Abstract
Diabetic macular edema (DME) is the most common cause of vision loss in patients with diabetic retinopathy with an increasing prevalence tied to the global epidemic in type 2 diabetes mellitus. Its pathophysiology starts with decreased retinal oxygen tension that manifests as retinal capillary hyperpermeability and increased intravascular pressure mediated by vascular endothelial growth factor (VEGF) upregulation and retinal vascular autoregulation, respectively. Spectral domain optical coherence tomography (SD-OCT) is the cornerstone of clinical assessment of DME. The foundation of treatment is metabolic control of hyperglycemia and blood pressure. Specific ophthalmic treatments include intravitreal anti-VEGF drug injections, intravitreal corticosteroid injections, focal laser photocoagulation, and vitrectomy, but a substantial fraction of eyes respond incompletely to all of these modalities resulting in visual loss and disordered retinal structure and vasculature visible on SD-OCT and OCT angiography. Efforts to close the gap between the results of interventions within randomized clinical trials and in real-world contexts, and to reduce the cost of care increasingly occupy innovation in the social organization of ophthalmic care of DME. Pharmacologic research is exploring other biochemical pathways involved in retinal vascular homeostasis that may provide new points of intervention effective in those cases unresponsive to current treatments.
Keywords: Diabetes, diabetic retinopathy, macular edema
Epidemiology and Risk Factors
Diabetic macular edema (DME) is the most common cause of visual loss in those with diabetic retinopathy and is increasing in prevalence globally.[1,2,3] The prevalence of DME in patients with diabetic retinopathy is 2.7%–11%[4,5,6,7,8] and it depends on the type of diabetes and the duration of the disease, but for both types 1 and 2 after 25-years duration, it approximates 30%. Systemic factors associated with DME include longer duration of diabetes, higher systolic blood pressure, and higher hemoglobin A1C. The sole ocular factor associated with DME is diabetic retinopathy severity as increasing severity is associated with increasing prevalence of DME.[9,10,11]
Genetics, Pathoanatomy, and Pathophysiology
The hypothesis that genetic risk and protective alleles exist for development of DME has not been tested with genome wide association studies of adequate size, but studies are underway.[12]
Anatomy
The capillaries in the macula are distributed in four strata within the inner retina with the exception of the single-level arrangement bordering the foveal avascular zone within the ganglion cell layer.[13] Farther from the fovea, the three additional levels of capillaries are found within the deep ganglion cell layer, inner plexiform layer/superficial inner nuclear layer, and deep inner nuclear layer, respectively.[14] These strata can be imaged by optical coherence tomography (OCT).[15]
Eighty percent of diabetes-related microaneurysms originate in the inner nuclear layer and its border zones[16] and are commonly found on the edges of nonperfused retina. Microaneurysms in DME do not preferentially cluster in any particular quadrant.[17] In DME, spectral domain (SD)-OCT angiography has shown microaneurysms and abnormal deep capillary networking in the superficial outer nuclear layer, a normally avascular zone.[18]
In center-involved diabetic macular edema (CIDME), the central macula is often thickest, an inversion of the normal morphology. In the foveal avascular zone, the only mechanism for extracellular fluid resorption is the retinal pigment epithelial (RPE) pump, which may explain the greater accumulation of edema fluid at this location.[19] An associated fundus sign is the appearance of the macular lipid star [Fig. 1]. In 15%–30% of cases of DME, a subfoveal serous retinal detachment is present. Although the explanation for the subfoveal location of fluid is conjectural, one theory posits an impaired RPE pump due to decreased subfoveal choroidal circulation.[20,21]
Figure 1.
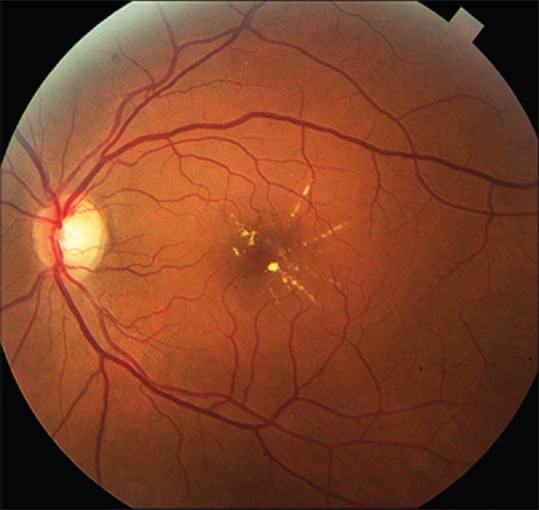
A macular lipid star is a common fundus sign in diabetic macular edema and indicates that the center of the macula is a preferential site for accumulation of extracellular fluid
Breakdown of the inner blood–retina barrier rather than outer blood–retina barrier breakdown is more important to the formation of DME.[22] Diabetes causes a redistribution of occludin in retinal vascular endothelium.[23] The Muller cells proliferate in epiretinal membranes that exert traction on microvessels and increase their permeability. Astrocytes, which wrap their end feet around microvessels, decrease their production of glial fibrillary acidic protein in diabetes, which may alter the blood–retina barrier.[23]
Diabetes-related abnormalities of the vitreoretinal interface may promote the development DME. During the process of vitreous separation, the macula and the disk may adhere to the posterior hyaloid more firmly, and traction may contribute to blood retinal barrier breakdown and provide a scaffold for cellular proliferation, which further increases traction on the macula.[24,25,26] In eyes with DME, the internal limiting membrane has more adherent cellular elements on its vitreous side, is thicker, and has more heparin sulfate proteoglycan compared with the internal limiting membrane from nondiabetic eyes. Fibromuscular cells found in epiretinal blocks of tissue biopsied at the time of vitrectomy for DME may be the basis for tangential traction on the retina with concomitant increases in capillary permeability.[25,26]
Physiology
In DME, the macula is thickened due to increased extracellular fluid derived from hyperpermeable retinal capillaries.[27] Prolonged hyperglycemia leads to reduced inner retinal oxygen tension, venous dilation, increased VEGF concentration within the retina, leukocyte stasis, and dysregulated growth factor levels, which together are associated with increased exudation of serum out of the retinal vasculature and into the extracellular space.[28,29] The RPE pump is overwhelmed by the exudation of serum and macular swelling results.[30,31]
A framework for understanding the pathophysiology of diabetic macular edema (DME) is the oxygen theory.[32] Prolonged periods of hyperglycemia lead to reduced perfusion of the inner retina and decreased inner retinal oxygen tension. The autoregulatory response of the retinal arterioles causes dilation, which leads to increased hydrostatic pressure in the intraretinal capillaries and venules as specified by Poiseuille's law.[31] The elevated intravascular pressure experienced by the capillaries may damage them.[31,32] Concomitantly, the decrease in retinal oxygen tension upregulates the synthesis of VEGF and other permeability factors, which increases microvasculature leakage. According to Starling's law, increased intravascular pressure and vascular permeability result in a net flow of water, ions, and macromolecules from the intravascular space into the extravascular space. Extracellular fluid is resorbed by re-entering the retinal vessels further downstream or through the choroid via the pumping action of the RPE.[32,33]
Many variables are suspected to modulate this process. The duration of diabetes and the integrated elevation of blood glucose reflected in the glycated hemoglobin (HbA1C) have proven pathophysiological importance. Retinal neurons and glial cells increase their production of VEGF, even before ophthalmoscopic evidence of capillary loss, associated with reduced occludin in capillary endothelial tight junctions.[23,34] Increased inflammation, characterized by leukostasis, accumulation of macrophages, intercellular adhesion molecule-1 activation (ICAM-1), and prostacyclin upregulation, is associated with capillary nonperfusion and breakdown of the blood–retina barrier.[29,35] Patients with DME have elevated vitreous levels of VEGF, ICAM-1, interleukin-6 (IL-6), and monocyte chemoattractant protein-1 compared with nondiabetic patients.[36] Inflammatory cytokines such as tumor necrosis factors alpha and beta, alpha 4 integrin, nitric oxide, and interleukin-1β mediate vascular permeability.[23,37,38,39] Many other small molecules and growth factors may contribute to the development of DME, although the details of the pertinent pathways are incompletely understood.[37,38,40,41] High lipid levels may cause endothelial dysfunction and increased vascular permeability through a local inflammatory response and higher levels of advanced glycation end products.[42] In addition to extracellular edema, intracellular edema may be relevant for DME. Dysregulated metabolism is associated with accumulation of intracellular osmotically active solutes that draw in water and cause cellular swelling.[31]
Decrease in subfoveal choroidal blood flow in type 2 diabetic patients with retinopathy may be relevant in the pathophysiology of DME. Eyes with DME have been reported to have a greater decrease in choroidal blood flow than eyes without DME, suggesting relative hypoxia of the RPE and outer retina, and consequent increased permeability of the outer blood retinal barrier.[20]
The vitreous may play a role in the pathogenesis of DME. Cross-linking and protein glycation are increased in the diabetic vitreous, which may explain instances of tangential macular traction that may induce DME.[43,44] Besides the direct effect of traction causing leakage from blood vessels or macular elevation with subretinal fluid, vitreous adherent to the macula may loculate mediators of vessel permeability in proximity to macular capillaries and may impede oxygenation of the retina, thereby causing venous dilation and increased edema via Starling's law or by upregulation of VEGF.[33,45,46,47,48,49]
This account of the pathophysiology of DME informs an understanding of how treatments for DME work [Fig. 2]. Grid laser increases the oxygenation of the inner retina both by reducing the number of oxygen-consuming photoreceptors and by shortening the diffusion pathway to the inner retina for oxygen originating in the choroid.[32,50] Focal photocoagulation presumably works by destroying leakage sources such as microaneurysms but may also improve RPE pumping of sodium ions and water outward toward the choroid.[32,51,52] Anti-VEGF drugs work by blocking the permeability inducing effects of VEGF.[53] Corticosteroids reduce expression of the VEGF gene, differentially regulate expression of the various VEGF receptors, and have other non-VEGF-mediated effects such as decreasing leukocyte recruitment and production of ICAM-1, and inhibiting collagenase induction that reduce the permeability of retinal microvessels.[54,55,56,57,58,59] Vitrectomy may work by increasing intravitreal and secondarily inner retinal oxygen levels, leading to downregulation of VEGF synthesis, which decreases the permeability of microvessels.[32,60] In addition, vitrectomy may open compartments of loculated cytokines and relieve traction exerted on the macula by an altered vitreous.[60,61]
Figure 2.
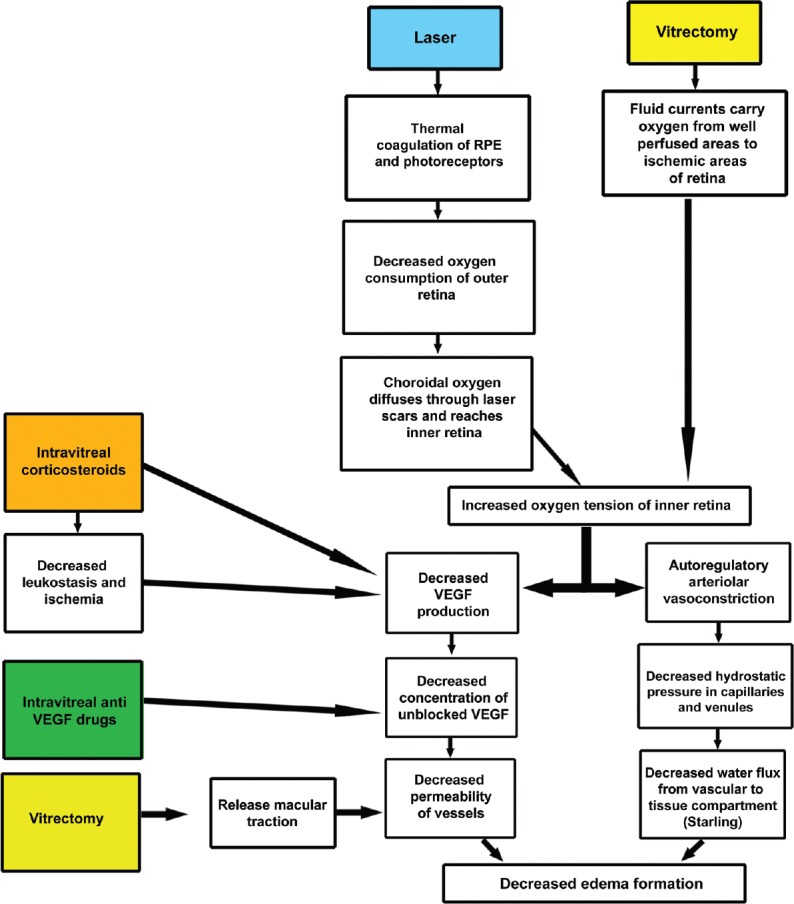
Schematic summarization of the mechanisms of action of the various treatments for diabetic macular edema. The color-filled blocks represent different treatment modalities for diabetic macular edema
Clinical Definitions
Diabetic macular edema
Retinal thickening within one disk diameter of the center of the macula or definite hard exudates in this region.[62]
Center-involved diabetic macular edema
DME in which the fovea is involved.
Clinically significant macular edema
The situation in which at least one of the following criteria is fulfilled:
Retinal thickening within 500 μm of the center of the macula
Hard exudates within 500 μm of the center of the macula with adjacent retinal thickening
One disk area of retinal thickening any part of which is within one disk diameter of the center of the macula.[63]
Focal and diffuse diabetic macular edema
These two terms have not been defined consistently in the literature.[64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] Focal edema is said to arise from microaneurysms, whereas diffuse edema is said to arise from generally dilated and hyperpermeable capillaries throughout the macula.[83] Focal DME has been reported to be more common than diffuse DME, but many cases of DME have mixed features making a clear distinction difficult.[51,80,84,85,86,87] Additional confusion may ensue because the term focal is used to describe a technique of applying laser directly to microaneurysms when treating DME with focal/grid photocoagulation.[63,88]
Other classifications of DME have been proposed. One scheme differentiates diffuse edema, cystoid macular edema, and serous retinal detachment based on OCT.[89] Attempts to correlate these subgroups to treatment outcomes have yielded inconsistent results, no consensus guidance exists on interventions for the proposed subtypes, and no DRCR network study has shown these DME classifications to help in clinical decision-making.[90]
Subclinical diabetic macular edema (SCDME)
The severity of DME may not reach the definition of CSME or CIDME. Clinical assessment of macular edema and OCT assessment of macular edema frequently disagree in this group of patients.[91,92] In addition, some eyes do not have clinically recognized DME, but macular thickening is detectable by OCT.[4] The term subclinical DME has been used to define both of these classes of DME that are less severe than clinically significant DME.[92,93]
Persistent diabetic macular edema
DME that has been treated without complete resolution is defined as persistent.[94,95,96,97] Persistent DME has been noted in a proportion of eyes treated by any modality, including focal laser photocoagulation, intravitreal injection of anti-VEGF drugs or corticosteroids, and vitrectomy. Different criteria have been applied for the number of treatments or duration of treatment required before applying the term. Some eyes have persistent edema despite all known treatments for DME.
Recurrent diabetic macular edema
Many cases exist in which DME resolves after treatment, but subsequently recurs.[98,99] Although DME can resolve spontaneously without treatment, and then recur, the term recurrent DME is used with reference to treated eyes with recurrences.
Methods of detection of DME
DME can be detected by stereoscopic slit-lamp examination using a fundus lens.[51,63,100] Direct ophthalmoscopy allows detection of lipid exudates but lacks stereopsis. Although lipid suggests associated macular thickening, the two findings are not synonymous; presence of lipid alone is an unreliable surrogate for DME.[101,102] Stereoscopic fundus photographs and fluorescein angiography can be used to assess the presence of DME but have been largely supplanted by OCT.[24,103,104,105]
The importance of OCT in diagnosing and managing DME cannot be overemphasized. The clinical diagnosis of DME as practiced in the Early Treatment Diabetic Retinopathy Study (ETDRS) era before OCT was beset by variability among clinicians.[51,106] In contrast, measurements made with OCT are highly reproducible.[107,108,109,110,111] In general, any change of macular thickness greater than 11% of a previous measurement exceeds OCT measurement variability and can be assumed to be a real change in macular thickness.[109] In addition to measurement variability, there is short-term fluctuation in macular thickness in DME. This refers to the variability noted over the course of days to even weeks when there is no trend in the changes.[112] Short-term fluctuation in DME is dependent on actual macular thickness and is larger than measurement variability.[113]
Of the many OCT indices that can be followed in the course of DME, the central subfield mean thickness (CST) is the best single measure.[114,115] It is more reproducible than center point thickness, yet is highly correlated (r = 0.99) with the latter.[114,115] Total macular volume (TMV) correlates somewhat less well with CST (r = 0.76), and there have been no conclusions drawn from analyzing TMV that would not have been drawn by studying CST instead.[94,104]
OCT was originally developed using time domain acquisition of images.[116] Subsequently instruments using spectral domain acquisition of images (SD-OCT) and swept-source OCT (SS-OCT) have been developed. SD-OCT and SS-OCT allow faster acquisition of images, denser sampling of the macula, and better imaging of the choroid and outer retina.[117,118,119,120] The normal values for SD-OCT and SS-OCT differ because the segmentation algorithms define the retina layers differently, and measurements are not interconvertible across instruments made by different companies.[118,119,121] The axial resolution of SD-OCT is 2–5 μm.[118,122] For the central subfield, the mean coefficient of variation of SD-OCT has been reported to be 0.66%.[118] The coefficient of repeatability for the central subfield thickness of SD-OCT is 5 μm.[123]
OCT is good for objectively measuring macular thickness, but macular thickening is only modestly correlated with visual acuity (r = −0.52) perhaps due to variable duration of edema and ischemia.[23,124] Photoreceptor outer segment length, defined as the length between the ellipsoid zone and the RPE, and outer retinal layer thickness, defined as the length between the external limiting membrane (ELM) and the RPE, correlate better with visual acuity (r = −0.81 and −0.65 to −0.8787, respectively).[125,126,127] Disorganization of the inner retinal layers (DRIL), defined as lack of definition of boundaries between ganglion cell-inner plexiform layer or inner-nuclear-outer-plexiform layers in ≥ 50% of the 1 mm central subfield, has been associated with worse visual acuity and less response to injections with bevacizumab or ranibizumab.[128,129,130] On average, each additional 100 μm of DRIL is associated with 6 ETDRS letters lost.[130]
Besides its usefulness in the detection of macular edema, OCT has value in following DME over time. SD-OCT provides enough detail regarding the outer retina that correlations of intactness of structures with visual outcomes are possible. Increased disruption of the ELM and ellipsoid zone (EZ) are associated with worse visual acuity outcomes.[131,132]
Natural History
The ETDRS provided natural history data regarding DME. Over 3 years of follow-up, the rate of moderate visual loss (15 letters on the ETDRS chart) was 8% per year.[63] Rates of visual loss increased according to the baseline visual acuity, with worse seeing eyes losing vision at a higher rate.[63] Rates of visual loss also increased according to baseline retinopathy severity, with eyes having more severe retinopathy losing vision at higher rates than eyes with less severe retinopathy.[63] Rates of visual acuity gain of at least 6 ETDRS letters in untreated eyes with DME and visual acuity of ≤ 20/40 over three years of follow-up were 20%–25%.[63] Of eyes with DME less severe than CSME (one subset of what has been termed subclinical DME) and observed without treatment, 22% and 25% progressed to CIDME at 1 and 3 years of follow-up, respectively.[63] In the OCT era, 31% of eyes with SCDME progressed to CSME over a median follow-up of 14 months.[93]
Chronic, untreated DME and refractory DME can lead to subretinal fibrosis, particularly if hard exudates are present, and by more subtle RPE pigmentary changes.[133,134,135,136,137]
Treatments
Metabolic control and effects of drugs
Recognition of the risk factors for DME led to randomized clinical trials of better blood pressure control in attempts to reduce the prevalence of the condition. The Diabetes Control and Complications Trial showed that tight blood glucose control in patients with type 1 diabetes reduced the cumulative incidence of macular edema at 9-year follow-up by 29% and reduced the application of focal laser treatment for DME by half.[138,139] The United Kingdom Prospective Diabetes Study was an analogous randomized clinical trial of patients with type 2 diabetes. It showed that tighter blood glucose control reduced the requirement for laser treatment at 10 years by 29%, compared with looser control; 78% of the laser treatments were for DME.[140] It also showed that a mean systolic blood pressure reduction of 10 mm Hg and a diastolic blood pressure reduction of 5 mm Hg over a median follow-up of 8.4 years led to a 35% reduction in retinal laser treatments, of which 78% were for DME.[141]
Increased serum cholesterol levels are associated with increased severity and risk of retinal hard exudates.[142,143] Patients with abnormally elevated triglycerides and HDL cholesterol had worse visual acuity outcomes after focal/grid photocoagulation than did patients with normal levels in one small prospective study.[144]
Thiazolidinediones are oral agents used to treat type 2 diabetes. They are peroxisome proliferator-activated receptor γ agonists that work by enhancing insulin sensitivity. Pioglitazone and rosiglitazone are members of this class of drugs in common use. They have been associated with peripheral edema, pulmonary edema, and/or congestive heart failure, especially when used in combination with insulin. Plasma VEGF levels are higher in patients on thiazolidinediones than in patients not on these drugs.[145] Case reports and retrospective database cohort studies suggest that they can be associated with new or worsened DME as well, but in the ACCORD study, use of thiazolidinediones was not associated with prevalence of DME at baseline or incidence of DME over 4 years of follow-up.[146,147]
Improved control of diabetes, hypertension, and serum lipids is frequently underemphasized by the ophthalmologist because changes in systemic disease management are usually made by the internist, yet there is an intimate connection between these changes and retinal effects. A multifactorial intervention aimed at reducing glycated hemoglobin, elevated blood pressure, and elevated serum lipids can produce measurable effects in macular thickness in as little as 6 weeks and forms a rational foundation on which to apply specific ophthalmic interventions.[148]
Specific Ophthalmic Treatments
Focal/grid laser photocoagulation
The ETDRS demonstrated superior visual outcomes with focal/grid laser for CSME compared with the natural history. Laser thus became the standard of care over the next 30 years.[63] Treatments were repeated at 4-month intervals if CSME persisted and treatable lesions or untreated, thickened, and nonperfused retina remained. The average patient received between three and four focal/grid laser treatments. ETDRS style focal/grid photocoagulation for DME has potential side effects including paracentral scotomas, subretinal fibrosis, and secondary choroidal neovascularization.[134,149,150,151,152]
The technique of focal/grid argon laser treatment has been modified over time. The most significant changes are embodied in the DRCR.net protocols that employ focal/grid photocoagulation. Rather than burns that can vary from 50 to 200 μm, all contemporary burns are 50 μm and they are less intense.[153] Yellow wavelength laser is acceptable in addition to green, but blue-green is not used because of concern over absorption by macular luteal pigment. Use of a guiding fluorescein angiogram is optional.[51,74,149,154] On average, for mild CIDME with CST in the range of 300–350 μm, one can expect that focal/grid laser will produce ~25 μm of macular thinning at the usual first follow-up interval of 3–4 months. For every 100 μm of additional baseline macular thickening above this threshold, one can expect that focal/grid laser will yield approximately 10 μm of additional macular thinning at the 3-to 4-month follow-up visit.[19] Visual acuity at this follow-up visit is, on average, unchanged from baseline.[154,155,156,157]
Subthreshold Laser Photocoagulation
Besides focal/grid suprathreshold laser treatment, diode laser micropulse laser has been used in case series and small randomized clinical trials.[158,159,160] Its advantages are absence of RPE scarring, no subsequent choroidal neovascularization, and elimination of paracentral visual field scotomas.[160,161] The disadvantages are that there is no visible endpoint for treatment, making it difficult to determine where treatment has and has not been given, and that there is no standardized, consensus set of treatment parameters or guidelines with respect to treatment within the foveal avascular zone. In addition, the reduction in macular edema after subthreshold laser photocoagulation occurs with a slower time course and more treatments are necessary to achieve elimination of edema.[160]
Intravitreal Injections of Corticosteroids
Corticosteroids were first used to treat DME in 2001.[162] Triamcinolone, dexamethasone, and fluocinolone have been used in many forms, including particulate suspensions, viscoelastic mixtures, and solid slow-release devices.[113,163,164,165,166] Many dosages and intervals between triamcinolone injections have been tried.[167] Although enthusiasm for serial intravitreal triamcinolone injections was initially high, protocol B of the DRCR network showed that focal laser led to superior visual acuity outcomes at 3 years relative to either triamcinolone 1 or 4 mg.[157,163] Since then, therapy with corticosteroids has taken a secondary role to anti-VEGF therapy. Side effects of cataract in phakic eyes and intraocular pressure elevation have accompanied all steroids studied, although to varying degrees.[157,168]
Slowly dissolving intravitreal dexamethasone implants (Ozurdex®, Allergan, Irvine, CA, USA) are effective in treating DME although the visual acuity gains are generally less than with anti-VEGF injections.[90,169] In a 3-year randomized controlled trial, the 0.7 mg dexamethasone implant was associated with ≥ 15 letter improvement in best corrected visual acuity (BCVA) in 22.2% of patients compared to 12.0% in the sham group.[169] Over three years in phakic patients, 59.2% of eyes required cataract surgery; 41.5% of eyes required the use of ocular hypotensive therapy.[169] The long-term visual outcome of intravitreal dexamethasone implant therapy correlates with the 3-month treatment response.[170]
Intravitreal fluocinolone acetonide implants (Iluvien®, Alimera, Alpharetta, GA, USA) last 3 years and, unlike the dexamethasone implant, do not dissolve. In the FAME trial, patients with persistent DME despite macular laser were randomized to low-dose (0.2 μg/day), high-dose (0.5 μg/day), or sham treatment. The percentage of eyes gaining at least 15 ETDRS letters at 24 months was 28.7% compared with 16.2% in the sham group. Cataract surgery was required in 74.9% of the low-dose fluocinolone group compared with 23.1% in the sham group. Glaucoma developed in 1.6% of eyes compared with 0.5% of sham eyes.[171]
Intravitreal Injections of Anti-VEGF Drugs
Anti-VEGF drugs include aptamers (pegaptanib), antibodies to VEGF (bevacizumab), antibody fragments to VEGF (ranibizumab), and fusion proteins, which combine a receptor for VEGF with the Fc fragment of an immunoglobulin (aflibercept and conbercept). The antibodies and fusion proteins bind all isoforms of VEGF-A; fusion proteins additionally bind VEGF-B and placental growth factor. Fusion proteins have higher affinity for VEGF and the potential for less frequent injection frequency in the treatment of DME.[172,173]
Bevacizumab (Avastin®, Genentech, S. San Francisco, CA, USA/Roche, Basel, SW) is Food and Drug Administration (FDA)-approved for treatment of advanced solid cancers, but is widely used off-label in the treatment of DME. It is much less expensive than the FDA-approved ocular anti-VEGF drugs.[174] Ziv-aflibercept (Zaltrap®, Regeneron, Tarrytown, NY, USA) is systemically formulated aflibercept in a buffered solution with a higher osmolarity (1,000 mOsm/L) than aflibercept.[175] In a rabbit model, intravitreal injection of ziv-aflibercept did not affect serum or intraocular osmolarity, and human studies are beginning to be published.[172,173]
The first anti-VEGF drug used to treat DME was pegaptanib (Macugen®, Bausch and Lomb, Rochester, NY, USA), which selectively blocks the 165-isoform of VEGF.[176] Its promise was superseded by superior results obtained with anti-VEGF drugs that blocked all isoforms of VEGF. The efficacy of bevacizumab and ranibizumab were proven in randomized controlled clinical trials in 2010 and that of aflibercept in 2014.[177,178,179] A prospective, randomized, comparative effectiveness trial of these three drugs showed no difference in efficacy of the three drugs in eyes with center-involved DME and visual acuity of 20/40 or better at 1 or 2 years of follow-up.[174] However, in eyes with visual acuity of 20/50 or worse, aflibercept was superior to ranibizumab and bevacizumab at 1 year, whereas at 2 years, aflibercept was no longer superior to ranibizumab but remained superior to bevacizumab.[174,180] An example illustrating effectiveness of aflibercept, persistence of DME, and SD-OCT correlates of suboptimal visual acuity outcomes is shown in Fig. 3.
Figure 3.
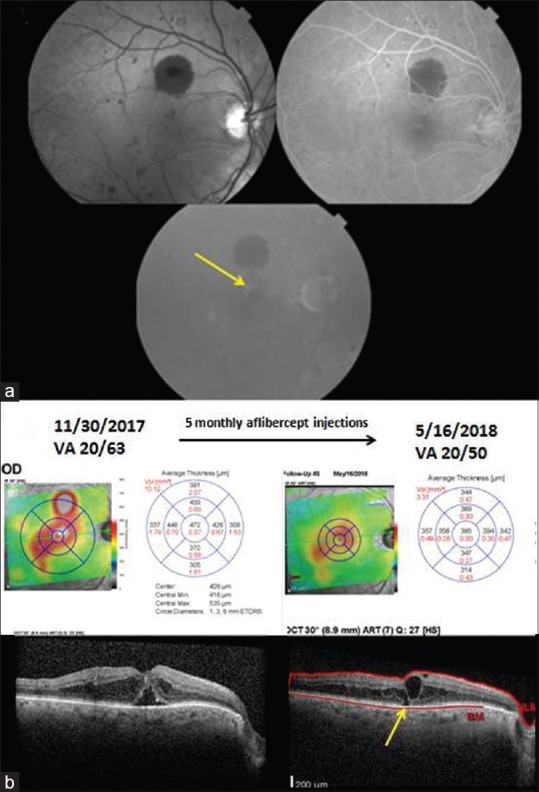
Images of a 77-year-old man with type 2 diabetes mellitus, who developed diabetic macular edema of the left eye that reduced visual acuity to 20/63. (a) Red free fundus photography shows microaneurysms and one large blot hemorrhage above the fovea. Fluorescein angiography shows multiple hyperfluorescent microaneurysms in the mid-phase, and late leakage above the fovea in the late frame. (b) The spectral domain-optical coherence tomography on November 30, 2017 shows center-involved diabetic macular edema and subfoveal fluid. After 5 monthly aflibercept injections, the edema has decreased, but persistent edema is present. An ellipsoid zone defect (yellow arrow) is apparent
Approaches aimed at increasing the intravitreal concentration of anti-VEGF agents have not proved beneficial. The READ-3 clinical trial examining two doses of ranibizumab (0.5 and 2.0 mg) in DME showed that at 2 years, the 0.5 mg dose was associated with a better visual outcome.[181,182] Focal laser added from the outset to anti-VEGF does not improve visual acuity outcomes relative to its use in a deferred manner if incomplete drying of the macula occurs with anti-VEGF therapy.[183] Randomized clinical trials demonstrate that these general results apply across various racial and ethnic groups.[174,184] As a result, in 2018, serial anti-VEGF intravitreal injection monotherapy is the standard of care for treating DME in developed countries.
Although serial injections of anti-VEGF drugs are first-line therapy for DME, some patients do not respond or respond incompletely. In the RISE and RIDE trials, persistent macular thickening was found in 20%–25% of patients.[178] A secondary analysis of protocol T comparing intravitreal aflibercept, bevacizumab, and ranibizumab for CI-DME found that persistent DME through 24 weeks was found in 31.6%, 65.6%, and 41.5% of eyes receiving aflibercept, bevacizumab, and ranibizumab, respectively.[97] Despite their incomplete responses, the visual acuity outcomes of eyes with chronic persistent DME were similar to those of eyes with complete resolution of edema.[97] Similar results were found in a secondary analysis of protocol I comparing intravitreal ranibizumab with prompt or deferred focal laser to intravitreal triamcinolone with prompt focal laser for CI-DME.[185]
A concomitant effect of anti-VEGF treatment for DME is improvement in retinopathy severity or slowing of the rate of progression of retinopathy. This effect has been noted with ranibizumab and aflibercept.[179] For aflibercept, there is an association between baseline retinopathy severity and proportion of patients achieving ≥ 2-step severity score improvement.[186] Another concomitant effect is thinning of the choroid.[187,188,189] In treatment naïve CIDME, 3–6 months of bevacizumab or ranibizumab was associated with choroidal thinning.[190,191]
No better results have been reported than those of RISE and RIDE using a monthly injections regimen. In the READ-2 trial, when less than monthly injection frequency after 2 years was succeeded by 1 year of monthly injections, additional statistically significant improvement in visual acuity was attainable (mean of 3.1 additional ETDRS letters).[192] However, RESOLVE, RESTORE, and DRCR network protocols I and T have demonstrated that similar outcomes can be achieved with monthly injections for 3–4 months followed by OCT and visual acuity guided prn follow-up treatment that decreases the number of injections required to produce the visual outcome.[193,194] Despite safety concerns that intravitreal anti-VEGF drugs could raise the risk of cardiovascular complications in patients with diabetes, there is no consistent evidence that this is the case.[174,194,195,196]
Bevacizumab is more cost-effective in treating DME than ranibizumab or aflibercept.[197,198] Medicare reimbursement for anti-VEGF drugs varies widely. In 2012, Medicare reimbursement was $50 for bevacizumab and $1,903 for ranibizumab.[174] The unit dose cost of aflibercept approximates that for ranibizumab for the treatment of macular degeneration, but the smaller approved dose of ranibizumab (0.3 mg) in the US means that the cost of ranibizumab is approximately 60% that of aflibercept. The cost differences arise because bevacizumab is not approved for intraocular use by the FDA, whereas ranibizumab and aflibercept are FDA-approved for intraocular use. Factors that influence which drugs are used include patient-factors and physician-factors. Patient-factors include Medigap insurance coverage and out-of-pocket costs. Physician-factors include Medicare drug repayment policies, industry economic incentives, and risks associated with compounding of bevacizumab.[199]
Combined Intravitreal Anti-VEGF and Corticosteroid Injections
Combination intravitreal bevacizumab and triamcinolone has not been found to improve outcomes compared with intravitreal bevacizumab monotherapy.[200] The addition of an intravitreal dexamethasone sustained release device to a regimen of ranibizumab injections did not improve visual acuity outcomes at 24 weeks, although macular thinning was greater than with intravitreal ranibizumab (IVR) alone.[96]
Vitrectomy
The idea that vitreomacular adhesion might promote DME arose from the observation that eyes with DME have a lower prevalence of posterior vitreous detachment than eyes without DME.[24] The subsequent observation that resolution of DME could occur after posterior vitreous detachment strengthened the plausibility that surgical induction of a vitreomacular separation might improve DME.[201,202] With the advent of OCT, vitreomacular adhesion was shown to be a risk factor for DME.[87] Vitrectomy for DME was first reported in 1992.[203] Since then, many small retrospective and prospective case series, several small clinical trials, but no large, multi-centered, randomized, controlled trials of the approach have been published.[46,49,61,94,95,98,99,136,204,205,206,207,208,209,210,211,212,213,214,215] Vitrectomy was introduced for eyes with a taut posterior hyaloid adherent to the macula, often associated with shallow traction macular detachment, which had failed previous focal/grid laser.[45,48,99,136,203,216] Later, it was explored as a therapy for eyes with an attached but non-thickened, non-taut posterior hyaloid or for eyes with persistent DME despite previous focal laser or intravitreal triamcinolone injection regardless of the status of the posterior hyaloid [Figs. 4 and 5].[95,99,204,205,207,215,217] Most recently, the treatment has been studied as a potential primary therapy in eyes with more severe edema and greater visual acuity loss at presentation.[46,136,208,210,213,215,218,219] The relative frequencies of the various candidate groups have been reported as follows: refractory DME in eyes with attached but non-taut posterior hyaloid 68%, refractory DME in eyes with posterior vitreous detachment 22%, refractory DME in eyes with a taut posterior hyaloid 5%, and refractory DME in eyes with an epiretinal membrane 5%.[220]
Figure 4.
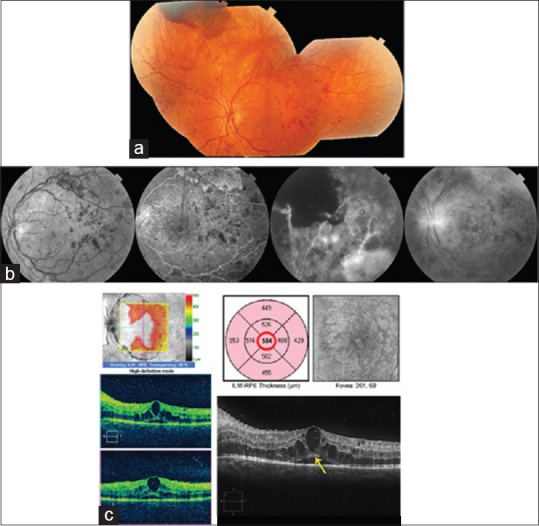
Images of the left eye of a 21-year-old woman with type 1 diabetes mellitus with proliferative diabetic retinopathy and center-involved diabetic macular edema. Her best corrected visual acuity was 20/40. Because of documented poor adherence to scheduled clinic visits, vitrectomy rather than serial anti-VEGF injection therapy was chosen. (a) New vessels were present on the disc and in the midperiphery of all quadrants with a preretinal hemorrhage superiorly. (b) Fluorescein angiography shows leakage from new vessels and areas of capillary nonperfusion in the midperiphery. (c) Spectral domain-optical coherence tomography shows center-involved intraretinal fluid and subfoveal fluid. The ellipsoid zone is intact (yellow arrow)
Figure 5.
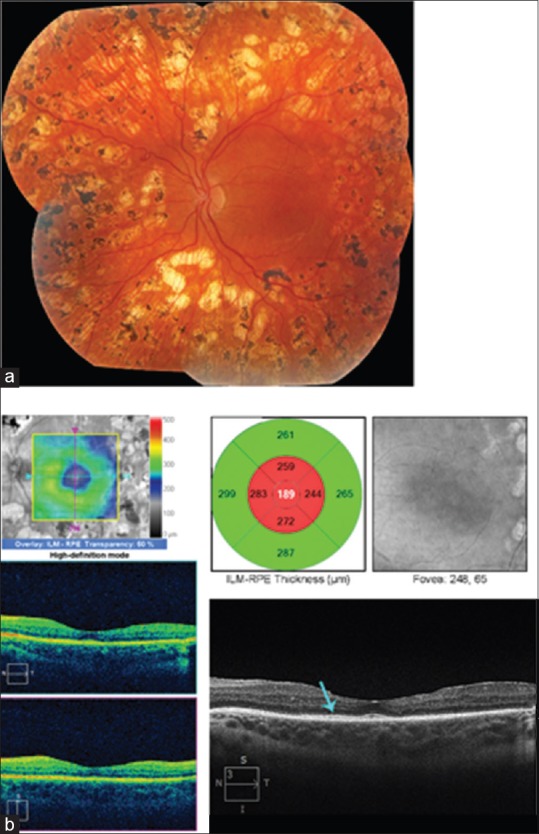
Postoperative images from the same patient shown in Fig. 4. (a) Following a single preoperative bevacizumab injection (to limit intraoperative bleeding), vitrectomy, internal limiting membrane peeling, and panretinal photocoagulation, the new vessels have regressed. (b) Five years later the center-involved diabetic macular edema remains resolved with no subsequent treatments. The best corrected visual acuity was 20/25.
A controversy exists regarding the effects of vitrectomy for DME. Several groups of investigators have reported data to suggest that vitrectomy reduces macular thickening but does not improve visual acuity.[135,211,215,218,220] Others report improved visual acuities simultaneous with decreases in macular thickening or lagging behind macular thinning by a few months.[94,136,208,221] Others report improved visual acuity in cases with macular traction, but no visual improvement in cases without traction.[48,222]
The largest prospective observational study with standardized data collection was performed by the DRCR network. In this study, which was not a randomized trial, 241 eyes were followed to a primary outcome visit at 6 months. Baseline median CSMT was 491 μm, interquartile range (IQR) (356, 586). Baseline visual acuity was 57 letters, IQR (45, 66). At 6 months follow-up, the median change in CSMT was -97 μm, IQR (−8, +10). The median change in ETDRS letter score was + 1 letter, IQR (−8, +10).
As has been reported for all other treatments for DME, recurrence of edema after initial improvement, incomplete resolution of macular thickening, and failure to respond at all to treatment also occur with vitrectomy, but the rates of these undesirable outcomes may be reduced compared with focal laser and intravitreal triamcinolone injections alone.[4,9,10,28]
Although there is general acceptance that vitrectomy has a role in the management of at least some cases of DME, there is also consensus that it has no role in many cases, including cases of mild edema with minimal visual compromise and in cases with large submacular hard exudates, in which chronic RPE atrophic changes limit the potential for improvement even after specifically removing these exudates through small retinotomies.[137,223] A prospective, multicenter, randomized clinical trial is needed to define the role of vitrectomy surgery in the management of DME.
Discrepancy between Outcomes in Randomized Controlled Trials and Real-World Conditions
The outcomes obtained in the treatment of DME in Randomized Controlled Trials (RCTs) and under real-world conditions are different. In real-world conditions, inferior visual acuity gains associated with less frequent intravitreal injections have been reported, a relationship that has been consistently noted internationally.[224,225,226,227,228,229,230] There are many factors that possibly explain the discrepancy. In clinical trials, patients are preselected for their commitment to complete the schedule of visits, costs are borne in most cases by the entity performing the study, and subsidies for travel are often provided. In real life, lack of time and means could contribute to lower treatment intensity, the nonmedical costs for patients are onerous especially for patients of lower economic means and who are motivated not to miss work for doctor visits, and the need to manage other comorbidities.[227,228,231] Both non-elderly and elderly patients with DME have higher rates of comorbidity and loss of work time and personal time compared with diabetic patients without DME.[232] For example, non-elderly patients with DME had an average of 24.7 annual days with healthcare visits compared with 14.4 for age-matched controls with diabetes but no DME.[232] The average direct medical cost ratio, adjusted for age, sex, race, geographic region, and comorbidity, for Medicare patients with DME over 3 years was 1.31 times that for diabetic controls without DME.[233] In a retrospective claims analysis of 2,733 newly diagnosed patients with DME conducted over the interval 2008 through 2010, the mean annual numbers of bevacizumab injections were 2.2, 2.5, and 3.6 for the years 2008, 2009, and 2010, respectively, fewer than in major clinical trials of anti-VEGF agents.[227] Similarly, in a retrospective study of 121 eyes of 110 patients with a new diagnosis of DME receiving anti-VEGF injection therapy for the first time between 2007 and 2012 from the Geisinger Health System database, a mean of 3.1 ± 2.4 injections per study eye were given in the first year of treatment. The mean change in corrected visual acuity was 4.7 ± 12.3 approximate ETDRS letters, where approximate ETDRS letters are calculated from Snellen visual acuity. Higher numbers of anti-VEGF injections in the first 12 months after diagnosis correlate with improved visual outcomes, implying that real-world outcomes usually lag those in RCTs.[227] Other factors that may contribute to the discrepancies include lack of protocol refractions in many real-world visits, and the variability in treatment regimens and follow-up used by real-world clinicians compared with standardized regimens in clinical trials.[228]
In a German study looking at pooled anti-VEGF injections for DME, the mean change in VA at 12 months was -1.3 letters with a median of 6 injections.[225] Both the number of injections and the visual acuity outcomes are inferior to those reported in RCTs. In a study of Medicare claims data from 2008 through 2010, the mean number of claims per year for anti-VEGF injections for DME was 3.1–4.6.[226] A US commercial database claims study over 2008 through 2010 reported mean numbers of bevacizumab injections for DME varying from 2.2 to 3.6.[227] By comparison, the number of injections of ranibizumab in RISE and RIDE was 12 in the first year, and of ranibizumab, bevacizumab, or aflibercept was 9–10 in DRCR protocol T.[174,178] A Danish study of IVR for DME at 12 months reported a median number of injections of 5 and a median change in BCVA of +5 ETDRS letters.[229] An Italian study of IVR for eyes with unilateral DME reported a mean ± SD number of injections of 4.15 ± 1.99 over 18 months of follow-up with a worsening of visual acuity on average.[230]
New Directions
Genetic mutations that render patients more or less susceptible to DME as a complication of diabetes mellitus are likely to be defined. The physiological pathways contributing to DME and not mediated by VEGF are a likely focus of future research. Using OCT and OCT angiography, it should be possible to define at almost histological levels the retinal changes occurring in DME and determine, which if any changes associate with visual outcomes. Clinical trials of new drugs initiated by drug companies and comparative effectiveness research by organizations like the DRCR network will provide an evidential basis for rational therapy. Efforts to close the gap between randomized clinical trials and real-world outcomes and to reduce the cost of care will draw increasing attention.
Summary of Key Points
The prevalence of DME is increasing worldwide, mainly because of increasing type 2 diabetes.
Understanding retinal anatomy helps in analyzing clinical presentations of DME based on the effects of the avascularity of the central macula, the locations of the microvessels in the inner retinal layers, the importance of the pigment epithelial layer, and the role of the vitreoretinal interface.
The oxygen theory of DME is the most comprehensive pathophysiologic schema and VEGF is the single most important mediator in that pathway, although not the sole mediator.
OCT is critical in managing DME.
Macular thickening has an imperfect correlation with visual acuity probably due to factors currently difficult to assess such as duration of edema and degree of macular ischemia.
Metabolic control of blood glucose, blood pressure, and serum lipids is the foundation of therapy for DME, and specific ocular treatments are most effective when this foundation is optimized first.
Serial injections of anti-VEGF drugs are first-line therapy for DME. Focal/grid laser, intravitreal injections of corticosteroids, and vitrectomy have secondary roles in particular cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, Linton KL. The beaver dam eye study. Retinopathy in adults with newly discovered and previously diagnosed diabetes mellitus. Ophthalmology. 1992;99:58–62. doi: 10.1016/s0161-6420(92)32011-1. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Moss SE, Klein BE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. XI. The incidence of macular edema. Ophthalmology. 1989;96:1501–10. doi: 10.1016/s0161-6420(89)32699-6. [DOI] [PubMed] [Google Scholar]
- 4.Xie XW, Xu L, Wang YX, Jonas JB. Prevalence and associated factors of diabetic retinopathy. The Beijing eye study 2006. Graefes Arch Clin Exp Ophthalmol. 2008;246:1519–26. doi: 10.1007/s00417-008-0884-6. [DOI] [PubMed] [Google Scholar]
- 5.Rubino A, Rousculp MD, Davis K, Wang J, Girach A. Diagnosed diabetic retinopathy in France, Italy, Spain, and the United Kingdom. Prim Care Diabetes. 2007;1:75–80. doi: 10.1016/j.pcd.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141:446–55. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varma R, Torres M, Peña F, Klein R, Azen SP Los Angeles Latino Eye Study Group. Prevalence of diabetic retinopathy in adult Latinos: The Los Angeles Latino eye study. Ophthalmology. 2004;111:1298–306. doi: 10.1016/j.ophtha.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105:1801–15. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 10.Lattanzio R, Brancato R, Pierro L, Bandello F, Iaccher B, Fiore T, et al. Macular thickness measured by optical coherence tomography (OCT) in diabetic patients. Eur J Ophthalmol. 2002;12:482–7. doi: 10.1177/112067210201200606. [DOI] [PubMed] [Google Scholar]
- 11.Browning DJ, Fraser CM, Clark S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology. 2008;115:533–900. doi: 10.1016/j.ophtha.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 12.Kuo JZ, Wong TY, Rotter JI. Challenges in elucidating the genetics of diabetic retinopathy. JAMA Ophthalmol. 2014;132:96–107. doi: 10.1001/jamaophthalmol.2013.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasaki M, Inomata H. Relation between superficial capillaries and foveal structures in the human retina. Invest Ophthalmol Vis Sci. 1986;27:1698–705. [PubMed] [Google Scholar]
- 14.Snodderly DM, Weinhaus RS. Retinal vasculature of the fovea of the squirrel monkey, Saimiri sciureus: Three-dimensional architecture, visual screening, and relationships to the neuronal layers. J Comp Neurol. 1990;297:145–63. doi: 10.1002/cne.902970111. [DOI] [PubMed] [Google Scholar]
- 15.Hagag AM, Pechauer AD, Liu L, Wang J, Zhang M, Jia Y, et al. OCT angiography changes in the 3 parafoveal retinal plexuses in response to hyperoxia. Ophthalmol Retina. 2018;2:329–36. doi: 10.1016/j.oret.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore J, Bagley S, Ireland G, McLeod D, Boulton ME. Three dimensional analysis of microaneurysms in the human diabetic retina. J Anat. 1999;194(Pt 1):89–100. doi: 10.1046/j.1469-7580.1999.19410089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sachdev N, Gupta V, Abhiramamurthy V, Singh R, Gupta A. Correlation between microaneurysm closure rate and reduction in macular thickness following laser photocoagulation of diabetic macular edema. Eye (Lond) 2008;22:975–7. doi: 10.1038/sj.eye.6702801. [DOI] [PubMed] [Google Scholar]
- 18.Querques G, Bandello F, Souied EH. Abnormal deep retinal capillary networking and microaneurysms in the outer nuclear layer of diabetic eyes. Ophthalmology. 2014;121:803–40. doi: 10.1016/j.ophtha.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Browning DJ, editor. Diabetic Retinopathy, Evidence-Based Management. 1st ed. Ch. 7. New York: Springer Inc; 2010. Diabetic macular edema. [Google Scholar]
- 20.Nagaoka T, Kitaya N, Sugawara R, Yokota H, Mori F, Hikichi T, et al. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004;88:1060–3. doi: 10.1136/bjo.2003.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fryczkowski AW, Hodes BL, Walker J. Diabetic choroidal and iris vasculature scanning electron microscopy findings. Int Ophthalmol. 1989;13:269–79. doi: 10.1007/BF02280087. [DOI] [PubMed] [Google Scholar]
- 22.Vinores SA, McGehee R, Lee A, Gadegbeku C, Campochiaro PA. Ultrastructural localization of blood-retinal barrier breakdown in diabetic and galactosemic rats. J Histochem Cytochem. 1990;38:1341–52. doi: 10.1177/38.9.2117624. [DOI] [PubMed] [Google Scholar]
- 23.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: More than meets the eye. Surv Ophthalmol. 2002;47(Suppl 2):S253–62. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 24.Nasrallah FP, Jalkh AE, Van Coppenolle F, Kado M, Trempe CL, McMeel JW, et al. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95:1335–9. doi: 10.1016/s0161-6420(88)33004-6. [DOI] [PubMed] [Google Scholar]
- 25.Gandorfer A. Role of vitreous in diabetic macular edema. Retina. 2012;32(Suppl 2):S211–5. doi: 10.1097/IAE.0b013e31825bc704. [DOI] [PubMed] [Google Scholar]
- 26.Gandorfer A, Rohleder M, Grosselfinger S, Haritoglou C, Ulbig M, Kampik A, et al. Epiretinal pathology of diffuse diabetic macular edema associated with vitreomacular traction. Am J Ophthalmol. 2005;139:638–52. doi: 10.1016/j.ajo.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger D, Fink-Cohen S, Gaton DD, Priel E, Yassur Y. Non-retinovascular leakage in diabetic maculopathy. Br J Ophthalmol. 1995;79:728–31. doi: 10.1136/bjo.79.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y, et al. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1) Am J Pathol. 2000;156:1733–9. doi: 10.1016/S0002-9440(10)65044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–41. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sander B, Larsen M, Moldow B, Lund-Andersen H. Diabetic macular edema: Passive and active transport of fluorescein through the blood-retina barrier. Invest Ophthalmol Vis Sci. 2001;42:433–8. [PubMed] [Google Scholar]
- 31.Lund-Andersen H. Mechanisms for monitoring changes in retinal status following therapeutic intervention in diabetic retinopathy. Surv Ophthalmol. 2002;47(Suppl 2):S270–7. doi: 10.1016/s0039-6257(02)00386-7. [DOI] [PubMed] [Google Scholar]
- 32.Stefánsson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006;51:364–80. doi: 10.1016/j.survophthal.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Kristinsson JK, Gottfredsdóttir MS, Stefánsson E. Retinal vessel dilatation and elongation precedes diabetic macular oedema. Br J Ophthalmol. 1997;81:274–8. doi: 10.1136/bjo.81.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin RH, Frank RN, Kennedy A, Eliott D, Puklin JE, Abrams GW, et al. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997;38:36–47. [PubMed] [Google Scholar]
- 35.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP, et al. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–52. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116:73–9. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Kent D, Vinores SA, Campochiaro PA. Macular oedema: The role of soluble mediators. Br J Ophthalmol. 2000;84:542–5. doi: 10.1136/bjo.84.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel JI, Hykin PG, Cree IA. Diabetic cataract removal: Postoperative progression of maculopathy – Growth factor and clinical analysis. Br J Ophthalmol. 2006;90:697–701. doi: 10.1136/bjo.2005.087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–58. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardner TW, Eller AW, Friberg TR, D’Antonio JA, Hollis TM. Antihistamines reduce blood-retinal barrier permeability in type I (insulin-dependent) diabetic patients with nonproliferative retinopathy. A pilot study. Retina. 1995;15:134–40. [PubMed] [Google Scholar]
- 41.Kaiser PK. Antivascular endothelial growth factor agents and their development: Therapeutic implications in ocular diseases. Am J Ophthalmol. 2006;142:660–8. doi: 10.1016/j.ajo.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 42.Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes. 2004;53:2883–92. doi: 10.2337/diabetes.53.11.2883. [DOI] [PubMed] [Google Scholar]
- 43.Sebag J, Buckingham B, Charles MA, Reiser K. Biochemical abnormalities in vitreous of humans with proliferative diabetic retinopathy. Arch Ophthalmol. 1992;110:1472–6. doi: 10.1001/archopht.1992.01080220134035. [DOI] [PubMed] [Google Scholar]
- 44.Sebag J, Balazs EA. Pathogenesis of cystoid macular edema: An anatomic consideration of vitreoretinal adhesions. Surv Ophthalmol. 1984;28(Suppl):493–8. doi: 10.1016/0039-6257(84)90231-5. [DOI] [PubMed] [Google Scholar]
- 45.Kaiser PK, Riemann CD, Sears JE, Lewis H. Macular traction detachment and diabetic macular edema associated with posterior hyaloidal traction. Am J Ophthalmol. 2001;131:44–9. doi: 10.1016/s0002-9394(00)00872-2. [DOI] [PubMed] [Google Scholar]
- 46.Otani T, Kishi S. A controlled study of vitrectomy for diabetic macular edema. Am J Ophthalmol. 2002;134:214–9. doi: 10.1016/s0002-9394(02)01548-9. [DOI] [PubMed] [Google Scholar]
- 47.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: A possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139:302–10. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 48.Massin P, Duguid G, Erginay A, Haouchine B, Gaudric A. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003;135:169–77. doi: 10.1016/s0002-9394(02)01837-8. [DOI] [PubMed] [Google Scholar]
- 49.Kadonosono K, Itoh N, Ohno S. Perifoveal microcirculation before and after vitrectomy for diabetic cystoid macular edema. Am J Ophthalmol. 2000;130:740–4. doi: 10.1016/s0002-9394(00)00575-4. [DOI] [PubMed] [Google Scholar]
- 50.Yu DY, Cringle SJ, Su E, Yu PK, Humayun MS, Dorin G, et al. Laser-induced changes in intraretinal oxygen distribution in pigmented rabbits. Invest Ophthalmol Vis Sci. 2005;46:988–99. doi: 10.1167/iovs.04-0767. [DOI] [PubMed] [Google Scholar]
- 51.Browning DJ. Diabetic macular edema: A critical review of the early treatment diabetic retinopathy study (ETDRS) series and subsequent studies. Comp Ophthalmol Update. 2000;1:69–83. [Google Scholar]
- 52.Wallentén KG, Malmsjö M, Andréasson S, Wackenfors A, Johansson K, Ghosh F, et al. Retinal function and PKC alpha expression after focal laser photocoagulation. Graefes Arch Clin Exp Ophthalmol. 2007;245:1815–24. doi: 10.1007/s00417-007-0646-x. [DOI] [PubMed] [Google Scholar]
- 53.Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–13. [PubMed] [Google Scholar]
- 54.Zhang X, Bao S, Lai D, Rapkins RW, Gillies MC. Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes. 2008;57:1026–33. doi: 10.2337/db07-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao H, Qiao X, Gao R, Mieler WF, McPherson AR, Holz ER, et al. Intravitreal triamcinolone does not alter basal vascular endothelial growth factor mRNA expression in rat retina. Vision Res. 2004;44:349–56. doi: 10.1016/j.visres.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 56.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341:309–15. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 57.Tamura H, Miyamoto K, Kiryu J, Miyahara S, Katsuta H, Hirose F, et al. Intravitreal injection of corticosteroid attenuates leukostasis and vascular leakage in experimental diabetic retina. Invest Ophthalmol Vis Sci. 2005;46:1440–4. doi: 10.1167/iovs.04-0905. [DOI] [PubMed] [Google Scholar]
- 58.Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, et al. Transcriptional interference between c-jun and the glucocorticoid receptor: Mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–15. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 59.Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005;80:249–58. doi: 10.1016/j.exer.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Stefánsson E, Landers MB., 3rd How does vitrectomy affect diabetic macular edema? Am J Ophthalmol. 2006;141:984. doi: 10.1016/j.ajo.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 61.Lewis H. The role of vitrectomy in the treatment of diabetic macular edema. Am J Ophthalmol. 2001;131:123–5. doi: 10.1016/s0002-9394(00)00660-7. [DOI] [PubMed] [Google Scholar]
- 62.Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1987;94:761–74. doi: 10.1016/s0161-6420(87)33527-4. [DOI] [PubMed] [Google Scholar]
- 63.Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study research group. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 64.Krepler K, Wagner J, Sacu S, Wedrich A. The effect of intravitreal triamcinolone on diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol. 2005;243:478–81. doi: 10.1007/s00417-004-1059-8. [DOI] [PubMed] [Google Scholar]
- 65.Negi AK, Vernon SA, Lim CS, Owen-Armstrong K. Intravitreal triamcinolone improves vision in eyes with chronic diabetic macular oedema refractory to laser photocoagulation. Eye (Lond) 2005;19:747–51. doi: 10.1038/sj.eye.6701636. [DOI] [PubMed] [Google Scholar]
- 66.Tunc M, Onder HI, Kaya M. Posterior sub-Tenon's capsule triamcinolone injection combined with focal laser photocoagulation for diabetic macular edema. Ophthalmology. 2005;112:1086–91. doi: 10.1016/j.ophtha.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 67.Ciardella AP, Klancnik J, Schiff W, Barile G, Langton K, Chang S, et al. Intravitreal triamcinolone for the treatment of refractory diabetic macular oedema with hard exudates: An optical coherence tomography study. Br J Ophthalmol. 2004;88:1131–6. doi: 10.1136/bjo.2004.041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knudsen LL. Retrobulbar injection of methylprednisolone in diffuse diabetic macular edema. Retina. 2004;24:905–9. doi: 10.1097/00006982-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 69.Avci R, Kaderli B. Intravitreal triamcinolone injection for chronic diabetic macular oedema with severe hard exudates. Graefes Arch Clin Exp Ophthalmol. 2006;244:28–35. doi: 10.1007/s00417-005-0069-5. [DOI] [PubMed] [Google Scholar]
- 70.Jonas JB, Kreissig I, Söfker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121:57–61. [PubMed] [Google Scholar]
- 71.McDonald HR, Schatz H. Grid photocoagulation for diffuse macular edema. Retina. 1985;5:65–72. doi: 10.1097/00006982-198500520-00001. [DOI] [PubMed] [Google Scholar]
- 72.Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 73.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: Results from the pan-american collaborative retina study group at 6-month follow-up. Ophthalmology. 2007;114:743–50. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 74.Bailey CC, Sparrow JM, Grey RH, Cheng H. The national diabetic retinopathy laser treatment audit. I. Maculopathy. Eye (Lond) 1998;12(Pt 1):69–76. doi: 10.1038/eye.1998.13. [DOI] [PubMed] [Google Scholar]
- 75.Kumar A, Sinha S, Azad R, Sharma YR, Vohra R. Comparative evaluation of vitrectomy and dye-enhanced ILM peel with grid laser in diffuse diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2007;245:360–8. doi: 10.1007/s00417-006-0456-6. [DOI] [PubMed] [Google Scholar]
- 76.Abu el Asrar AM, Morse PH. Laser photocoagulation control of diabetic macular oedema without fluorescein angiography. Br J Ophthalmol. 1991;75:97–9. doi: 10.1136/bjo.75.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aylward GW. Progressive changes in diabetics and their management. Eye (Lond) 2005;19:1115–8. doi: 10.1038/sj.eye.6701969. [DOI] [PubMed] [Google Scholar]
- 78.Zein WM, Noureddin BN, Jurdi FA, Schakal A, Bashshur ZF. Panretinal photocoagulation and intravitreal triamcinolone acetonide for the management of proliferative diabetic retinopathy with macular edema. Retina. 2006;26:137–42. doi: 10.1097/00006982-200602000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Bresnick GH. Diabetic macular edema. A review. Ophthalmology. 1986;93:989–97. doi: 10.1016/s0161-6420(86)33650-9. [DOI] [PubMed] [Google Scholar]
- 80.Gaucher D, Sebah C, Erginay A, Haouchine B, Tadayoni R, Gaudric A, et al. Optical coherence tomography features during the evolution of serous retinal detachment in patients with diabetic macular edema. Am J Ophthalmol. 2008;145:289–96. doi: 10.1016/j.ajo.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 81.Bardak Y, Cekiç O, Tiğ SU. Comparison of ICG-assisted ILM peeling and triamcinolone-assisted posterior vitreous removal in diffuse diabetic macular oedema. Eye (Lond) 2006;20:1357–9. doi: 10.1038/sj.eye.6702152. [DOI] [PubMed] [Google Scholar]
- 82.Zander E, Herfurth S, Bohl B, Heinke P, Herrmann U, Kohnert KD, et al. Maculopathy in patients with diabetes mellitus type 1 and type 2: Associations with risk factors. Br J Ophthalmol. 2000;84:871–6. doi: 10.1136/bjo.84.8.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verma LK, Vivek MB, Kumar A, Tewari HK, Venkatesh P. A prospective controlled trial to evaluate the adjunctive role of posterior subtenon triamcinolone in the treatment of diffuse diabetic macular edema. J Ocul Pharmacol Ther. 2004;20:277–84. doi: 10.1089/1080768041725308. [DOI] [PubMed] [Google Scholar]
- 84.Berman DH, Friedman EA. Partial absorption of hard exudates in patients with diabetic end-stage renal disease and severe anemia after treatment with erythropoietin. Retina. 1994;14:1–5. doi: 10.1097/00006982-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 85.Greenstein VC, Chen H, Hood DC, Holopigian K, Seiple W, Carr RE, et al. Retinal function in diabetic macular edema after focal laser photocoagulation. Invest Ophthalmol Vis Sci. 2000;41:3655–64. [PubMed] [Google Scholar]
- 86.Greenstein VC, Holopigian K, Hood DC, Seiple W, Carr RE. The nature and extent of retinal dysfunction associated with diabetic macular edema. Invest Ophthalmol Vis Sci. 2000;41:3643–54. [PubMed] [Google Scholar]
- 87.Gaucher D, Tadayoni R, Erginay A, Haouchine B, Gaudric A, Massin P, et al. Optical coherence tomography assessment of the vitreoretinal relationship in diabetic macular edema. Am J Ophthalmol. 2005;139:807–13. doi: 10.1016/j.ajo.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 88.Browning DJ, Altaweel MM, Bressler NM, Bressler SB, Scott IU Diabetic Retinopathy Clinical Research Network. Diabetic macular edema: What is focal and what is diffuse? Am J Ophthalmol. 2008;146:649–55. doi: 10.1016/j.ajo.2008.07.013. 655.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127:688–93. doi: 10.1016/s0002-9394(99)00033-1. [DOI] [PubMed] [Google Scholar]
- 90.Kaldırım H, Yazgan S, Atalay K, Gurez C, Savur F. Intravitreal dexamethasone implantation in patients with different morphological diabetic macular edema having insufficient response to ranibizumab. Retina. 2018;38:986–92. doi: 10.1097/IAE.0000000000001648. [DOI] [PubMed] [Google Scholar]
- 91.Browning DJ, McOwen MD, Bowen RM, Jr, O’Marah TL. Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology. 2004;111:712–5. doi: 10.1016/j.ophtha.2003.06.028. [DOI] [PubMed] [Google Scholar]
- 92.Brown JC, Solomon SD, Bressler SB, Schachat AP, DiBernardo C, Bressler NM, et al. Detection of diabetic foveal edema: Contact lens biomicroscopy compared with optical coherence tomography. Arch Ophthalmol. 2004;122:330–5. doi: 10.1001/archopht.122.3.330. [DOI] [PubMed] [Google Scholar]
- 93.Browning DJ, Fraser CM. The predictive value of patient and eye characteristics on the course of subclinical diabetic macular edema. Am J Ophthalmol. 2008;145:149–54. doi: 10.1016/j.ajo.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 94.Patel JI, Hykin PG, Schadt M, Luong V, Fitzke F, Gregor ZJ, et al. Pars plana vitrectomy for diabetic macular oedema: OCT and functional correlations. Eye (Lond) 2006;20:674–80. doi: 10.1038/sj.eye.6701945. [DOI] [PubMed] [Google Scholar]
- 95.Rosenblatt BJ, Shah GK, Sharma S, Bakal J. Pars plana vitrectomy with internal limiting membranectomy for refractory diabetic macular edema without a taut posterior hyaloid. Graefes Arch Clin Exp Ophthalmol. 2005;243:20–5. doi: 10.1007/s00417-004-0958-z. [DOI] [PubMed] [Google Scholar]
- 96.Maturi RK, Glassman AR, Liu D, Beck RW, Bhavsar AR, Bressler NM, et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: A DRCR network phase 2 randomized clinical trial. JAMA Ophthalmol. 2018;136:29–38. doi: 10.1001/jamaophthalmol.2017.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bressler NM, Beaulieu WT, Glassman AR, Blinder KJ, Bressler SB, Jampol LM, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: A secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136:257–69. doi: 10.1001/jamaophthalmol.2017.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stolba U, Binder S, Gruber D, Krebs I, Aggermann T, Neumaier B, et al. Vitrectomy for persistent diffuse diabetic macular edema. Am J Ophthalmol. 2005;140:295–301. doi: 10.1016/j.ajo.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 99.Recchia FM, Ruby AJ, Carvalho Recchia CA. Pars plana vitrectomy with removal of the internal limiting membrane in the treatment of persistent diabetic macular edema. Am J Ophthalmol. 2005;139:447–54. doi: 10.1016/j.ajo.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 100.Brun SC, Bressler SB, Maguire MG. A comparison of fundus biomicroscopy and 90 diopter lens examination in the detection of diabetic macular edema. Invest Ophthalmol Vis Sci. 1993;34:72. [Google Scholar]
- 101.Rudnisky CJ, Tennant MT, de Leon AR, Hinz BJ, Greve MD. Benefits of stereopsis when identifying clinically significant macular edema via teleophthalmology. Can J Ophthalmol. 2006;41:727–32. doi: 10.3129/i06-066. [DOI] [PubMed] [Google Scholar]
- 102.Bresnick GH, Mukamel DB, Dickinson JC, Cole DR. A screening approach to the surveillance of patients with diabetes for the presence of vision-threatening retinopathy. Ophthalmology. 2000;107:19–24. doi: 10.1016/s0161-6420(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 103.Gangnon RE, Davis MD, Hubbard LD, Aiello LM, Chew EY, Ferris FL, 3rd, et al. A severity scale for diabetic macular edema developed from ETDRS data. Invest Ophthalmol Vis Sci. 2008;49:5041–7. doi: 10.1167/iovs.08-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davis MD, Bressler SB, Aiello LP, Bressler NM, Browning DJ, Flaxel CJ, et al. Comparison of time-domain OCT and fundus photographic assessments of retinal thickening in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49:1745–52. doi: 10.1167/iovs.07-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gupta A. Reply to letter. Ind J Ophthalmol. 1997;45:260–1. [Google Scholar]
- 106.Emanuele N, Klein R, Moritz T, Davis MD, Glander K, Anderson R, et al. Comparison of dilated fundus examinations with seven-field stereo fundus photographs in the veterans affairs diabetes trial. J Diabetes Complications. 2009;23:323–9. doi: 10.1016/j.jdiacomp.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 107.Browning DJ. Interobserver variability in optical coherence tomography for macular edema. Am J Ophthalmol. 2004;137:1116–7. doi: 10.1016/j.ajo.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 108.Browning DJ, Fraser CM. Intraobserver variability in optical coherence tomography. Am J Ophthalmol. 2004;138:477–9. doi: 10.1016/j.ajo.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 109.Krzystolik MG, Strauber SF, Aiello LP, Beck RW, Berger BB, et al. Diabetic Retinopathy Clinical Research Network. Reproducibility of macular thickness and volume using zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114:1520–5. doi: 10.1016/j.ophtha.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Massin P, Vicaut E, Haouchine B, Erginay A, Paques M, Gaudric A, et al. Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol. 2001;119:1135–42. doi: 10.1001/archopht.119.8.1135. [DOI] [PubMed] [Google Scholar]
- 111.Koozekanani D, Roberts C, Katz SE, Herderick EE. Intersession repeatability of macular thickness measurements with the humphrey 2000 OCT. Invest Ophthalmol Vis Sci. 2000;41:1486–91. [PubMed] [Google Scholar]
- 112.Browning DJ, Fraser CM, Propst BW. The variation in optical coherence tomography-measured macular thickness in diabetic eyes without clinical macular edema. Am J Ophthalmol. 2008;145:889–93. doi: 10.1016/j.ajo.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 113.Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: Preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–24. doi: 10.1016/j.ophtha.2003.05.037. [DOI] [PubMed] [Google Scholar]
- 114.Browning DJ, Glassman AR, Aiello LP, Bressler NM, Bressler SB, Danis RP, et al. Optical coherence tomography measurements and analysis methods in optical coherence tomography studies of diabetic macular edema. Ophthalmology. 2008;115:1366–71. doi: 10.1016/j.ophtha.2007.12.004. 1371.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chan A, Duker JS. A standardized method for reporting changes in macular thickening using optical coherence tomography. Arch Ophthalmol. 2005;123:939–43. doi: 10.1001/archopht.123.7.939. [DOI] [PubMed] [Google Scholar]
- 116.Hee MR, Puliafito CA, Wong C, Duker JS, Reichel E, Rutledge B, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995;113:1019–29. doi: 10.1001/archopht.1995.01100080071031. [DOI] [PubMed] [Google Scholar]
- 117.Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49:4290–6. doi: 10.1167/iovs.08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kakinoki M, Sawada O, Sawada T, Kawamura H, Ohji M. Comparison of macular thickness between cirrus HD-OCT and stratus OCT. Ophthalmic Surg Lasers Imaging. 2009;40:135–40. doi: 10.3928/15428877-20090301-09. [DOI] [PubMed] [Google Scholar]
- 119.Legarreta JE, Gregori G, Punjabi OS, Knighton RW, Lalwani GA, Puliafito CA, et al. Macular thickness measurements in normal eyes using spectral domain optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2008;39:S43–9. doi: 10.3928/15428877-20080715-02. [DOI] [PubMed] [Google Scholar]
- 120.Wang JC, Laíns I, Providência J, Armstrong GW, Santos AR, Gil P, et al. Diabetic choroidopathy: Choroidal vascular density and volume in diabetic retinopathy with swept-source optical coherence tomography. Am J Ophthalmol. 2017;184:75–83. doi: 10.1016/j.ajo.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 121.Willoughby AS, Chiu SJ, Silverman RK, Farsiu S, Bailey C, Wiley HE, et al. Platform-independent cirrus and spectralis thickness measurements in eyes with diabetic macular edema using fully automated software. Transl Vis Sci Technol. 2017;6:9. doi: 10.1167/tvst.6.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Drexler W. Cellular and functional optical coherence tomography of the human retina: The Cogan lecture. Invest Ophthalmol Vis Sci. 2007;48:5339–51. doi: 10.1167/iovs.07-0895. [DOI] [PubMed] [Google Scholar]
- 123.Ibrahim MA, Sepah YJ, Symons RC, Channa R, Hatef E, Khwaja A, et al. Spectral- and time-domain optical coherence tomography measurements of macular thickness in normal eyes and in eyes with diabetic macular edema. Eye (Lond) 2012;26:454–62. doi: 10.1038/eye.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, et al. Diabetic Retinopathy Clinical Research Network. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Forooghian F, Stetson PF, Meyer SA, Chew EY, Wong WT, Cukras C, et al. Relationship between photoreceptor outer segment length and visual acuity in diabetic macular edema. Retina. 2010;30:63–70. doi: 10.1097/IAE.0b013e3181bd2c5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eliwa TF, Hussein MA, Zaki MA, Raslan OA. Outer retinal layer thickness as good visual predictor in patients with diabetic macular edema. Retina. 2018;38:805–11. doi: 10.1097/IAE.0000000000001599. [DOI] [PubMed] [Google Scholar]
- 127.Wong RL, Lee JW, Yau GS, Wong IY. Relationship between outer retinal layers thickness and visual acuity in diabetic macular edema. Biomed Res Int 2015. 2015:981471. doi: 10.1155/2015/981471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132:1309–16. doi: 10.1001/jamaophthalmol.2014.2350. [DOI] [PubMed] [Google Scholar]
- 129.Fickweiler W, Schauwvlieghe AM, Schlingemann RO, Maria Hooymans JM, Los LI, Verbraak FD, et al. Predictive value of optical coherence tomographic features in the bevacizumab and ranibizumab in patients with diabetic macular edema (Brdme) study. Retina. 2018;38:812–9. doi: 10.1097/IAE.0000000000001626. [DOI] [PubMed] [Google Scholar]
- 130.Das R, Spence G, Hogg RE, Stevenson M, Chakravarthy U. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol. 2018;136:202–8. doi: 10.1001/jamaophthalmol.2017.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muftuoglu IK, Mendoza N, Gaber R, Alam M, You Q, Freeman WR, et al. Integrity of outer retinal layers after resolution of central involved diabetic macular edema. Retina. 2017;37:2015–24. doi: 10.1097/IAE.0000000000001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chhablani JK, Kim JS, Cheng L, Kozak I, Freeman W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2012;250:1415–20. doi: 10.1007/s00417-012-1968-x. [DOI] [PubMed] [Google Scholar]
- 133.Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137:675–82. doi: 10.1016/j.ajo.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 134.Fong DS, Segal PP, Myers F, Ferris FL, Hubbard LD, Davis MD, et al. Subretinal fibrosis in diabetic macular edema. ETDRS report 23. Early treatment diabetic retinopathy study research group. Arch Ophthalmol. 1997;115:873–7. doi: 10.1001/archopht.1997.01100160043006. [DOI] [PubMed] [Google Scholar]
- 135.Figueroa MS, Contreras I, Noval S. Surgical and anatomical outcomes of pars plana vitrectomy for diffuse nontractional diabetic macular edema. Retina. 2008;28:420–6. doi: 10.1097/IAE.0b013e318159e7d2. [DOI] [PubMed] [Google Scholar]
- 136.Harbour JW, Smiddy WE, Flynn HW, Jr, Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121:405–13. doi: 10.1016/s0002-9394(14)70437-4. [DOI] [PubMed] [Google Scholar]
- 137.Takaya K, Suzuki Y, Mizutani H, Sakuraba T, Nakazawa M. Long-term results of vitrectomy for removal of submacular hard exudates in patients with diabetic maculopathy. Retina. 2004;24:23–9. doi: 10.1097/00006982-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 138.Progression of retinopathy with intensive versus conventional treatment in the diabetes control and complications trial. Diabetes control and complications trial research group. Ophthalmology. 1995;102:647–61. doi: 10.1016/s0161-6420(95)30973-6. [DOI] [PubMed] [Google Scholar]
- 139.Early worsening of diabetic retinopathy in the diabetes control and complications trial. Arch Ophthalmol. 1998;116:874–86. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- 140.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 141.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK prospective diabetes study group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 142.Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin epidemiologic study of diabetic retinopathy. XIII. Relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98:1261–5. doi: 10.1016/s0161-6420(91)32145-6. [DOI] [PubMed] [Google Scholar]
- 143.Chew EY, Klein ML, Ferris FL, 3rd, Remaley NA, Murphy RP, Chantry K, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early treatment diabetic retinopathy study (ETDRS) report 22. Arch Ophthalmol. 1996;114:1079–84. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 144.Kremser BG, Falk M, Kieselbach GF. Influence of serum lipid fractions on the course of diabetic macular edema after photocoagulation. Ophthalmologica. 1995;209:60–3. doi: 10.1159/000310581. [DOI] [PubMed] [Google Scholar]
- 145.Emoto M, Anno T, Sato Y, Tanabe K, Okuya S, Tanizawa Y, et al. Troglitazone treatment increases plasma vascular endothelial growth factor in diabetic patients and its mRNA in 3T3-L1 adipocytes. Diabetes. 2001;50:1166–70. doi: 10.2337/diabetes.50.5.1166. [DOI] [PubMed] [Google Scholar]
- 146.Ambrosius WT, Danis RP, Goff DC, Jr, Greven CM, Gerstein HC, Cohen RM. Lack of association between thiazolidinediones and macular edema in type 2 diabetes: The ACCORD eye substudy. Arch Ophthalmol. 2010;128:312–8. doi: 10.1001/archophthalmol.2009.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gower EW, Lovato JF, Ambrosius WT, Chew EY, Danis RP, Davis MD, et al. Lack of longitudinal association between thiazolidinediones and incidence and progression of diabetic eye disease: The ACCORD eye study. Am J Ophthalmol. 2018;187:138–47. doi: 10.1016/j.ajo.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Singh R, Abhiramamurthy V, Gupta V, Gupta A, Bhansali A. Effect of multifactorial intervention on diabetic macular edema. Diabetes Care. 2006;29:463–4. doi: 10.2337/diacare.29.02.06.dc05-1931. [DOI] [PubMed] [Google Scholar]
- 149.Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991;109:1549–51. doi: 10.1001/archopht.1991.01080110085041. [DOI] [PubMed] [Google Scholar]
- 150.Guyer DR, D’Amico DJ, Smith CW. Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992;113:652–6. doi: 10.1016/s0002-9394(14)74789-0. [DOI] [PubMed] [Google Scholar]
- 151.Han DP, Mieler WF, Burton TC. Submacular fibrosis after photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992;113:513–21. doi: 10.1016/s0002-9394(14)74722-1. [DOI] [PubMed] [Google Scholar]
- 152.Lewis H, Schachat AP, Haimann MH, Haller JA, Quinlan P, von Fricken MA, et al. Choroidal neovascularization after laser photocoagulation for diabetic macular edema. Ophthalmology. 1990;97:503–10. doi: 10.1016/s0161-6420(90)32574-5. [DOI] [PubMed] [Google Scholar]
- 153.Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early treatment diabetic retinopathy study report number 2. Early treatment diabetic retinopathy study research group. Ophthalmology. 1987;94:761–74. doi: 10.1016/s0161-6420(87)33527-4. [DOI] [PubMed] [Google Scholar]
- 154.Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, et al. Diabetic Retinopathy Clinical Research Network. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–7. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Browning DJ, Fraser CM, Powers ME. Comparison of the magnitude and time course of macular thinning induced by different interventions for diabetic macular edema: Implications for sequence of application. Ophthalmology. 2006;113:1713–9. doi: 10.1016/j.ophtha.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 156.Chew E, Strauber S, Beck R, Aiello LP, Antoszyk A, et al. Diabetic Retinopathy Clinical Research Network. Randomized trial of peribulbar triamcinolone acetonide with and without focal photocoagulation for mild diabetic macular edema: A pilot study. Ophthalmology. 2007;114:1190–6. doi: 10.1016/j.ophtha.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–9. doi: 10.1016/j.ophtha.2008.06.015. 1449.e1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88:1173–9. doi: 10.1136/bjo.2003.040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:74–80. doi: 10.1136/bjo.2004.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Akduman L, Olk RJ. Subthreshold (invisible) modified grid diode laser photocoagulation in diffuse diabetic macular edema (DDME) Ophthalmic Surg Lasers. 1999;30:706–14. [PubMed] [Google Scholar]
- 161.Luttrull JK, Sramek C, Palanker D, Spink CJ, Musch DC. Long-term safety, high-resolution imaging, and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina. 2012;32:375–86. doi: 10.1097/IAE.0b013e3182206f6c. [DOI] [PubMed] [Google Scholar]
- 162.Jonas JB, Söfker A. Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am J Ophthalmol. 2001;132:425–7. doi: 10.1016/s0002-9394(01)01010-8. [DOI] [PubMed] [Google Scholar]
- 163.Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M, et al. Intravitreal triamcinolone for refractory diabetic macular edema: Two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533–8. doi: 10.1016/j.ophtha.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 164.Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–7. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 165.Audren F, Lecleire-Collet A, Erginay A, Haouchine B, Benosman R, Bergmann JF, et al. Intravitreal triamcinolone acetonide for diffuse diabetic macular edema: Phase 2 trial comparing 4 mg vs.2 mg. Am J Ophthalmol. 2006;142:794–99. doi: 10.1016/j.ajo.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 166.Avitabile T, Longo A, Reibaldi A. Intravitreal triamcinolone compared with macular laser grid photocoagulation for the treatment of cystoid macular edema. Am J Ophthalmol. 2005;140:695–702. doi: 10.1016/j.ajo.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 167.Lam DS, Chan CK, Mohamed S, Lai TY, Li KK, Li PS, et al. A prospective randomised trial of different doses of intravitreal triamcinolone for diabetic macular oedema. Br J Ophthalmol. 2007;91:199–203. doi: 10.1136/bjo.2006.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Maturi RK, Pollack A, Uy HS, Varano M, Gomes AM, Li XY, et al. Intraocular pressure in patients with diabetic macular edema treated with dexamethasone intravitreal implant in the 3-year mead study. Retina. 2016;36:1143–52. doi: 10.1097/IAE.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 169.Boyer DS, Yoon YH, Belfort R, Jr, Bandello F, Maturi RK, Augustin AJ. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–14. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 170.Al-Khersan H, Hariprasad SM, Chhablani J Dex Implant Study Group. Early response to intravitreal dexamethasone implant therapy in diabetic macular edema may predict visual outcome. Am J Ophthalmol. 2017;184:121–8. doi: 10.1016/j.ajo.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 171.Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–3500. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 172.Andrade GC, Dias JR, Maia A, Farah ME, Meyer CH, Rodrigues EB, et al. Intravitreal injections of ziv-aflibercept for diabetic macular edema: A pilot study. Retina. 2016;36:1640–5. doi: 10.1097/IAE.0000000000001000. [DOI] [PubMed] [Google Scholar]
- 173.de Oliveira Dias JR, Badaró E, Novais EA, Colicchio D, Chiarantin GM, Matioli MM, et al. Preclinical investigations of intravitreal ziv-aflibercept. Ophthalmic Surg Lasers Imaging Retina. 2014;45:577–84. doi: 10.3928/23258160-20141118-15. [DOI] [PubMed] [Google Scholar]
- 174.Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, et al. Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Andrade GC, de Oliveira Dias JR, Maia A, Farah ME, Meyer CH, Rodrigues EB, et al. Intravitreal ziv-aflibercept for diabetic macular edema: 48-week outcomes. Ophthalmic Surg Lasers Imaging Retina. 2018;49:245–50. doi: 10.3928/23258160-20180329-06. [DOI] [PubMed] [Google Scholar]
- 176.Cunningham ET, Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D’Amico DJ. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112:1747–57. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 177.Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: Report 3. Arch Ophthalmol. 2012;130:972–9. doi: 10.1001/archophthalmol.2012.393. [DOI] [PubMed] [Google Scholar]
- 178.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 179.Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122:2044–52. doi: 10.1016/j.ophtha.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 180.Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–9. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Do DV, Sepah YJ, Boyer D, Callanan D, Gallemore R, Bennett M, et al. Month-6 primary outcomes of the READ-3 study (Ranibizumab for edema of the mAcula in diabetes-protocol 3 with high dose) Eye (Lond) 2015;29:1538–44. doi: 10.1038/eye.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sepah YJ, Sadiq MA, Boyer D, Callanan D, Gallemore R, Bennett M, et al. Twenty-four-month outcomes of the ranibizumab for edema of the macula in diabetes – Protocol 3 with high dose (READ-3) study. Ophthalmology. 2016;123:2581–7. doi: 10.1016/j.ophtha.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 183.Diabetic Retinopathy Clinical Research Network. Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077.e5. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, et al. The REVEAL study: Ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology. 2015;122:1402–15. doi: 10.1016/j.ophtha.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 185.Bressler SB, Ayala AR, Bressler NM, Melia M, Qin H, Ferris FL, 3rd, et al. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol. 2016;134:278–85. doi: 10.1001/jamaophthalmol.2015.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dhoot DS, Baker K, Saroj N, Vitti R, Berliner AJ, Metzig C, et al. Baseline factors affecting changes in diabetic retinopathy severity scale score after intravitreal aflibercept or laser for diabetic macular edema: Post hoc analyses from VISTA and VIVID. Ophthalmology. 2018;125:51–6. doi: 10.1016/j.ophtha.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 187.Kim JT, Lee DH, Joe SG, Kim JG, Yoon YH. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:3378–84. doi: 10.1167/iovs.12-11503. [DOI] [PubMed] [Google Scholar]
- 188.Chae JB, Joe SG, Yang SJ, Lee JY, Sung KR, Kim JY, et al. Effect of combined cataract surgery and ranibizumab injection in postoperative macular edema in nonproliferative diabetic retinopathy. Retina. 2014;34:149–56. doi: 10.1097/IAE.0b013e3182979b9e. [DOI] [PubMed] [Google Scholar]
- 189.Lee SH, Kim J, Chung H, Kim HC. Changes of choroidal thickness after treatment for diabetic retinopathy. Curr Eye Res. 2014;39:736–44. doi: 10.3109/02713683.2013.867064. [DOI] [PubMed] [Google Scholar]
- 190.Yiu G, Manjunath V, Chiu SJ, Farsiu S, Mahmoud TH. Effect of anti-vascular endothelial growth factor therapy on choroidal thickness in diabetic macular edema. Am J Ophthalmol. 2014;158:745–5100. doi: 10.1016/j.ajo.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nourinia R, Ahmadieh H, Nekoei E, Malekifar P, Tofighi Z. Changes in central choroidal thickness after treatment of diabetic macular edema with intravitreal bevacizumab correlation with central macular thickness and best-corrected visual acuity. Retina. 2018;38:970–5. doi: 10.1097/IAE.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 192.Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Sophie R, et al. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol. 2013;131:139–45. doi: 10.1001/2013.jamaophthalmol.91. [DOI] [PubMed] [Google Scholar]
- 193.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 194.Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): A 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yanagida Y, Ueta T. Systemic safety of ranibizumab for diabetic macular edema: Meta-analysis of randomized trials. Retina. 2014;34:629–35. doi: 10.1097/IAE.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 196.Lang GE, Berta A, Eldem BM, Simader C, Sharp D, Holz FG, et al. Two-year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: Interim analysis of the RESTORE extension study. Ophthalmology. 2013;120:2004–12. doi: 10.1016/j.ophtha.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 197.Stein JD, Newman-Casey PA, Kim DD, Nwanyanwu KH, Johnson MW, Hutton DW, et al. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology. 2013;120:1835–42. doi: 10.1016/j.ophtha.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pershing S, Enns EA, Matesic B, Owens DK, Goldhaber-Fiebert JD. Cost-effectiveness of treatment of diabetic macular edema. Ann Intern Med. 2014;160:18–29. doi: 10.7326/M13-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wu CM, Wu AM, Greenberg PB, Yu F, Lum F, Coleman AL, et al. Frequency of bevacizumab and ranibizumab injections for diabetic macular edema in medicare beneficiaries. Ophthalmic Surg Lasers Imaging Retina. 2018;49:241–4. doi: 10.3928/23258160-20180329-05. [DOI] [PubMed] [Google Scholar]
- 200.Soheilian M, Garfami KH, Ramezani A, Yaseri M, Peyman GA. Two-year results of a randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus laser in diabetic macular edema. Retina. 2012;32:314–21. doi: 10.1097/IAE.0b013e31822f55de. [DOI] [PubMed] [Google Scholar]
- 201.Hikichi T, Fujio N, Akiba J, Azuma Y, Takahashi M, Yoshida A, et al. Association between the short-term natural history of diabetic macular edema and the vitreomacular relationship in type II diabetes mellitus. Ophthalmology. 1997;104:473–8. doi: 10.1016/s0161-6420(97)30289-9. [DOI] [PubMed] [Google Scholar]
- 202.Yamaguchi Y, Otani T, Kishi S. Resolution of diabetic cystoid macular edema associated with spontaneous vitreofoveal separation. Am J Ophthalmol. 2003;135:116–8. doi: 10.1016/s0002-9394(02)01855-x. [DOI] [PubMed] [Google Scholar]
- 203.Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99:753–9. doi: 10.1016/s0161-6420(92)31901-3. [DOI] [PubMed] [Google Scholar]
- 204.Ikeda T, Sato K, Katano T, Hayashi Y. Improved visual acuity following pars plana vitrectomy for diabetic cystoid macular edema and detached posterior hyaloid. Retina. 2000;20:220–2. doi: 10.1097/00006982-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 205.La Heij EC, Hendrikse F, Kessels AG, Derhaag PJ. Vitrectomy results in diabetic macular oedema without evident vitreomacular traction. Graefes Arch Clin Exp Ophthalmol. 2001;239:264–70. doi: 10.1007/s004170000251. [DOI] [PubMed] [Google Scholar]
- 206.Pendergast SD, Hassan TS, Williams GA, Cox MS, Margherio RR, Ferrone PJ, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130:178–86. doi: 10.1016/s0002-9394(00)00472-4. [DOI] [PubMed] [Google Scholar]
- 207.Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol. 1996;122:258–60. doi: 10.1016/s0002-9394(14)72018-5. [DOI] [PubMed] [Google Scholar]
- 208.Terasaki H, Kojima T, Niwa H, Piao CH, Ueno S, Kondo M, et al. Changes in focal macular electroretinograms and foveal thickness after vitrectomy for diabetic macular edema. Invest Ophthalmol Vis Sci. 2003;44:4465–72. doi: 10.1167/iovs.02-1313. [DOI] [PubMed] [Google Scholar]
- 209.Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: The role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol. 2001;132:369–77. doi: 10.1016/s0002-9394(01)01050-9. [DOI] [PubMed] [Google Scholar]
- 210.Yanyali A, Nohutcu AF, Horozoglu F, Celik E. Modified grid laser photocoagulation versus pars plana vitrectomy with internal limiting membrane removal in diabetic macular edema. Am J Ophthalmol. 2005;139:795–801. doi: 10.1016/j.ajo.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 211.Patel JI, Hykin PG, Schadt M, Luong V, Fitzke F, Gregor ZJ, et al. Pars plana vitrectomy with and without peeling of the inner limiting membrane for diabetic macular edema. Retina. 2006;26:5–13. doi: 10.1097/00006982-200601000-00002. [DOI] [PubMed] [Google Scholar]
- 212.Yamamoto T, Hitani K, Tsukahara I, Yamamoto S, Kawasaki R, Yamashita H, et al. Early postoperative retinal thickness changes and complications after vitrectomy for diabetic macular edema. Am J Ophthalmol. 2003;135:14–9. doi: 10.1016/s0002-9394(02)01819-6. [DOI] [PubMed] [Google Scholar]
- 213.Asami T, Terasaki H, Kachi S, Nakamura M, Yamamura K, Nabeshima T, et al. Ultrastructure of internal limiting membrane removed during plasmin-assisted vitrectomy from eyes with diabetic macular edema. Ophthalmology. 2004;111:231–7. doi: 10.1016/j.ophtha.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 214.Patel JI, Hykin PG, Schadt M, Luong V, Bunce C, Fitzke F, et al. Diabetic macular oedema: Pilot randomised trial of pars plana vitrectomy vs. macular argon photocoagulation. Eye (Lond) 2006;20:873–81. doi: 10.1038/sj.eye.6702012. [DOI] [PubMed] [Google Scholar]
- 215.Hartley KL, Smiddy WE, Flynn HW, Jr, Murray TG. Pars plana vitrectomy with internal limiting membrane peeling for diabetic macular edema. Retina. 2008;28:410–9. doi: 10.1097/IAE.0b013e31816102f2. [DOI] [PubMed] [Google Scholar]
- 216.Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000;20:126–33. [PubMed] [Google Scholar]
- 217.Yang CM. Surgical treatment for severe diabetic macular edema with massive hard exudates. Retina. 2000;20:121–5. [PubMed] [Google Scholar]
- 218.Mochizuki Y, Hata Y, Enaida H, Yoshiyama K, Miyazaki M, Ueno A, et al. Evaluating adjunctive surgical procedures during vitrectomy for diabetic macular edema. Retina. 2006;26:143–8. doi: 10.1097/00006982-200602000-00003. [DOI] [PubMed] [Google Scholar]
- 219.Landers MB, 3rd, Graversen VA, Stewart MW. Early vitrectomy for DME: Does it have a role? Sometimes Vitrectomy can be First-Line Treatment. Part 1 of 2. Retina Physician. 2013 [Google Scholar]
- 220.Thomas D, Bunce C, Moorman C, Laidlaw DA. A randomised controlled feasibility trial of vitrectomy versus laser for diabetic macular oedema. Br J Ophthalmol. 2005;89:81–6. doi: 10.1136/bjo.2004.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Higuchi A, Ogata N, Jo N, Wada M, Matsumura M. Pars plana vitrectomy with removal of posterior hyaloid face in treatment of refractory diabetic macular edema resistant to triamcinolone acetonide. Jpn J Ophthalmol. 2006;50:529–31. doi: 10.1007/s10384-006-0366-5. [DOI] [PubMed] [Google Scholar]
- 222.Shah SP, Laidlaw DA. Vitrectomy for diabetic macular edema. Am J Ophthalmol. 2006;141:225. doi: 10.1016/j.ajo.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 223.Laidlaw DA. Vitrectomy for diabetic macular oedema. Eye (Lond) 2008;22:1337–41. doi: 10.1038/eye.2008.84. [DOI] [PubMed] [Google Scholar]
- 224.Holekamp NM, Campbell J, Almony A, Ingraham H, Marks S, Chandwani H, et al. Vision outcomes following anti-vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol. 2018;191:83–91. doi: 10.1016/j.ajo.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 225.Wecker T, Ehlken C, Bühler A, Lange C, Agostini H, Böhringer D, et al. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol. 2017;101:353–9. doi: 10.1136/bjophthalmol-2016-308668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dugel PU, Layton A, Varma RB. Diabetic macular edema diagnosis and treatment in the real world: An analysis of medicare claims data (2008 to 2010) Ophthalmic Surg Lasers Imaging Retina. 2016;47:258–67. doi: 10.3928/23258160-20160229-09. [DOI] [PubMed] [Google Scholar]
- 227.Kiss S, Liu Y, Brown J, Holekamp NM, Almony A, Campbell J, et al. Clinical utilization of anti-vascular endothelial growth-factor agents and patient monitoring in retinal vein occlusion and diabetic macular edema. Clin Ophthalmol. 2014;8:1611–21. doi: 10.2147/OPTH.S60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, et al. Real-world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom National Health Service setting. Am J Ophthalmol. 2016;172:51–7. doi: 10.1016/j.ajo.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 229.Brynskov T, Laugesen CS, Sørensen TL. Intravitreal ranibizumab for diabetic macular oedema: 1-year experiences in a clinical setting. Acta Ophthalmol. 2013;91:e243–4. doi: 10.1111/aos.12014. [DOI] [PubMed] [Google Scholar]
- 230.Menchini U, Bandello F, De Angelis V, Ricci F, Bonavia L, Viola F, et al. Ranibizumab for visual impairment due to diabetic macular edema: Real-world evidence in the Italian population (PRIDE study) J Ophthalmol 2015. 2015:324841. doi: 10.1155/2015/324841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Smiddy RA, Smiddy WE. Nonmedical costs and implications for patients seeking vitreoretinalcare. Retina. 2014;34:1882–7. doi: 10.1097/IAE.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 232.Wallick CJ, Hansen RN, Campbell J, Kiss S, Kowalski JW, Sullivan SD, et al. Comorbidity and health care resource use among commercially insured non-elderly patients with diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina. 2015;46:744–51. doi: 10.3928/23258160-20150730-09. [DOI] [PubMed] [Google Scholar]
- 233.Shea AM, Curtis LH, Hammill BG, Kowalski JW, Ravelo A, Lee PP, et al. Resource use and costs associated with diabetic macular edema in elderly persons. Arch Ophthalmol. 2008;126:1748–54. doi: 10.1001/archopht.126.12.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]


