D1 and PCNA levels after transfection of the siLRG1 plasmid. Cyclin D1 is one of the most important proteins regulating the cell cycle. It can bind to and activate the unique cyclin-dependent kinase CDK4 during G1 to promote cell cycle progression from G1 to S, which promotes cell proliferation [20]. Cyclin D1 is involved in the immortalization of tumor cells [21]. PCNA is involved in cellular DNA synthesis. PCNA is not significantly expressed in G0–G1 phase cells, but in the late G1 phase, its expression increased significantly, while in S phase it reached a peak [22]. PCNA is a sensitive indicator of cell cycle response [22]. This suggests that after downregulating siLRG1 expression, KASUMI-1 cells were arrested at G0/G1 by downregulating Cyclin D1 levels, and the response was decreased in PCNA levels. Caspase is the main executive protein of apoptosis, and Caspase-3 is the most important member of the Caspase family [23]; its activity is regulated by Bcl-2 and Bax [24]. Various studies have shown that the inhibition of apoptosis in tumor cells is related to Bcl-2 and Bax [25,26], and it can also be used as a predictor of prognosis in patients with tumors [27,28]. The results of this study indicate that after downregulation of the LRG1 gene, pro-Caspase-3 can be hydrolyzed to cleaved-Caspase-3 by downregulating Bcl-2 and upregulating Bax levels, thereby promoting apoptosis.
To further study the mechanism of action of the LRG1 gene on leukemia cell cycle and apoptosis, we investigated the JAK-STAT pathway. Upregulation of the JAK-STAT pathway is prevalent in tumors and occurs through a process of activation of JAK by cytokines and receptors, which makes it close to STAT and tyrosine phosphorylation of STATs. The STATs are separated from the receptors to form dimers that translocate to the nucleus to identify the target sequence and regulate gene expression [13,14]. There is a close relationship between STAT3 and hematological tumors in the STAT family [29]. The results of the present study show that downregulation of the LRG1 gene can significantly inhibit the phosphorylation of JAK and STAT. This suggests that LRG1 mainly acts on the signal transduction upstream of the JAK-STAT pathway. By decreasing the phosphorylation level of JAK, the level of STAT phosphorylation is decreased, thereby inhibiting gene expression and inhibiting cell proliferation. Low activated STAT level can also promote cell apoptosis by inhibiting the level of survivin, Bcl-2, and other anti-apoptotic proteins [30–32].
Conclusions
In conclusion, silencing the LRG1 gene blocks KASUMI-1 cells in G0/G1 and promotes apoptosis, which may be due to the downregulation of LRG1 gene expression and inhibiting signal transduction of the JAK/STAT pathway, thereby regulating cyclins and cell damage. The relationship between death-associated proteins suggests that the LRG1 gene may become a new target for the treatment of AML.
Footnotes
Source of support: Project 1: Exploring the Therapeutic Mechanism of Small Dose Rituximab Conjuncted with Prednisone on Adult Warm-reactive Autoimmune Hemolytic Anemia (AIHA) Contract No.: Guizhou Science Cooperation No. LH[2016]7185. Project 2: Clinical Research on Dickkopf-1and Myeloid Leukemia TZJF-2010-099 Molecular Mechanism of NF-κB Signaling Pathway and Acute Lymphoblastic Leukemia Glucocorticoid Resistance Funder name: Guizhou Science Cooperation Grant No. SY[2011]3050
References
- 1.Ofran Y, Rowe JM. Acute myeloid leukemia in adolescents and young adults: Challenging aspects. Acta Haematol. 2014;132(3–4):292–97. doi: 10.1159/000360200. [DOI] [PubMed] [Google Scholar]
- 2.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008. Blood. 2013;121(15):2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juliusson G, Lazarevic V, Horstedt AS, et al. Acute myeloid leukemia in the real world: Why population-based registries are needed. Blood. 2012;119(17):3890–99. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haupt H, Baudner S. [Isolation and characterization of an unknown, leucine-rich 3.1-S-alpha2-glycoprotein from human serum (author’s transl)]. Hoppe Seylers Z Physiol Chem. 1977;358(6):639–46. [in German] [PubMed] [Google Scholar]
- 5.Serada S, Fujimoto M, Terabe F, et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis. 2012;18(11):2169–79. doi: 10.1002/ibd.22936. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz A, Alagozlu H, Ozdemir O, Arici S. Effects of the chemokine receptor 5 (CCR5)-Delta32 mutation on hepatitis C virus-specific immune responses and liver tissue pathology in HCV infected patients. Hepat Mon. 2014;14(7):e11283. doi: 10.5812/hepatmon.11283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Abraham S, McKenzie JAG, et al. LRG1 promotes angiogenesis by modulating endothelial TGF-beta signalling. Nature. 2013;499(7458):306–11. doi: 10.1038/nature12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladd JJBT, Johnson MM, Zhang Q. Increased plasma levels of the APC-interacting protein MAPRE1, LRG1 and IGFBP2 preceding a diagnosis of colorectal cancer in women. Cancer Prev Res (Phila) 2012;5(4):9. doi: 10.1158/1940-6207.CAPR-11-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Xie X, Nie S, et al. Altered expression of sialylated glycoproteins in ovarian cancer sera using lectin-based ELISA assay and quantitative glycoproteomics analysis. J Proteome Res. 2013;12(7):3342–52. doi: 10.1021/pr400169n. [DOI] [PubMed] [Google Scholar]
- 10.Wu RS, Yu CS, Liu KC, et al. Citosol (thiamylal sodium) triggers apoptosis and affects gene expressions of murine leukemia RAW 264.7 cells. Hum Exp Toxicol. 2012;31(8):771–79. doi: 10.1177/0960327111429137. [DOI] [PubMed] [Google Scholar]
- 11.Wang CH, Li M, Liu LL, et al. LRG1 expression indicates unfavorable clinical outcome in hepatocellular carcinoma. Oncotarget. 2015;6(39):42118–29. doi: 10.18632/oncotarget.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong D, Zhao S, He G, et al. Stable knockdown of LRG1 by RNA interference inhibits growth and promotes apoptosis of glioblastoma cells in vitro and in vivo. Tumour Biol. 2015;36(6):4271–78. doi: 10.1007/s13277-015-3065-3. [DOI] [PubMed] [Google Scholar]
- 13.Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32(21):2601–13. doi: 10.1038/onc.2012.347. [DOI] [PubMed] [Google Scholar]
- 14.Vera J, Rateitschak K, Lange F, et al. Systems biology of JAK-STAT signalling in human malignancies. Prog Biophys Mol Biol. 2011;106(2):426–34. doi: 10.1016/j.pbiomolbio.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Tibes R, Bogenberger JM, Geyer HL, Mesa RA. JAK2 inhibitors in the treatment of myeloproliferative neoplasms. Expert Opin Investig Drugs. 2012;21(12):1755–74. doi: 10.1517/13543784.2012.721352. [DOI] [PubMed] [Google Scholar]
- 16.Yan S, Li Z, Thiele CJ. Inhibition of STAT3 with orally active JAK inhibitor, AZD1480, decreases tumor growth in neuroblastoma and pediatric sarcomas in vitro and in vivo. Oncotarget. 2013;4(3):433–45. doi: 10.18632/oncotarget.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng AC, Eisenberg JM, Heath RJ, et al. Human leucine-rich repeat proteins: A genome-wide bioinformatic categorization and functional analysis in innate immunity. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4631–38. doi: 10.1073/pnas.1000093107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobe B, Deisenhofer J. Proteins with leucine-rich repeats. Curr Opin Struct Biol. 1995;5(3):409–16. doi: 10.1016/0959-440x(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhong D, He G, Zhao S, et al. LRG1 modulates invasion and migration of glioma cell lines through TGF-beta signaling pathway. Acta Histochem. 2015;117(6):551–58. doi: 10.1016/j.acthis.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Baldin V, Lukas J, Marcote MJ, et al. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7(5):812–21. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa H, Zukerberg L, Togawa K, et al. Human cyclin D1 oncogene and esophageal squamous cell carcinoma. Cancer. 1995;76(4):541–49. doi: 10.1002/1097-0142(19950815)76:4<541::aid-cncr2820760402>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.Strzalka W, Ziemienowicz A. Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Ann Bot. 2011;107(7):1127–40. doi: 10.1093/aob/mcq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–76. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 24.Khemtemourian L, Sani MA, Bathany K, et al. Synthesis and secondary structure in membranes of the Bcl-2 anti-apoptotic domain BH4. J Pept Sci. 2006;12(1):58–64. doi: 10.1002/psc.686. [DOI] [PubMed] [Google Scholar]
- 25.Ong YL, McMullin MF, Bailie KE, et al. High bax expression is a good prognostic indicator in acute myeloid leukaemia. Br J Haematol. 2000;111(1):182–89. doi: 10.1046/j.1365-2141.2000.02315.x. [DOI] [PubMed] [Google Scholar]
- 26.Reed JC, Miyashita T, Takayama S, et al. BCL-2 family proteins: Regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996;60(1):23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Kohler T, Schill C, Deininger MW, et al. High Bad and Bax mRNA expression correlate with negative outcome in acute myeloid leukemia (AML) Leukemia. 2002;16(1):22–29. doi: 10.1038/sj.leu.2402340. [DOI] [PubMed] [Google Scholar]
- 28.Gil J, Ramsey D, Szmida E, et al. The BAX gene as a candidate for negative autophagy-related genes regulator on mRNA levels in colorectal cancer. Med Oncol. 2017;34(2):16. doi: 10.1007/s12032-016-0869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuda T, Nakamura T, Nakao K, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18(15):4261–69. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauane M, Gopalkrishnan RV, Lebedeva I, et al. Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. J Cell Physiol. 2003;196(2):334–45. doi: 10.1002/jcp.10309. [DOI] [PubMed] [Google Scholar]
- 31.Dalgic CT, Kaymaz BT, Ozkan MC, et al. Investigating the Role of JAK/STAT Pathway on Dasatinib-Induced Apoptosis for CML Cell Model K562. Clin Lymphoma Myeloma Leuk. 2015;15(Suppl):S161–66. doi: 10.1016/j.clml.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Selvi N, Kaymaz BT, Gunduz C, et al. Bortezomib induces apoptosis by interacting with JAK/STAT pathway in K562 leukemic cells. Tumour Biol. 2014;35(8):7861–70. doi: 10.1007/s13277-014-2048-0. [DOI] [PubMed] [Google Scholar]