Abstract
A series of eighteen azomethines has been synthesized by the reaction of appropriate primary aromatic amines with aryl and/or heteroaryl carboxaldehydes. The synthesized azomethines have been evaluated for their in vitro antileishmanial, antibacterial and antifungal activities. The results revealed some antifungal activity of most of the synthesized compounds, whereas the antileishmaniasis activity results highlighted that all synthesized azomethines inhibited parasite growth and most of them showed highly potent action towards Leishmania major promastigotes. No remarkable bactericidal activities were observed.
Keywords: azomethines, primary aromatic amines, hetaryl carboxyladehyes, antileishmanial activity, antifungal activity, antibacterial activity
Introduction
Although the last century has been characterized by a drastic lowering in the mortality caused by infectious diseases, some diseases still represent a dreadful menace to human health and therefore, for a more efficient control, require the steady development of new, more powerful and inexpensive drugs. Leischmaniasis, considered a serious public health concern in many countries, is one of these diseases. It is now endemic in many parts of Pakistan and Balochistan and the Upper Sindh are vulnerable to cutaneous leishmaniasis. The World Health Organization (WHO) has identified leishmaniasis as a major and increasing public health problem [1]. In spite of the social and economic importance of leishmaniasis as a tropical infectious disease, efforts directed towards the discovery of new drugs against it remain undeveloped [2,3]. Moreover, the drugs currently in use are expensive, require long-term treatment [4], display high liver and heart toxicities, develop clinical resistance after few weeks of treatment and currently contribute to increase leishmaniasis-AIDS co-infections in some countries [5,6]. Azomethines are characterized by the –CH=N- (imino group) which has special importance in elucidating the mechanism of transmination and racemization in biological system [7,8,9]. Azomethines have high potential chemical permutation possibilities and show diuretic [10], anticancer [11,12,13,14], antibacterial [15,16,17,18,19], and antifungal activities [20,21,22]. This class of compounds has also exhibited activity against a wide range of organisms and is known to have medicinal importance and is used in drug design [23,24,25]. It has been also reported that when some aldehydes were functionalized by condensation with various amines, the resulting azomethines had antiparasitic activities [26].
Results and Discussion
The present work is a continuation of our ongoing programme devoted to the utilization of simple and readily obtainable materials in the synthesis of different biologically active compounds [27,28,29,30,31,32,33], aiming at the same time to seek a cure for the cutaneous antileishmaniasis facing our society in Pakistan by presenting some cheap azomethines that might be useful in antileishmanial and antifungal drug development.
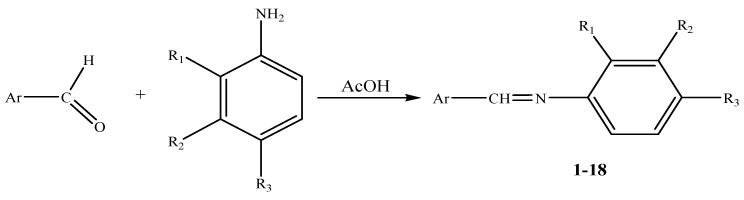
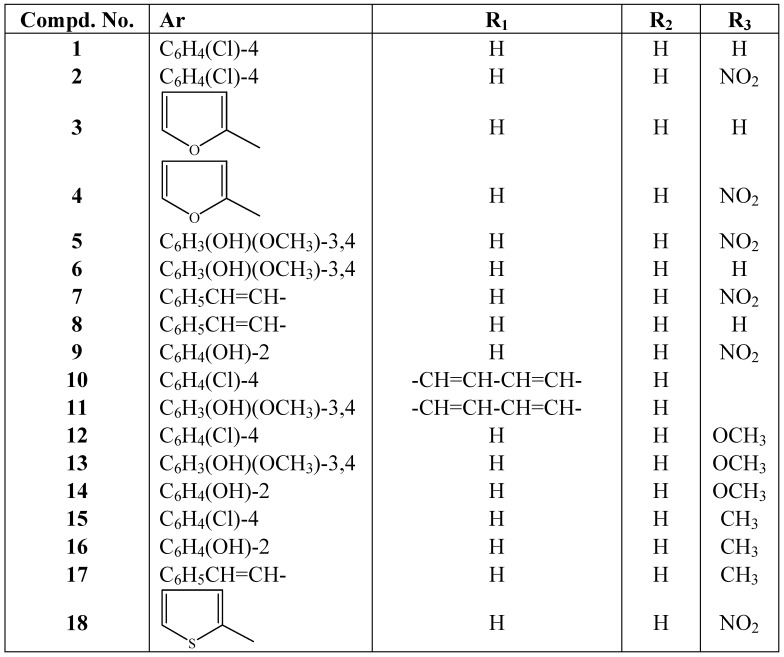
Reaction of various substituted anilines with aromatic and/or heterocyclic aldehydes in hot methanol containing a catalytic amount of glacial acetic acid afforded the target azomethines 1-18 in good yields (55–87%). (cf. Scheme 1).
Scheme 1.
General synthetic route.
The structural features of the synthesized azomethines were determined from the corresponding IR, 1H-NMR, 13C-NMR and mass spectroscopy data, listed in Table 1, Table 2, Table 3 and Table 4. All compounds showed in the IR spectra a strong absorption band at 1,592–1,637 cm−1, typical of the stretching vibrations of the C=N functionality, as well as the bands corresponding to C-H stretching vibrations.
Table 1.
Physical and select IR data of the synthesized azomethines 1-18.
| Compd. | Color | Moleculara formula (Mol. Mass) |
Mp (°C) (recryst. solvent)b | Yield (%) |
IR (cm−1) | ||
|---|---|---|---|---|---|---|---|
| N=C | Azomethine C-H |
Substituent | |||||
| 1 | Pale yellow | C13H10ClN [34] (215.05) |
72-4 (L.P) |
82 | 1621 | 2874, 1352 |
- |
| 2 | Yellow | C13H9ClN2O2 [35] (260.04) |
140-42 (L.P) |
74 | 1625 | 1333 | 1505, 1376(NO2) |
| 3 | Brown | C11H9NO [36] (171.07) |
106 d (B) |
79 | 1605 | 2850, 1328 | 3360(OH) |
| 4 | Brown | C11H8N2O3 [37] (216.05) |
196-98 (EtOH) |
68 | 1592 | 1285 | 1535, 1339(NO2) |
| 5 | Pale orange | C14H12N2OH (272.08) |
148-50 (L.P) |
64 | 1628 | 1325 | 3354–3480(OH) 1544, 1342 (NO2) |
| 6 | Pale yellow | C14H13NO2 (227.09) |
155-57 (L.P) |
72 | 1621 | 1388 | 3088 broad(OH) |
| 7 | Orange | C15H12N2O2 (252.09) |
133d (MeOH) |
65 | 1592 | 1300 | 1512, 1335(NO2) |
| 8 | Pale brown | C15H13N (207.10) |
100-02 (MeOH) |
66 | 1623 | 1375 | - |
| 9 | Orange | C13H10N2O3 (242.07) |
162-64 (L.P) |
59 | 1632 | 1338 | 1562, 1347 (NO2), 3150 (OH) |
| 10 | Pale brown | C17H12ClN (265.07) |
107-09 (MeOH) |
78 | 1637 | 1391 | - |
| 11 | Brown | C18H15NO2 (277.11) |
114-16 (B) |
76 | 1621 | 1381 | 3048(OH) |
| 12 | Pale Brown | C14H12ClNO (245.06) |
136-37 (MeOH) |
87 | 1629 | 2838, 1364 | 2838, 2895 (CH3) |
| 13 | Pale Brown | C15H15NO3 ( 257.11) |
257-59 (MeOH) |
70 | 1621 | 2954, 1378 | 3000 (broad OH), 2954, 2982 (CH3) |
| 14 | Greenish White | C14H13NO2 (227.09) |
85-87 (MeOH) |
67 | 1637 | 2964, 1362 | 3610(OH), 2964, 2996 (CH3) |
| 15 | White | C14H12ClN (229.07) |
127-29 (MeOH) |
79 | 1622 | 2929, 1354 | 2854, 2925 (CH3) |
| 16 | White | C14H13NO (211.10) |
96-98 (MeOH) |
67 | 1615 | 2920, 1366 | 3100 (OH), 2920 (CH3) |
| 17 | Pale Orange | C16H15N [38] (221.12) |
76-77 (MeOH) |
71 | 1627 | 2912, 1379 | 2912 (CH3) |
| 18 | Pale Yellow | C11H8N2O2S [39] (232.03) |
135-37 (MeOH) |
55 | 1612 | 1323 | 1543, 1341 (NO2) |
a references are given for known products, the others are new; b L.P = Light Petroleum b.p. 60–80 °C, B = benzene, EtOH = ethanol, MeOH = methanol.
Table 2.
1H-NMR of azomethines 5-16 (DMSO-d6) (δ, ppm).
| Compd. | 1H-NMR |
|---|---|
| 5 | 9.45 (s, 1H, -OH, exchangeable), 8.36 (s, 1H, benzylidenimine H), 8.08-6.90 (m, 7H, Ar-H), 3.80 (s, 3H, -OCH3). |
| 6 | 9.75 (s, 1H, -OH, exchangeable), 8.43 (s, 1H, -CH=N), 7.52-6.88 (m, 8H, Ar-H), 3.84 (s, 3H, -OCH3). |
| 7 | 8.10 (m, 1H, -CH=N), 7.60-6.95 (m, 11H, Ar-H and olefinic H). |
| 8 | 8.25 (d, 1H, benzylideimin H, J = 5.36 Hz), 7.54-7.07 (m, 12H, Ar-H and olefinic H). |
| 9 | 12.27 (s, 1H, OH, exchangeable), 8.99 (s, 1H, -CH=N), 8.32-6.98 (m, 7H, Ar-H). |
| 10 | 8.73 (s, 1H, benzylidenimine H), 8.09-7.23 (m, 11H, Ar-H). |
| 11 | 9.76 (s, 1H, OH, exchangeable), 8.53 (s, 1H, benzylidenimine H), 8.29-6.92 (m, 10H, Ar-H), 3.89 (s, 3H, -OCH3). |
| 12 | 8.64 (s, 1H, -CH=N), 7.93-6.97 (m, 8H, Ar-H), 3.77 (s, 3H, -OCH3). |
| 13 | 9.60 (s, 1H, OH, exchangeable), 8.44 (s, 1H, -CH=N), 7.49-6.86 (m, 7H, Ar-H), 3.83 and 3.76 (2s, 6H, 2 -OCH3). |
| 14 | 13.38 (s, 1H, OH, exchangeable), 8.59 (s, 1H, benzylidenimine H), 7.36-6.89 (m, 7H, Ar-H), 3.82 (s, 3H, -OCH3) |
| 15 | 8.41 (s, 1H, -CH=N),7.83-7.11 (m, 8H, Ar-H), 2.36 (s, 3H, -CH3) |
| 16 | 13.19 (s, 1H, OH, exchangeable), 8.94 (s, 1H, -CH=N), 7.63-6.93 (m, 7H, Ar-H), 2.33 (s, 3H, -CH3). |
Table 3.
13C-NMR (DMSO-d6) ( δ, ppm) of azomethines 5-16.
| Compd | 13C-NMR |
|---|---|
| 5 | 160.0, 154.2, 152.4, 147.3, 146.3, 131.0, 125.5, 125.2, 123.2, 115.9, 112.3, 56.1. |
| 6 | 160.2, 152.4, 152.0, 147.3, 131.1, 129.9, 127.2, 125.5, 122.3, 115.9, 112.3, 56.1. |
| 7 | 163.7, 158.6, 146.4, 135.2, 133.3, 128.5, 128.5, 127.9, 125.2, 123.2, 119.8. |
| 8 | 163.7, 152.4, 135.2, 133.3, 130.0, 128.6, 128.5, 127.9, 127.2, 122.3, 119.9. |
| 9 | 161.1, 160.0, 154.2, 146.4, 132.4, 132.1, 125.2, 123.2, 121.4, 120.5, 117.8. |
| 10 | 160.0, 151.9, 136.6, 135.0, 134.5, 130.5, 128.9, 128.6, 128.3, 127.8, 126.8, 126.3, 115.2. |
| 11 | 160.0, 152.2, 151.9, 147.9, 147.3, 135.0, 131.1, 128.5, 128.3, 127.8, 126.7, 126.3, 125.5, 115.9, 115.1, 112.3, 56.1. |
| 12 | 160.0, 159.1, 144.3, 136.5, 134.5, 130.6, 128.8, 122.1, 115.6, 55.8. |
| 13 | 159.9, 159.1, 152.4, 147.3, 144.3, 131.1, 125.5, 115.9, 115.6, 122.1, 112.3, 56.1, 55.8. |
| 14 | 161.1, 160.1, 159.1, 144.3, 132.4, 132.1, 122.1, 121.4, 120.4, 117.8, 115.5, 55.8. |
| 15 | 160.1, 149.1, 136.9, 136.5, 134.5, 130.6, 130.3, 128.9, 122.2, 21.3. |
| 16 | 161.1, 159.9, 149.1, 136.8, 132.4, 132.1, 130.3, 122.2, 121.4, 120.5, 117.7, 21.3. |
Table 4.
Mass spectra of azomethines 5-16.
| Compd. | m/z (% Relative abundance) |
|---|---|
| 5 | 272 (M+., 4.25), 227 (2.34), 139 (4.85), 138 (70.25), 107 (76.60), 92 (48.80), 91 (6.91), 65 (100.00). |
| 6 | 227 (M+., 98.26), 211 (21.66), 166 (14.45), 154 (7.12), 139 (5.12), 104 (22.42), 77 (100.00) |
| 7 | 252 (M+., 27.28), 251 (M-1⎤+., 83.41), 205 (56.73), 178 (4.88), 149 (4.88), 138 (54.55), 115 (70.16), 92 (48.50), 91 (47.95), 65 (100.00) |
| 8 | 207 (M+., 33.52), 206 (M-1⎤+., 100.00), 178 (3.25), 128 (13.57), 115 (18.72), 77 (90.33). |
| 9 | 242 (M+., 96.65), 212 (13.38), 195 (37.11), 167 (29.72), 151 (5.91), 120 (44.48), 76 (100.00) |
| 10 | 267 (M+2⎤+., 20.42), 266 (M+1⎤+., 20.79), 265 (M+., 60.68), 230 (2.89), 202 (3.48), 154 (23.00), 127 (100.00), 77 (29.47), 51 (9.14). |
| 11 | 277 (M+., 100.00), 261 (7.13), 216 (6.07), 204 (5.90), 178 (3.99), 154 (19.97), 127 (80.53), 101 (11.97), 85 (44.01), 83 (69.57), 77 (27.60). |
| 12 | 247 (M+2⎤+., 26.19), 246 (M+1⎤+., 15.23), 245 (M+., 77.99), 230 (100.00), 201 (10.17), 167 (27.80), 149 (5.64), 139 (10.67), 114 (4.26), 77 (35.29), 63 (45.11). |
| 13 | 257 (M+., 100.00), 242 (85.72), 227 (3.73), 198 (5.24), 170 (15.75), 154 (7.33), 134 (6.16), 115 (7.02), 77 (4.02), 64 (19.33). |
| 14 | 228 (M+1⎤+., 16.62), 227 (M+., 100.00), 212 (71.79), 183 (4.33), 154 (3.40), 128 (5.33), 105 (3.08), 77 (55.04), 65 (38.15). |
| 15 | 231 (M+2⎤+., 30.49), 230 (M+1⎤+., 33.41), 229 (M+., 90.60), 193 (2.89), 165 (4.57), 150 (2.05), 118 (22.99), 91 (100.00), 77 (9.79), 65 (61.60). |
| 16 | 211 (M+., 100.00), 210 (M-1⎤+., 76.87), 167 (6.29), 120 (12.86), 118 (8.68), 91 (80.65), 65 (74.49). |
The 1H-NMR spectra of compounds 1-18 contained multiple signals in the δ 6.90 to 8.08 ppm regions due to aromatic protons and a singlet at 8.43-9.75 ppm from the C-H protons of the CH=N groups. In the spectra of compounds 5, 6, 11, 12, 13 and 14, signals between δ 3.50 and 4.0 ppm were attributed to –OCH3 groups. The signals at δ 2.36 and 2.33 ppm which appeared as singlets in compounds 15 and 16 were assigned for methyl groups. In the DEPT spectra of compounds 1-18, there are no peaks at 189.0-196.0 ppm due to CHO group and the observed peaks were shifted to ~160.0-163.0 ppm which were attributable to HC=N group.
Antileishmaniasis activity
Antileishmanial activity of the compounds (1-18) was assayed by Zhai’s method [40] using a pre-established culture of L. major (Table 5) Parasites were cultured in medium M199 with 10% foetal bovine serum; 25 mM of HEPES, and 0.22 µg of penicillin and streptomycin, respectively, at 24 °C in a shaking incubator. Compounds 1, 6 and 16 have moderate activity against L major. Azomethines 4, 10, 14, 15, and 17 show good activity against L major and azomethines 9 and 12 show significant activity. The high in vitro antileishmaniasis activity of these compounds makes them promising leads for development of effective therapeutic agents.
Table 5.
% Inhibition of azomethines against L. major leishmania.
| Compound | L. major a |
|---|---|
| 1 | 0.77 ± 0.09 |
| 2 | - |
| 3 | - |
| 4 | 0.65 ± 0.01 |
| 5 | - |
| 6 | 0.73 ±0.16 |
| 7 | - |
| 8 | - |
| 9 | 0.59 ± 0.07 |
| 10 | 0.61 ± 0.27 |
| 11 | - |
| 12 | 0.57 ±0.11 |
| 13 | 0.63 ± 0.04 |
| 14 | 0.68 ±0.12 |
| 15 | 0.66 ±0.18 |
| 16 | 0.81 ± 0.03 |
| 17 | 0.62 ± 0.08 |
| 18 | - |
| Standard Drug IC50 (µg/mL± S.D) | |
| Amphotericin B | 0.56 ± 0.20 |
a percentage inhibition activity: 100 = (non-significant; 0.95−0.80 = low; 0.79−0.70 = Moderate; 0.69−0.60 = Good; below 0.59-0.56 = Significant activity).
Antifungal activity
The antifungal bioassay is screened by the Agar Tube Dilution Method [41,42], in which the test compounds were screened for activity against the following organisms: Candida albicans, Aspergillus flavus, Microsportum canis, Fusarium solani and Candida glabrata. Table 6 shows % inhibition of the synthesized azomethines. In all tests the linear growth in control was 100 mm. It is evident from the antifungal screening that:
-
(i)
None of the compounds are inactive against C. albicans or C. glabrata.
-
(ii)
Only compound 17 has moderate activity against A. flavus.
-
(iii)
Azomethines 4, 5, 12 and 14 have low activity against M. canis, whereas compound 9 has good antifungal activity against the same fungus.
-
(iv)
Azomethine 9 shows moderate activity against F. solani, whereas azomethines 4, 11, 17 and 18 have low activity against the same fungus.
Table 6.
% inhibition of azomethines against different types of fungi.
| Compound | C. albicans | A. flavus | M. canis | F. solani | C. glabrata |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 20a | 35 | 0 |
| 5 | 0 | 0 | 30 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 60 | 40 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 | 35 | 0 |
| 12 | 0 | 0 | 35 | 0 | 0 |
| 13 | 0 | 0 | 0 | 0 | 0 |
| 14 | 0 | 0 | 20 | 0 | 0 |
| 15 | 0 | 0 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | 0 |
| 17 | 0 | 40 | 0 | 15 | 0 |
| 18 | 0 | 0 | 0 | 20 | 0 |
| Std. Drug MIC (mg/mL) | |||||
| Miconazole | 110.8 | - | 98.4 | 73.25 | 110.28 |
| Amphotericin B | - | 20.0 | - | - | - |
a percentage inhibition activity: 0-39 = low (non-significant); 40-59 = Moderate; 60-69 = Good; Above 70 = Significant activity.
Antibacterial activity
The antibacterial activity of the synthesized azomethines was screened by the agar well diffusion method [43,44] against Gram-negative and Gram-positive bacteria, namely Escherchia coli, Bacillus subtilis, Shigella flexenari, Staphylococcus aureus, Pseudomonas aeruginosa and Salmonella typhi. Only compound 4 showed significant activity (25 mm inhibition zone) against E. coli and moderate activity (14 mm zone) against B. subtilis.
Experimental
General
All aromatic and heterocyclic aldehydes as well as primary aromatic amines were obtained commercially from the Aldrich Chemical Company. Solvents used were of analytical grade. M.p. were measured using a Stuart (UK) electric melting point apparatus. IR spectra were recorded with a Thermo 330 FTIR spectrometer. Mass spectra were measured on a MAT 312 Finnigan instrument operating at 70 eV. 1H- and 13C-NMR spectra were recorded on a Avance 400 Bruker instrument using TMS as internal standard and DMSO-d6 as solvent.
Chemistry: General procedure for synthesis of azomethines
An equimolar mixture of aryl and/or hetaryl carboxaldehyde with selected primary aryl amine in methyl alcohol (30 mL) containing few drops of glacial acetic acid was heated to reflux temperature for 3-4 hours then left overnight until the product precipitated. The product was filtered off, washed with light petroleum and recrystallized from the suitable solvent [cf. Table 1]. It is worthy to mention that anhydrous dry solvents for recrystallization were essential to avoid decomposition by hydrolysis. The crystalline solid that separated out was filtered by suction using water pump and washed with light petrol to afford the desired azomethines 1-18 in pure state.
Biological Screening: Antileishmaniasis Activity - Preparation of Samples
Each compound (1 mg) was dissolved in DMSO (1 mL) and Amphotercin B (1 mg) was also dissolved in DMSO (1 mL) as positive control. Parasites at log phase were centrifuged at 3,000 rpm for 3 minutes. Parasites were diluted in fresh culture medium to a final density of 2 × 106 cells/mL. In 96-well plates, 180 µL of medium was added in different wells. Twenty µL of the compound was added in medium and serially diluted. Parasite culture (100 µL) was added in all wells. Three rows were left for negative and positive controls. In the negative controls, DMSO was serially diluted in medium while the positive control contained varying concentrations of the standard antileishmanial compound Amphotercin B. The plates were incubated for 72 hours at 24 °C. The culture was examined microscopically on an improved neubaur counting chamber and IC50 values of compounds possessing antileishmanial activity were calculated. All assays were run in duplicate. IC50 of samples was determined by using the Prism software.
Antifungal activity
The concentration of the tested compounds was 200 ug/mL of DMSO. A reference antifungal and DMSO were used as positive and negative control, respectively. Incubation temperature was 27 °C and time 7 days. The inculation of fungus was carried out with a 4 mm diameter piece of fungus removed from a seven-day-old culture. The growth in the compound-amended media was determined by measuring linear growth (mm) and growth inhibition calculated with reference to the negative control and calculating the % inhibition of fungal growth:
Antibacterial Activity
The concentration of tested compound was 1 mg/mL of DMSO, whereas the concentration of the reference antibacterial drug imipenem was 10 mg/disc. Blank test showed that DMSO in the preparations of the test solutions does not affect the test organisms.
Conclusion
Eighteen azomethines were prepared by reacting an aromatic or heterocyclic aldehyde and an aromatic primary amine compound in a protic solvent like ethanol or methanol heated to reflux temperature in presence of few drops of acetic acid as a catalyst. The acidic catalyst leads to the increase of the electrophilicity of the caronyl function of the carboxalehyde containing reactant. The results revealed some order of fungicidal activities of most of the synthesized compounds whereas the results of leishmanicidal activity highlighted that all synthesized azomethines inhibited the growth of parasite and most of them showed high potent action towards leishmania major promastigotes. No remarkable bactericidal activities have been recorded. Azomethines are characterized by the –CH=N- (imino group) which has special importance in elucidating the mechanism of transmination and racemization in biological system. Azomethines have high potential chemical permutation and show diuretic, anticancer, antibacterial, and antifungal activities. This class of compounds also exhibited activity against a wide range of organisms and are known to have medicinal importance and used in drugs design. It has been also reported that some aldehydes when were functionalized by condensation with various amines, the produced azomethines had antiparasitic activities. It is evident that Azomethine compounds 1, 6 and 16 have moderate activity against L major. Azomethines 4, 10, 14, 15, and 17 show good activity against L major and azomethine 9 and 12, shows significant activity against L.major. The high in vitro Anti leishmaniasis activity of these compounds makes them a promising lead for development of effective therapeutic agents.It is clear that the synthesize of Azomethines showed only compound 17 has moderated activity against A flavus. Azomethine 4,5,12 and 14 have low activity against M. Canis whereas compound 9 has good anti fungal activity against same fungus. Azomethine 9 shows a moderate activity against F. Solani whereas azomehtines 4,11,17 and 18 have low activity against the same fungus. In bacteria among the 18 synthesize of azomethines tested only compound 4 showed significant activity against E.coli and low activity against B. subtilis.
Acknowledgments
This work was supported by University of Balochistan, Quetta and Higher Education Commission (HEC), Islamabad, Pakistan.
Footnotes
Sample Availability: Samples of the compounds 1-18 are available from the authors.
References
- 1.Modabber F. Tropical Disease Research Progress 1991–1992. World Health Organization; Geneva, Switzerland: 1993. Tropical Disease Research Progress Leishmaniasis; pp. 77–87. [Google Scholar]
- 2.Croft S.L. The current status of antiparasite chemotherapy. Parasitology. 1997;114:3–15. [PubMed] [Google Scholar]
- 3.Engers H.D., Bergquist R., Modabber F. Progress on vaccines against parasites. Dev. Biol. Stand. 1996;87:73–84. [PubMed] [Google Scholar]
- 4.Murray H.W. Treatment of visceral leishmaniasis in 2004. Am. J. Trop. Med. Hyg. 2004;71:787–794. [PubMed] [Google Scholar]
- 5.Croft S.L. Recent developments in the chemotherapy of leishmaniasis. Trends Pharmacol. Sci. 1988;9:376–381. doi: 10.1016/0165-6147(88)90258-1. [DOI] [PubMed] [Google Scholar]
- 6.Berman J.D. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 1988;10:560–586. doi: 10.1093/clinids/10.3.560. [DOI] [PubMed] [Google Scholar]
- 7.Lau K.Y., Mayr A., Cheung K.K. Synthesis of transition metal isocyanide complexes containing hydrogen bonding sites in peripheral locations. Inorg. Chim. Acta. 1999;285:223–232. doi: 10.1016/S0020-1693(98)00352-1. [DOI] [Google Scholar]
- 8.Ispir E., toroglu S., karyaldiz A. Synthesis, characterization, antimicrobial and genotoxic activities of new Schiff bases and their complexes. Transition Met. Chem. 2008;33:953–960. doi: 10.1007/s11243-008-9135-2. [DOI] [Google Scholar]
- 9.Ispir E., Kurtoglu M., Purtas F. Synthesis and antimicrobial activity of new Schiff bases having the –SiOR group (R=CH3 or CH2CH3), and their transition metal complexes. Trans. Met. Chem. 2005;30:1042–1047. doi: 10.1007/s11243-005-6311-5. [DOI] [Google Scholar]
- 10.Supuran C.T., Barboiu M., Luca C., Pop E., Brewster M.E., Dinculescu A. Carbonic anhydrase activators. Part 14. Syntheses of mono and bis pyridinium salt derivatives of 2-amino-5-(2-aminoethyl)- and 2-amino-5-(3-aminopropyl)-1,3,4-Thiadiazole and their interaction with isozyme II. Eur. J. Med. Chem. 1996;31:597–606. doi: 10.1016/0223-5234(96)89555-9. [DOI] [Google Scholar]
- 11.Sharma K.P., Jolly V.S., Phatak P. Schiff bases and their derivatives as potential anticancer agents. Ultra Scient. Phys. Sci. 1998;10:263–266. [Google Scholar]
- 12.Kuzamin V.E., Artemenko A.G., Lozytska R.N., Fedtchouk A.S., Lozitsky V.P., Muratov E.N., Mescheriakov A.K. Investigation of anticancer activity of macrocyclic Schiff bases by means of 4D-QSAR based on simplex representation of molecular structure. SAR QSAR Environ. Res. 2005;16:219–230. doi: 10.1080/10659360500037206. [DOI] [PubMed] [Google Scholar]
- 13.Shingare M.S., Ingle D.B. Synthesis of pyrimidine Schiff bases as anticancer agents. J. Indian Chem. Soc. 1976;53:1036–1037. [Google Scholar]
- 14.Shkawat D.R., Sabins S.S., Deliwala C.V. Potential anticancer agents, Schiff bases from p-(3-azaspiro[5,5]undec-3-yl)benzaldehydes. Bull. Haffkine Inst. 1973;1:35–39. [Google Scholar]
- 15.More S.V., Dongarkhadekar D.V., Chavan R.N., Jadhav W.N., Bhusare S.R., Pawar R.P. Synthesis and antibacterial activity of new Schiff bases, 4-thiazolidinones and 2-azetidinones. J. Indian Chem. Soc. 2002;79:768–769. doi: 10.1002/chin.200326142. [DOI] [Google Scholar]
- 16.Bhendkar A.K., Vijay K., Raut A.W. Synthesis of some novel Schiff bases of 2-aminopyrimidine and their antimicrobial activity. Acta Ciencia India Chem. 2004;30:29–32. [Google Scholar]
- 17.Vaghasiya Y.K., Nair R.S., Baluja M., Chanda S.S. Synthesis, structural determination and antibacterial activity of compounds derived from vanillin and 4-aminoantipyrine. J. Serb. Chem. Soc. 2004;69:991–998. doi: 10.2298/JSC0412991V. [DOI] [Google Scholar]
- 18.Vashi K., Naik H.B. Synthesis of novel Schiff base and azetidinone derivatives and their antibacterial activity. Eur. J. Chem. 2004;1:272–276. doi: 10.1155/2004/158924. [DOI] [Google Scholar]
- 19.Rhodes R.C., Hall H., Beesley S.R., Jenkins J.G., Collins D.C., Zheng P. Therapeutic Potentiation of the immune system by costimulatory Schiff base-forming drugs. Nature. 1995;377:71–75. doi: 10.1038/377071a0. [DOI] [PubMed] [Google Scholar]
- 20.Safwat H.M., Ragab F.A., Eid N.M., Abdel G.M. Synthesis, anti-tumor and antimicrobial activities of 3-chloro-9- (p-N-substituted sulfamoylphenylaminoethylene) acridines. Egyptian J. Pharm. Sci. 1988;29:99–110. [Google Scholar]
- 21.Mtrei R., Yadawe M., Patil S.A. Synthesis of biologically active p-bis(amino-5-mercapto-1,2,4-triazol-3-yl)benzene and its Schiff base: a new class of bis-triazole. Orient. J. Chem. 1996;12:101–102. [Google Scholar]
- 22.Hossain M.E., Allam M.N., Begum J., Akbar M.A., Uddin M.N., Smith F.E., Hynes R.C. The preparation, characterization, crystal structure and biological activities of some Cu(II) complexes of the 2-benzoyl pyridine Schiff bases of S-methyl-and S-benzyldithiocarbazate. Inorg. Chim. Acta. 1996;249:207–213. doi: 10.1016/0020-1693(96)05098-0. [DOI] [Google Scholar]
- 23.Khan S.A., Siddiqui A.A., Shibeer B. Analgesic activity of isatin derivatives. Asian J. Chem. 2002;14:1117–1118. [Google Scholar]
- 24.Verma M., Pandeya S.N., Singh K.N., Stables J.P. Anticonvulsant activity of Schiff bases of isatin derivatives. Acta Pharm. 2004;54:49–56. [PubMed] [Google Scholar]
- 25.Sari N., Arslan S., Logoglu E., Sakiyan L. Antibacterial Activities of some Amino acid-Schiff bases. GUJ Sci. 2003;16:283–288. [Google Scholar]
- 26.Rathelot P., Azas N., El-Kashef H., Delmas F., Di Giorgio C., Timon-David P., Maldonado J., Vanelle P. 1,3-Diphenylpyrazoles: synthesis and antiparasitic activities of azomethine derivatives. Eur. J. Med. Chem. 2002;37:671–679. doi: 10.1016/S0223-5234(02)01388-0. [DOI] [PubMed] [Google Scholar]
- 27.Madkour H., Farag A., Ramses S., Ibrahiem N. Synthesis and fungicidal activity of new imidazoles from 2-(chloromethyl)-1 H –benzimidazole. Phosphor. Sulfur Phosphorous Silicon. 2006;181:255–265. doi: 10.1080/104265090970241. [DOI] [Google Scholar]
- 28.Madkour H.M.F., Afify A.A.E., Abdalha A.A., Elsayed G.A., Salem M.S. Synthetic utility of enaminonitrile moiety in heterocyclic synthesis: Synthesis of some new thienopyrimidines. Phosphor. Sulfur Phosphorous Silicon. 2009;184:719–732. [Google Scholar]
- 29.Nofal Z.M., El-Zahar M.I., Salem M.A.I., Madkour H.M.F. Synthesis and chemoprophylactic effect of novel coumarin derivatives. Egypt J. Chem. 2005;48:587–604. [Google Scholar]
- 30.Madkour H.M.F., Mahmoud M.R., Sakr A.M., Habashy M.M. Synthesis and antibacterial activity of new 4H-pyrano [3,2-h]quinolines and fused derivatives. Sci. Pharm. 2001;69:33–52. doi: 10.1002/chin.200131148. [DOI] [Google Scholar]
- 31.Madkour H.M.F., Salem M.A.I., Soliman E.A., Mahmoud N.F.H. A facile one-pot synthesis and antibacterial activity of aziridines and thiazines from 1,3-diarylprop-2-enones. Phosphor. Sulfur Phosphorous Silicon. 2001;170:15–28. doi: 10.1080/10426500108040582. [DOI] [Google Scholar]
- 32.Salem M.A.I., Madkour H.M.F., Soliman E.A., Mahmoud N.F.H. Synthesis of bactericides via carbon nucleophilic addition on 1,3-diarylprop-2-enones as michael acceptors. Heterocycles. 2000;53:1129–1143. doi: 10.3987/COM-98-8232. [DOI] [Google Scholar]
- 33.Mahmoud M.R., Madkour H.M.F. Reactions of 3-3-chlorophenyl-1-4-(3,4-methylenedioxybenzylidene)aminophenylprop-2-enone with some nucleophiles. Synth. Commun. 1996;26:3799–3808. doi: 10.1080/00397919608003796. [DOI] [Google Scholar]
- 34.Sobanov A.A., Zolotukhin A.V., Galkina I.V., Galkin V.I., Cherkasov R.A. Kinetics and mechanism of the Pudovik reaction in the azomethine series: III. Acid-catalyzed hydrophosphorylation of imines. Russ. J. Gen. Chem. 2006;76:421–429. doi: 10.1134/S1070363206030121. [DOI] [Google Scholar]
- 35.Neuvonen H., Neuvonen K., Fülöp F. Substituent cross-interaction effects on the electronic character of the C=N bridging group in substituted benzylidene anilines − Models for molecular cores of mesogenic compounds. A 13C-NMR study and comparison with theoretical results. J. Org. Chem. 2006;71:3141–3148. doi: 10.1021/jo0600508. [DOI] [PubMed] [Google Scholar]
- 36.Montalvo-Gonza´lez R., Ariza-Castolo A. Molecular structure of di-aryl-aldimines by multinuclear magnetic resonance and X-ray diffraction. J. Mol. Struct. 2003;655:375–389. doi: 10.1016/S0022-2860(03)00279-5. [DOI] [Google Scholar]
- 37.Subudhi B.B., Satpathy S., Bhatta D., Pradhan D. Synthesis of some 2-(R, R')-3-(p-nitro phenyl)-thiazolidinone as potent antifungal agents. J. Teach. Res. Chem. 2005;12:42–45. [Google Scholar]
- 38.Sain B., Thyagarajan G., Sandhu J.S. Cycloaddition reactions of meso-ionic oxazolone with cinnamaldehyde anils. Can. J. Chem. 1980;58:2034–2037. doi: 10.1139/v80-323. [DOI] [Google Scholar]
- 39.Youssef M.S.K. Synthesis of antimicrobial heteroaryl-substituted-1,2,3-triazolines via the reaction of diazomethane with anils and mixed azines of thiophenealdehyde and/or isatin. J. Chem. Technol. Biotechnol. 1981;31:363–367. doi: 10.1002/jctb.280310150. [DOI] [Google Scholar]
- 40.Zhai L., Chen M., Blom J., Teander T.G., Christensen S.B., Karazmi A. The antileishmanial acitivity of novel oxygenated chalcones and their mechanism of action. Antimicrob. Agents Chemother. 1999;43:793–803. doi: 10.1093/jac/43.6.793. [DOI] [PubMed] [Google Scholar]
- 41.Choudhary M.I., Dur-e-Shahwar Parveen Z., Jabbar A., Ali I., Atta-Ur-Rahman Antifungal steroidal lactones from Withania coagulance. Phytochemistry. 1995;40:1243–1246. doi: 10.1016/0031-9422(95)00429-b. [DOI] [PubMed] [Google Scholar]
- 42.Janaki S., Vijayasekaram V. Antifungal activities of aglaia roxburghiana (W & A) Biomedicine. 1998;18:86–89. [Google Scholar]
- 43.Alves T.M.A., Silva A.F., Brandao M., Grandi T.S.M., Smania E.F.A., Smania A., Jr., Zani C.L. Antibacterial xanthones from Kielmeyera variabilis mart. (Clusiaceae) Mem. Inst. Oswaldo. Cruz. 2000;95:367–373. doi: 10.1590/S0074-02762000000300012. [DOI] [PubMed] [Google Scholar]
- 44.Stepanović S., Antić N., Dakić I., Švabić-Vlahović M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 2003;158:353–357. doi: 10.1078/0944-5013-00215. [DOI] [PubMed] [Google Scholar]