Abstract
A previous study implied that long intergenic non-coding RNA 1410 (LINC01410) promotes angiogenesis and metastasis of gastric cancer. However, the role of LINC01410 in colon cancer (CC) has remained elusive. In the present study, LINC01410 was identified to be highly expressed in CC tissues compared to adjacent normal tissues. It was indicated that high expression of LINC01410 in CC tissues was associated with poor prognosis. Further functional study suggested that LINC01410 knockdown significantly reduced the proliferation and invasive capacity of HT-29 and SW620 cells, and inhibited the cell cycle. Regarding the mechanism, LINC01410 was indicated to serve as a sponge for microRNA (miR)-3128, as evidenced by a luciferase reporter assay. Furthermore, knockdown of LINC01410 significantly increased the levels of miR-3128. In addition, miR-3128 was markedly downregulated in CC tissues compared with that in adjacent normal tissues. A rescue assay revealed that inhibition of miR-3128 significantly abrogated the effects of LINC01410 knockdown on CC cell proliferation and invasion. In conclusion, the present study demonstrated that LINC01410 functions as an oncogene in CC, at least in part by directly inhibiting miR-3128.
Keywords: long intergenic non-coding RNA 1410, colon cancer, proliferation, invasion, microRNA-3128
Introduction
Colon cancer (CC) is the third most common cancer type and the fourth leading cause of cancer-associated mortality worldwide (1). Although numerous advances have been made regarding the diagnosis and therapeutic treatment of CC in recent years, the rate of recurrence or death within five years has not improved (2). The 5-year survival rate of CC patients remains unsatisfactory (3). Liver or lung metastasis is frequent in CC patients, and is one of the major causes of CC-associated mortality (4). Hence, it is necessary to elucidate the underlying mechanisms of CC development and metastasis, with the aim of developing novel biomarkers and therapeutic targets for CC treatment in the clinic.
Long non-coding RNAs (lncRNAs) belong to the family of ncRNA, accounting for >80% of human genomic transcripts (5). LncRNAs have a length of >200 nucleotides and do not encode any protein (6). Accumulating evidence suggests that lncRNAs possess key functions in multiple biological processes, including cell development, migration and propagation (7,8). A growing number of studies prove that certain lncRNAs may serve as potential diagnostic or prognostic biomarkers (9). Various lncRNAs are aberrantly expressed in cancer and their levels are correlated with the clinicopathological characteristics of the patients. For instance, downregulation of XLOC_010588 has been reported to restrain CC progression (10). Furthermore, lncRNA UICLM increases CC metastasis via the sequester of microRNA (miR)-215 to promote Zinc finger E-Box binding homeobox 2 expression (11). However, most lncRNAs remain to be identified and functionally validated.
A previous study indicated that long intergenic ncRNA 1410 (LINC01410) enhances gastric cancer metastasis (12). However, it has remained elusive whether LINC01410 exerts any role in CC. In the present study, it was demonstrated that LINC01410 is upregulated in CC tissues compared with that in adjacent normal tissues. Furthermore, high expression of LINC01410 in CC tissues was associated with poor prognosis. Mechanistically, it was demonstrated that LINC01410 sequesters miR-3128. Through rescue assays, it was further revealed that inhibition of miR-3128 abrogated the inhibitory effect of LINC01410 silencing on the proliferation and invasion of CC cell lines. Taken together, the present study illustrated that LINC01410 serves as an oncogene in CC by sponging miR-3128, suggesting LINC01410 may be a promising therapeutic target.
Materials and methods
Clinical specimens and cell lines
The NCM460 colon epithelial cell line and a panel of CC cell lines (HT-29, HCT116, SW620 and LoVo) were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China). NCM460 was maintained in McCoy's 5a medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). CC cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Hyclone; GE, Healthcare, Little Chalfont, UK) containing 10% FBS. The 53 CC and adjacent normal tissues (obtained 2 cm away from the lesions) were collected from Chenzhou No. 1 People's Hospital (Chenzhou, China). None of the patients received any chemotherapy or radiotherapy treatment prior to sample collection. The study was approved by the Institutional Research Ethics Committee of Chenzhou No. 1 People's Hospital (Chenzhou, China) and written informed consent was obtained from each subject.
Cell transfection
miR-3128 mimics (5′-UCUGGCAAGUAAAAAACUCUCAU-3′), miR-control (5′-ACAUCUGCGUAAGAUUCGAGUCUA-3′), miR-3128 inhibitors (5′-AUGAGAGUUUUUUACUUGCCAGA-3′) and control inhibitors (5′-GCGUAACUAAUACAUCGGAUUCGU-3′) were purchased from GenePharma (Shanghai, China). CC cells were transfected with 100 nM miR-3128 mimics, miR-control, miR-3128 inhibitors or control inhibitors using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols. For LINC01410 knockdown, small interfering RNA (5′-GCAGCACCAUAUGAGAAGU-3′) oligonucleotides targeting LINC01410 and a scrambled oligo as a negative control were purchased from GenePharma. At 24 h after transfection, the cells were collected and the depletion efficiency was validated through reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis.
Cell cycle analysis
A total of 1×106 CC cells were fixed with 70% ethanol overnight at 4°C, collected by centrifugation and stained with propidium iodide solution (50 mg/ml in PBS) supplemented with 0.25 mg/ml RNase A (Sigma-Aldrich; Merck KGaA; Darmstadt, Germany). After incubation for 15 min at 4°C, the cells were analyzed by flow cytometry (FACS Canto II; BD Biosciences, Franklin Lakes, NJ, USA).
Cell proliferation assay
A Cell Counting Kit (CCK)-8 (Dojindo, Kumamoto, Japan) was utilized to assess cell proliferation according to the manufacturer's protocols. In brief, 2×103 CC cells were seeded onto 96-well plates, cultured for 24, 48 or 72 h and stained with 10 µl CCK-8 reagent. After incubation for 1 h at 37°C, the absorbance at 450 nm was determined using a SUNRISE Microplate Reader (Tecan, Maennedorf, Switzerland).
Transwell invasion assay
Matrigel® (BD Biosciences) pre-coated invasion chambers (8 µm; PSET010R5; EMD Millipore, Billerica, MA, USA) were used for the Transwell invasion assay. A total of 4×104 CC cells were seeded into the upper chamber with serum-free DMEM. The lower chamber was supplemented with 500 µl DMEM containing 10% FBS. After incubation for 24 h, the cells that had invaded to the lower side of the membrane were fixed with 4% paraformaldehyde for 30 min at 25°C and stained with 0.1% crystal violet for 30 min at 25°C.
RT-qPCR
Total RNA was extracted from 1×107 cultured cells or tumor tissues using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Subsequently, 2 µg of total RNA was reverse-transcribed with random primers (Invitrogen; Thermo Fisher Scientific Inc.) and SuperScript IV Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific Inc.) according to the manufacturer's protocols. The reaction was performed with incubation at 42°C for 1 h, and the enzyme was subsequently inactivated by incubation at 85°C for 5 min. The complementary DNA then amplified by real-time PCR with gene-specific primers and the Fast SYBR Green Master Mix (Applied Biosystems; Thermo Fisher Scientific Inc.). The thermocycling conditions were as follows: Denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 sec and elongation at 60°C for 1 min. The expression of lncRNA or miR-3128 was normalized to that of U6 and relative gene expression levels were calculated using the 2−∆∆Cq method (13). The primer sequences utilized were as follows: LINC01410 forward, 5′-GTGACAAGAATGGCCCAAGC-3′ and reverse, 5′-ACTGTGCACCTGTTACACCA-3′; miR-3128 forward, 5′-AACGAGACGACGACAGAC-3′ and reverse, 5′-TCTGGCAAGTAAAAAACTCTCAT-3′; U6 forward, 5′-AACGAGACGACGACAGAC-3′ and reverse, 5′-GCAAATTCGTGAAGCGTTCCATA-3′.
Luciferase reporter assay
miR-3128 was predicted as a target of LINC01410 using an online tool (http://mirdb.org/miRDB/index.html). The LINC01410 sequence containing the putative wild-type or mutant binding site of miR3128 was constructed into the pRL-CMV Renilla luciferase reporter plasmid (Promega Corporation, Madison, WI, USA). miR-3128 mimics or negative miR-control, and pRL-CMV Renilla luciferase reporter [wild-type (WT)-LINC01410 or Mutant-LINC01410] was co-transfected into CC cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h, luciferase activity was measured by using a luciferase assay kit (Promega Corporation). Firefly luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 5 (GraphPad Inc., La Jolla, CA, USA). Student's t-test and one-way analysis of variance followed by Tukey's post-hoc test were used to analyze differences between 2 or multiple groups, respectively, for statistical significance. Pearson's correlation analysis was used to determine the correlations. The Kaplan-Meier method was used to draw survival curves and the log-rank test was used to determine statistical significance. P<0.05 was considered to indicate a statistically significant difference.
Results
LINC01410 is upregulated in CC tissues
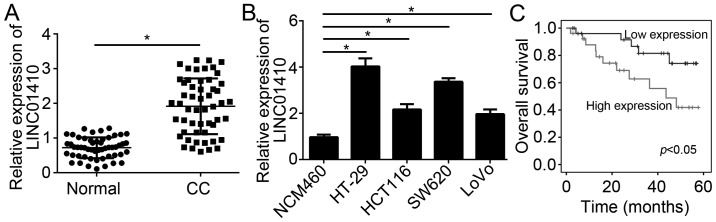
First, the expression patterns of LINC01410 in tissues from CC patients and in CC cell lines were assessed by RT-qPCR. The results indicated that LINC01410 expression was significantly upregulated in CC tissues compared with that in adjacent normal tissues (Fig. 1A). Furthermore, the expression of LINC01410 was also markedly upregulated in CC cell lines (HT-29, HCT116, SW620 and LoVo cells) compared with that in the NCM460 normal colon epithelial cells (Fig. 1B). To determine whether LINC01410 may serve as a biomarker for the prognosis of CC patients, a Kaplan-Meier curve analysis was performed with CC patients stratified into a LINC01410 low expression group (n=29) and a LINC01410 high expression group (n=26; median value of LINC01410 was used as a cutoff). The results indicated that high expression of LINC01410 in CC patients was linked with poor prognosis (Fig. 1C) The association between LINC01410 expression and clinical characteristics of the CC patients is presented in Table I. As presented, LINC01410 expression was positively associated with advanced stage and lymph node metastasis. Taken together, these results indicate that LINC01410 may be involved in CC progression.
Figure 1.
LINC01410 is upregulated in CC tissues. (A) Relative expression of LINC01410 in CC tissues and adjacent normal tissues determined by reverse transcription-quantitative polymerase chain reaction analysis. (B) Relative expression of LINC01410 in CC cell lines and a healthy reference cell line. (C) High expression of LINC01410 in CC patients was associated poor survival according to Kaplan-Meier analysis. *P<0.05 vs. the control group. LINC01410, long intergenic non-coding RNA 1410; CC, colon cancer.
Table I.
Differences in the distribution of colon cancer patients (n=53) with high/low expression of long intergenic non-coding RNA 1410 regarding various clinicopathological characteristics.
| Clinicopathologic parameters | Low (n=27) (%) | High (n=26) (%) | P-value |
|---|---|---|---|
| Age (years) | 0.414 | ||
| ≤65 | 16 (30.2) | 12 (22.6) | |
| >65 | 11 (20.8) | 14 (26.4) | |
| Sex | 0.275 | ||
| Male | 17 (32.1) | 12 (22.6) | |
| Female | 10 (18.9) | 14 (26.4) | |
| Tumor size (cm) | 0.785 | ||
| ≤5 | 14 (26.4) | 15 (28.3) | |
| >5 | 13 (24.5) | 11 (20.8) | |
| TNM stage | 0.028 | ||
| I/II | 19 (35.8) | 10 (18.9) | |
| III/IV | 8 (15.1) | 16 (30.2) | |
| Lymph node metastasis | 0.008 | ||
| No | 23 (43.4) | 13 (24.5) | |
| Yes | 4 (7.6) | 13 (24.5) |
P-values were calculated using the Chi-square test. TNM, tumor-nodes-metastasis.
LINC01410 knockdown inhibits CC cell proliferation and invasion
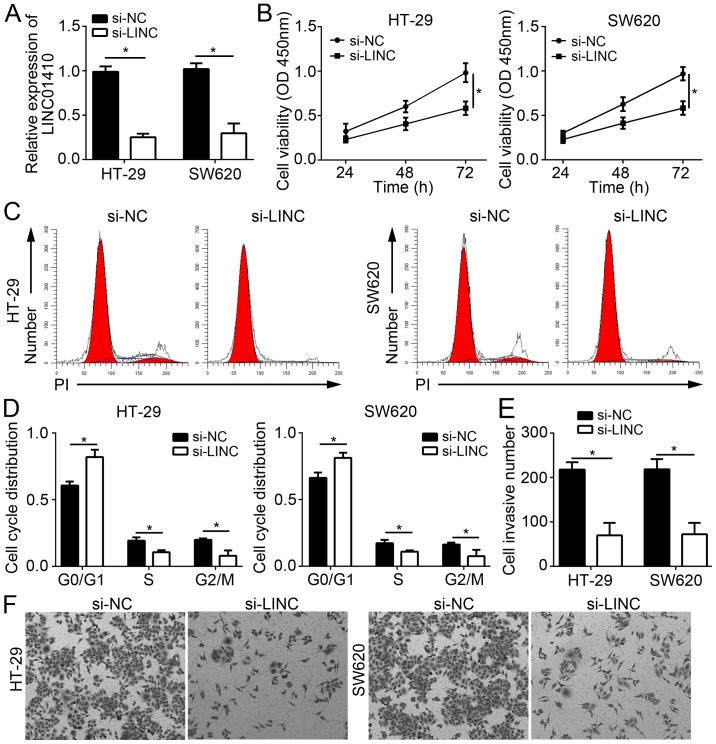
To determine the function of LINC01410 in CC, the HT-29 and SW620 cell lines were selected for the subsequent experiments. LINC01410 was knocked down in HT-29 and SW620 cells by transfection with si-LINC01410 (Fig. 2A). CCK8 assays were then performed, the results of which demonstrated that LINC01410 knockdown significantly inhibited the proliferation of HT-29 and SW620 cells (Fig. 2B). Cell cycle progression is a major factor for cell proliferation. Therefore, the effect of LINC01410 on cell cycle progression in CC cells was examined. It was demonstrated that LINC01410 knockdown significantly reduced the cell population in S and G2/M phase and increased that in G0/G1 phase (Fig. 2C and D). Tumor metastasis is frequently associated with recurrence and increases in the malignant behavior, as well as the progression of CC. Hence, the role of LINC01410 in CC cell invasion, a process linked with metastasis, was next evaluated. Transwell assays indicated that knockdown of LINC01410 significantly suppressed cell invasion (Fig. 2E and F). In conclusion, the present results demonstrated that LINC01410 has as oncogenic role and may be involved in the progression of CC.
Figure 2.
LINC01410 knockdown inhibits CC cell proliferation and invasion. (A) Relative expression of LINC01410 in HT-29 and SW620 cells transfected with si-NC or si-LINC. (B) Cell Counting Kit-8 assays were used to detect the proliferation of HT-29 and SW620 cells transfected with si-NC or si-LINC. (C and D) The cell cycle distribution of HT-29 and SW620 cells transfected with si-NC or si-LINC was determined by fluorescence-assisted cell sorting. (E and F) The invasive capacity of HT-29 and SW620 cells transfected with si-NC or si-LINC was examined by Transwell assays (magnification, ×100). *P<0.05 vs. the indicated group. LINC01410, long intergenic non-coding RNA 1410; CC, colon cancer; si-LINC, small interfering RNA targeting LINC01410; si-NC, scrambled control siRNA; OD, optical density.
LINC01410 serves as a sponge for miR-3128
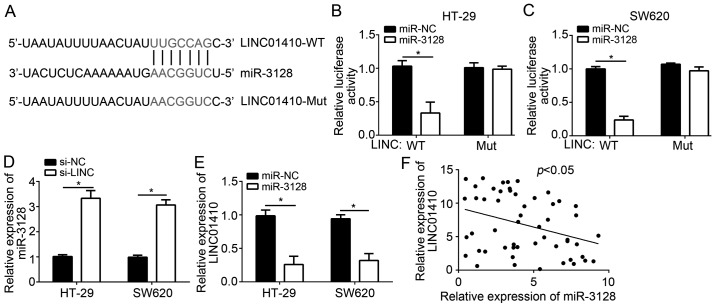
lncRNAs have been reported to act as sponges for miRNAs. To investigate the molecular mechanisms by which LINC01410 exerts its oncogenic role in CC cells, target miRNAs of LINC01410 were predicted by a bioinformatics analysis. miR-3128 ranked at the top among all candidate miRs, and a potential binding site for miR-3128 was identified in the 3′-UTR of LINC01410 (Fig. 3A). To confirm this prediction, a luciferase reporter assay was performed. The results illustrated that overexpression of miR-3128 significantly inhibited the luciferase activity of WT-LINC01410 in HT-29 and SW620 cells (Fig. 3B and C), whereas mutation of the binding site in LINC01410 abolished this effect. Furthermore, knockdown of LINC01410 significantly enhanced the levels of miR-3128 (Fig. 3D). Of note, an inverse correlation between LINC01410 and miR-3128 was observed in CC tissues (Fig. 3E). Taken together, these results demonstrated that LINC01410 serves as a sponge for miR-3128 in CC cells.
Figure 3.
LINC01410 serves as a sponge for miR-3128. (A) The binding site of miR-3128 in the 3′-UTR of LINC01410 was determined using an online bioinformatics tool (http://mirdb.org/miRDB/index.html). (B and C) A luciferase reporter assay was used to confirm the direct interaction between LINC01410 and miR-3128 in HT-29 and SW620 cells. (D) Knockdown of LINC01410 increased the levels of miR-3128 in HT-29 and SW620 cells. (E and F) Inverse correlation between miR-3128 and LINC01410 in colon cancer tissues determined by reverse transcription-quantitative polymerase chain reaction analysis. *P<0.05 vs. the control group. LINC01410, long intergenic non-coding RNA 1410; miR, microRNA; WT, wild-type; Mut, mutated; si-LINC, small interfering RNA targeting LINC01410; si-NC, scrambled control siRNA.
Inhibition of miR-3128 rescues the effects of LINC01410 knockdown
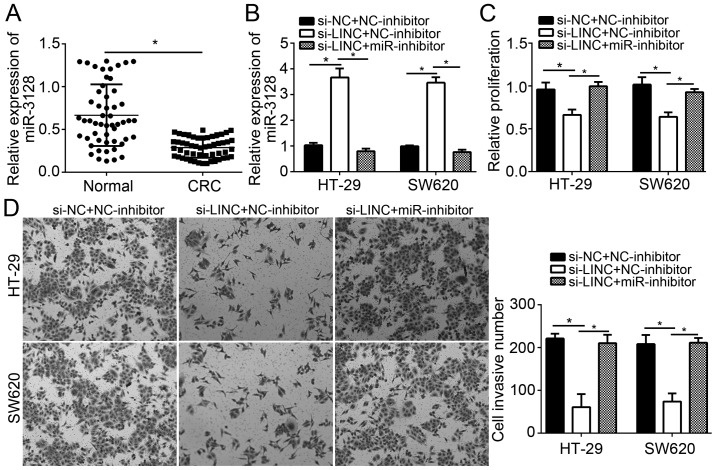
To the best of our knowledge, the present study was the first to explore the role of miR-3128 in CC. To determine the role of miR-3128 and whether LINC01410 exerts its oncogenic effects in CC via sponging miR-3128, the expression of miR-3128 in CC tissues was first examined by RT-qPCR. The results indicated that miR-3128 expression was significantly downregulated in CC tissues (Fig. 4A), which suggested that decreases in miR-3128 may be involved in CC progression. Subsequently, rescue assays via restoration of miR-3128 expression in LINC01410-depleted CC cells were performed. HT-29 and SW480 cells were transduced with si-LINC01410 alone or in combination with miR-3128 inhibitors. It was revealed that miR-3128 levels were increased in LINC01410-depleted HT-29 and SW620 cells, and that this was effectively abrogated by co-transfection with miR-3128 inhibitors (Fig. 4B). The cells were then subjected to CCK8 and Transwell assays, the results of which indicated that LINC01410 knockdown significantly inhibited CC cell proliferation and invasion, while simultaneous inhibition of miR-3128 abrogated the inhibitory effects of LINC01410 knockdown on cell proliferation and invasion (Fig. 4C and D). Taken together, these results suggested that LINC01410 promotes CC progression via sponging miR-3128.
Figure 4.
Inhibition of miR-3128 rescues the effects of LINC01410 knockdown. (A) Relative expression of miR-3128 in colon cancer tissues and adjacent normal tissues. (B) Relative expression of miR-3128 in HT-29 and SW620 cells transfected with the indicated plasmids. (C) A Cell Counting Kit-8 assay was used to determine the proliferation HT-29 and SW620 cells transfected with the indicated plasmids. (D) Transwell assays were used to assess the invasion of HT-29 and SW620 cells transfected with the indicated plasmids. *P<0.05 vs. the control group. LINC01410, long intergenic non-coding RNA 1410; miR, microRNA; si-LINC, small interfering RNA targeting LINC01410; NC, negative control/scrambled.
Discussion
An increasing number of studies have reported the roles of aberrant lncRNA expression in cancer and the correlation between lncRNAs and tumor development and progression (14,15). In the present study, it was demonstrated that LINC01410 expression was increased in CC tissues and cell lines. Knockdown of LINC01410 markedly suppressed CC cell growth, inhibited entry into the cell cycle and impaired the invasive capacity of the cells. Furthermore, miR-3128 was identified as a direct target of LINC01410 and it was demonstrated that the inhibition of proliferation and invasion by LINC01410 knockdown was abrogated by miR-3128 inhibitors. The present study demonstrated that LINC01410 is essential for CC growth and may be a potential prognostic biomarker.
Certain lncRNAs have been demonstrated to have critical roles in various human cancer types and regulate multiple biological processes. For instance, lncRNA PVT1 upregulates sex-determining region Y box (SOX)2 levels to enhance breast cancer proliferation. (16). lncRNA distal-less homeobox 6-antisense RNA 1 (AS1) contributes to renal cell carcinoma proliferation (17). lncRNA SOX21-AS1 was reported to enhance lung adenocarcinoma cell growth (18). lncRNA X inactive specific transcript (XIST) promotes bladder cancer proliferation and metastasis by sponging miR-124 (19). A recent study demonstrated that LINC01410 promotes gastric cancer angiogenesis and metastasis by miR-532/neutrophil cytosolic factor 2/nuclear factor-κB signaling (12). However, the role of LINC01410 in CC has remained largely elusive. In the present study, LINC01410 was identified as an oncogene in CC by regulating cell proliferation and invasion. CCK8 assays indicated that LINC01410 knockdown significantly inhibited CC cell growth in vitro. Cell cycle arrest is a major cause of tumor growth suppression. Of note, in the present study, it was demonstrated that LINC01410 knockdown significantly inhibited CC cell growth and made the cells resume a quiescent state in G0/G1 phase, which suggested that LINC01410 promotes CC proliferation at least partially by regulating the cell cycle. Furthermore, the Transwell assay revealed that LINC01410 knockdown markedly repressed the invasion of CC cells. These results suggested that LINC01410 contributes to CC progression and may be a promising therapeutic target for CC treatment. Although the Kaplan Meyer analysis and the association analysis in Table I have implied that LINC01410 may also serve an oncogenic role in vivo, a xenograft nude mouse experiment would be required to validate this. In addition, whether LINC01410 is involved in the regulation of the response of CC to drugs requires further exploration.
Various lncRNAs have been demonstrated to have roles in tumorigenesis and regulate the expression of associated genes by serving as a sponge of their miRs (20). For instance, lncRNA XIST controls bladder cancer tumorigenicity through sponging miR-200c (21). lncRNA homeobox A11-AS inhibits miR-124 to promote uveal melanoma progression (22). lncRNA prostate cancer-associated transcript 7 inhibits miR-134-5p to promote nasopharyngeal carcinoma growth (23). The bioinformatics analysis performed in the present study suggested that LINC01410 acts as a sponge for miR-3128. Through a luciferase reporter assay, the direct interaction between LINC01410 and miR-3128 in CC cells was validated. Furthermore, it was demonstrated that LINC01410 knockdown significantly promoted miR-3128 expression, while overexpression of miR-3128 inhibited LINC01410 expression in CC cells. To the best of our knowledge, the function of miR-3128 in cancer has not been previously investigated, and the present study was the first to indicate that miR-3128 was downregulated in CC tissues compared with that in adjacent normal tissues. In addition, inhibition of miR-3128 significantly abrogated the suppressive effects of LINC01410 knockdown on CC cell proliferation and invasion, which suggested that miR-3128 serves as a tumor suppressor in CC. However, the present study did not investigate the downstream signaling of the LINC01410/miR-3128 axis that is involved in the promotion of CC progression, which requires investigation in the future.
In conclusion, the present study was the first to demonstrate that LINC01410 promoted CC proliferation and invasion by inhibiting miR-3128. The present results suggested that LINC01410 may be a potential therapeutic target for CC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
JL and MZ initiated and designed the present study, analyzed and interpreted the results and wrote the manuscript. YG, XL, XY and FX performed various experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Regarding the use of human samples, the protocol of the present study was approved by the Institutional Ethics Committee of Chenzhou No. 1 People's Hospital (Chenzhou, China) and all patients enrolled provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Karsa LV, Lignini TA, Patnick J, Lambert R, Sauvaget C. The dimensions of the CRC problem. Best Pract Res Clin Gastroenterol. 2010;24:381–396. doi: 10.1016/j.bpg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19–27. [PMC free article] [PubMed] [Google Scholar]
- 3.Shan Z, An N, Qin J, Yang J, Sun H, Yang W. Long non-coding RNA Linc00675 suppresses cell proliferation and metastasis in colorectal cancer via acting on miR-942 and Wnt/beta-catenin signaling. Biomed Pharmacother. 2018;101:769–776. doi: 10.1016/j.biopha.2018.02.123. [DOI] [PubMed] [Google Scholar]
- 4.Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: Metastases to a single organ. World J Gastroenterol. 2015;21:11767–11776. doi: 10.3748/wjg.v21.i41.11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai KW, Lo YH, Liu H, Yeh CY, Chen YZ, Hsu CW, Chen WS, Wang JH. Linc00659, a long noncoding RNA, acts as novel oncogene in regulating cancer cell growth in colorectal cancer. Mol Cancer. 2018;17:72. doi: 10.1186/s12943-018-0821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B, Ye B, Yang L, Zhu X, Huang G, Zhu P, Du Y, Wu J, Qin X, Chen R, et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat Immunol. 2017;18:499–508. doi: 10.1038/ni.3712. [DOI] [PubMed] [Google Scholar]
- 7.Ye B, Liu B, Yang L, Zhu X, Zhang D, Wu W, Zhu P, Wang Y, Wang S, Xia P, et al. LncKdm2b controls self-renewal of embryonic stem cells via activating expression of transcription factor Zbtb3. EMBO J. 2018;37:pii: e97174. doi: 10.15252/embj.201797174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, He X, Li S, Xu X, Chen X, Zhu H. Long Non-coding RNA ucoo2kmd.1 regulates CD44-dependent cell growth by competing for miR-211-3p in colorectal cancer. PLoS One. 2016;11:e0151287. doi: 10.1371/journal.pone.0151287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saus E, Brunet-Vega A, Iraola-Guzman S, Pegueroles C, Gabaldon T, Pericay C. Long Non-coding RNAs As potential novel prognostic biomarkers in colorectal cancer. Front Genet. 2016;7:54. doi: 10.3389/fgene.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Zhao L, Zhang Y, Guan L, Zhang H, Zhou H, Gao T, Miao P, Sun M. Downregulation of the long non-coding RNA XLOC_010588 inhibits the invasion and migration of colorectal cancer. Oncol Rep. 2018;39:1619–1630. doi: 10.3892/or.2018.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen DL, Lu YX, Zhang JX, Wei XL, Wang F, Zeng ZL, Pan ZZ, Yuan YF, Wang FH, Pelicano H, et al. Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression. Theranostics. 2017;7:4836–4849. doi: 10.7150/thno.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JX, Chen ZH, Chen DL, Tian XP, Wang CY, Zhou ZW, Gao Y, Xu Y, Chen C, Zheng ZS, et al. LINC01410-miR-532-NCF2-NF-kB feedback loop promotes gastric cancer angiogenesis and metastasis. Oncogene. 2018;37:2660–2675. doi: 10.1038/s41388-018-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Li T, Xu C, Cai B, Zhang M, Gao F, Gan J. Expression and clinicopathological significance of the lncRNA HOXA11-AS in colorectal cancer. Oncol Lett. 2016;12:4155–4160. doi: 10.3892/ol.2016.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun M, Nie FQ, Wang ZX, De W. Involvement of lncRNA dysregulation in gastric cancer. Histol Histopathol. 2016;31:33–39. doi: 10.14670/HH-11-655. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Zhou J, Wang Z, Wang P, Li S. Upregulation of SOX2 activated LncRNA PVT1 expression promotes breast cancer cell growth and invasion. Biochem Biophys Res Commun. 2017;493:429–436. doi: 10.1016/j.bbrc.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Zeng X, Hu Z, Ke X, Tang H, Wu B, Wei X, Liu Z. Long noncoding RNA DLX6-AS1 promotes renal cell carcinoma progression via miR-26a/PTEN axis. Cell Cycle. 2017;16:2212–2219. doi: 10.1080/15384101.2017.1361072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X, Huang C, He X, Liu X, Ji J, Zhang E, Wang W, Guo R. A novel long Non-coding RNA, SOX21-AS1, indicates a poor prognosis and promotes lung adenocarcinoma proliferation. Cell Physiol Biochem. 2017;42:1857–1869. doi: 10.1159/000479543. [DOI] [PubMed] [Google Scholar]
- 19.Xiong Y, Wang L, Li Y, Chen M, He W, Qi L. The long Non-coding RNA XIST interacted with MiR-124 to modulate bladder cancer growth, invasion and migration by targeting androgen receptor (AR) Cell Physiol Biochem. 2017;43:405–418. doi: 10.1159/000480419. [DOI] [PubMed] [Google Scholar]
- 20.Qi H, Wen B, Wu Q, Cheng W, Lou J, Wei J, Huang J, Yao X, Weng G. Long noncoding RNA SNHG7 accelerates prostate cancer proliferation and cycle progression through cyclin D1 by sponging miR-503. Biomed Pharmacother. 2018;102:326–332. doi: 10.1016/j.biopha.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Xu R, Zhu X, Chen F, Huang C, Ai K, Wu H, Zhang L, Zhao X. LncRNA XIST/miR-200c regulates the stemness properties and tumourigenicity of human bladder cancer stem cell-like cells. Cancer Cell Int. 2018;18:41. doi: 10.1186/s12935-018-0540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Q, Zhao N, Zha G, Wang H, Tong Q, Xin S. LncRNA HOXA11-AS exerts oncogenic functions by repressing p21 and miR-124 in uveal melanoma. DNA Cell Biol. 2017;36:837–844. doi: 10.1089/dna.2017.3808. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Tao Z, Qu J, Zhou X, Zhang C. Long non-coding RNA PCAT7 regulates ELF2 signaling through inhibition of miR-134-5p in nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2017;491:374–381. doi: 10.1016/j.bbrc.2017.07.093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.