Abstract
Twenty-five percent of diabetes-related vision loss stems from complications of proliferative diabetic retinopathy (PDR). Panretinal photocoagulation has been the preferred treatment of high-risk PDR for decades and more recently intravitreal injections of drugs that inhibit the actions of vascular endothelial growth factor have become popular. But despite these treatments PDR may progress uncontrollably to advanced pathologies such as traction retinal detachments (TRDs), combined traction/rhegmatogenous retinal detachments (TRD/RRDs), vitreous hemorrhages, rubeosis iridis, and traction maculopathies, which produce mild-to-severe loss of vision. TDR have long been the most common indication for PDR-related vitreoretinal surgery. Vitrectomy surgery is indicated for recent (<6 months duration) TRD involving the macula, progressive TRD that threatens the macula, and recent data suggest that chronic macula-involving TRDs (>6 months duration) may also benefit. Combined TRD/RRD represents a particularly challenging surgical condition but advances in surgical instrumentation, dissection techniques, and post-operative tamponade have produced excellent success rates. The recent development of small-gauge vitrectomy systems has persuaded most surgeons to switch platforms since these appear to produce shorter surgical times and quicker post-operative recoveries. Pre-operative injections of bevacizumab are frequently administered for persistent neovascularization to facilitate surgical dissection of pre-retinal fibrosis and reduce the incidence of post-operative hemorrhages. Recent trends toward earlier surgical intervention and expanded indications are likely to continue as surgical instrumentation and techniques are further developed.
Keywords: Diabetes, diabetic retinopathy, tractional retinal detachment, vitrectomy
Diabetic retinopathy (DR) is the leading cause of new blindness in patients aged 20–74 years in industrialized nations.[1,2] It has been estimated that 93 million people throughout the world have DR, with approximately one-third of them having diabetic macular edema (DME). DME is responsible for 75% of DR-related vision loss but advances in laser photocoagulation[3] and intravitreal pharmacotherapy, particularly corticosteroids[4] and drugs that inhibit the actions of vascular endothelial growth factor (VEGF)[5,6,7] have enabled physicians to stabilize retinopathy, decrease macular edema, and improve visual acuity (VA) in the majority of affected patients.
Vision loss in the other 25% of patients with DR stems from complications of proliferative diabetic retinopathy (PDR). Approximately 17 million patients throughout the world have PDR[8] and without treatment more than half of the patients with high-risk PDR – based on the classification system developed for the Diabetic Retinopathy Study – will be blind within 5 years.[9] When panretinal photocoagulation (PRP) of the retina is performed prior to the development of severe PDR-related complications [vitreous hemorrhage and traction retinal detachment (TRD)], the incidence of severe vision loss decreases by about 50%.[10] The Early Treatment of Diabetic Retinopathy Study (ETDRS) showed that 5% of patients with PDR will require vitreous surgery despite having received what appeared to be adequate PRP.[11]
The first pars plana vitrectomy was performed in 1970 on an eye with a non-clearing vitreous hemorrhage and the VA improved from 2/200 to 20/50.[12] A subsequent series of cases from 1977 described the following indications for diabetic vitrectomy: non-clearing vitreous hemorrhages (70%); TRD (20%); and combined traction/rhegmatogenous retinal detachment (TRD/RRD) (10%).[13] Between 1980 and 2004, VA improvements in eyes with TRD were limited because of what was believed to be excessively long durations of macular detachment prior to surgery.[14] Since then the benefits and safety of vitrectomy have steadily risen and the threshold for performing vitrectomy has decreased. Indications for vitrectomy have expanded during recent years to include severe fibrovascular proliferation, dense vitreous hemorrhage with rubeosis, ghost cell glaucoma, dense pre-macular hemorrhage, and a taut hyaloid with DME.[15,16,17,18,19,20,21,22,23,24]
Even with advances in surgical techniques, improvements in instrumentation, and broadening of surgical indications, TRD remains the most common reason for vitrectomy in patients with PDR. This manuscript will discuss current techniques, strategies, and results in patients with TRD who undergo vitreoretinal surgery.
Pathophysiology
The mechanisms leading to the development of DR are complex and remain incompletely understood. Based on the observation that several biochemical pathways (activation of protein kinase C, increased flux through the hexosamine pathway, increased intracellular formation of advanced glycation end-products, and increased polyol pathway flux) are dysregulated in patients with DM and DR, Brownlee proposed the “unifying theory” for the development of DR.[25] He noted that each of these pathways interferes with electron transfer through the mitochondrial cytochrome chain resulting in the accumulation of superoxide ions. Oxidative stress creates a pro-inflammatory state that upregulates the synthesis of various chemokines and cytokines. These molecules promote the development of DME by breaking down the blood-retinal barrier and the development of PDR by stimulating the growth of pre-retinal proliferative tissue.
Oxidative stress and retinal ischemia upregulate the production of angiogenic factors, particularly VEGF, and several chemokines.[26] Increased levels of nitric oxide (NO) pathway metabolites (citrulline and arginine) have been found in the vitreous of eyes with RRD and TRD[27] and excess NO creates toxic free radicals that may inhibit mitochondrial function and cause cell death by damaging DNA.[28] Other pro-inflammatory and growth factor molecules found in eyes with PDR include the following: chemokine (C-C) ligand 2 [CCL2; monocyte chemotaxis protein (MCP)-1], CCL4, CCL11, CCL17, CCL19, CXCL9, CXCL10, TGF-β1,2,3,[29] interleukin (IL)-1β, IL-6, IL-8, erythropoietin, adiponectin, sICAM-1, and sVCAM-1.
The vitreomacular interface is key to the development of PDR as evidenced by the protective effect of posterior vitreous detachment. TRD represents an advanced form of PDR that results from neovascular growth from existing retinal vasculature into the vitreomacular interface with an accompanying vestment of fibrotic tissue and contractile elements. Growth factors create a biochemical environment favorable for angiogenesis. Neovascular buds grow from the larger retinal blood vessels into the potential space between the internal limiting membrane and the posterior hyaloid, and by using the hyaloid as a scaffold they eventually invade the cortical vitreous, thereby creating firm adhesions between the hyaloid and the inner retina.[30,31] The co-development of contractile fibrous tissue results in anteroposterior and tangential traction on the fibrovascular complex and thinned ischemic retina by the vitreous. Excessive traction frequently causes the fragile new vessels to bleed into the vitreous and/or pre-retinal space, and the retina to deform and detach.
The retinal pigment epithelium (RPE) pump produces negative pressure in the subretinal space creating a concave retinal configuration between tractionally elevated areas with heavy pre-retinal fibrosis. Retinal elevation is highest at loci of anteroposterior vitreoretinal traction and beneath broader areas of tangential traction.
A combined TRD/RRD has a convex or bullous configuration because liquefied vitreous moves through a full thickness retinal break into the subretinal space [Figs. 1 and 2]. Whereas TRDs are limited to areas of fibrosis and vitreoretinal traction, combined TRD/RRD is usually characterized by a detachment that extends to the ora serrata. Tangential traction from broad areas of fibrosis may create full-thickness retinal breaks that convert a purely traction detachment to one with a rhegmatogenous component.[32] Most cases of TRD/RRD have widespread and tightly adherent plaque-like pre-retinal proliferation, partial PVDs, and retinal folds. A few eyes develop flap tears but oval breaks near areas of extensive fibrosis are more commonly seen.[33] Fibrosis sometimes increases following PRP, perhaps because of a decrease in VEGF levels and upregulation of connective tissue growth factor (CTGF), which sometimes leads to the formation of breaks near heavy laser photocoagulation. Older studies reported that TRD/RRDs accounted for 17–35% of diabetic eyes undergoing vitrectomy[22,34] but with earlier surgical intervention and new indications for surgery, these eyes now account for a smaller proportion of surgeries.[35,36]
Figure 1.
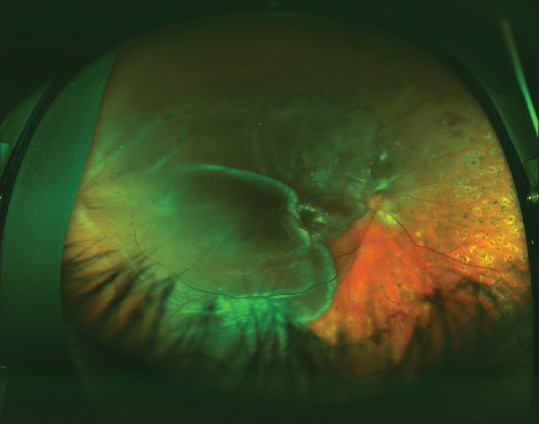
This traction/rhegmatogenous retinal detachment involves the entire temporal retina. Note the bullous or convex configuration of the retina and the extension of the detachment to the ora serrata
Figure 2.
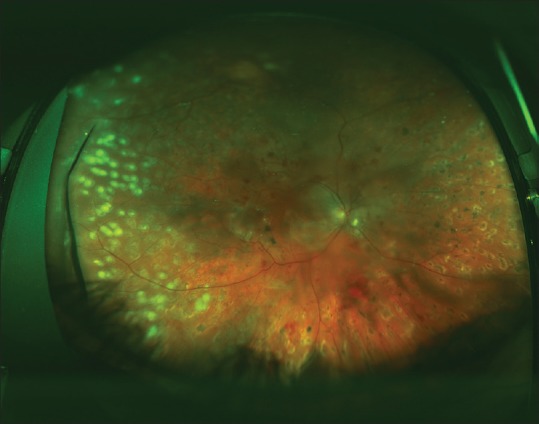
One month after vitrectomy surgery with the use of silicone oil, the retina remains completely flat. A retinal break could not be identified pre-operatively but during surgery a full-thickness retinal hole was found superotemporal to the macula. Six months later, the best corrected visual acuity was 20/400, compared to counting fingers @ 4’ pre-operatively
Management
Surgical techniques
When care for a patient with PDR and TRD has been assumed, optimization of systemic health should be the first consideration. Not only can improvement in systemic health stabilize DR in some patients, but also it may prevent the development of serious systemic adverse events because PDR has been associated with increased all-cause mortality.[37,38] Establishing euglycemia together with improving the management of associated systemic risk factors may avoid the need for future surgery in patients with PDR. Nephropathy and hypertension may worsen retinopathy[39,40] and should be stabilized. Careful coordination with anesthesiologists for perioperative control of hypertension can reduce problems with intraretinal bleeding. Anti-VEGF drugs and corticosteroids may also stabilize DR and allow surgery to be deferred. Optimal control of systemic conditions helps patients get medically cleared for surgery and may decrease the risk of post-operative complications, but unfortunately these interventions will not reverse the damage already present in most eyes with TRDs or change their need for surgery.[41]
Patients having extramacular traction retinal detachments may need de novo or supplemental PRP to reduce neovascular activity, wall-off the detached area, and thereby reduce the probability of spread of detachment into the macula.
The primary goals of vitrectomy are to clear medial opacities and stabilize the proliferative process.[42] Vitrectomy is also thought to increase retinal blood flow by decreasing the resistive index [resistive index = (systolic red blood cell velocity − diastolic red blood cell velocity)/systolic red blood cell velocity].[43] The primary indications for diabetic vitrectomy were established in the 1980s and remain equally valid today (removal of non-clearing media opacities and relief of vitreoretinal traction).[44]
A TRD that has recently involved the macula continues to be the most common indication for vitrectomy and despite improvements in instrumentation and techniques, it remains a challenging surgery. TRD is usually classified as follows: TRD recently involving the macula; extra-macular TRD; and long-standing macular TRD. Prompt surgical intervention is indicated for a recent macular detachment, whereas surgery is not routinely performed for a peripheral RD or a chronic macular RD. Older studies showed that chronic TRDs had worse visual outcomes than acute macula-involving TRDs.[14,45] Patients with a macula-involving TRD usually experience rapid loss of vision when the photoreceptors are pulled away from the RPE. In early surgical series, TRD constituted 20% of diabetic vitrectomies[13] but this proportion has risen to 40% in more recent studies.[22] In eyes with TRD, vitrectomy techniques are used to remove fibrous membranes, relieve anterior–posterior and tangential traction, and allow the retina to spontaneously re-attach. In the absence of a retinal break, vitreous tamponade is not necessary.
In eyes with chronic macular detachments, the retina is frequently atrophic and the fibrous membranes are often strongly adherent. Detachments of >6 months duration may be accompanied by photoreceptor degeneration, which often prevents return of useful vision.[32]
The pathophysiology of TRD depends on vitreoretinal adhesions emanating from vascular epicenters so a detailed assessment of the posterior hyaloid configuration is important for the planning of surgery. Eyes with broader areas of vitreoretinal adhesion may have higher rates of membrane reproliferation and poorer visual outcomes.[46] Vitreous should be dissected from the retinal as far into the periphery as possible but complete removal may be impossible in phakic eyes. Some surgeons believe that eyes which require peripheral dissection should undergo combined cataract extraction/vitrectomy to facilitate access to the equatorial retina. An encircling band with a segmental buckle may be considered to support areas of residual traction.
Apparent elevation of the macula in PDR may represent either TRD or tractional retinoschisis. Schisis can be resolved in 50% of patients with surgery but VA improvements are usually modest.[47] Observation may be the best approach in eyes with stable tractional retinoschisis.[48]
In the era of 20-gauge vitrectomy instruments, surgical techniques for TRD included viscodissection, membrane segmentation,[49] segmentation with membrane delamination,[50] “en-bloc” excision of membranes using the attached posterior hyaloid as an “extra hand”,[51,52] “modified en-bloc excision” of membranes using a bimanual technique,[53] and “total en-bloc excision” in which the glial ring and posterior hyaloid membrane are removed together from the posterior pole with a hook.[54] Ancillary instrumentation necessary for the performance of these cases included chandeliers, illuminated instruments, illuminated infusions, vertical and horizontal scissors, tissue manipulators (combination of diathermy, aspiration, and illumination), mechanized scissors, spatulas, scrapers, diamond knives, and viscoelastic injectors.[34,55]
Common intraoperative complications included bleeding, iatrogenic breaks, and sclerotomy-associated complications (vitreous incarceration, fibrovascular ingrowth, and retinal tears). Bleeding from segmented fibrovascular tissue was common because pre-operative anti-angiogenic drugs were not available, and the intraocular pressure (IOP) during the surgery could not be adequately controlled because valved cannulas were not available and machines could not automatically control IOP. Iatrogenic breaks occurred frequently because the large cutting port of the 20-gauge vitrector combined with its relatively low cutting rate created high, excessive tissue movement, traction on the retina, and subsequent tears and breaks. Retinal tears often occurred at sclerotomy sites because cannulas were not used, and multiple exchanges of instruments through the sclerotomies caused traction on the vitreous base. The larger sclerotomies also allowed for fibrovascular ingrowth into very ischemic eyes.
Small-gauge vitrectomy system – 23, 25, and 27 – have become increasingly popular and offer advantages over traditional 20-gauge instruments. Small-gauge systems reduce operating time, patient discomfort, conjunctival trauma, and shorten recovery times.[56]
Microincisional vitrectomy systems, together with valved cannulas and pre-operative anti-angiogenics to reduce the vascularity of fibrovascular tissue, have enabled the development of new surgical techniques.[57,58] The smaller vitrectomy probes, particularly the 27-gauge and 25-gauge probes, with cutting rates of 10,000 cuts/min, together with the proximity of the cutting port to the tip of the instrument (50% of the distance compared to 20-gauge), allow segmentation and controlled removal of most pre-retinal membranes, with minimum movement of the underlying retina.[59] The reduced diameter of the probes allows for access into tight tissue planes and the use of blunt dissection techniques. Hypersonic vitrectomy instrumentation, which works by hypersonic liquefaction of vitreous adjacent to a probe tip oscillating at 1.7 million cycles per minute, may reduce iatrogenic breaks further, but is too new for comprehensive assessment.
Some surgeons still prefer 20-gauge bimanual membrane dissection in eyes with broader areas of vitreoretinal attachment, whereas others contend that 23-gauge systems have all the wound advantages of 25-gauge, combined with better fluidics, better flow rates, improved aspiration, and a more efficient core vitrectomy.[60] As with most surgical instrumentation, decisions regarding which system to use are usually based on the complexity of the case and the preference of the surgeon.
Vitreoschisis makes identification of remaining posterior hyaloid difficult, so many surgeons use triamcinolone to identify residual cortical vitreous.
As surgical instrumentation and skills have improved, more complicated cases are being operated on[61] and the threshold for surgery has decreased.[42,43,62,63,64,65,66] Advances in vitreoretinal surgery include wide-field viewing systems, multiport illumination systems,[67] perfluorocarbon liquids, and silicone oil.[45] The use of silicone oil, as opposed to long-acting gas tamponade, may accelerate visual rehabilitation, maintain tamponade, prevent hypotony,[68] and decrease the incidence of recurrent bleeding or rubeosis.[50,69,70] With current instruments, injection of 1000 centistokes oil can be performed in only 2 min. Oil has been used in both the primary and secondary repairs of complex detachments, and its use has been suggested for eyes with advanced PDR and intractable proliferative vitreoretinopathy (PVR).[71] Oil may prevent or reverse rubeosis iridis by preventing VEGF from diffusing to the iris from the retina.[50] Eyes with persistent or recurrent RD after TRD vitrectomy often develop intraocular inflammation, hypotony, and corneal folds, but with silicone oil, the eyes often remain quiet. Therefore, oil should be considered in eyes that are likely to fail after vitrectomy.[72]
Disadvantages of oil include cataract development, need for removal to achieve complete visual rehabilitation,[73] development of band keratopathy (particularly with oil-cornea touch),[74] and emulsification with resultant IOP elevation.[75] Oil removal is usually an elective procedure in a stable eye performed to avoid these oil-related complications. Risks of premature oil removal include recurrent retinal detachment, vitreous hemorrhage, and phthisis.
A thin layer of blood beneath silicone oil may not promote peri-silicone proliferation, but a thick hemorrhage may compartmentalize growth factors and lead to re-growth of membranes and recurrent detachments.[76] A large hemorrhage under oil should prompt a re-operation with blood removal and replacement of the oil.
Intravitreal heparin may be injected to remove pre-retinal clotted blood[64] and low-molecular-weight heparin (enoxaparin) has been added to the infusion bottle to reduce the formation of post-vitrectomy fibrin.[77]
Good visual and anatomic outcomes have been reported with combined cataract extraction and vitrectomy,[78,79,80,81,82] and compared to crystalline lens sparing vitrectomy combined surgery requires fewer re-operations.[83] Combined surgery offers the advantage of a single trip to the operating room, faster recovery, earlier improvement in vision, better post-operative visualization of the retina, and earlier treatment of the fellow eye. Disadvantages include longer surgical times and increased post-vitrectomy inflammation and fibrin formation.[65] Combined surgery is not recommended for patients with severe ischemia, iris neovascularization, and eyes with severe TRD.[63,65] Combined lensectomy/vitrectomy surgeries have been associated with a higher incidence (up to 4×) of neovascular glaucoma (NVG).[40] In high risk eyes, cataract extraction can be performed at a later date without the removal of silicone oil.
In a retrospective study of 251 eyes, those undergoing vitrectomy with delayed cataract extraction had similar visual outcomes at 4 years as those undergoing combined surgery. Cataract progression occurred in 64% of eyes, and cataract surgery was subsequently performed in 39% of phakic eyes with a median time from vitrectomy to cataract extraction of 22 months. A strong trend (P = 0.07) toward NVG development was noted in the combined surgery group.[84]
An example of a case of macula-involving TRD in a patient with PDR is shown in Figs. 3-5.
Figure 3.
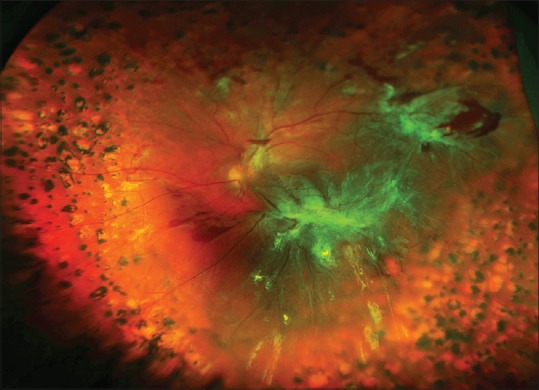
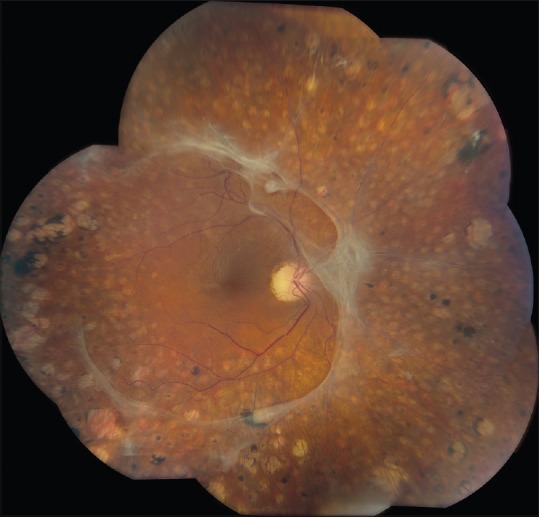
Traction retinal detachment of the left eye of a 32-year-old patient with type 1 diabetes mellitus. The visual acuity was 20/160. The right eye was blind for the past 5 years following failed surgery for traction retinal detachment elsewhere. Pre-retinal fibrous plaques are present inferior to the macula and along the superotemporal arcade. Some pre-retinal hemorrhage is present. Panretinal photocoagulation scars are present
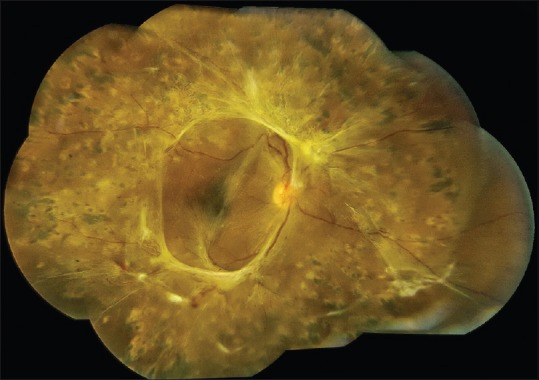
Figure 5.
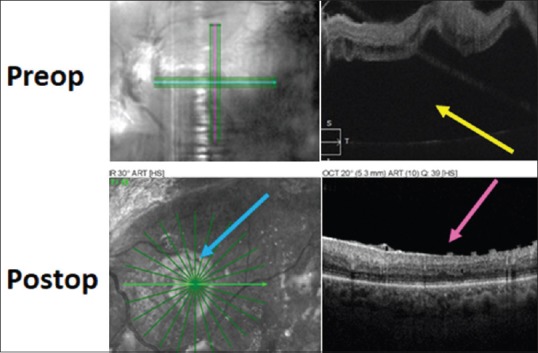
Pre-operative (top) and post-operative (bottom) optical coherence tomography images in the case of traction retinal detachment involving the macula shown in Figures 1 and 2. The yellow arrow shows subfoveal fluid pre-operatively and the fluid is gone post-operatively. The pink arrow shows loss of the foveal depression in the post-operative macula, which is a common finding. The blue arrow shows the light reflex at the silicone oil-retina interface
Figure 4.
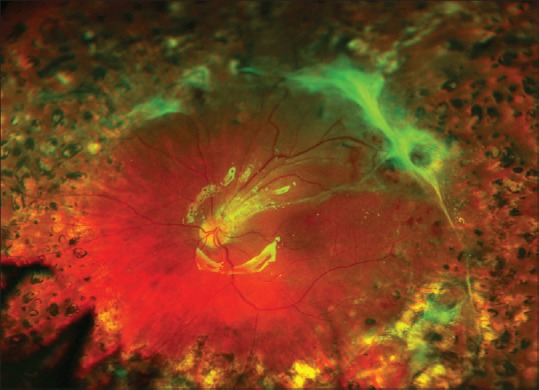
Appearance of the fundus 7 months after vitrectomy, membrane peeling, supplemental laser photocoagulation, and silicone oil injection because of an iatrogenic retinal break at the time of surgery. The visual acuity is 20/50. Not all of the fibrous plaques were removed at the time of surgery. It was judged that more aggressive peeling, sectioning, and attempts at removal would cause more iatrogenic retinal breaks. This is a common intraoperative judgment that must be made
Clinical Indications and Results
The Diabetic Retinopathy Vitrectomy Study (DRVS) is the only prospective, randomized trial of vitrectomy in diabetic patients. The DRVS validated the superiority of vitrectomy over observation, and given the overwhelming evidence in support of vitrectomy for complications of diabetes and the subsequent widespread adoption and improvement of surgical techniques, a similar trial is unlikely to be performed. In the DRVS, 36% of eyes had “moderate or severe retinal elevations” with an additional 34% having “questionable or definite elevations” before surgery.[44] The trial did not include eyes with macula-involving TRDs so extrapolation of its results to this now common indication should be done with caution. Subsequent diabetic vitrectomy studies have reported that 32%, 38%, and 46% of eyes were operated on for TRDs, with 12%, 10%, and 20% of them progressing to hand motion or less VA.[36,73,85] A more recent study of diabetic vitrectomies showed a similar TRD rate of 32%, but only 3% of eyes had poor visual outcomes after surgery.[42] Eyes best suited for vitrectomy are those with both fibrous proliferation and moderate NV in which PRP has already been performed or cannot be performed because of vitreous hemorrhage.[86]
Favorable factors for visual recovery after vitrectomy for macula-involving TRD include brief duration of detachment, presence of previous PRP, and the absence of vitreous hemorrhage and severe neovascularization.[32,34,40,73,87,88,89,90,91,92] Macular re-attachment has been achieved in 66–88% of eyes and VA improved in 49–75% of eyes after surgery,[34,40,52,86,89,90,93] with final VA of > 5/200 in 59–80% of eyes.[14,40] More recent rates of anatomic success have increased to 83–92.6%, with fewer than 25% of eyes suffering vision loss.[14,45,52,94,95,96,97,98] Among these studies, differences in entry criteria, relative proportions of TRD and combined detachment,[34,45,98,99] and use of silicone oil[100] make direct comparisons difficult. Studies show that VA after vitrectomy fluctuates within the first 6 months but tends to stabilize at 1 year and does not change from years 3 through 5.[101] In contrast with results from the DRVS, progression to no light perception (NLP) is unusual with current surgical techniques.
Predictors for poor visual outcomes include iris neovascularization and NVG,[102,103] vitreopapillary traction,[104] poor initial VA (<5/200), and the presence of TRD or RRD. Having at least one of these factors increases the risk of poor final VA by 1.5- to 3.9-fold.[101] Poor VA is usually due to persistent retinal folds, macular ischemia, cystoid macular edema, or photoreceptor damage. Center point thickness correlates weakly with post-operative VA but integrity of the external limiting membrane and ellipsoid zone are strongly correlated.[105,106,107,108]
Post-operative macular ischemia is the most sensitive correlate of poor VA following vitrectomy.[42,109] Other post-operative correlates for poor visual outcome include vitreous hemorrhage[42] and iris neovascularization.[42] About one-third of patients will develop recurrent, post-operative vitreous hemorrhage from vitreous base neovascularization, neovascularization near the sclerotomy sites, or diffusion from peripheral vitreous blood clots.[110,111,112] Some authors advocate cryotherapy to sclerotomy sites and anterior retina.[111,112] In a retrospective chart review of 114 eyes, total retinal ablation from the equator to the ora serrata combined with meticulous shaving of the vitreous base decreased the incidence of post-operative hemorrhage to 4.38%.[113]
Surgery is not usually performed for extramacular TRDs because vision is rarely affected and progression to macula-involving TRD often does not occur [Fig. 6]. Progression to macular involvement ranges from 14 to 15% at 1 year and 21 to 23% at 2 years.[44,114] A progressive extramacular TRD that threatens the macula may benefit from surgery[115] and many surgeons have expanded their surgical indications to include TRDs that threaten the macula or cause distortion since these patients frequently have metamorphopsia [Fig. 7], loss of central visual field, or reduction in VA.[116,117,118]
Figure 6.
This extrafoveal tractional retinal detachment is characterized by fibrosis with complete involution of neovascular vessels. No progression of the detachment occurred during the course of 2 years
Figure 7.
This extrafoveal traction retinal detachment has created traction lines through the fovea, giving the patient metamorphopsia. Vitrectomy was performed because of the visual symptoms
In a retrospective analysis of 114 primary vitrectomies performed over a 5-year period, 38% of patients underwent fellow eye surgery at a mean of 1.6 years after the first surgery. At baseline, 14% of patients were already blind in the fellow eye. The presence of a TRD or TRD/RRD without vitreous hemorrhage was a risk factor for fellow eye vitrectomy (odds ratio: 5.5). Additional scatter laser photocoagulation after baseline did not affect the need for subsequent vitrectomy. By 8–12 years after initial vitrectomy, 57% of patients were dead (mean time to death: 4.3 years) with hypertension and cataract in the fellow eye associated with increased mortality; regular use of aspirin; and shorter duration of diabetes were associated with decreased mortality. Good VA in at least one eye was maintained in many patients, with 54% having VA of at least 6/12 and 83% having VA of at least 6/60.[119]
In another retrospective analysis of 434 eyes undergoing primary diabetic vitrectomy, fellow eyes underwent vitrectomy at rates of 24% (1 year), 34% (3 years), and 36% (5 years). Younger patients had a higher rate of fellow eye surgery and the mean time to fellow eye surgery was 10.5 months.[120]
In a respective study of 44 eyes (33 patients) undergoing vitrectomy for central TRD, mean VA improved from 20/800 to 20/160 (P = 0.02) after 10 months. Most patients had long-standing macular detachments (median of 120 days). In 86.3% of eyes the retina was fully attached at the final examination. Advanced age (>50 years), iris neovascularization, type 2 DM, macula-involving TRD of >30 days, and VA <20/200 were associated with worse final visual outcomes. Silicone oil was removed in only 23% of eyes with most eyes tolerating long-term oil tamponade.[14]
In a series of 45 eyes with long-term silicone oil tamponade, 85.2% had anatomic success at 3 months and the best corrected visual acuity (BCVA) was stable or improved in 89%. After an average follow-up of over 2 years, breaks with adjacent unreleased traction were the only factor associated with final success (P = 0.024). Oil-related complications included peri-silicone proliferation (9), NGV (4), oil migration into the anterior chamber (9), and pupillary block (5).[72]
In a 3-year retrospective review of 42 diabetic vitrectomies in Latino patients 63% had macula-involving TRDs. Most eyes requiring post-operative tamponade received gas but 29% were treated with silicone oil. None of the eyes treated with oil developed retinal detachments; 16% of eyes treated with gas developed detachments but the numbers were too small for statistical significance. Eyes treated with gas had marginally better pre-operative and post-operative VAs. Six eyes had oil removed 3–6 months after surgery but none developed retinal detachments.[121]
In a series of combined TRD/RRDs, only 17.5% had identifiable breaks before surgery but 82.5% had breaks identified during surgery. After core vitrectomy and penetration of the posterior hyaloid, delamination and segmentation techniques were used to remove fibrovascular membranes, and avascular pre-retinal membranes were removed with forceps. Silicone oil (5000 centistokes) was used in 57% of eyes, particularly those with large, multiple breaks, retinotomies, possible undiscovered breaks, and residual traction, and 92% had long-term retinal re-attachment. A 5 mm encircling episcleral band was placed between the equator and ora serrata in every case. VA improved in 70% of eyes, was unchanged in 15% and worsened in 15%. A post-operative VA of 20/400 or better was achieved in 47% and pre-operative VA was the only factor predictive of final VA.[122] In no case was silicone oil removed but other studies have suggested that removal of oil is accompanied by better VA.[123]
In a series of eyes with TRD of at least 6 months duration, 37.5% of eyes suffered iatrogenic breaks but 90.6% were attached with one surgery and 87.5% had stable VA or one line improvement. Factors associated with better vision included younger age (<50 years), good pre-operative VA (>20/400), and good macular perfusion. These VA results compare favorably to those from studies with more acute TRDs and have emboldened surgeons to operate on more chronic TRDs.[124]
Post-operative epiretinal membranes (ERMs) are found in 21.7–52.8% of eyes despite extensive vitrectomies and numerous attempts to remove all vitreoretinal traction.[108,125,126] Risk factors for ERM formation after diabetic vitrectomy include active PDR, high-grade fibrovascular proliferation, post-operative hemorrhage, and residual fibrovascular stumps.[127] Vitreoschisis commonly occurs in eyes with TRDs,[128,129] making intraoperative identification and complete removal of the posterior hyaloid difficult. Residual cortical vitreous on the inner surface of the retina serves as a scaffold for further fibrous proliferation. Removing the internal limiting membrane (ILM) during vitrectomy serves to also remove adherent cortical vitreous and may decrease the post-operative growth of ERM.[130] For this reason, more surgeons are routinely performing ILM removal during diabetic vitrectomies.[108]
Recurrent RD after primary vitrectomy for PDR is usually severe and may render the eye inoperable.[32] Iatrogenic breaks during fibrovascular dissection are common during surgery for complicated TRDs (29%) and are more likely to be associated with a poor outcome than are peripheral breaks or dialyses,[131] but data suggest that only breaks associated with adjacent traction limit anatomic success. If removal of residual traction is not possible, segmental buckling surgery should be considered. Retinectomies should be used with caution because of concern that large retinal breaks will be created and bleeding may further complicate the surgery. The incidences of peripheral breaks during posterior hyaloid removal (6%)[132] and dialyses from sclerotomy sites (8%)[133] are much lower than posterior iatrogenic breaks and contribute much less to surgical failure.
Small-gauge instrumentation
The introduction of small-gauge vitrectomy instrumentation has changed most vitreoretinal surgeons’ approach to TRDs. The narrow diameter of the instruments and trans-scleral cannulas limit the development of scissors and multi-functional instruments, but they enable surgeons to safely, effectively, and quickly remove most fibrovascular membranes with the vitrectomy probe. A lift-and-shave technique with only the 25- and 27-gauge vitrector was used in 42 eyes with TRDs, with 90% of eyes achieving improved VA.[134] Significantly more posterior and sclerotomy-related breaks occur with 20-gauge (18.8%) compared to 23-gauge (7%) vitrectomy instruments but rates of retinal detachment are the same.[132]
In a retrospective study of 14 eyes with TRD operated with 25-gauge instruments and silicone oil, mean VA improved from 3.0 LogMAR to 1.6 LogMAR and only 14.2% developed retinal detachments. None of the sclerotomies required suturing and hypotony was seen in 21.4% of eyes 2 h after surgery but all resolved at 1 month. The authors stated that surgical manipulation of membranes and vitreoretinal adhesions outside the posterior pole were more challenging with 25-gauge instruments and they recommended against 25-gauge vitrectomy when adhesions extend to the periphery. They suggest the use of “hybrid” vitrectomies with switching from 25-gauge to 20-gauge instruments to perform lensectomies and fibrovascular dissection over the peripheral retina.[135]
In a retrospective study of 403 eyes with TRDs, 87.6% were attached after one surgery with similar success rates among eyes operated with 20-, 23-, and 25-gauge systems. BCVA improved by two or more lines in 56.3% of eyes. Eyes receiving silicone oil tamponade had lower single surgery re-attachment rates (77.6 vs. 87.6%; P = 0.013) and higher rates of vision loss (34.7 vs. 19.9%; P < 0.0001), but were more likely to have had combined TRD/RRDs (47.0 vs. 21.3%; P < 0.0001) and macula-involving detachments (74.5 vs. 60.0%; P < 0.0001).[136]
Bevacizumab
Pre-operative intravitreal injections of bevacizumab to cause involution of neovascularization was first described by Chen.[137] Pre-operative bevacizumab has been touted to decrease intraoperative bleeding,[138,139] post-operative hemorrhage,[139,140] and surgical times.[141,142] Some surgeons claim that bevacizumab improves surgical outcomes[143] but others state that final VAs are not affected.[144]
Bevacizumab administered 1–14 days before surgery improved the ease of surgery in complex cases of TRD or vitreous hemorrhage.[143] Indications for injection were macula-involving TRD with active NV [Fig. 8], rubeosis with vitreous hemorrhage that prevented more PRP, high risk features for the development of rubeosis following surgery (severe ischemia or a fellow eye that developed rubeosis after vitrectomy). Fourteen of 18 eyes had improved VA but 38.8% experienced hemorrhages post-operatively. The authors noted significant regression of the fibrovascular complexes without progression and thickening of the fibrous component or worsening of the TRD. Bevacizumab appeared to simplify surgery because the fibrovascular complex could be separated from the retina without bleeding. They reported that delamination was easier to perform but acknowledged that this was difficult to objectively evaluate. They suggested that intraoperative bevacizumab and PRP be performed in cases with severe PDR.
Figure 8.
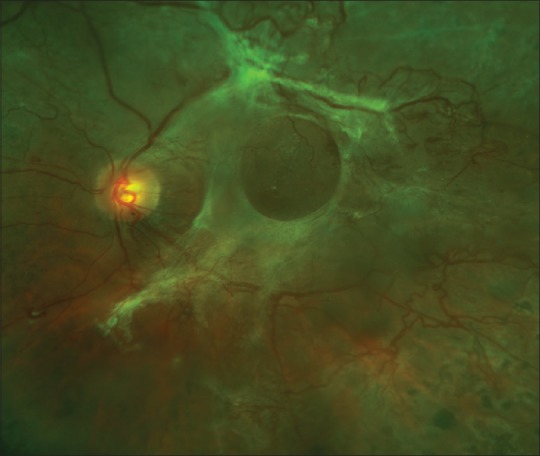
This fovea-involving traction retinal detachment is accompanied by active neovascularization. Bevacizumab was injected intravitreally 3 days prior to surgery to decrease the risk of intraoperative bleeding
Other surgeons report that bevacizumab injections should be carefully considered in eyes with macular-threatening traction. Of 38 eyes with PDR that received pre-operative bevacizumab, two eyes suffered worsening retinal traction 1.5 and 2 months later.[145] In another series, 11 eyes developed progressive traction from 3–31 days (mean of 13) after bevacizumab injections.[146] The authors speculate that the risk of progressive traction – referred to as the “crunch” syndrome - is related to severity of the fibrous tissue.
It has been suggested that VEGF blockade upregulates CTGF production by vascular endothelial cells[147] but supporting data have been difficult to obtain. Following pre-operative bevacizumab in eyes with PDR, VEGF levels were decreased and CTGF levels were also slightly decreased. The authors point out that decreased VEGF may downregulate CTGF and that the growth factors responsible for the angiogenic switch from neovascularization to fibrosis are not known. Vitrectomy is usually performed within 1 week after bevacizumab injection to take advantage of the drug's full anti-angiogenic effect and to minimize the chance of progressive traction. Surgeons using bevacizumab pre-operatively should be ready to go to the operating room on short notice because a TRD could be converted to a TRD/RRD. For this reason, bevacizumab injections should be delayed until medical clearance for surgery has been obtained.[148]
A retrospective study compared eyes receiving bevacizumab before small-incision vitrectomy (23- or 25-gauge) with results from a previous cohort of 20-gauge surgeries. The groups achieved comparable anatomic success rates and VAs, but the small-gauge cohort experienced shorter durations of surgery (by 40 min). Phacoemulsification with the insertion of an acrylic IOL was performed in most eyes. For membranes that were broadly adherent to the retina, a bimanual technique with intraocular forceps and vitrector, with assistance of a chandelier light system, was used. The vitreous base was shaved with the assistance of scleral depression. Rapid progression of TRD was noted in 18% of eyes after bevacizumab and this was more commonly seen in eyes with ring-shaped fibrovascular proliferation and absence of previous PRP.[149]
New Developments
Relieving vitreomacular traction by pharmacologically inducing a posterior vitreous detachment has been attempted for decades, but successes have been modest. Ocriplasmin is approved for the treatment of symptomatic vitreomacular traction but success rates in non-diabetic eyes are at best 50%. Phase III trials in patients with DME are underway but strong adhesive forces between the posterior hyaloid and the internal limiting membrane in patients with DR calls into question the likelihood of success.
The use of small-gauge vitrectomy instrumentation has spread throughout the world as surgeons enjoy the benefits of 23-, 25-, and 27-gauge instruments. Further refinements in vitrectors, light sources, and laser probes will occur, and scissors and multi-functional instruments are likely to be developed.
Intraoperative optical coherence tomography (OCT) has been used in eyes undergoing vitrectomy, enabling the identification of tissue planes beneath fibrovascular membranes and the presence of residual membranes for removal.
Recently introduced heads-up displays provide physicians with virtual views of the posterior pole. Robotic-assisted surgery is commonly performed by general, urologic, cardiothoracic, gynecologic, and orthopedic surgeons, and pilot studies have shown that this instrumentation can be used by corneal surgeons with good results. It is not clear what advantages this technology may offer vitreoretinal surgeons but studies are likely to be performed.
Conclusion
The treatment of TRDs and TRD/RRDs has improved significantly over the past four decades. Effective surgical management of these conditions remains challenging, however, and requires careful pre-operative planning, excellent surgical skills, prudent judgment, and careful post-operative management. Because each case presents with different anatomy, each requires a unique individualized approach. Surgical results have improved in recent years and with the continued development of techniques and instrumentation, we believe this trend will continue.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–63. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Blindness caused by diabetes – massachusetts, 1987-1994. MMWR Morb Mortal Wkly Rep. 1996;45:937–41. [PubMed] [Google Scholar]
- 3.Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study research group. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 4.Boyer DS, Yoon YH, Belfort R, Jr, Bandello F, Maturi RK, Augustin AJ. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–14. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, et al. Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–77.e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–54. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris FL., 3rd Results of 20 years of research on the treatment of diabetic retinopathy. Prev Med. 1994;23:740–2. doi: 10.1006/pmed.1994.1127. [DOI] [PubMed] [Google Scholar]
- 10.Photocoagulation treatment of proliferative diabetic retinopathy: The second report of diabetic retinopathy study findings. Ophthalmology. 1978;85:82–106. doi: 10.1016/s0161-6420(78)35693-1. [DOI] [PubMed] [Google Scholar]
- 11.Flynn HW, Jr, Chew EY, Simons BD, Barton FB, Remaley NA, Ferris FL., 3rd Pars plana vitrectomy in the early treatment diabetic retinopathy study. ETDRS report number 17. The early treatment diabetic retinopathy study research group. Ophthalmology. 1992;99:1351–7. doi: 10.1016/s0161-6420(92)31779-8. [DOI] [PubMed] [Google Scholar]
- 12.Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: A pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:813–20. [PubMed] [Google Scholar]
- 13.Aaberg TM. Vitrectomy for diabetic retinopathy. In: Freeman HM, Hirose T, Schepens CL, editors. Vitreous Surgery and Advances in Fundus Diagnosis and Treatment. New York: Appleton-Century-Crofts; 1977. pp. 297–313. [Google Scholar]
- 14.La Heij EC, Tecim S, Kessels AG, Liem AT, Japing WJ, Hendrikse F, et al. Clinical variables and their relation to visual outcome after vitrectomy in eyes with diabetic retinal traction detachment. Graefes Arch Clin Exp Ophthalmol. 2004;242:210–7. doi: 10.1007/s00417-003-0815-5. [DOI] [PubMed] [Google Scholar]
- 15.Brucker AJ, Michels RG, Green WR. Pars plana vitrectomy in the management of blood-induced glaucoma with vitreous hemorrhage. Ann Ophthalmol. 1978;10:1427–37. [PubMed] [Google Scholar]
- 16.Campbell DG, Simmons RJ, Grant WM. Ghost cells as a cause of glaucoma. Am J Ophthalmol. 1976;81:441–50. doi: 10.1016/0002-9394(76)90299-3. [DOI] [PubMed] [Google Scholar]
- 17.Campbell DG, Simmons RJ, Tolentino FI, McMeel JW. Glaucoma occurring after closed vitrectomy. Am J Ophthalmol. 1977;83:63–9. doi: 10.1016/0002-9394(77)90193-3. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg RS, Peyman GA, Huamonte FU. Elevation of intraocular pressure after pars plana vitrectomy. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1976;200:157–61. doi: 10.1007/BF00414365. [DOI] [PubMed] [Google Scholar]
- 19.Wilensky JT, Goldberg MF, Alward P. Glaucoma after pars plana vitrectomy. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83:114–21. [PubMed] [Google Scholar]
- 20.O’Hanley GP, Canny CL. Diabetic dense premacular hemorrhage. A possible indication for prompt vitrectomy. Ophthalmology. 1985;92:507–11. doi: 10.1016/s0161-6420(85)34014-9. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay RC, Knobloch WH, Cantrill HL. Timing of vitrectomy for active proliferative diabetic retinopathy. Ophthalmology. 1986;93:283–9. doi: 10.1016/s0161-6420(86)33742-4. [DOI] [PubMed] [Google Scholar]
- 22.Aaberg TM, Abrams GW. Changing indications and techniques for vitrectomy in management of complications of diabetic retinopathy. Ophthalmology. 1987;94:775–9. doi: 10.1016/s0161-6420(87)33528-6. [DOI] [PubMed] [Google Scholar]
- 23.Harbour JW, Smiddy WE, Flynn HW, Jr, Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121:405–13. doi: 10.1016/s0002-9394(14)70437-4. [DOI] [PubMed] [Google Scholar]
- 24.Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99:753–9. doi: 10.1016/s0161-6420(92)31901-3. [DOI] [PubMed] [Google Scholar]
- 25.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 26.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 27.Diederen RM, La Heij EC, Deutz NE, Kessels AG, van Eijk HM, Hendrikse F, et al. Increased nitric oxide (NO) pathway metabolites in the vitreous fluid of patients with rhegmatogenous retinal detachment or diabetic traction retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2006;244:683–8. doi: 10.1007/s00417-005-0141-1. [DOI] [PubMed] [Google Scholar]
- 28.Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10:179–90. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- 29.Dai Y, Wu Z, Wang F, Zhang Z, Yu M. Identification of chemokines and growth factors in proliferative diabetic retinopathy vitreous. Biomed Res Int 2014. 2014:486386. doi: 10.1155/2014/486386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis MD. Vitreous contraction in proliferative diabetic retinopathy. Arch Ophthalmol. 1965;74:741–51. doi: 10.1001/archopht.1965.00970040743003. [DOI] [PubMed] [Google Scholar]
- 31.Faulborn J, Bowald S. Microproliferations in proliferative diabetic retinopathy and their relationship to the vitreous: Corresponding light and microscopic studies. Graefes Arch Clin Exp Ophthalmol. 1985;223:130. doi: 10.1007/BF02148888. [DOI] [PubMed] [Google Scholar]
- 32.Eliott D, Lee MS, Abrams GW. Proliferative diabetic retinopathy: Principles and techniques of surgical treatment. In: Ryan SJ, editor. Retina. 4th ed. Amsterdam, The Netherlands: Elsevier Inc; 2006. pp. 2413–49. [Google Scholar]
- 33.Tasman W. Retinal detachment secondary to proliferative diabetic retinopathy. Arch Ophthalmol. 1972;87:286–9. doi: 10.1001/archopht.1972.01000020288010. [DOI] [PubMed] [Google Scholar]
- 34.Thompson JT, de Bustros S, Michels RG, Rice TA. Results and prognostic factors in vitrectomy for diabetic traction-rhegmatogenous retinal detachment. Arch Ophthalmol. 1987;105:503–7. doi: 10.1001/archopht.1987.01060040073036. [DOI] [PubMed] [Google Scholar]
- 35.Kakehashi A, Trempe CL, Fujio N, McMeel JW, Schepens CL. Retinal breaks in diabetic retinopathy: Vitreoretinal relationships. Ophthalmic Surg. 1994;25:695–9. [PubMed] [Google Scholar]
- 36.Yang CM. Surgical treatment for diabetic retinopathy: 5-year experience. J Formos Med Assoc. 1998;97:477–84. [PubMed] [Google Scholar]
- 37.Miettinen H, Haffner SM, Lehto S, Rönnemaa T, Pyörälà K, Laakso M, et al. Retinopathy predicts coronary heart disease events in NIDDM patients. Diabetes Care. 1996;19:1445–8. doi: 10.2337/diacare.19.12.1445. [DOI] [PubMed] [Google Scholar]
- 38.Klein R, Moss SE, Klein BE, DeMets DL. Relation of ocular and systemic factors to survival in diabetes. Arch Intern Med. 1989;149:266–72. [PubMed] [Google Scholar]
- 39.Aiello LP, Cahill MT, Wong JS. Systemic considerations in the management of diabetic retinopathy. Am J Ophthalmol. 2001;132:760–76. doi: 10.1016/s0002-9394(01)01124-2. [DOI] [PubMed] [Google Scholar]
- 40.Rice TA, Michels RG, Rice EF. Vitrectomy for diabetic rhegmatogenous retinal detachment. Am J Ophthalmol. 1983;95:34–44. doi: 10.1016/0002-9394(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 41.Cunha-Vaz JG. Medical treatment of retinopathy of type-2 diabetes. Ophthalmologica. 2004;218:291–6. doi: 10.1159/000079469. [DOI] [PubMed] [Google Scholar]
- 42.Mason JO, 3rd, Colagross CT, Haleman T, Fuller JJ, White MF, Feist RM, et al. Visual outcome and risk factors for light perception and no light perception vision after vitrectomy for diabetic retinopathy. Am J Ophthalmol. 2005;140:231–5. doi: 10.1016/j.ajo.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 43.Sullu Y, Hamidova R, Beden U, Yakupov K, Canbaz S, Danaci M, et al. Effects of pars plana vitrectomy on retrobulbar haemodynamics in diabetic retinopathy. Clin Exp Ophthalmol. 2005;33:246–51. doi: 10.1111/j.1442-9071.2005.01013.x. [DOI] [PubMed] [Google Scholar]
- 44.Two-year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Diabetic retinopathy vitrectomy study (DRVS) report #1. Ophthalmology. 1985;92:492–502. doi: 10.1016/s0161-6420(85)34002-2. [DOI] [PubMed] [Google Scholar]
- 45.Castellarin A, Grigorian R, Bhagat N, Del Priore L, Zarbin MA. Vitrectomy with silicone oil infusion in severe diabetic retinopathy. Br J Ophthalmol. 2003;87:318–21. doi: 10.1136/bjo.87.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eliott D. Vitreoretinal Attachments in Proliferative Diabetic Retinopathy: Effect on Outcome. Vail Vitrectomy Meeting Vail, CO. 2004 [Google Scholar]
- 47.Su CC, Yang CH, Yeh PT, Yang CM. Macular tractional retinoschisis in proliferative diabetic retinopathy: Clinical characteristics and surgical outcome. Ophthalmologica. 2014;231:23–30. doi: 10.1159/000355078. [DOI] [PubMed] [Google Scholar]
- 48.Chao DL, Flynn HW., Jr Stability of macular traction in involutional diabetic retinopathy over a 5-year course. Ophthalmic Surg Lasers Imaging Retina. 2015;46:131–3. doi: 10.3928/23258160-20150101-25. [DOI] [PubMed] [Google Scholar]
- 49.Meredith TA, Kaplan HJ, Aaberg TM. Pars plana vitrectomy techniques for relief of epiretinal traction by membrane segmentation. Am J Ophthalmol. 1980;89:408–13. doi: 10.1016/0002-9394(80)90012-4. [DOI] [PubMed] [Google Scholar]
- 50.Charles S. Vitreous Microsurgery. Baltimore: Williams and Wilkins; 2002. pp. 107–20. [Google Scholar]
- 51.Abrams GW, Williams GA. “En bloc” excision of diabetic membranes. Am J Ophthalmol. 1987;103:302–8. [PubMed] [Google Scholar]
- 52.Williams DF, Williams GA, Hartz A, Mieler WF, Abrams GW, Aaberg TM, et al. Results of vitrectomy for diabetic traction retinal detachments using the en bloc excision technique. Ophthalmology. 1989;96:752–8. doi: 10.1016/s0161-6420(89)32813-2. [DOI] [PubMed] [Google Scholar]
- 53.Han DP, Murphy ML, Mieler WF. A modified en bloc excision technique during vitrectomy for diabetic traction retinal detachment. Results and complications. Ophthalmology. 1994;101:803–8. doi: 10.1016/s0161-6420(94)31255-3. [DOI] [PubMed] [Google Scholar]
- 54.Kakehashi A. Total en bloc excision: A modified vitrectomy technique for proliferative diabetic retinopathy. Am J Ophthalmol. 2002;134:763–5. doi: 10.1016/s0002-9394(02)01680-x. [DOI] [PubMed] [Google Scholar]
- 55.Chaudhry NA, Lim ES, Saito Y, Mieler WF, Liggett PE, Filatov V, et al. Early vitrectomy and endolaser photocoagulation in patients with type I diabetes with severe vitreous hemorrhage. Ophthalmology. 1995;102:1164–9. doi: 10.1016/s0161-6420(95)30895-0. [DOI] [PubMed] [Google Scholar]
- 56.Lakhanpal RR, Humayun MS, de Juan E, Jr, Lim JI, Chong LP, Chang TS. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology. 2005;112:817–24. doi: 10.1016/j.ophtha.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 57.Berk Ergun S, Toklu Y, Cakmak HB, Raza S, Simsek S. The effect of intravitreal bevacizumab as a pretreatment of vitrectomy for diabetic vitreous hemorrhage on recurrent hemorrhage. Semin Ophthalmol. 2015;30:177–80. doi: 10.3109/08820538.2013.835847. [DOI] [PubMed] [Google Scholar]
- 58.Arevalo JF, Sanchez JG, Saldarriaga L, Berrocal MH, Fromow-Guerra J, Morales-Canton V, et al. Retinal detachment after bevacizumab. Ophthalmology. 2011;118:2304.e3–7. doi: 10.1016/j.ophtha.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 59.Dugel PU, Abulon DJ, Dimalanta R. Comparison of attraction capabilities associated with high-speed, dual-pneumatic vitrectomy probes. Retina. 2015;35:915–20. doi: 10.1097/IAE.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 60.Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25:208–11. doi: 10.1097/00006982-200502000-00015. [DOI] [PubMed] [Google Scholar]
- 61.Thompson MJ, Ip MS. Diabetic macular edema: A review of past, present, and future therapies. Int Ophthalmol Clin. 2004;44:51–67. doi: 10.1097/00004397-200404440-00006. [DOI] [PubMed] [Google Scholar]
- 62.Helbig H, Sutter FK. Surgical treatment of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2004;242:704–9. doi: 10.1007/s00417-004-0977-9. [DOI] [PubMed] [Google Scholar]
- 63.Lahey JM, Francis RR, Kearney JJ, Cheung M. Combining phacoemulsification and vitrectomy in patients with proliferative diabetic retinopathy. Curr Opin Ophthalmol. 2004;15:192–6. doi: 10.1097/01.icu.0000120676.27548.0e. [DOI] [PubMed] [Google Scholar]
- 64.Imamura Y, Kamei M, Minami M, Ueki M, Ikeda T. Heparin-assisted removal of clotting preretinal hemorrhage during vitrectomy for proliferative diabetic retinopathy. Retina. 2005;25:793–5. doi: 10.1097/00006982-200509000-00020. [DOI] [PubMed] [Google Scholar]
- 65.Treumer F, Bunse A, Rudolf M, Roider J. Pars plana vitrectomy, phacoemulsification and intraocular lens implantation. Comparison of clinical complications in a combined versus two-step surgical approach. Graefes Arch Clin Exp Ophthalmol. 2005;3:1–8. doi: 10.1007/s00417-005-0146-9. [DOI] [PubMed] [Google Scholar]
- 66.Kono T, Shiga S, Takesue Y, Sakamoto T. Long-term results of pars plana vitrectomy combined with filtering surgery for neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2005;36:211–6. [PubMed] [Google Scholar]
- 67.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes 4 years after a trial of intensive therapy. N Engl J Med. 2000;342L:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morse LS, McCuen BW., 2nd The use of silicone oil in uveitis and hypotony. Retina. 1991;11:399–404. doi: 10.1097/00006982-199111040-00006. [DOI] [PubMed] [Google Scholar]
- 69.Lucke KH, Foerster MH, Laqua H. Long-term results of vitrectomy and silicone oil in 500 cases of complicated retinal detachments. Am J Ophthalmol. 1987;104:624–33. doi: 10.1016/0002-9394(87)90176-0. [DOI] [PubMed] [Google Scholar]
- 70.Brourman ND, Blumenkranz MS, Cox MS, Trese MT. Silicone oil for the treatment of severe proliferative diabetic retinopathy. Ophthalmology. 1989;96:759–64. doi: 10.1016/s0161-6420(89)32828-4. [DOI] [PubMed] [Google Scholar]
- 71.Riedel KG, Gabel VP, Neubauer L, Kampik A, Lund OE. Intravitreal silicone oil injection: Complications and treatment of 415 consecutive patients. Graefes Arch Clin Exp Ophthalmol. 1990;228:19–23. doi: 10.1007/BF02764284. [DOI] [PubMed] [Google Scholar]
- 72.Shen YD, Yang CM. Extended silicone oil tamponade in primary vitrectomy for complex retinal detachment in proliferative diabetic retinopathy: A long-term follow-up study. Eur J Ophthalmol. 2007;17:954–60. doi: 10.1177/112067210701700614. [DOI] [PubMed] [Google Scholar]
- 73.Thompson JT, Auer CL, de Bustros S, Michels RG, Rice TA, Glaser BM, et al. Prognostic indicators of success and failure in vitrectomy for diabetic retinopathy. Ophthalmology. 1986;93:290–5. doi: 10.1016/s0161-6420(86)33741-2. [DOI] [PubMed] [Google Scholar]
- 74.La Heij EC, Hendrikse F, Kessels AG. Results and complications of temporary silicone oil tamponade in patients with complicated retinal detachments. Retina. 2001;21:107–14. doi: 10.1097/00006982-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Gonvers M. Temporary silicone oil tamponade in the treatment of complicated diabetic retinal detachments. Graefes Arch Clin Exp Ophthalmol. 1990;228:415–22. doi: 10.1007/BF00927253. [DOI] [PubMed] [Google Scholar]
- 76.Pearson RV, McLeod D, Gregor ZJ. Removal of silicone oil following diabetic vitrectomy. Br J Ophthalmol. 1993;77:204–7. doi: 10.1136/bjo.77.4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lane RG, Jumper JM, Nasir MA, MacCumber MW, McCuen BW., 2nd A prospective, open-label, dose-escalating study of low molecular weight heparin during repeat vitrectomy for PVR and severe diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2005;243:701–5. doi: 10.1007/s00417-004-0912-0. [DOI] [PubMed] [Google Scholar]
- 78.Peyman GA, Huamonte F, Goldberg MF. Management of cataract in patients undergoing vitrectomy. Am J Ophthalmol. 1975;80:30–6. doi: 10.1016/0002-9394(75)90864-8. [DOI] [PubMed] [Google Scholar]
- 79.Kokame GT, Flynn HW, Jr, Blankenship GW. Posterior chamber intraocular lens implantation during diabetic pars plana vitrectomy. Ophthalmology. 1989;96:603–10. doi: 10.1016/s0161-6420(89)32842-9. [DOI] [PubMed] [Google Scholar]
- 80.Benson WE, Brown GC, Tasman W, McNamara JA. Extracapsular cataract extraction, posterior chamber lens insertion, and pars plana vitrectomy in one operation. Ophthalmology. 1990;97:918–21. doi: 10.1016/s0161-6420(90)32481-8. [DOI] [PubMed] [Google Scholar]
- 81.Koenig SB, Han DP, Mieler WF, Abrams GW, Jaffe GJ, Burton TC, et al. Combined phacoemulsification and pars plana vitrectomy. Arch Ophthalmol. 1990;108:362–4. doi: 10.1001/archopht.1990.01070050060031. [DOI] [PubMed] [Google Scholar]
- 82.Treumer F, Bunse A, Rudolf M, Roider J. Pars plana vitrectomy, phacoemulsification and intraocular lens implantation. Comparison of clinical complications in a combined versus two-step surgical approach. Graefes Arch Clin Exp Ophthalmol. 2006;244:808–15. doi: 10.1007/s00417-005-0146-9. [DOI] [PubMed] [Google Scholar]
- 83.Schiff WM, Barile GR, Hwang JC, Tseng JJ, Cekiç O, Del Priore LV, et al. Diabetic vitrectomy: Influence of lens status upon anatomic and visual outcomes. Ophthalmology. 2007;114:544–50. doi: 10.1016/j.ophtha.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 84.Silva PS, Diala PA, Hamam RN, Arrigg PG, Shah ST, Murtha TL, et al. Visual outcomes from pars plana vitrectomy versus combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation in patients with diabetes. Retina. 2014;34:1960–8. doi: 10.1097/IAE.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 85.Smiddy WE, Feuer W, Irvine WD, Flynn HW, Jr, Blankenship GW. Vitrectomy for complications of proliferative diabetic retinopathy Functional outcomes. Ophthalmology. 1995;102:1688–95. doi: 10.1016/s0161-6420(95)30808-1. [DOI] [PubMed] [Google Scholar]
- 86.Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Clinical application of results of a randomized trial – Diabetic retinopathy vitrectomy study report 4. The diabetic retinopathy vitrectomy study research group. Ophthalmology. 1988;95:1321–34. doi: 10.1016/s0161-6420(88)33014-9. [DOI] [PubMed] [Google Scholar]
- 87.Aaberg TM. Pars plana vitrectomy for diabetic traction retinal detachment. Ophthalmology. 1981;88:639–42. doi: 10.1016/s0161-6420(81)34973-2. [DOI] [PubMed] [Google Scholar]
- 88.Gardner TW, Blankenship GW. Proliferative diabetic retinopathy: Principles and techniques of surgical treatment. In: Ryan SJ, editor. Retina. 2nd ed. St. Louis: Mosby; 1994. pp. 515–39. [Google Scholar]
- 89.Peyman GA, Huamonte FU, Goldberg MF, Sanders DR, Nagpal KC, Raichand M, et al. Four hundred consecutive pars plana vitrectomies with the vitrophage. Arch Ophthalmol. 1978;96:45–50. doi: 10.1001/archopht.1978.03910050009002. [DOI] [PubMed] [Google Scholar]
- 90.Tolentino FI, Freeman HM, Tolentino FL. Closed vitrectomy in the management of diabetic traction retinal detachment. Ophthalmology. 1980;87:1078–89. doi: 10.1016/s0161-6420(80)35115-4. [DOI] [PubMed] [Google Scholar]
- 91.Blankenship GW. Preoperative prognostic factors in diabetic pars plana vitrectomy. Ophthalmology. 1982;89:1246–9. doi: 10.1016/s0161-6420(82)34654-0. [DOI] [PubMed] [Google Scholar]
- 92.Ho T, Smiddy WE, Flynn HW., Jr Vitrectomy in the management of diabetic eye disease. Surv Ophthalmol. 1992;37:190–202. doi: 10.1016/0039-6257(92)90137-i. [DOI] [PubMed] [Google Scholar]
- 93.Aaberg TM. Clinical results in vitrectomy for diabetic traction retinal detachment. Am J Ophthalmol. 1979;88:246–53. doi: 10.1016/0002-9394(79)90473-2. [DOI] [PubMed] [Google Scholar]
- 94.Steinmetz RL, Grizzard WS, Hammer ME. Vitrectomy for diabetic traction retinal detachment using the multiport illumination system. Ophthalmology. 2002;109:2303–7. doi: 10.1016/s0161-6420(02)01291-5. [DOI] [PubMed] [Google Scholar]
- 95.Tao Y, Jiang YR, Li XX, Gao L, Jonas JB. Long-term results of vitrectomy without endotamponade in proliferative diabetic retinopathy with tractional retinal detachment. Retina. 2010;30:447–51. doi: 10.1097/IAE.0b013e3181d374a5. [DOI] [PubMed] [Google Scholar]
- 96.Rahimy E, Pitcher JD, 3rd, Gee CJ, Kreiger AE, Schwartz SD, Hubschman JP, et al. Diabetic tractional retinal detachment repair by vitreoretinal fellows in a county health system. Retina. 2015;35:303–9. doi: 10.1097/IAE.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 97.Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D, et al. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:365–8. doi: 10.1136/bjo.2007.124495. [DOI] [PubMed] [Google Scholar]
- 98.Imamura Y, Minami M, Ueki M, Satoh B, Ikeda T. Use of perfluorocarbon liquid during vitrectomy for severe proliferative diabetic retinopathy. Br J Ophthalmol. 2003;87:563–6. doi: 10.1136/bjo.87.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maturi RK, Merrill PT, Lomeo MD, Diaz-Rohena R, Khan M, Lambert HM, et al. Perfluoro-N-octane (PFO) in the repair of complicated retinal detachments due to severe proliferative diabetic retinopathy. Ophthalmic Surg Lasers. 1999;30:715–20. [PubMed] [Google Scholar]
- 100.Azen SP, Scott IU, Flynn HW, Jr, Lai MY, Topping TM, Benati L. Silicone oil in the repair of complex retinal detachments. A prospective observational multicenter study. Ophthalmology. 1998;105:1587–97. doi: 10.1016/S0161-6420(98)99023-6. [DOI] [PubMed] [Google Scholar]
- 101.Nakazawa M, Kimizuka Y, Watabe T, Kato K, Watanabe H, Yamanobe S, et al. Visual outcome after vitrectomy for diabetic retinopathy. A five-year follow-up. Acta Ophthalmol (Copenh) 1993;71:219–23. doi: 10.1111/j.1755-3768.1993.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 102.de Bustros S, Thompson JT, Michels RG, Rice TA. Vitrectomy for progressive proliferative diabetic retinopathy. Arch Ophthalmol. 1987;105:196–9. doi: 10.1001/archopht.1987.01060020050026. [DOI] [PubMed] [Google Scholar]
- 103.McCuen BW, 2nd, Rinkoff JS. Silicone oil for progressive anterior ocular neovascularization after failed diabetic vitrectomy. Arch Ophthalmol. 1989;107:677–82. doi: 10.1001/archopht.1989.01070010695029. [DOI] [PubMed] [Google Scholar]
- 104.Kroll P, Wiegand W, Schmidt J. Vitreopapillary traction in proliferative diabetic vitreoretinopathy. Br J Ophthalmol. 1999;83:261–4. doi: 10.1136/bjo.83.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dooley I, Laviers H, Papavasileiou E, Mckechnie C, Zambarakji H. Spectral domain ocular coherence tomography findings pre- and post vitrectomy with fibrovascular membrane delamination for proliferative diabetic retinopathy. Eye (Lond) 2016;30:34–9. doi: 10.1038/eye.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murakami T, Uji A, Ogino K, Unoki N, Yoshitake S, Dodo Y, et al. Macular morphologic findings on optical coherence tomography after microincision vitrectomy for proliferative diabetic retinopathy. Jpn J Ophthalmol. 2015;59:236–43. doi: 10.1007/s10384-015-0382-4. [DOI] [PubMed] [Google Scholar]
- 107.Shah VA, Brown JS, Mahmoud TH. Correlation of outer retinal microstucture and foveal thickness with visual acuity after pars plana vitrectomy for complications of proliferative diabetic retinopathy. Retina. 2012;32:1775–80. doi: 10.1097/IAE.0b013e318255068a. [DOI] [PubMed] [Google Scholar]
- 108.Im JC, Kim JH, Park DH, Shin JP. Structural changes of the macula on optical coherence tomography after vitrectomy for proliferative diabetic retinopathy. Ophthalmologica. 2017;238:186–95. doi: 10.1159/000477826. [DOI] [PubMed] [Google Scholar]
- 109.Ishida M, Takeuchi S. Long-term results of vitrectomy for complications of proliferative diabetic retinopathy. Nippon Ganka Gakkai Zasshi. 2001;105:457–62. [PubMed] [Google Scholar]
- 110.Soto-Pedre E, Hernaez-Ortega MC, Vazquez JA. Risk factors for postoperative hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmic Epidemiol. 2005;12:335–41. doi: 10.1080/09286580500227068. [DOI] [PubMed] [Google Scholar]
- 111.Hershberger VS, Augsburger JJ, Hutchins RK, Raymond LA, Krug S. Fibrovascular ingrowth at sclerotomy sites in vitrectomized diabetic eyes with recurrent vitreous hemorrhage: Ultrasound biomicroscopy findings. Ophthalmology. 2004;111:1215–21. doi: 10.1016/j.ophtha.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 112.Yeh PT, Yang CM, Yang CH, Huang JS. Cryotherapy of the anterior retina and sclerotomy sites in diabetic vitrectomy to prevent recurrent vitreous hemorrhage: An ultrasound biomicroscopy study. Ophthalmology. 2005;112:2095–102. doi: 10.1016/j.ophtha.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 113.Mason JO, 3rd, Colagross CT, Vail R. Diabetic vitrectomy: Risks, prognosis, future trends. Curr Opin Ophthalmol. 2006;17:281–5. doi: 10.1097/01.icu.0000193098.28798.18. [DOI] [PubMed] [Google Scholar]
- 114.Charles S, Flinn CE. The natural history of diabetic extramacular traction retinal detachment. Arch Ophthalmol. 1981;99:66–8. doi: 10.1001/archopht.1981.03930010068003. [DOI] [PubMed] [Google Scholar]
- 115.Packer AJ. Vitrectomy for progressive macular traction associated with proliferative diabetic retinopathy. Arch Ophthalmol. 1987;105:1679–82. doi: 10.1001/archopht.1987.01060120077029. [DOI] [PubMed] [Google Scholar]
- 116.Bresnick GH, Haight B, de Venecia G. Retinal wrinkling and macular heterotopia in diabetic retinopathy. Arch Ophthalmol. 1979;97:1890–5. doi: 10.1001/archopht.1979.01020020338010. [DOI] [PubMed] [Google Scholar]
- 117.Bresnick GH, Smith V, Pokorny J. Visual function abnormalities in macular heterotopia caused by proliferative diabetic retinopathy. Am J Ophthalmol. 1981;92:85–102. doi: 10.1016/s0002-9394(14)75912-4. [DOI] [PubMed] [Google Scholar]
- 118.Michael JC, de Venecia G, Bresnick GH. Macular heterotopia in proliferative diabetic retinopathy. Arch Ophthalmol. 1994;112:1455–9. doi: 10.1001/archopht.1994.01090230069022. [DOI] [PubMed] [Google Scholar]
- 119.Vote BJ, Gamble GD, Polkinghorne PJ. Auckland proliferative diabetic vitrectomy fellow eye study. Clin Exp Ophthalmol. 2004;32:397–403. doi: 10.1111/j.1442-9071.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 120.Hwang JC, Sharma AG, Eliott D. Fellow eye vitrectomy for proliferative diabetic retinopathy in an inner city population. Br J Ophthalmol. 2013;97:297–301. doi: 10.1136/bjophthalmol-2012-302233. [DOI] [PubMed] [Google Scholar]
- 121.Flaxel CJ, Dustin L, Kim J, Bekendam P, Row P. Outcome of diabetic vitrectomy in Latino population. Retina. 2007;27:1274–8. doi: 10.1097/IAE.0b013e31805d0bfb. [DOI] [PubMed] [Google Scholar]
- 122.Yang CM, Su PY, Yeh PT, Chen MS. Combined rhegmatogenous and traction retinal detachment in proliferative diabetic retinopathy: Clinical manifestations and surgical outcome. Can J Ophthalmol. 2008;43:192–8. doi: 10.3129/i08-007. [DOI] [PubMed] [Google Scholar]
- 123.Douglas MJ, Scott IU, Flynn HW., Jr Pars plana lensectomy, pars plana vitrectomy, and silicone oil tamponade as initial management of cataract and combined traction/rhegmatogenous retinal detachment involving the macula associated with severe proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging. 2003;34:270–8. [PubMed] [Google Scholar]
- 124.Abunajma MA, Al-Dhibi H, Abboud EB, Al Zahrani Y, Alharthi E, Alkharashi A, et al. The outcomes and prognostic factors of vitrectomy in chronic diabetic traction macular detachment. Clin Ophthalmol. 2016;10:1653–61. doi: 10.2147/OPTH.S98555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang CM, Yeh PT, Cheng SF, Yang CH, Chen MS. Macular appearance after diabetic vitrectomy for fibrovascular proliferation: An optical coherence tomography study. Acta Ophthalmol. 2010;88:193–8. doi: 10.1111/j.1755-3768.2008.01420.x. [DOI] [PubMed] [Google Scholar]
- 126.Meier P, Wiedemann P. Vitrectomy for traction macular detachment in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1997;235:569–74. doi: 10.1007/BF00947086. [DOI] [PubMed] [Google Scholar]
- 127.Hsu YR, Yang CM, Yeh PT. Clinical and histological features of epiretinal membrane after diabetic vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2014;252:401–10. doi: 10.1007/s00417-013-2479-0. [DOI] [PubMed] [Google Scholar]
- 128.Gandorfer A, Rohleder M, Grosselfinger S, Haritoglou C, Ulbig M, Kampik A, et al. Epiretinal pathology of diffuse diabetic macular edema associated with vitreomacular traction. Am J Ophthalmol. 2005;139:638–52. doi: 10.1016/j.ajo.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 129.Itakura H, Kishi S, Kotajima N, Murakami M. Persistent secretion of vascular endothelial growth factor into the vitreous cavity in proliferative diabetic retinopathy after vitrectomy. Ophthalmology. 2004;111:1880–4. doi: 10.1016/j.ophtha.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 130.Chang PY, Yang CM, Yang CH, Chen MS, Wang JY. Pars plana vitrectomy for diabetic fibrovascular proliferation with and without internal limiting membrane peeling. Eye (Lond) 2009;23:960–5. doi: 10.1038/eye.2008.334. [DOI] [PubMed] [Google Scholar]
- 131.Kamura Y, Sato Y, Deguchi Y, Yagi F. Iatrogenic retinal breaks during 20-gauge vitrectomy for proliferative diabetic retinopathy. Clin Ophthalmol. 2013;7:29–33. doi: 10.2147/OPTH.S38784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Issa SA, Connor A, Habib M, Steel DH. Comparison of retinal breaks observed during 23 gauge transconjunctival vitrectomy versus conventional 20 gauge surgery for proliferative diabetic retinopathy. Clin Ophthalmol. 2011;5:109–14. doi: 10.2147/OPTH.S16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oyakawa RT, Schachat AP, Michels RG, Rice TA. Complications of vitreous surgery for diabetic retinopathy. I. Intraoperative complications. Ophthalmology. 1983;90:517–21. doi: 10.1016/s0161-6420(83)34526-7. [DOI] [PubMed] [Google Scholar]
- 134.Berrocal MH. All-probe vitrectomy dissection techniques for diabetic tractional retinal detachments: Lift and shave. Retina. 2018;38(Suppl 1):S2–4. doi: 10.1097/IAE.0000000000001884. [DOI] [PubMed] [Google Scholar]
- 135.Altan T, Acar N, Kapran Z, Unver YB, Ozdogan S. Transconjunctival 25-gauge sutureless vitrectomy and silicone oil injection in diabetic tractional retinal detachment. Retina. 2008;28:1201–6. doi: 10.1097/IAE.0b013e3181853d3c. [DOI] [PubMed] [Google Scholar]
- 136.Storey PP, Ter-Zakarian A, Philander SA, Olmos de Koo L, George M, Humayun MS, et al. Visual and anatomical outcomes after diabetic traction and traction-rhegmatogenous retinal detachment repair. Retina. 2017 Aug 8; doi: 10.1097/IAE.0000000000001793. doi: 10.1097/IAE.0000000000001793. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 137.Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006;26:699–700. doi: 10.1097/01.iae.0000225351.87205.69. [DOI] [PubMed] [Google Scholar]
- 138.Hattori T, Shimada H, Nakashizuka H, Mizutani Y, Mori R, Yuzawa M, et al. Dose of intravitreal bevacizumab (Avastin) used as preoperative adjunct therapy for proliferative diabetic retinopathy. Retina. 2010;30:761–4. doi: 10.1097/IAE.0b013e3181c70168. [DOI] [PubMed] [Google Scholar]
- 139.Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: A randomized clinical trial. Ophthalmology. 2009;116:1943–8. doi: 10.1016/j.ophtha.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 140.Lo WR, Kim SJ, Aaberg TM, Sr, Bergstrom C, Srivastava SK, Yan J. Visual outcomes and incidence of recurrent vitreous hemorrhage after vitrectomy in diabetic eyes pretreated with bevacizumab (avastin) Retina. 2009;29:926–31. doi: 10.1097/IAE.0b013e3181a8eb88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rizzo S, Genovesi-Ebert F, Di Bartolo E, Vento A, Miniaci S, Williams G, et al. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR) Graefes Arch Clin Exp Ophthalmol. 2008;246:837–42. doi: 10.1007/s00417-008-0774-y. [DOI] [PubMed] [Google Scholar]
- 142.di Lauro R, De Ruggiero P, di Lauro R, di Lauro MT, Romano MR. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:785–91. doi: 10.1007/s00417-010-1303-3. [DOI] [PubMed] [Google Scholar]
- 143.Yeoh J, Williams C, Allen P, Buttery R, Chiu D, Clark B, et al. Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: A prospective case series. Clin Exp Ophthalmol. 2008;36:449–54. [PubMed] [Google Scholar]
- 144.Pokroy R, Desai UR, Du E, Li Y, Edwards P. Bevacizumab prior to vitrectomy for diabetic traction retinal detachment. Eye (Lond) 2011;25:989–97. doi: 10.1038/eye.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moradian S, Ahmadieh H, Malihi M, Soheilian M, Dehghan MH, Azarmina M, et al. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1699–705. doi: 10.1007/s00417-008-0914-4. [DOI] [PubMed] [Google Scholar]
- 146.Arevalo JF, Maia M, Flynn HW, Jr, Saravia M, Avery RL, Wu L. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:213–6. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]
- 147.Sohn EH, He S, Kim LA, Salehi-Had H, Javaheri M, Spee C, et al. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: Report no.1. Arch Ophthalmol. 2012;130:1127–34. doi: 10.1001/archophthalmol.2012.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiao C, Eliott D, Spee C, He S, Wang K, Mullins RF, et al. Apoptosis and angiofibrosis in diabetic tractional membranes after vascular endothelial growth factor inhibition: Results of a prospective trial. Report no. 2. Retina. 2017 Nov 22; doi: 10.1097/IAE.0000000000001952. doi: 10.1097/IAE.0000000000001952. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009;116:927–38. doi: 10.1016/j.ophtha.2008.11.005. [DOI] [PubMed] [Google Scholar]


