Abstract
Background/Introduction
Sarcoidosis is a multi-systemic disorder of unknown etiology, characterized by the presence of non-caseating granulomas in target organs. In 90% of cases, there is thoracic involvement. Fifty to seventy percent of pulmonary sarcoidosis patients will experience acute, self-limiting disease. For the subgroup of patients who develop persistent disease, no targeted therapy is currently available.
Aim
To investigate the potential of the single nucleotide polymorphism (SNP), Toll-like receptor 3 Leu412Phe (TLR3 L412F; rs3775291), as a causative factor in the development of and in disease persistence in pulmonary sarcoidosis. To investigate the functionality of TLR3 L412F in vitro in primary human lung fibroblasts from pulmonary sarcoidosis patients.
Design
SNP-genotyping and cellular assays, respectively, were used to investigate the role of TLR3 L412F in the development of persistent pulmonary sarcoidosis.
Methods
Cohorts of Irish sarcoidosis patients (n = 228), healthy Irish controls (n = 263) and a secondary cohort of American sarcoidosis patients (n = 123) were genotyped for TLR3 L412F. Additionally, the effect of TLR3 L412F in primary lung fibroblasts from pulmonary sarcoidosis patients was quantitated following TLR3 activation in the context of cytokine and type I interferon production, TLR3 expression and apoptotic- and fibroproliferative-responses.
Results
We report a significant association between TLR3 L412F and persistent clinical disease in two cohorts of Irish and American Caucasians with pulmonary sarcoidosis. Furthermore, activation of TLR3 in primary lung fibroblasts from 412 F-homozygous pulmonary sarcoidosis patients resulted in reduced IFN-β and TLR3 expression, reduced apoptosis- and dysregulated fibroproliferative-responses compared with TLR3 wild-type patients.
Discussion/Conclusion
This study identifies defective TLR3 function as a previously unidentified factor in persistent clinical disease in pulmonary sarcoidosis and reveals TLR3 L412F as a candidate biomarker.
Introduction
Sarcoidosis is a multi-systemic disorder of unknown cause which is characterized by the presence of non-caseating granulomas in target organs. Ninety percent of sarcoidosis cases have thoracic involvement.1,2 The highest annual incidence of sarcoidosis has been observed in northern European countries (5–40 cases per 100 000 people).3 Phenotypically, sarcoidosis follows either an acute or chronic course. Up to 70% of patients present with acute sarcoidosis and experience self-limiting disease which will resolve within 1–2 years.4 In contrast, 30–50% of patients will develop persistent pulmonary sarcoidosis for which no approved treatments are currently available and corticosteroid use is the standard, non-specific treatment method.4
Although mechanisms underlying the development of sarcoidosis are currently unknown, it hypothesized to be caused by an aberrant host immune response to unknown environmental antigens in genetically pre-disposed individuals. A number of alterations in sarcoidosis patients’ immune responses have been reported including, an exaggerated Th1 response, increased Th17 activity, attenuated regulatory T cell responses and oligoclonal expansion of CD4+ T cell responses, which is consistent with chronic antigenic stimulation.5–9
To date, no specific pathogen has been identified as a causative factor in sarcoidosis. Several studies have a role for mycobacterial or propionibacterial organisms in the pathogenesis of sarcoidosis. Specifically, a meta-analysis of studies carried out between 1980 and 2006 demonstrated that 26% of all sarcoidosis tissues had evidence of mycobacterial nucleic acids.10 In the context of viral infection, seroepidemiological studies have demonstrated significant levels of antibodies to Epstein–Barr virus (EBV), rubella and parainfluenza 3 in sarcoidosis.11 However, no correlation could be made between viral antibody titre and stage of disease or activity.11
In this study, we investigated the role of defective TLR3 in the development of persistent clinical disease in pulmonary sarcoidosis. TLR3 has previously been shown to bind dsRNA from viruses, bacteria and helminths, respectively, in addition to mRNA released from necrotic cells.2,12–15 Specifically, here we investigated the role of the TLR3 polymorphism, Leu412Phe (TLR3 Leu412Phe, L412F; rs3775291) as a causative factor in the development of and in disease persistence in pulmonary sarcoidosis, respectively. Previously, we identified a role for TLR3 L412F in accelerated disease progression and increased risk of mortality in idiopathic pulmonary fibrosis (IPF).16TLR3 L412F has also been implicated as a causative factor in a number of autoimmune and inflammatory diseases such as diabetes, systemic lupus erythematosus and rheumatoid arthritis,17–19 as well as a variety of cancers.20–24TLR3 L412F has also been demonstrated to have either a protective or pathogenic effect in viral infection.25,26
In this study, we report a significant association between development of a persistent clinical phenotype in pulmonary sarcoidosis and the TLR3 L412F variant in cohorts of Irish and American Caucasians, respectively. Furthermore, activation of TLR3 in vitro in primary human lung fibroblasts from 412 F-homozygous patients resulted in decreased TLR3 and IFN-β expression, reduced apoptosis and dysregulated proliferation, respectively, compared with fibroblasts from TLR3 wild-type patients. Our findings imply that defective TLR3 promotes a persistent disease phenotype in sarcoidosis and reveals TLR3 L412F as a candidate prognostic biomarker in this interstitial lung disease.
Materials and methods
Study subjects
A cohort of Irish Caucasian pulmonary sarcoidosis patients (n = 228; Table 1) was recruited from St Vincent’s University Hospital, Elm Park, Dublin 4 (SVUH). A cohort of Irish Caucasian healthy volunteers (n = 263) was additionally recruited as a control group. Genomic DNA was obtained from the American cohort of sarcoidosis patients attending the specialized Sarcoidosis Clinic at Johns Hopkins University School, Baltimore, MA, USA (n = 123; Table 1).
Table 1.
Study demographics for Irish and American pulmonary sarcoidosis cohorts, and TLR3 L412F (rs3775291) genotypes
| Irish sarcoidosis cases | American sarcoidosis cases | |||||
|---|---|---|---|---|---|---|
| No. | 228 | 123 | ||||
| Sex, M/F | 109/119 | 53/70 | ||||
| Median age, year range | 32 (16–66) | 42 (18–72) | ||||
| Leu/Leu | Leu/Phe | Phe/Phe | Leu/Leu | Leu/Phe | Phe/Phe | |
| No. | 127 | 85 | 16 | 91 | 28 | 4 |
| Sex, M/F | 53/74 | 48/37 | 8/8 | 37/54 | 13/15 | 3/1 |
| Median age, year range | 30 (17–63) | 32 (16–63) | 36 (24–66) | 42 (18–65) | 44 (28–75) | 35 (23–38) |
Diagnosis of pulmonary sarcoidosis and classification of persistent disease
Irish and American pulmonary sarcoidosis patients were diagnosed at initial hospital presentation by the same physician (S.C.D. and D.R.M), respectively, and followed-up for at least 2 years (Supplementary Methods). Patients at 2 years follow-up were classified as having either ‘persistent’ disease or ‘non-persistent’ disease based on a modification of a system previously described2 (Supplementary Methods).
TLR3 L412F genotyping
TLR3 L412F genotyping was carried out as described by us previously in a parallel study investigating the role of the TLR3 L412F in IPF16 (Supplementary Methods).
Analysis of TLR3 L412F functionality in primary human lung fibroblasts from pulmonary sarcoidosis patients
Primary fibroblast cell lines were isolated from lung biopsies of sarcoidosis patients (supplied by SVUH) and cultured as described previously27 (Supplementary Methods). Methodology pertaining to the analysis of the effects of TLR3 L412F on fibroblast-apoptosis, -proliferation and -cytokine/interferon production, respectively, is detailed in the Supplementary Methods Section.
Statistical analysis
All statistical analyses were carried out using GraphPad Instat Software (GraphPad Software Inc. CA, USA). Statistical analyses of genotype and allele frequencies were performed using two-tailed χ2 tests (3 x 2 χ2 tests for independence and trend, respectively, or 2 x 2 χ2 test where appropriate) or 2 x 2 Fisher’s exact tests if the χ2 test was inappropriate. Forward, stepwise logistic regression analysis was carried out to obtain corrected P-values for appropriate confounders. One-way analysis of variance was used to test for statistical significance (two-tailed analysis) between experimental groups of three. Multiple comparisons between groups were then assessed using the Tukey–Kramer post-hoc test (for parametric analysis) or Dunn’s post-hoc test (for non-parametric analysis). Statistical significance was recorded at P < 0.05.
Results
TLR3 L412F (rs3775291) is not associated with development of pulmonary sarcoidosis in Irish patients
In this study, we tested for an association between TLR3 L412F and development of pulmonary sarcoidosis in an Irish case-control study of 263 control subjects and 228 sarcoidosis cases (Table 2). L412F genotype frequencies in the Irish control group and Irish sarcoidosis group were determined to be consistent with Hardy–Weinberg Equilibrium (controls: P = 0.32; cases: P = 0.72). No significant association was found between development of sarcoidosis and the L412F genotypes [P = 0.6326; Odds ratio (OR) for 412 F (Phe) carriers: 1.105 (95% CI: 0.774–1.578); OR for Phe/Phe homozygotes (Phe/Phe): 0.801 (CI: 0.387–1.660); Table 2] or allele frequency [P = 0.8942; OR: 1.003 (CI: 0.774–1.372); Table 2]. This indicates that the variant allele does not strongly promote development of the disease in an Irish sarcoidosis population.
Table 2.
TLR3 L412F (rs3775291) polymorphism frequencies in pulmonary sarcoidosis: Irish case-control and disease persistence studies
| Irish case-control study |
Irish disease persistence study |
|||||||
|---|---|---|---|---|---|---|---|---|
| Status | Leu/Leu | Leu/Phe | Phe/Phe | Status | Leu/Leu | Leu/Phe | Phe/Phe | |
| Control subjects | 140 | 108 | 15 | Persistent | 52 | 39 | 13 | |
| (n = 263) | (0.53) | (0.41) | (0.06) | (n = 104) | (0.50) | (0.37) | (0.13) | |
| Irish Sarcoidosis cases | 127 | 85 | 16 | Non-persistent | 75 | 46 | 3 | |
| (n = 228) | (0.56) | (0.37) | (0.07) | (n = 124) | (0.61) | (0.37) | (0.02) | |
| P-values | P-values | |||||||
| Genotype | a0.6326 | Genotype | f0.0095 g(0.0157) | |||||
| Trend | b0.8350 | Trend | h0.0133 | |||||
| Allele | c0.8942 | Allele | i0.0166 | |||||
| OR (95% CI) | OR (95% CI) | |||||||
| Phe carrier | d1.105 (0.774–1.578) | Phe carrier | j1.531(0.904–2.592) | |||||
| Phe/Phe homozygote | e0.801 (0.387–1.660) | Phe/Phe homozygote | k5.762 (1.594–20.284) | |||||
aIrish Case-Control Study: L412F genotype frequencies did not differ significantly between control subjects and sarcoidosis cases: χ2 test for independence (2 d.f.) and
bχ2 test for trend (1 d.f.). OR and 95% confidence interval (CI) for
dPhe carriers (Leu/Phe and Phe/Phe) and
ePhe/Phe homozygotes.
cAllele frequencies did not differ significantly between subjects and cases [χ2 test: P = 0.8942; OR: 1.003 (CI: 0.774–1.372)]. Irish Disease Persistence Study: a significant association was observed between TLR3 L412F and disease persistence in Irish sarcoidosis cases. L412F genotypes were compared using:
fχ2 test for independence and
hχ2 test for trend, respectively.
gLogistic regression analysis was performed to calculate the adjusted P-values for the confounding factors: age at diagnosis, gender and erythema nodosum positivity. OR (CI) for
jPhe carriers and
kPhe/Phe homozygotes.
iAllele frequencies were compared using a χ2 test [OR: 1.713 (CI: 1.122–2.617)].
TLR3 L412F promotes a persistent clinical phenotype in Irish patients with pulmonary sarcoidosis
We then tested for an association between TLR3 L412F and disease persistence in sarcoidosis. Patients were defined as having either ‘persistent’ (n = 104) or ‘non-persistent’ (n = 124) disease at 2 years post-diagnosis according to established criteria2 (Table 2). We observed a significant association between development of a persistent disease phenotype in Irish sarcoidosis patients and 412 F homozygosity [P = 0.0095; OR for Phe/Phe homozygotes: 5.762 (CI: 1.594–20.284), Table 2] and F allele frequency [P = 0.0166; OR: 1.713 (CI: 1.122–2.617); Table 2]. This suggests that TLR3 412 F homozygosity may be a useful prognostic biomarker in sarcoidosis.
TLR3 L412F is associated with disease persistence in American Caucasians with pulmonary sarcoidosis
To test for replication of the association between TLR3 L412F and disease persistence in Irish sarcoidosis patients, we carried out a validation study in an American cohort of sarcoidosis patients. We genotyped 123 genomic DNA samples from patients attending a tertiary referral centre for sarcoidosis (Table 1). We found a significant association between L412F heterozygosity [P = 0.0432; OR for Phe carriers: 3.535 (CI: 1.246–10.029); Table 3] and F allele frequency [P = 0.0221; OR: 2.836 (CI: 1.129–7.122); Table 3], respectively, and development of persistent disease in the overall American population. Further analysis of individual Caucasian American and African American cohorts revealed a significant association between disease persistence and 412 F heterozygote genotype [P = 0.0114, OR for Phe carriers: 4.4 (CI: 1.411–13.717); Table 3] and allele frequency [P = 0.0205, OR: 3.175 (CI: 1.207–8.351); Table 3], respectively, in Caucasian Americans but not African Americans.
Table 3.
Replication study: TLR3 L412F (rs3775291) genotype and disease persistence in American pulmonary sarcoidosis cases
| Status | Leu/Leu | Leu/Phe | Phe/Phe |
|---|---|---|---|
| Persistent | 55 | 24 | 3 |
| (n = 82) | (0.67) | (0.29) | (0.04) |
| Non-persistent | 36 | 4 | 1 |
| (n = 41) | (0.88) | (0.10) | (0.02) |
| P-values (All US cases) | OR (95% CI; All US cases) | ||
| Genotype | a0.0432 b(0.0077) | Phe carrier | e3.535 (1.246–10.029) |
| Trend | c0.0278 | Phe/Phe homozygote | f1.519 (0.153–15.082) |
| Allele | d0.0221 | ||
| P-values (Ethnicity) | Caucasians (n = 71) | African Americans (n = 52) | |
| Genotype | g0.0114 | h0.3070 | |
| Allele | i0.0205 | j0.3204 | |
| OR (95% CI) | Caucasians | African Americans | |
| Phe carrier | k4.400 (1.411–13.717) | l4.761 (0.2466–91.939) | |
| Phe/Phe homozygote | m1.902 (0.188–19.290) | n4.761 (0.2466–91.939) | |
aA significant association was detected between TLR3 L412F and disease persistence in an American cohort of sarcoidosis patients attending a tertiary referral centre. L412F genotypes were compared within the overall American (US) cohort using: 3 x 2 χ2 test for independence (2 d.f.) and
c3 x 2 χ2 test for trend (1 d.f.), respectively. OR (95% CI) for
ePhe carriers and
fPhe/Phe homozygotes in overall US cohort.
dAllele frequencies were compared using a 2 x 2 Fisher’s Exact test (OR: 2.836; 95% CI: 1.129–7.122).
bLogistic regression analysis was performed to calculate the adjusted P-values, using the confounding factors: age at diagnosis, gender and race. A significant risk of disease persistence was conferred by L412F genotype b(P = 0.0077). However, a significant risk of disease persistence was also conferred by Caucasian ancestry (P = 0.0295) compared with African American ancestry. Therefore, further analysis was carried out for individual Caucasian and African American populations, respectively. A significant association was found between disease persistence and genotype in Caucasians but not African Americans:
g,hGenotype frequencies and
i,jallele frequencies were compared using 2 x 2 Fisher’s Exact tests. OR (95% CI) for
k,lPhe carriers and
m,nPhe/Phe homozygotes for Caucasians and African Americans, respectively.
Primary lung fibroblasts from TLR3 L412F homozygote sarcoidosis patients produce reduced IL-8 and IFN-β, and have reduced TLR3 expression
In order to elucidate the mechanisms underlying the association between development of persistent disease and TLR3 412 F, we investigated TLR3 function in primary human fibroblasts from TLR3 412 F wild-type (Leu/Leu) vs. homozygous (Phe/Phe) sarcoidosis patients with a persistent disease phenotype. Following TLR3 activation by Poly(I:C) treatment, variant Phe/Phe fibroblasts had significantly reduced IL-8 production (Figure 1A; NF-κB-readout) and IFN-β expression (Figure 1B; IRF3-readout), respectively. These findings conferred with the authors who first described the polymorphism and who reported that it resulted in defective TLR3 function via reduced NF-κB and IRF3 signalling.28 In addition, following TLR3 activation by Poly(I:C) treatment, variant Phe/Phe fibroblasts also had blunted TLR3 mRNA expression compared with wild-type fibroblasts (Figure 1C). We have also shown additionally using FACS that the upregulation of extracellular TLR3 following Poly(I:C) treatment on Phe/Phe primary lung fibroblasts was blunted compared with wild-type cells (Supplementary Figure S1A). In contrast, levels of intracellular TLR3 expression on wild-type and Phe/Phe fibroblasts were comparable following Poly(I:C) treatment (Supplementary Figure S1B).
Figure 1.
Effect of TLR3 L412F on TLR3-induced IL-8, IFN-β mRNA and TLR3 mRNA in Leu/Leu and Phe/Phe primary human lung fibroblasts from pulmonary sarcoidosis patients. TLR3 L412F attenuates Poly(I:C)-driven (A) IL-8 production, (B) IFN-β mRNA and (C) TLR3 mRNA expression in primary human lung fibroblasts from Phe/Phe sarcoidosis patients compared with Leu/Leu patients at 24 h post-treatment, as quantitated by ELISA and QPCR analysis, respectively. (A–C) *P < 0.05, **P < 0.01, ***P < 0.001: Poly(I:C) 100 μg/ml compared with Medium-only at 24 h post-treatment. ++P < 0.01: Poly(I:C) 100 μg/ml in fibroblasts from Phe/Phe (n = 3) compared with Leu/Leu patients (n = 3). Results shown are the mean ± SEM of (B, C) three or (A) six replicates from a representative of (B) two or (A, C) three separate experiments.
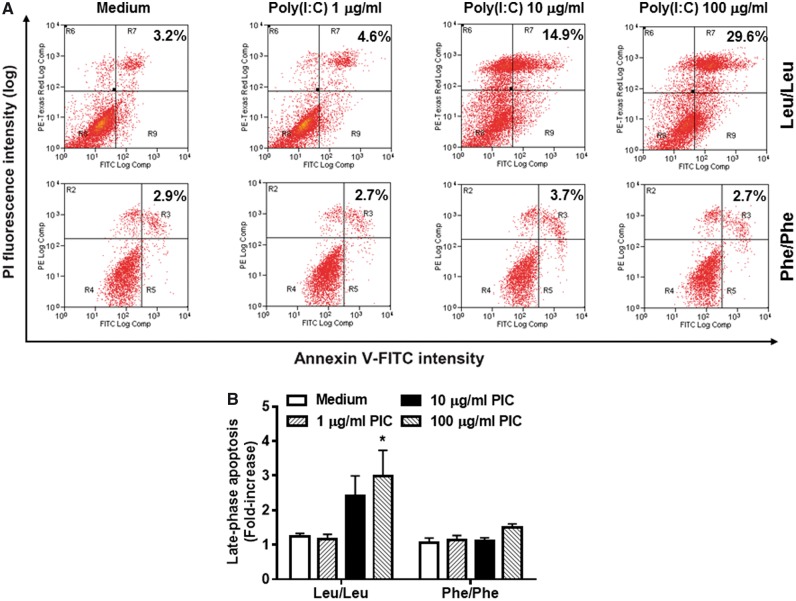
TLR3 L412F inhibits apoptosis and dysregulates proliferation in primary lung fibroblasts from homozygote sarcoidosis patients
Here, we assessed the induction of TLR3-induced apoptosis in Leu/Leu and Phe/Phe fibroblasts and found a significantly reduced ability of Phe/Phe fibroblasts to undergo late-phase apoptosis compared with wild-type cells (Figure 2A and B). Furthermore, we also observed a decreased ability of Phe/Phe cells to reduce their proliferation in response to Poly(I:C) compared with Leu/Leu cells (Figure 3A). Interestingly, a comparable level of reduction in fibroproliferation was observed following treatment of primary human lung fibroblasts for 24 h with 1000 IU/ml IFN-β in cells from Leu/Leu and Phe/Phe patients (Figure 3B). This result suggests that the dysregulated fibroproliferation seen in TLR3 defective, Phe/Phe fibroblasts may be due to their reduced ability to produce IFN-β.
Figure 2.
Effect of TLR3 L412F on TLR3-induced apoptotic responses in Leu/Leu and Phe/Phe primary human lung fibroblasts from pulmonary sarcoidosis patients. A significant increase in Poly(I:C)-induced (A, B) late-phase apoptosis in Leu/Leu, but not Phe/Phe, fibroblasts following 24 h treatment, as quantitated by Annexin V/Propidium Iodide staining using FACS analysis. (B) *P < 0.05, Poly(I:C) 100 μg/ml compared with Medium-only. ++P < 0.01: Treatment with 100 μg/ml Poly(I:C) in fibroblasts from Phe/Phe (n = 3) compared with Leu/Leu (n = 3). Results shown are the mean ± SEM of (B) six or (A) five replicates from a representative of (A, B) three separate experiments.
Figure 3.
Effect of TLR3 L412F on TLR3-induced proliferative responses in Leu/Leu and Phe/Phe primary human lung fibroblasts from pulmonary sarcoidosis patients. (A) Poly(I:C) treatment significantly reduces proliferation of Leu/Leu fibroblasts following 24 h treatment with 1-100 μg/ml Poly(I:C), as quantitated using 3 H-thymidine incorporation. The fold-decrease in Poly(I:C)-induced proliferation at 100 μg/ml at 24 h is significantly more in Leu/Leu compared with Phe/Phe fibroblasts, as quantitated using 3 H-thymidine incorporation. (B) Reconstitution of Phe/Phe cells with recombinant human IFN-β (1000 IU/ml) leads to an equivalent reduction in cell proliferation in Leu/Leu and Phe/Phe cells. (A, B) *P < 0.05, **P < 0.01, ***P < 0.001: Poly(I:C) 100 μg/ml compared with Medium-only. ++P < 0.01: Treatment with 100 μg/ml Poly(I:C) in fibroblasts from Phe/Phe (n = 3) compared with Leu/Leu patients (n = 3). Results shown are the mean ± SEM of (B) six or (A) five replicates from a representative of (A, B) three separate experiments.
Discussion
In this study, we investigated the role of defective TLR3 in the pathogenesis of pulmonary sarcoidosis. Specifically, we investigated the role of the TLR3 polymorphism, TLR3 L412F (rs3775291), as a causative factor in the development of, and in disease persistence in pulmonary sarcoidosis, respectively. Previously, we identified a role for TLR3 L412F in accelerated disease progression and increased risk of mortality in IPF.16 In this study, we established that TLR3 L412F was not associated with development of pulmonary sarcoidosis but was significantly associated with disease persistence. Irish patients who presented with sarcoidosis, and who were TLR3 412 F-homozygous, were significantly more likely to develop persistent disease. Therefore, these findings suggest that TLR3 L412F plays a broader role in interstitial lung disease and that its pathogenic effects are not limited to IPF.
In this study, the association between TLR3 L412F and disease persistence in Irish pulmonary sarcoidosis patients was validated in a modest-sized American cohort of patients, attending a tertiary referral centre. American pulmonary sarcoidosis patients who had one copy of the variant allele were almost five times more likely to develop persistent disease. Interestingly, there was also a significant association between disease persistence and race. When Caucasian American and African American populations were analysed individually, a significant association was found between the 412 F allele and disease persistence in Caucasian Americans but not in African Americans. This may reflect specific genetic backgrounds in Caucasian Americans compared to African Americans which results in different spectrums of disease presentations in both populations. These novel results merit further investigation using larger cohort-size.
Here, we also report that primary lung fibroblasts from TLR3 412 F-homozygous pulmonary sarcoidosis patients had reduced IL-8, IFN-β and TLR3 production or expression, reduced fibroblast apoptosis and dysregulated fibroproliferative responses compared with cells from wild-type patients, following TLR3-activation. Our findings imply that defective TLR3 promotes a persistent disease phenotype in sarcoidosis by dysregulating apoptotic and fibroproliferative processes via an IFN-β-dependent process. Thus, this study identifies defective TLR3 function as a previously undescribed factor in the development of persistent clinical disease in pulmonary sarcoidosis and reveals the TLR3 signalling pathway as a novel therapeutic target in its treatment (see Supplementary Figure S2 for schematic of proposed mechanism). The strength of this study lies in its exploration of the role of the change in primary lung fibroblast function in disease persistence in pulmonary sarcoidosis patients, in the context of the TLR3 L412F polymorphism. In this study, primary lung fibroblasts from three patients from each respective genotype were utilized (i.e. TLR3 Leu/Leu wild-type and TLR3 Phe/Phe homozygote patients). In future studies to investigate the role of TLR3 L412F further in the pathogenesis of persistent pulmonary sarcoidosis, and as a candidate prognostic marker, additional lung fibroblasts from wild-type and homozygote patients will be recruited.To date, no specific pathogen has been identified as a causative factor in sarcoidosis. Several studies have a role for mycobacterial or propionibacterial organisms in the pathogenesis of sarcoidosis. Previously, a human herpes 8 open reading frame DNA was detected in a significantly higher proportion of sarcoid-compared with non-sarcoid lung tissue.29 However, the role of these viruses, and of EBV particularly, in the etiology of sarcoidosis remains speculative.11,29 TLR3 was originally identified as an anti-viral receptor and was shown to bind viral dsRNA.12 More recently, the role for TLR3 in microbial infection has been expanded. TLR3 is now known to additionally bind dsRNA from bacteria and helminths.13,14 It has also been shown to bind mRNA which has been released from necrotic cells during infection and inflammation.15 In this study, the defective function associated with the effects of TLR3 L412F in cells from 412 F-homozygous patients would provide a mechanism by which bacterial or viral infection could promote a persistent clinical phenotype in sarcoidosis.
In this study, we also observed that reduced IFN-β expression or production is one of the mechanisms by which TLR3 L412F mediates its effects. In the context of IFN-β, this has been shown to directly induce apoptosis in cells following TLR3 activation in an autocrine manner.30 Interestingly, the addition of IFN-β to in vitro cultures of our 412 F-homozygous cells in our study resulted in a restoration to a wild-type proliferative phenotype. With the current therapeutic use of IFN-β in a variety of cancers and autoimmune disorders, our data would support the therapeutic targeting of sarcoidosis patients exhibiting the 412 F-homozygous genotype with IFN-β. Therefore, we suggest that treatment of 412 F-homozygous sarcoidosis patients with persistent clinical disease with recombinant IFN-β may represent a novel treatment regimen. Other authors have reported the induction of IFN-α-induced sarcoidosis in a patient being treated for hepatitis C and the development of sarcoidosis in multiple sclerosis and myeloma patients, respectively, following recombinant IFN-β treatment.31–34 However, previously, Charlier et al. examined a series of four patients with sarcoidosis, treated by IFN-α or IFN-β for viral hepatitis or multiple sclerosis. Interestingly, no recurrence or exacerbation of sarcoidosis had occurred at 4 years of follow-up. This study series suggests that type I IFNs do not exacerbate sarcoidosis in remission and this makes their use possible if indicated. However, their effect in persistent forms of the disease needs further evaluation.35
Sarcoidosis can follow a variable clinical course. Historically, it is well recognized that presentation with erythema nodosum and bilateral hilar adenopathy on chest radiograph has a better prognosis (15% risk of chronicity and progression) compared to presentation with bilateral chest radiograph infiltrates (40% chance of progression). However, we are unable to stratify, with a high degree of accuracy, the prognosis of individual patients at presentation. This is a significant clinical unmet need in clinical practice and in clinical trials design. It would be of significant advantage in clinical trials if we could enrich patient recruitment favoring a more aggressive clinical phenotype. This would offer the best opportunity of assessing whether specific, proposed therapies are clinically efficacious or not. This study reveals TLR3 L412F as a candidate prognostic biomarker in pulmonary sarcoidosis.
Conclusion
In conclusion, this study reveals for the first time that defective TLR3 function, and specifically TLR3 L412F-homozygosity, are important factors in driving a persistent clinical phenotype in pulmonary sarcoidosis patients. This study also suggests that restoration of TLR3-function may provide a novel therapeutic strategy in the treatment of sarcoidosis.
Author contributions
G.C., M.S. and E.H. performed experiments. I.K. collated patients’ clinical data. L.M., A.T., C.O.R. and D.N.O.D. processed clinical samples. S.L.K. and D.R.M. provided patient samples. U.G.K., D.C.S., A.G.B. and P.G.F. contributed to experimental design. C.M.H. provided patient samples and contributed to experimental design. M.E.A conceived of, designed and performed experiments, analysed data and wrote the paper. S.C.D. conceived of experiments, analysed data and contributed to manuscript drafting.
Supplementary Material
Acknowledgement
The authors thank Dr Clare O’ Connor (UCD) for her assistance with the statistical analyses.
Supplementary material
Supplementary material is available at QJMED online.
Funding
This work was supported by grants from the Health Research Board (HRB) Ireland (HRA-POR-2011-49) and the Irish Lung Foundation (ILF) awarded to S.C.D. and M.E.A.
Conflict of interest: None declared.
References
- 1. Iannuzzi MC, Rybicki BA, Teirstein AS.. Sarcoidosis. N Engl J Med 2007; 357:2153–65. [DOI] [PubMed] [Google Scholar]
- 2. Statement on Sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–55. [DOI] [PubMed] [Google Scholar]
- 3. Pietinalho A, Hiraga Y, Hosoda Y, Lofroos AB, Yamaguchi M, Selroos O.. The frequency of sarcoidosis in Finland and Hokkaido, Japan. A comparative epidemiological study. Sarcoidosis 1995; 12:61–7. [PubMed] [Google Scholar]
- 4. Chen ES, Moller DR.. Etiologies of sarcoidosis. Clin Rev Allergy Immunol 2015; 49:6–18. [DOI] [PubMed] [Google Scholar]
- 5. Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P.. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol 1996; 156:4952–60. [PubMed] [Google Scholar]
- 6. Moller DR. Cells and cytokines involved in the pathogenesis of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16:24–31. [PubMed] [Google Scholar]
- 7. Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, Teolato S, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 2011; 66:144–50. [DOI] [PubMed] [Google Scholar]
- 8. Rappl G, Pabst S, Riemann D, Schmidt A, Wickenhauser C, Schutte W, et al. Regulatory T cells with reduced repressor capacities are extensively amplified in pulmonary sarcoid lesions and sustain granuloma formation. Clin Immunol 2011; 140:71–83. [DOI] [PubMed] [Google Scholar]
- 9. Grunewald J, Hultman T, Bucht A, Eklund A, Wigzell H.. Restricted usage of T cell receptor V alpha/J alpha gene segments with different nucleotide but identical amino acid sequences in HLA-DR3+ sarcoidosis patients. Mol Med 1995; 1:287–96. [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta D, Agarwal R, Aggarwal AN, Jindal SK.. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J 2007; 30:508–16. [DOI] [PubMed] [Google Scholar]
- 11. Byrne EB, Evans AS, Fouts DW, Israel HL.. A seroepidemiological study of Epstein-Barr virus and other viral antigens in sarcoidosis. Am J Epidemiol 1973; 97:355–63. [DOI] [PubMed] [Google Scholar]
- 12. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA.. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001; 413:732–8. [DOI] [PubMed] [Google Scholar]
- 13. Spelmink L, Sender V, Hentrich K, Kuri T, Plant L, Henriques-Normark B.. Toll-like receptor 3/TRIF-dependent IL-12p70 secretion mediated by Streptococcus pneumoniae RNA and its priming by influenza A virus coinfection in human dendritic cells. MBio 2016; 7:e00168–16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aksoy E, Zouain CS, Vanhoutte F, Fontaine J, Pavelka N, Thieblemont N, et al. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J Biol Chem 2005; 280:277–83. [DOI] [PubMed] [Google Scholar]
- 15. Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med 2008; 205:2609–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Dwyer DN, Armstrong ME, Trujillo G, Cooke G, Keane MP, Fallon PG, et al. The Toll-like receptor 3 L412F polymorphism and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2013; 188:1442–50. [DOI] [PubMed] [Google Scholar]
- 17. Assmann TS, Brondani L. d A, Bauer AC, Canani LH, Crispim D.. Polymorphisms in the TLR3 gene are associated with risk for type 1 diabetes mellitus. Eur J Endocrinol 2014; 170:519–27. [DOI] [PubMed] [Google Scholar]
- 18. Laska MJ, Troldborg A, Hansen B, Stengaard-Pedersen K, Junker P, Nexø BA, et al. Polymorphisms within Toll-like receptors are associated with systemic lupus erythematosus in a cohort of Danish females. Rheumatology (Oxford) 2014; 53:48–55. [DOI] [PubMed] [Google Scholar]
- 19. Laska MJ, Hansen B, Troldborg A, Lorenzen T, Stengaard-Pedersen K, Junker P, et al. A non-synonymous single-nucleotide polymorphism in the gene encoding Toll-like Receptor 3 (TLR3) is associated with sero-negative rheumatoid arthritis (RA) in a Danish population. BMC Res Notes 2014; 7:716.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng D, Hao Y, Zhou W, Ma Y.. Association between Toll-like receptor 3 polymorphisms and cancer risk: a meta-analysis. Tumour Biol 2014; 35:7837–46. [DOI] [PubMed] [Google Scholar]
- 21. Castro FA, Forsti A, Buch S, Kalthoff H, Krauss C, Bauer M, et al. TLR-3 polymorphism is an independent prognostic marker for stage II colorectal cancer. Eur J Cancer 2011; 47:1203–10. [DOI] [PubMed] [Google Scholar]
- 22. Dai J, Hu Z, Dong J, Xu L, Pan S, Jiang Y, et al. Host immune gene polymorphisms were associated with the prognosis of non-small-cell lung cancer in Chinese. Int J Cancer 2012; 130:671–6. [DOI] [PubMed] [Google Scholar]
- 23. Zeljic K, Supic G, Jovic N, Kozomara R, Brankovic-Magic M, Obrenovic M, et al. Association of TLR2, TLR3, TLR4 and CD14 genes polymorphisms with oral cancer risk and survival. Oral Dis 2014; 20:416–24. [DOI] [PubMed] [Google Scholar]
- 24. Chen D-N, Song C-G, Yu K-D, Jiang Y-Z, Ye F-G, Shao Z-M, et al. A prospective evaluation of the association between a single nucleotide polymorphism rs3775291 in toll-like receptor 3 and breast cancer relapse. PLoS One 2015; 10:e0133184.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sironi M, Biasin M, Cagliani R, Forni D, De Luca M, Saulle I, et al. A common polymorphism in TLR3 confers natural resistance to HIV-1 infection. J Immunol 2012; 188:818–23. [DOI] [PubMed] [Google Scholar]
- 26. Geng PL, Song LX, An H, Huang JY, Li S, Zeng XT.. Toll-like receptor 3 is associated with the risk of HCV infection and HBV-related diseases. Medicine (Baltimore) 2016; 95:e2302.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol 2008; 40:2174–82. [DOI] [PubMed] [Google Scholar]
- 28. Ranjith-Kumar CT, Miller W, Sun J, Xiong J, Santos J, Yarbrough I, et al. Effects of single nucleotide polymorphisms on Toll-like receptor 3 activity and expression in cultured cells. J Biol Chem 2007; 282:17696–705. [DOI] [PubMed] [Google Scholar]
- 29. Di Alberti L, Piattelli A, Artese L, Favia G, Patel S, Saunders N, et al. Human herpesvirus 8 variants in sarcoid tissues. Lancet 1997; 350:1655–61. [DOI] [PubMed] [Google Scholar]
- 30. Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T.. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol 2006; 176:4894–901. [DOI] [PubMed] [Google Scholar]
- 31. Trien R, Cooper CJ, Paez D, Colon E, Ajmal S, Salameh H.. Interferon-alpha-induced sarcoidosis in a patient being treated for hepatitis C. Am J Case Rep 2014; 15:235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oudghiri B, Benzagmout M, Boujraf S, Belahcen F, Ibrahimi A.. Multisystem sarcoidosis in a patient on interferon-alpha therapy for chronic hepatitis C. J Glob Infect Dis 2012; 4:128–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chakravarty SD, Harris ME, Schreiner AM, Crow MK.. Sarcoidosis triggered by interferon-Beta treatment of multiple sclerosis: a case report and focused literature review. Semin Arthritis Rheum 2012; 42:206–12. [DOI] [PubMed] [Google Scholar]
- 34. Bobbio-Pallavicini E, Valsecchi C, Tacconi F, Moroni M, Porta C.. Sarcoidosis following beta-interferon therapy for multiple myeloma. Sarcoidosis 1995; 12:140–2. [PubMed] [Google Scholar]
- 35. Charlier C, Nunes H, Trinchet JC, Roullet E, Mouthon L, Beaugrand M, et al. Evolution of previous sarcoidosis under type 1 interferons given for severe associated disease. Eur Respir J 2005; 25:570–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.