Abstract
Background
This study investigated the distribution and features of natural killer T (NKT) cells in the peripheral blood of diabetic patients, and their regulatory roles on vascular endothelial cells.
Material/Methods
Peripheral lymphocytes were isolated from diabetic patients. NKT cell distribution, proportion, and surface and intracellular markers were detected with flow cytometry. Peripheral blood-derived NKT cells were isolated and co-cultured with human umbilical vein endothelial cells (HUVECs). Proliferation and migration of HUVECs were assessed with the CCK-8 assay and the Transwell chamber assay.
Results
The ratios of CD3-CD56+ NK and CD3+CD56+ NKT cells in the peripheral blood of patients with type II diabetes were significantly elevated. The expression levels of NKp30, NKG2D, and NKp44 on the surface were increased in the CD3+CD56+ NKT cells, while the expression levels of NKG2A and 158b were significantly downregulated. The expression level of granzymes in the peripheral blood-derived NKT cells were not changed in patients with type II diabetes, but the expression levels of IFNγ and IL-4 were significantly increased. However, after co-culture with NKT cells derived from the peripheral blood of diabetic patients, the proliferation and migration of HUVECs were significantly inhibited, and was restored by treatment with IL-4 antibody. In addition, the IL-4 stimulus inhibited the proliferation and migration of HUVECs.
Conclusions
Peripheral blood NKT cells are increased and activated in diabetes. NKT cells inhibit the proliferation and migration of HUVECs by secreting IL-4, thereby inducing vascular injuries.
MeSH Keywords: Endothelial Cells; Killer Cells, Natural; Umbilical Veins
Background
Diabetes is a chronic disease that is commonly seen in clinical practice. It is a syndrome of metabolic disorders caused by the interaction between genetic and environmental factors [1,2]. Epidemiology studies have shown that diabetes has the highest incidence of any chronic disease worldwide, and is a huge threat to human health [3]. Diabetes is clinically divided into types I and II, and the incidence of type II diabetes is relatively higher [4]. Type II diabetes is mainly caused by insulin resistance and impaired islet function, which can cause glucose metabolism disorders and microvascular diseases, as well as a series of complications (such as retinopathy, diabetic feet, and diabetic nephropathy) [5,6]. Therefore, it is of great significance to investigate the diabetes-induced microvascular diseases to improve disease intervention and treatment. Microangiopathy mainly refers to the changes and dysfunctions of microvasculature, blood flow, and microvascular-related peripheral environment, caused by various factors (such as the matrix and neighboring cells) [7,8]. In clinical practice, microvascular diseases are divided into chronic and acute types. Acute microangiopathy is also known as acute systemic microangiopathy, such as shock and DIC [9]; however, the most typical diabetes-induced microangiopathy is chronic microangiopathy [10]. Numerous studies have found that endothelial cells play key roles in the pathogenesis of diabetic microangiopathy, and significant changes occur in the cell structure and function [11]. In diabetic retinopathy and glomerular lesions, vascular endothelial cells are damaged, which destroy the blood-retinal and blood-glomerular barriers, thickens the stroma, and induces fibrotic changes, eventually leading to retinal detachment or glomerular barrier dysfunction [12,13]. Injuries to vascular endothelial cells contribute to the basis of these complications.
In recent years, the relationship between the immune system and diabetes pathogenesis has attracted extensive attention. Studies have found that disorders of the immune system are closely related to the development of type I diabetes [14]. With the influence of certain factors, immune cells can be involved in the attack of β-cells in the islets, resulting in decreased insulin secretion and type I diabetes [15]. Abnormal proportion of Th1/2 cells has been found in type II diabetes, together with increased activity of natural killer T (NKT) cells, suggesting the involvement of immune cell abnormalities in the disease development [16,17]. The NKT cells belong to the heterogeneous T cell group, first reported in mice by Makino et al. in 1995. Later studies have found that these cells recognize the specific sugar lipid molecules presented by the CD1d molecules, as well as the polypeptides presented by non-major histocompatibility complexes. Therefore, NKT cells are also referred to as CD1d-dependent natural killer-like T cells [18,19]. T lymphocyte markers are expressed on the cell surface (CD3 and TCR), as well as the NK cellular markers (NKp30, NKG2D, CD56, and CD161). Therefore, CD3 and CD56 have long been used as the markers for NKT cells in related investigations [20]. NKT cells have been found to play dual roles in immune regulation, which can enhance or inhibit the immune responses in different disease processes [21]. Under the stimulation of antigens, different NKT cells can rapidly secrete cytokines with immune-regulating effects, affecting the differentiation of Th1/Th2 cells, or directly acting on target cells and regulating the biological functions [22]. NKT cells also express cell necrosis-related molecules that can kill tumor cells [23]. Although NKT cells are present in peripheral blood, their activities and involvement in the regulation of vascular endothelial cells are unclear. In this study, these issues were investigated in vitro and in the clinic.
Material and Methods
Study subjects and peripheral blood sample collection
A total of 41 patients with type II diabetes who were admitted to our hospital from January 2016 to December 2017 were included in this study. Another 30 health normal subjects were recruited as controls. A peripheral blood sample (5 ml) was collected from each patient and subject. In the blood sample, 2 ml was used for the lymphocyte isolation and the identification of NKT cells with flow cytometry, and the other 3 ml of peripheral blood sample was used for the isolation and purification of NKT cells. Inclusion criteria for type II diabetes were as follows: (1) based on the WHO criteria (1999), patients meeting the diagnostic criteria of the 75 g oral glucose tolerance test; (2) patients diagnosed as diabetic, orally administered with blood glucose-controlling drugs, for more than 1 year; and (3) patients previously diagnosed as diabetic, using insulin to control blood glucose for more than 1 year. Exclusion criteria included other endocrine diseases, cardiovascular and basic diseases, malignant tumors, autoimmune diseases, infectious diseases, pregnancy, and with heavy smoking at admission. Patients’ clinical information and pathological data were collected. Prior written and informed consent was obtained from every patient, and the study was approved by the local ethics review board.
Culture of human umbilical vein endothelial cells (HUVECs)
HUVECs were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured with low-glucose DMEM medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco). When 90% confluence was reached, the cells were passaged. HUVECs in the logarithmic growth phase were used for the following investigations. For the establishment of a high-glucose-induced cell model, the cells were cultured with high-glucose DMEM medium containing 10% FBS.
Isolation of peripheral blood mononuclear cells (PBMCs)
We collected 2 ml anticoagulated peripheral blood from healthy subjects. PBMCs were isolated with the Ficoll lymphocyte separation method, followed by adding 5× volume sterile PBS. After centrifugation at 1200 rpm for 6 min, the supernatant was discarded. The cells were re-suspended with PBS for further use.
Preparation of vascular endothelial cell condition medium
HUVECs were cultured with high-glucose DMEM medium containing 10% FBS in a 37°C 5% CO2 incubator for 48 h. The culture supernatant was collected and mixed with low-glucose DMEM medium containing 10% FBS (v: v of 1: 1) for co-culture.
Isolation, purification, and culture of NKT cells
The PBMCs were isolated as detailed above. Peripheral blood NKT cells were isolated with the CD3+CD56+ NKT Cell Isolation Kit (Miltenyi Biotech Company, Cologne, Germany), according to the manufacturer’s instructions. Briefly, the PBMCs were added into tube A, followed by adding 10 ml sterile PBS. After centrifugation at 250×g for 10 min, the supernatant was discarded. The cells were counted, and re-suspended by 400 μl PBS. The cells were incubated with 100 μl biotinylated anti-CD3+CD56+ NKT cell antibody in the dark at 4°C for 10 min. After washing with PBS, 100 μl avidin beads were added to incubate the cells in the dark at 4°C for 15 min. After washing, the cells were transferred into the 1.5-ml EP tube and subjected to the magnetic bead column for 15 min. The transparent liquid was transferred into a new EP tube, and the NKT cells were obtained. The NKT cells were cultured with RPMI 1640 medium containing 10% FBS and 100 IU IL-2 in a 37°C 5% CO2 incubator.
Experimental grouping
In the in vitro co-culture experiments, the peripheral blood NKT cell culture (NC) group was cultured only with RPMI 1640 medium containing 10% FBS and 100 U/ml IL-2. For the co-culture group, the culture supernatant from the high-glucose-induced HUVECs was collected as the condition culture medium, which was used to co-culture the healthy human peripheral blood NKT cells. The condition culture medium contained 100 U/ml IL-2, which was replaced every 24 h.
In the cytokine stimulation experiment, the recombinant protein IL-4 (R&D Systems, MN, USA) was added to the DMEM medium (with the final concentration of 0.5 ng/ml), containing 10% FBS. After co-cultured with HUVECs for 48 h, the HUVECs were collected for the following experiments.
Detection of NKT phenotype and function
According to the instructions for use of the fluorescent antibodies, 5 μl fluorescence-labeled antibody was added to incubate the cells at room temperature in the dark for 10 min. Flow cytometry was used to detect the NKT cell surface and intracellular markers. Mononuclear lymphocytes were circled by FSC/SSC, and the CD3+CD56+ cell populations were gated for further analysis. Normal human PBMCs or NKT cells were activated with 100 U/ml IL-2 for 24 h. After centrifugation at 1000 rpm for 5 min, the cells were collected, and the expression levels of NKG2D, NKP30, NKG2A, CD158b, CD4, CD8, TCRvα24, IL-4, perforin, granzyme, and Ki-67 were detected.
Assessment of vascular endothelial cell proliferation activity
The proliferation activity of the vascular endothelial cells was detected with the CCK-8 methods (Beyotime, Haimen, Jiangsu, China), according to the manufacturer’s instructions. Briefly, the vascular endothelial cells were planted onto the 96-well plate at the density of 2×103 cells/well. The 20 μl CCK-8 (5 g/L) was added into the wells at 0 h, 24 h, 48 h, and 72 h, respectively. On the final day, 150 μl CCK-8 reaction solution was added to incubate the cells at 37°C for 2 h. The absorbance at 490 nm was detected and the proliferation curves were plotted.
Cell cycle was detected with flow cytometry. Totally 1×106 HUVEC cells were collected from each group. After washing with PBS, the cell cycle was detected with the Cell Cycle Assay Kit (BD company). The cells were subsequently incubated with 200 μl solution A at room temperature for 10 min, 150 μl solution B at room temperature for 10 min, and then 120 μl solution C in the dark for 10 min. The detection data from flow cytometry were analyzed by Modfit software.
Detection of migration ability
Transwell assay was used to detect the invasion and metastasis capacity of HUVECs. Briefly, totally 1×105 cells were cultured in the upper Transwell chamber with 200 μl serum-free 1640 medium. A total of 500 μl of 1640 medium containing 20% FBS was added into the lower chamber. After 24 h, the cells were fixed with 4% formaldehyde at room temperature for 10 min. After washing with PBS, the cells in the upper chamber were wiped off, and Giemsa staining was then performed. Migrating cells were observed under the light microscope (200×). Five fields were randomly selected, and the cells penetrating the membrane were counted. Cell invasion and migration capacities were evaluated accordingly.
Statistical analysis
Data are expressed as mean ±SD. GraphPad Prism 7.0 software was used for statistical analysis. One-way ANOVA was used for multiple comparisons, while the t test was performed for pair-wise comparison. P<0.05 was considered as statistically significant.
Results
Ratio of CD3+CD56+ NK cells in peripheral blood of diabetic patients
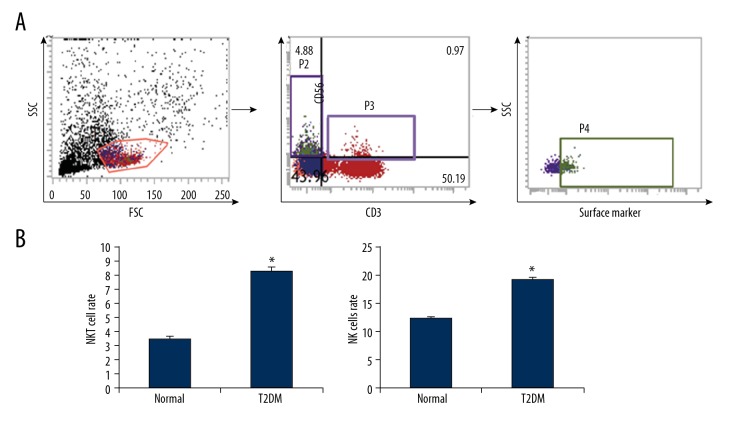
The NK cells in the peripheral blood was analyzed with fluorescence-labeled CD3 and CD56, and the CD3+CD56+ cells were recognized as the NKT cells. Our results showed that, in the normal control subjects, the percentage of NKT cells in the peripheral blood was 3.5±0.21%, which was significantly elevated in the patients with type II diabetes (8.31±0.27%) (P<0.05). CD3 and CD56 also represented the screening labels for NK cells, and the NK cell percentage was also analyzed. Our results showed that the ratio of CD3-CD56+ NK cells in the peripheral blood of the type II diabetic patients was 19.2±0.38%, which was significantly higher than in the normal control subjects (12.4±0.17%) (P<0.05) (Figure 1). Taken together, these results suggest that the ratios of NK cells and NKT cells in the peripheral blood were significantly increased in the type II diabetic patients, indicating activation of the natural immune system in the disease.
Figure 1.
Proportions of NKT and NK cells in peripheral blood from diabetic patients. (A) The NKT and NK cells in the peripheral blood of patients with type II diabetes were identified with flow cytometry. (B) Statistical analysis of NKT and NK cells. Compared with the normal control group, * P<0.05.
Phenotype analysis of CD3+CD56+ NKT cells in peripheral blood of diabetic patients
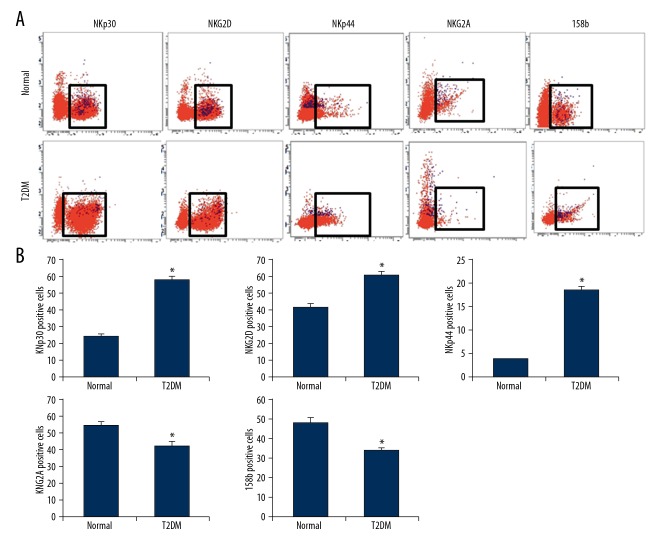
NKT cells have been found to have the features of NK cells, which also express the activating and inhibiting receptors on the NK cell surface. Therefore, the expression and distribution of peripheral blood NKT cell surface receptors (NKp30, NKp44, NKG2D, NKG2A, and 158b) in peripheral blood of type II diabetic patients were analyzed using fluorescent antibodies. Our results showed that the NKp30-positive rate in the NKT cells from the peripheral blood of diabetic patients (54.9±2.4%) was significantly higher than in the normal control subjects (36.1±1.6%) (P<0.05). The NKG2D-positive rate in the NKT cells from the peripheral blood of diabetic patients (61.2±1.8%) was significantly higher than in the normal control subjects (41.9±2.1%) (P<0.05). The NKp44-positive rate in the NKT cells from the peripheral blood of diabetic patients (18.4±0.78%) was significantly higher than in the normal control subjects (3.8±0.12%) (P<0.05). For the inhibiting receptors, the NKG2A-positive rate in the NKT cells from the peripheral blood of diabetic patients (42.7±2.5%) was significantly lower than in the normal control subjects (55.3±1.9%) (P<0.05). The 158b-positive rate in the NKT cells from the peripheral blood of diabetic patients (33.7±1.2%) was significantly higher than in the normal control subjects (47.9±2.6%) (P<0.05) (Figure 2). These results suggest that the NKT cells in the peripheral blood were upregulated in patients with type II diabetes.
Figure 2.
Surface receptor distribution on NKT cell in diabetes. (A) Expression levels of NKp30, NKG2D, NKp44, NKG2A, and 158b on the surface of NKT cells in patients with type II diabetes were analyzed with flow cytometry. (B) Statistical analysis of these surface receptor expression levels. Compared with the normal control group, * P<0.05.
CD4+ and CD8+ typing of peripheral blood NKT cells in diabetic patients
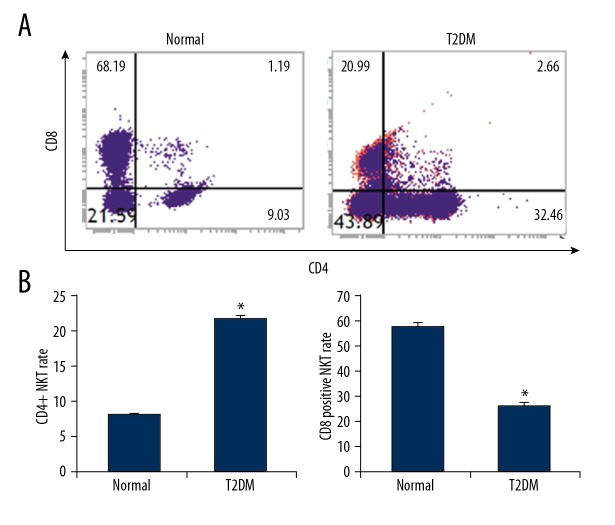
Based on the expression of CD4 and CD8, the CD3+CD56+ NKT cells were divided into CD4+, CD8+, and CD4−CD8− subtypes, with different functions. Therefore, the ratio of CD4+CD8+ NKT cells in the peripheral blood from the diabetic patients showed that, in the peripheral blood from the normal control subjects, the CD4+ NKT ratio was 7.95±0.29%, which was significantly higher than in the diabetic patients (21.7±0.39%). Moreover, the ratio of CD8+ NKT cells in the peripheral blood from the diabetic patients (25.3±1.75%) was significantly lower than in the normal control subjects (57.3±1.63%) (P<0.05). In addition, the ratio of CD4−CD9− NKT cells in the peripheral blood from the diabetic patients (51.8±2.1%) was significantly higher than in the normal control subjects (35.3±1.3%) (P<0.05) (Figure 3). These results suggest that the CD4+ cell proportion was elevated in the peripheral blood of the type II diabetic patients, while the CD8+ cell proportion declined, suggesting the changed immune regulatory function of NKT cells.
Figure 3.
Subtype analysis of NKT cells in peripheral blood of diabetes. (A) NKT subtypes in the peripheral blood of diabetic patients were analyzed with flow cytometry. (B) Statistical analysis of CD4+ and CD8+ NKT cells. Compared with the normal control group, * P<0.05.
Detection of NKT intracellular markers in peripheral blood of diabetic patients
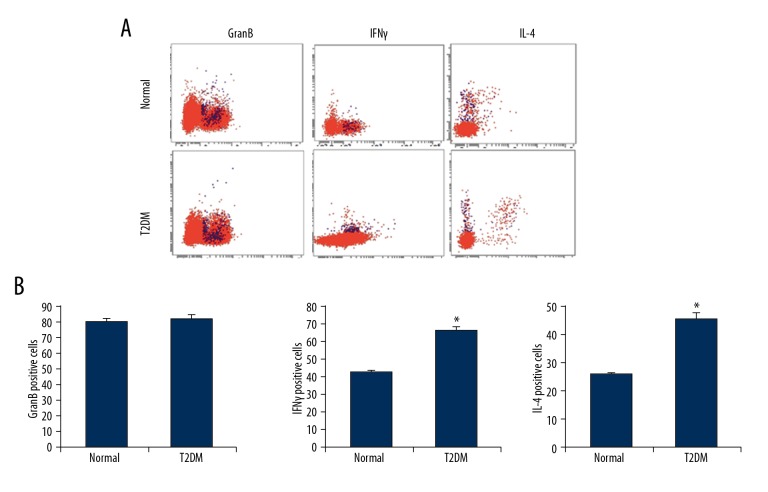
NKT cells are similar to NK cells, and they can exert direct cell-killing effects through granzymes and IFNγ. Like T cells, NKT cells can regulate the immune function through secreting cytokines. It has been reported that NKT cells secret TH2 cytokines such as IL-4, participating in the regulation of inflammatory response. Therefore, the expression of these markers in NKT cells was detected by flow cytometry. Our results showed that the granzyme-positive rate in the NKT cells in the peripheral blood of diabetic patients was 82.6±2.5%, the IFNγ-positive rate was 66.3±1.80%, and the IL-4-positive rate was 45.8±1.76%. In contrast, in the normal control subjects, the granzyme-positive rate in NKT cells in peripheral blood was 80.4±2.1% (P>0.05 compared with diabetic patients), the IFNγ-positive rate was 42.8±0.85% (P<0.05), and the IL-4-positive rate was 25.7±0.58% (P<0.05) (Figure 4). These results suggest that the NKT cells in the peripheral blood were significantly activated in the diabetic patients, with enhanced secretion ability of cytokines.
Figure 4.
Expression of effectors and cytokines in the NKT cells in the peripheral blood of diabetes. (A) Expression levels of GranB, IFNγ, and IL-4 in the NKT cells in the peripheral blood of diabetic patients were detected with flow cytometry. (B) Statistical analysis of GranB, IFNγ, and IL-4. Compared with the normal control group, * P<0.05.
Effects of peripheral blood NKT cells on biological function of endothelial cells
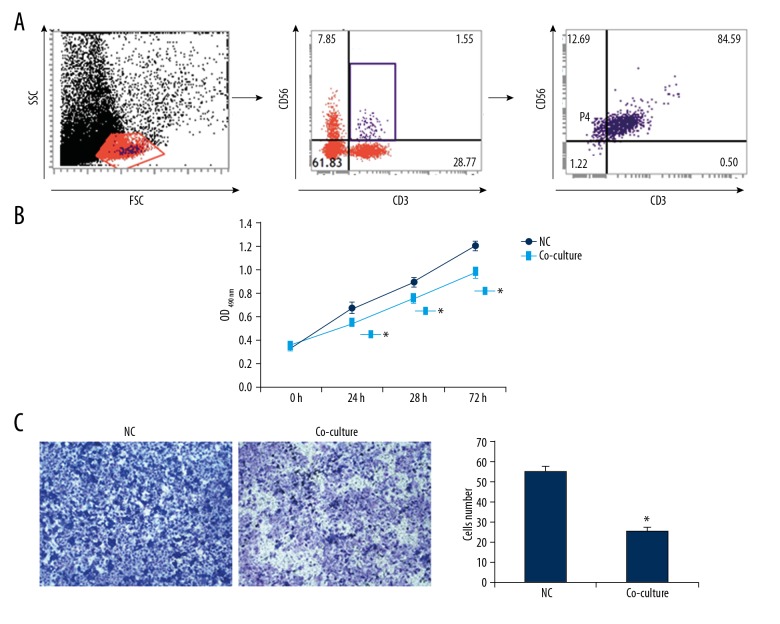
To further study whether endothelial cells play roles in NKT cell activation, the peripheral blood-derived NKT cells were isolated from diabetic patients, which were co-cultured with HUVECs for 48 h, and the biological functions of endothelial cells were assessed. Our results from the CCK-8 assay showed that, after co-culturing with the peripheral blood-derived NKT cells for 24 h, 48 h, and 72 h, the OD490 nm values for the HUVECs were significantly lower than in the control groups (P<0.05). Moreover, our results from the Transwell assay showed that, after the co-culture, the migrating HUVECs were 25.7±1.8, which was significantly less than in the normal HUVECs (55.7±2.8) (P<0.05) (Figure 5). These results suggest that the peripheral blood-derived NKT cells from diabetic patients can significantly inhibit the proliferation and migration abilities of vascular endothelial cells.
Figure 5.
Effects of peripheral blood-derived NKT cells on HUVECs. HUVECs were co-cultured with peripheral blood-derived NKT cells. (A) NKT cell gating strategy and identification. (B) Proliferation of HUVECs was assessed with the CCK-8 assay. (C) Migration of HUVECs was assessed with the Transwell chamber assay. Compared with the normal control group, * P<0.05.
IL-4 mediates inhibiting effects of NKT cells on proliferation and migration of vascular endothelial cells
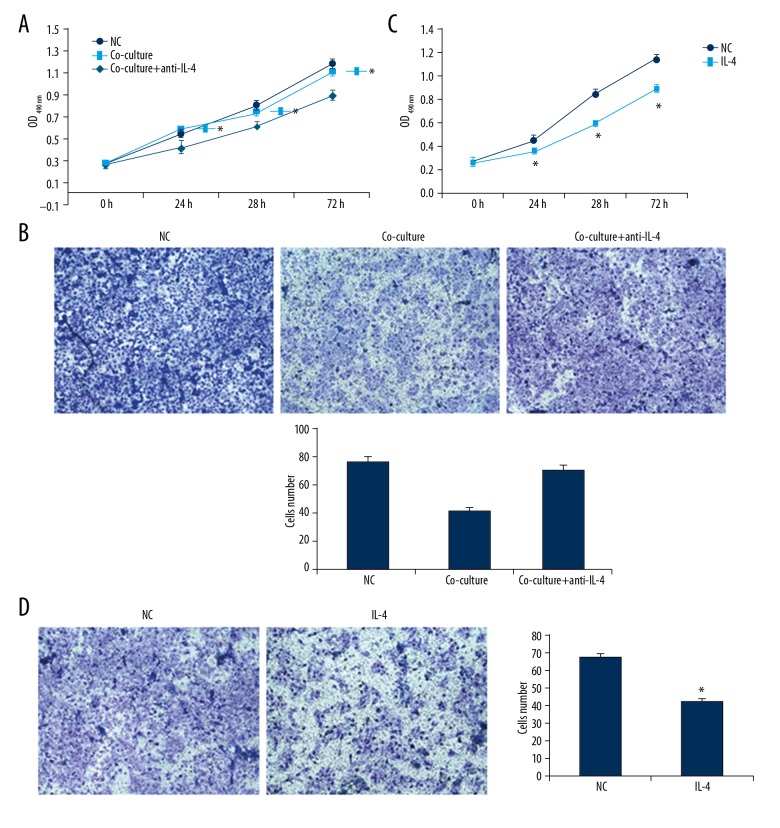
IL-4 is an inflammation-related cytokine secreted by immune cells, which plays an important role in the regulation of cellular biological functions. Our previous studies have shown that IL-4 is highly expressed in NKT cells derived from diabetic patients. Therefore, the IL-4 antibody was added to the co-culture system of NKT cells and HUVECs for 48 h. Our results from the CCK-8 assay and the Transwell test showed that, after adding the IL-4 antibody, the proliferation and migration abilities of HUVECs were significantly elevated compared with the simple co-culture group (P<0.05). To further clarify the role of IL-4, the HUVECs were cultured with the complete medium containing IL-4. Our results showed that IL-4 inhibited the proliferation and migration abilities of HUVECs (Figure 6), showing that NKT cells derived from peripheral blood of diabetic patients can inhibit the proliferation and migration of vascular endothelial cells via secreting IL-4.
Figure 6.
Involvement of IL-4 in the effects of diabetic peripheral blood-derived NKT cells on HUVECs. (A, B) HUVECs were co-cultured with peripheral blood-derived NKT cells treated with IL-4 antibody. The proliferation and migration of HUVECs were detected with CCK-8 (A) and the Transwell chamber (B) assays. (C, D) HUVECs were treated with IL-4 stimulus. The proliferation and migration of HUVECs were detected with CCK-8 (C) and the Transwell chamber (D) assays. Compared with the normal control group, * P<0.05.
Discussion
In China, the prevalence of diabetes in adults is as high as 11.6%, of which more than 90% is type II diabetes [24]. An important cause of the type II diabetes is the insulin resistance, which is closely related to lipid disorders and obesity [25]. Many studies have found that type II diabetes is associated with low-level inflammatory response, with abnormal activation of multiple immune cells [26]. In addition, the microvascular endothelial cell lesions are one of the underlying lesions of diabetes, based on which the diabetic complications are induced (such as retinopathy, diabetic feet, and kidney disease) [27]. However, there is little published data on the relationship between the immune activation and endothelial cell injury in diabetes. In this study, our results showed that the number and activities of NKT cells in the peripheral blood of the diabetic patients were significantly elevated, and these cells can secret IL-4 to inhibit the proliferation and migration of vascular endothelial cells, affecting the regulation of endothelial dysfunction.
The natural immune system is the first line of defense against microbial infections and tumors [28]. NKT cells have received attention in recent years and were shown to play important roles in immune regulation and tumor therapy [29]. NKT cells are heterogeneous immune cells that have features similar to those of NK and T cells. NKT cells express NK cell-activating and -inhibiting receptors on the surface [30]. The activities of NKG2D, NKp30, NKp44, NKG2A, and 158b are regulated by their respective ligands. When the receptor expression is enhanced, the NKT cells are activated, which releases perforin and granzyme B, killing the target cells [31]. However, the NKT cells also have TCR, as do T cells, which is CD1d-limiting [32]. Studies have found that, after TCR binds to ligands, the NKT cells are activated and secrete many cytokines (such as IFN-γ, IL-4, and IL-17), participating in the downstream immune cascade and the regulation of Th1/2 type conversion [33]. There are functional abnormalities in the immune cells of diabetic patients, including the imbalanced Th1/2 ratio, macrophages involved in vascular remodeling, and abnormal activation of NK cells in types I and II diabetes [34]. However, the activities and functions of NKT cells in the pathogenesis of diabetes remain unclear. Our study shows that the ratios of CD3+CD56+ NKT cells in peripheral blood of type II diabetic patients were significantly elevated, and the ratios of CD3−CD56+ NK cells were also increased. These results suggest the activated status of the natural immune system in patients with type II diabetes.
The expression and distribution of NKT receptors on the cell surface were further analyzed. Our results showed that the expression levels of the activating receptors NKp30, NKp44, and NKG2D were significantly increased on the NKT cell surface in the peripheral blood from diabetic patients, while the expression levels of inhibiting receptors NKG2A and 158b were significantly downregulated. These results suggest the enhanced activities of NKT cells in the type II diabetes. The NKT cells were further divided into CD4+, CD8+, and CD4−CD8− subtypes. Our results showed that the numbers of CD4+ and CD4−CD8− NKT cells were significantly increased in the peripheral blood of diabetic patients, while the number of CD8+ NKT cells significantly declined. Studies have shown that CD4+ NKT cells respond to TCR stimulation by producing large amounts of IL-4 and IFNγ, thus regulating immune responses. Our results showed that IL-4 and IFNγ were highly expressed in the NKT cells in peripheral blood from diabetic patients, while no significant changes were observed in the expression of granzyme. These results show that the ratio and activity of NKT cells in the peripheral blood of diabetic patients were significantly increased, mostly the CD4+ and CD4−CD8− subtypes, with fewer CD8+ subtypes. Moreover, the secretions of IL-4 and IFNγ were also upregulated.
The vascular endothelium is a natural barrier in the inner wall of blood vessels, which has important functions in the protection of blood vessels, and in the regulation of blood coagulation system, blood pressure, and secretion [35]. Studies have shown that damages and dysfunctions of vascular endothelial cells directly affect the remodeling and vasoconstriction of blood vessels. Vascular endothelial cells can secrete NO, which regulates blood vessel vasomotion and thus affects blood pressure [36]. Vascular endothelial cells can also secrete cytokines and participate in inflammatory regulation of vascular lesions [37]. In diabetes, the high-glucose peripheral blood environment can induce vascular endothelial cell damage. Under normal circumstances, vascular endothelial cells can repair themselves by proliferating or migrating to the lesions [38]. Whether inflammation-related cytokines regulate the biological behavior of vascular endothelial cell proliferation and migration is not yet clear. In this study, our results showed that IL-4 was highly expressed in the peripheral blood-derived NKT cells in patients with diabetes. IL-4 is a Th2 differentiation-related cytokine that can induce the differentiation of Th cells into Th2 cells. In recent years, IL-4 has been shown to affect and regulate vascular endothelial cells [39]. Kim et al. [40] found that IL-4 can inhibit the proliferation of umbilical vein endothelial cells by regulating the expression of p53, p21, cyclin D1, and cyclin E. Our results showed that, after co-cultured with peripheral blood-derived NKT cells from diabetic patients, the proliferation and migration abilities of HUVECs were significantly inhibited. In order to confirm that IL-4 mediated this process, the IL-4 antibody was applied, which remarkably restored the proliferation and migration abilities of HUVECs. IL-4 recombinant protein was added to culture the HUVECs, showing that the proliferation and migration abilities of HUVECs were significantly inhibited. These results indicate that the peripheral blood-derived NKT cells from diabetic patients inhibit HUVEC proliferation and migration via the IL-4 pathway.
Conclusions
In conclusion, our results show that the proportion and activity of NKT cells in the peripheral blood of diabetic patients were significantly increased, and their subtypes were significantly changed. The NKT cells inhibited the proliferation and migration abilities of vascular endothelial cells by secreting IL-4, and thus participated in the vascular endothelial cell damage.
Acknowledgements
We thank Director Tie Zhang (Department of Endocrinology, Tai’an Central Hospital, Tai’an, Shandong, China) for kind assistance in study design, experiment performance, data collection and analysis, and manuscript preparation.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Abouzed TK, Contreras MDM, Sadek KM, et al. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in Wistar rats in relation to their metabolite fingerprint. Diabetes Res Clin Pract. 2018;140:253–64. doi: 10.1016/j.diabres.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 2.Roy C, Tremblay PY, Anassour-Laouan-Sidi E, et al. Risk of gestational diabetes mellitus in relation to plasma concentrations of amino acids and acylcarnitines: A nested case-control study. Diabetes Res Clin Pract. 2018;140:183–90. doi: 10.1016/j.diabres.2018.03.058. [DOI] [PubMed] [Google Scholar]
- 3.Zamstein O, Sheiner E, Wainstock T, et al. Maternal gestational diabetes and long-term respiratory related hospitalizations of the offspring. Diabetes Res Clin Pract. 2018;140:200–7. doi: 10.1016/j.diabres.2018.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Pierce JS, Jordan SS, Arnau RC. Development and validation of the pediatric diabetes routines questionnaire for adolescents. J Clin Psychol Med Settings. 2018 doi: 10.1007/s10880-018-9563-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Yu CG, Yuan SS, Yang LY, et al. Angiopoietin-like 3 is a potential biomarker for retinopathy in type II diabetic patients. Am J Ophthalmol. 2018;191:34–41. doi: 10.1016/j.ajo.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 6.Cirillo E, Kutmon M, Gonzalez Hernandez M, et al. From SNPs to pathways: Biological interpretation of type II diabetes (T2DM) genome wide association study (GWAS) results. PLoS One. 2018;13:e0193515. doi: 10.1371/journal.pone.0193515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huo X, Zhang J, Guo X, et al. Gender difference in the association of early- vs. late-onset type II Diabetes with non-fatal microvascular disease in China: A cross-sectional study. Front Endocrinol. 2018;9:15. doi: 10.3389/fendo.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollia C, Antonopoulos AS, Siasos G, et al. Associations between adiponectin gene variability, pro-inflammatory and angiogenetic markers: Implications for microvascular disease development in type II diabetes mellitus? Curr Vasc Pharmacol. 2018 doi: 10.2174/1570161116666180108113825. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Levelt E, Piechnik SK, Liu A, et al. Adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type II diabetes mellitus without obstructive coronary artery disease. J Cardiovasc Magn Reson. 2017;19:81. doi: 10.1186/s12968-017-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee J, Nema V, Dhas Y, Mishra N. Role of microRNAs in type II diabetes and associated vascular complications. Biochimie. 2017;139:9–19. doi: 10.1016/j.biochi.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Bulum T, Blaslov K, Duvnjak L. The use of anthropometric measurements of obesity in prediction of microvascular complications in obese type II diabetic patients. Acta Clin Croati. 2016;55:217–23. doi: 10.20471/acc.2016.55.02.06. [DOI] [PubMed] [Google Scholar]
- 12.Kang WL, Xu GS. Corrigendum: Atrasentan increased the expression of klotho by mediating miR-199b-5p and prevented renal tubular injury in diabetic nephropathy. Sci Rep. 2018;8:46965. doi: 10.1038/srep46965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uil M, Scantlebery AML, Butter LM, et al. Combining streptozotocin and unilateral nephrectomy is an effective method for inducing experimental diabetic nephropathy in the ‘resistant’ C57Bl/6J mouse strain. Sci Rep. 2018;8:5542. doi: 10.1038/s41598-018-23839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canibano-Hernandez A, Saenz Del Burgo L, Espona-Noguera A, et al. Current advanced therapy cell-based medicinal products for type-1-diabetes treatment. Int J Pharm. 2018;543:107–20. doi: 10.1016/j.ijpharm.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 15.Smith MJ, Simmons KM, Cambier JC. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat Rev Nephrol. 2017;13:712–20. doi: 10.1038/nrneph.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih YL, Lu HF, Hsiao CW, et al. Distribution of cytotoxic T lymphocyte-associated antigen-4 promoter polymorphisms in taiwanese patients with type II diabetes mellitus. Int J Med Sci. 2018;15:395–402. doi: 10.7150/ijms.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prattichizzo F, De Nigris V, Spiga R, et al. Inflammageing and metaflammation: The yin and yang of type II diabetes. Ageing Res Rev. 2018;41:1–17. doi: 10.1016/j.arr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Nishioka Y, Masuda S, Tomaru U, Ishizu A. CD1d-restricted type II NKT cells reactive with endogenous hydrophobic peptides. Front Immunol. 2018;9:548. doi: 10.3389/fimmu.2018.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, Zhu S, Wang L, et al. Memory follicular helper invariant NKT cells recognize lipid antigens on memory B cells and elicit antibody recall responses. J Immunol. 2018;200:3117–27. doi: 10.4049/jimmunol.1701026. [DOI] [PubMed] [Google Scholar]
- 20.de Mingo Pulido A, de Gregorio E, Chandra S, et al. Differential role of cathepsins S and B in hepatic APC-mediated NKT cell activation and cytokine secretion. Front Immunol. 2018;9:391. doi: 10.3389/fimmu.2018.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekar D, Govene L, Del Rio ML, et al. Downregulation of BTLA on NKT cells promotes tumor immune control in a mouse model of mammary carcinoma. Int J Mol Sci. 2018;19(3) doi: 10.3390/ijms19030752. pii: E752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podbielska M, O’Keeffe J, Hogan EL. Autoimmunity in multiple sclerosis: Role of sphingolipids, invariant NKT cells and other immune elements in control of inflammation and neurodegeneration. J Neurol Sci. 2018;385:198–214. doi: 10.1016/j.jns.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Torina A, Guggino G, La Manna MP, Sireci G. The janus face of NKT cell function in autoimmunity and infectious diseases. Int J Mol Sci. 2018;19(2) doi: 10.3390/ijms19020440. pii: E440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D, Zhang R, Liu Y, et al. CD36 gene variants is associated with type II diabetes mellitus through the interaction of obesity in rural Chinese adults. Gene. 2018;659:155–59. doi: 10.1016/j.gene.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Aslam M, Rathi S, et al. Association of vitamin D levels and type II diabetes mellitus in asian indians is independent of obesity. Exp Clin Endocrinol Diabetes. 2018;126(9):553–58. doi: 10.1055/s-0043-124076. [DOI] [PubMed] [Google Scholar]
- 26.Verboven K, Wouters K, Gaens K, et al. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci Rep. 2018;8:4677. doi: 10.1038/s41598-018-22962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan Y, Liu J, Xu Y, et al. Factors that influence pancreatic beta cell function and insulin resistance in newly diagnosed type II diabetes patients: A sub-analysis of the MARCH trial. Diabetes Ther. 2018;9:743–52. doi: 10.1007/s13300-018-0393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto FY, Yin F, Rossi W, Jr, et al. beta-1,3 glucan derived from Euglena gracilis and Algamune enhances innate immune responses of red drum (Sciaenops ocellatus L.) Fish Shellfish Immunol. 2018;77:273–79. doi: 10.1016/j.fsi.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang N, Zhang M, Liu RT, et al. Statins reduce the expressions of Tim-3 on NK cells and NKT cells in atherosclerosis. Eur J Pharmacol. 2018;821:49–56. doi: 10.1016/j.ejphar.2017.12.050. [DOI] [PubMed] [Google Scholar]
- 30.Gaya M, Barral P, Burbage M, et al. Initiation of antiviral B cell immunity relies on innate signals from spatially positioned NKT cells. Cell. 2018;172:517–33.e20. doi: 10.1016/j.cell.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teyton L. New directions for natural killer T cells in the immunotherapy of cancer. Front Immunol. 2017;8:1480. doi: 10.3389/fimmu.2017.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sag D, Ozkan M, Kronenberg M, Wingender G. Improved detection of cytokines produced by invariant NKT cells. Sci Rep. 2017;7:16607. doi: 10.1038/s41598-017-16832-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKnight CG, Morris SC, Perkins C, et al. NKT cells contribute to basal IL-4 production but are not required to induce experimental asthma. PLoS One. 2017;12:e0188221. doi: 10.1371/journal.pone.0188221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thanapati S, Ganu MA, Tripathy AS. Differential inhibitory and activating NK cell receptor levels and NK/NKT-like cell functionality in chronic and recovered stages of chikungunya. PLoS One. 2017;12:e0188342. doi: 10.1371/journal.pone.0188342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chai YC, Mendes LF, van Gastel N, et al. Fine-tuning pro-angiogenic effects of cobalt for simultaneous enhancement of vascular endothelial growth factor secretion and implant neovascularization. Acta Biomater. 2018;72:447–60. doi: 10.1016/j.actbio.2018.03.048. [DOI] [PubMed] [Google Scholar]
- 36.Tabecka-Lonczynska A, Mytych J, Solek P, et al. Vascular endothelial growth factor (VEGF-A) and fibroblast growth factor (FGF-2) as potential regulators of seasonal reproductive processes in male European bison (Bison bonasus, Linnaeus 1758) Gen Comp Endocrinol. 2018;263:72–79. doi: 10.1016/j.ygcen.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Bartakova A, Kuzmenko O, Alvarez-Delfin K, et al. A cell culture approach to optimized human corneal endothelial cell function. Invest Ophthalmol Vis Sci. 2018;59:1617–29. doi: 10.1167/iovs.17-23637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morohoshi K, Mochinaga R, Watanabe T, et al. 16 kDa vasoinhibin binds to integrin alpha5 beta1 on endothelial cells to induce apoptosis. Endocr Connect. 2018;7:630–36. doi: 10.1530/EC-18-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoya M, Nagamatsu T, Fujii T, et al. Impact of Th1/Th2 cytokine polarity induced by invariant NKT cells on the incidence of pregnancy loss in mice. Am J Reprod Immunol. 2018:79. doi: 10.1111/aji.12813. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Cheon IS, Won YJ, et al. IL-4 inhibits cell cycle progression of human umbilical vein endothelial cells by affecting p53, p21(Waf1), cyclin D1, and cyclin E expression. Mol Cells. 2003;16:92–96. [PubMed] [Google Scholar]