Abstract
BACKGROUND: Premorbid antithrombotic medication may worsen intracranial injury and outcome after traumatic brain injury (TBI). Routine laboratory tests are insufficient to evaluate platelet activity.
OBJECTIVE: To profile the spectrum of platelet inhibition, as measured by aspirin and P2Y12 response unit assays, in a TBI population on antiplatelet therapy.
METHODS: This single-center, prospective cohort study included patients presenting to our institution between November 2010 and January 2015 with a clinical history of TBI. Serum platelet reactivity levels were determined immediately on admission and analyzed using the aspirin and P2Y12 response unit assays; test results were reported as aspirin response units and P2Y12 response units. We report congruence between assay results and clinical history as well as differences in assay results between types of antiplatelet therapy.
RESULTS: A sample of 317 patients was available for analysis, of which 87% had experienced mild TBI, 7% moderate, and 6% severe; the mean age was 71.5 years. The mean aspirin response units in patients with a history of any aspirin use was 456 ± 67 (range, 350-659), with 88% demonstrating therapeutic platelet inhibition. For clopidogrel, the mean P2Y12 response unit was 191 ± 70 (range, 51-351); 77% showed therapeutic response.
CONCLUSION: Rapid measurement of antiplatelet function using the aspirin and P2Y12 response assays indicated as many as one fourth of patients on antiplatelet therapy do not have platelet dysfunction. Further research is required to develop guidelines for the use of these assays to guide platelet transfusion in the setting of TBI.
Keywords: Aspirin, Aspirin response unit, Clopidogrel, Platelet dysfunction, P2Y12 response unit, Traumatic brain injury
ABBREVIATIONS
- ARU
aspirin response unit
- GCS
glasgow coma scale
- PRU
P2Y12 response unit
- TBI
traumatic brain injury
As the population ages, it is more common to encounter patients with traumatic brain injury (TBI) who are on antithrombotic therapy for preexisting illnesses. Premorbid use of antithrombotic medications such as warfarin, aspirin, and clopidogrel may increase the risk and severity of traumatic intracranial hemorrhage and may predispose patients to enlargement of these posttraumatic lesions.1,2 Standard laboratory analyses are used in patients with TBI to evaluate the degree of anticoagulation due to vitamin K antagonists (ie, warfarin) as well as guide goal-directed reversal of the anticoagulatory effects of these medications to acceptable subtherapeutic levels.3-7 Unfortunately, these routine coagulation tests are insufficient to evaluate platelet activity and guide hemostatic therapy in TBI patients on antiplatelet therapy.
Recently, point-of-care aspirin response unit (ARU) and P2Y12 response unit (PRU) assays for evaluating aspirin- and thienopyridine-induced platelet inhibition have become widely available and have undergone extensive external validation in the cardiac literature.8-15 The assays enable platelet function testing for patients taking aspirin, P2Y12 inhibitors (clopidogrel, ticlopidine, or prasugrel), and IIb/IIIa inhibitors (abciximab or eptifibatide)8-12 and has demonstrated nearly 100% sensitivity and 96% specificity for the detection of antiplatelet function.16 However, there are currently insufficient data demonstrating the utility of the ARU and PRU assays in assessing platelet function in patients with TBI. In the current study, we evaluated the ARU and PRU assays for the detection of platelet inhibition in TBI patients on premorbid antiplatelet agents.
METHODS
Study Population
The study was performed under a Quality Assurance project through the Department of Neurosurgery. Deidentified data were extracted, including patient age, sex, mechanism of injury, Glasgow Coma Scale score, Marshall score, and history of aspirin or clopidogrel use. Patients eligible for this analysis were adults presenting to our institution between November 2010 and January 2015 within 24 hours of a traumatic injury and having a positive clinical screen for TBI necessitating a noncontrast head computed tomography according to American College of Emergency Physicians/Centers for Disease Control and Prevention evidence-based joint practice guidelines.17 Patients were excluded from analysis if they were younger than 16 years of age, had missing admission computed tomography head scans, had a known bleeding diathesis, were taking anticoagulation therapy (eg, warfarin, dabigatran), and/or in whom antithrombotic agent use was unknown. Patients were also excluded if antiplatelet reversal (eg, platelet transfusion, 1-desamino-8-d-arginine vasopressin administration) was performed at an outside hospital.
Sample Collection and Measurement of Platelet Function
Platelet dysfunction was evaluated using the VerifyNow assay (Accumetrics, San Diego, CA). During collection of whole-blood samples for standard laboratory tests during presentation to the emergency department, 2 additional tubes of 2 mL of whole blood were collected in standard citrated blood draw collection tubes for the ARU and PRU assays and analyzed using standard, previously described procedures.9,18 An ARU count <550 was considered therapeutic, whereas a level >550 was considered nontherapeutic and indicated aspirin resistance in patients taking aspirin.16,19 A PRU count <240 was considered therapeutic, and a level >240 was considered nontherapeutic.20,21
Statistical Analysis
Continuous demographic characteristics were assessed for normality using the Kolmogorov-Smirnov test; normally distributed data were analyzed by a t test, and the remainders were compared using the Wilcoxon rank sum test. Values are reported as mean ± SD; significant associations between outcomes and predictors are reported as odds ratios with 95% confidence interval. An acceptable type I error was set a priori at α = .05 for all statistical tests. All data were analyzed using STATA 13.1 (StataCorp, College Station, Texas).
RESULTS
Baseline Characteristics
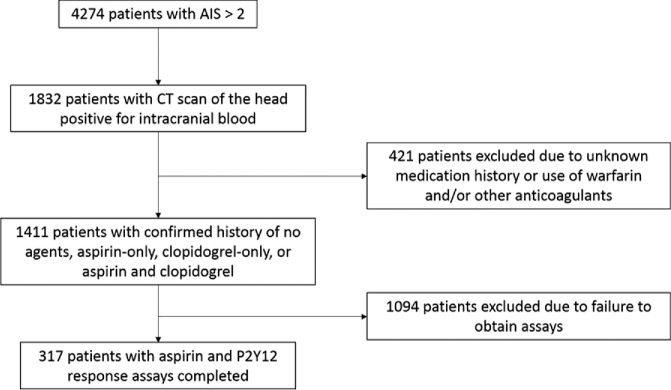
A total of 317 patients were included in this analysis (Figure). The mean patient age was 71.5 ± 15.4 years, and 56.5% of patients were male (Table 1). The median Glasgow Coma Scale (GCS) score on admission across all patients was 15 (interquartile range, 14-15). TBI severity on admission was 87% mild (GCS score, 13-15), 7% moderate (GCS score, 9-12), and 6% severe (GCS score ≤8). Intracranial severity as measured by the Marshall score was 2 (interquartile range, 1-2). Mechanisms of injury were 78% fall (either ground level or from a height), 18% motor vehicle accident, and 4% other.
FIGURE.
Patient selection flow diagram. AIS, Abbreviated Injury Score; CT, computed tomography.
TABLE 1.
Baseline Characteristicsa
| Overall | |
|---|---|
| Subjects, N (%) | 317 (100) |
| Age, y, mean ± SD | 71.5 ± 15.4 |
| Sex, male, % | 57 |
| Mechanism of injury, % | |
| Fall (ground level or from height) | 78 |
| Motor vehicle accident | 18 |
| Other | 4 |
| Injury severity | |
| Initial GCS score, median (IQR) | 15 (14-15) |
| Severe TBI (GCS score ≤8), % | 6 |
| Marshall score, median (IQR) | 2 (1-2) |
aGCS, Glasgow Coma Scale; IQR, interquartile range; TBI, traumatic brain injury.
Table 2 reports the distribution of antiplatelet agents in the cohort. The most common antiplatelet therapy was aspirin only (53% of the cohort). Seventeen percent of the cohort was taking a combination of aspirin and clopidogrel, and 5% were taking clopidogrel only. Overall, 69% of the cohort was reported to be taking any aspirin, whereas 21% were taking any clopidogrel. Twenty-six percent of the cohort was not taking any antiplatelet agent at the time of injury.
TABLE 2.
Distribution of Antiplatelet Agents
| Overall | |
|---|---|
| No agent, n (%) | 82 (26) |
| Aspirin only, n (%) | 167 (53) |
| Clopidogrel only, n (%) | 15 (5) |
| Both agents, n (%) | 53 (17) |
| Any aspirin,a n (%) | 220 (69) |
| Any clopidogrel,b n (%) | 68 (21) |
| Any antiplatelet, n (%) | 235 (74) |
| Total | 317 |
aPatients taking either aspirin only or dual therapy.
bPatients taking either clopidogrel only or dual therapy.
ARU and PRU Assay Results
Table 3 demonstrates the results of the ARU and PRU assays compared with the reported clinical history of antiplatelet use. The mean ARU for all patients was 478 ± 82 (range, 350-659), with 79% demonstrating therapeutic platelet inhibition. For clopidogrel, the mean PRU for all patients was 218 ± 74 (range, 51-482), and 62% showed therapeutic response. Eighty-eight percent of patients reporting any aspirin use were therapeutic on ARU, whereas 77% of those on any clopidogrel were therapeutic on PRU; these were significantly greater than those not on antiplatelet therapy (P < .001 and P = .003, respectively). No significant difference in admission PRU was found between patients who took any aspirin and those taking no agent (P = .16). However, a significant difference in ARUs was found between patients who were taking any clopidogrel and those not taking any agent (P < .001). Approximately 26% of our patients were not taking an antiplatelet agent, yet 52% and 54% in this cohort had therapeutic assays for aspirin and clopidogrel, respectively.
TABLE 3.
Admission Aspirin Response Unit and P2Y12 Response Unit Results Stratified by Antiplatelet Therapya
| % Therapeutic, ARU | ARU, Mean (SD) | P, ARU | % Therapeutic, PRU | PRU, Mean SD, | P, PRU | |
|---|---|---|---|---|---|---|
| No agent | 52 | 536 (90) | 54 | 229 (76) | ||
| Aspirin only | 87 | 460 (68) | <.001b | 53 | 237 (65) | .54 |
| Clopidogrel only | 63 | 518 (97) | .59 | 73 | 210 (64) | .39 |
| Both agents | 92 | 439 (61) | <.001b | 78 | 185 (72) | .002b |
| Any aspirin (aspirin only + both) | 88 | 456 (67) | <.001b | 65 | 212 (73) | .15 |
| Any clopidogrel (clopidogrel only + both) | 88 | 451 (72) | <.001b | 77 | 191 (70) | .003b |
| Any antiplatelet agent | 87 | 457 (69) | <.001b | 66 | 212 (72) | .14 |
| All patients | 79 | 478 (82) | 62 | 218 (74) |
aARU, aspirin response unit; PRU, P2Y12 response unit.
bIndicates a significant result.
Aspirin-only, clopidogrel-only, and both agent comparisons were made against no agent. For any aspirin, any clopidogrel, and any antiplatelet agent, comparisons were made against patients not taking the particular agent. The Student t test used for all comparisons of assay values.
DISCUSSION
Many techniques for measuring platelet function have been developed, such as platelet aggregometry, thromboelastography platelet mapping, flow cytometry, Platelet Function Analyzer-100, and urinary 11-dehyrothromboxane B2.22,23 Platelet aggregometry is considered the historic gold standard. Previous measurements of platelet function were hindered by large-sample volume requirements, sample preparation requirements including centrifuging or pipetting, requirement of specialized personnel, long delays until results, prohibitively expensive equipment, or nonspecific results in the case of measuring thromboxane B2 and its metabolites.22,23 The ARU and PRU assays can be considered true point-of-care tests, given that they do not have these issues, although there is uncertainty over whether platelet count or hemoglobin level can influence assay results.11,22,24
We demonstrated in our cohort of patients that a clinical history of any aspirin or any clopidogrel use is associated with a therapeutic PRU result. However, a substantial proportion of patients with no history of antiplatelet therapy had therapeutic assay results as well (52% with therapeutic ARU and 54% with therapeutic PRU). Our finding of an incongruence between assay results and clinical history is not uncommon. It is well established in the neurosurgical and cardiac literature that a subset of patients with a reported history of antiplatelet therapy will be found to have normal platelet function. In a pair of systematic reviews, Hovens et al25 and Snoep et al26 report that approximately 1 in 4 patients taking aspirin will show biochemical resistance, and 1 in 5 patients taking clopidogrel will be resistant. Conversely, platelet dysfunction in patients without a history of antiplatelet use in the setting of TBI is not uncommon.27,28 Genetic polymorphisms of platelet and cytochrome P-450 enzymes, chronic illness, brain trauma–specific changes in the inflammatory milieu, consumption of platelet granule contents, antiplatelet medications, and other factors likely influence platelet function at the time of injury.29-32 Our results indicate that the majority of patients had therapeutic ARU and PRU results regardless of a history of platelet use. Both our study and that of Bachelani et al27 used cutoff thresholds for therapeutic platelets based on the cardiac literature (550 for ARUs and 240 for PRUs), which may not be valid in the TBI population. Future investigation of meaningful thresholds for the ARU and PRU assays in the TBI population using outcomes may be warranted. Previous studies investigating the relationship between a history of antiplatelet therapy and outcome in TBI have had conflicting results, which may be explained by a multitude of factors affecting platelet function.33-37
The ARU and PRU assays have been validated in the cardiac literature to reveal platelet dysfunction and predict future adverse events.8,9,12-15,38 We show in this study its feasibility in the TBI population. Further work to validate the utility of the ARU and PRU assays in the TBI population as a prognostic and management tool is required.
Disclosures
Support for this research was provided by the Walter L. Copeland Fund of The Pittsburgh Foundation, the University of Pittsburgh Clinical Scientist Training Program (CSTP), and the Clinical and Translational Science Institute (UL1 TL1TR000005). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
We thank the research nurses at the University of Pittsburgh Brain Trauma Research Center, the neurosurgery residents at the University of Pittsburgh, and Benjamin E. Zusman for enrolling patients and collecting data for this study.
REFERENCES
- 1. Lavoie A, Ratte S, Clas D et al. . Preinjury warfarin use among elderly patients with closed head injuries in a trauma center. J Trauma. 2004;56(4):802-807. [DOI] [PubMed] [Google Scholar]
- 2. Franko J, Kish KJ, O'Connell BG, Subramanian S, Yuschak JV. Advanced age, and preinjury warfarin anticoagulation increase the risk of mortality after head trauma. J Trauma. 2006;61(1):107-110. [DOI] [PubMed] [Google Scholar]
- 3. Mayer SA, Davis SM, Skolnick BE et al. . Can a subset of intracerebral hemorrhage patients benefit from hemostatic therapy with recombinant activated factor VII? Stroke. 2009;40(3):833-840. [DOI] [PubMed] [Google Scholar]
- 4. Pabinger I, Brenner B, Kalina U et al. . Prothrombin complex concentrate (Beriplex P/N) for emergency anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost. 2008;6(4):622-631. [DOI] [PubMed] [Google Scholar]
- 5. Preston FE, Laidlaw ST, Sampson B, Kitchen S. Rapid reversal of oral anticoagulation with warfarin by a prothrombin complex concentrate (Beriplex): efficacy and safety in 42 patients. Br J Haematol. 2002;116(3):619-624. [DOI] [PubMed] [Google Scholar]
- 6. Lankiewicz MW, Hays J, Friedman KD, Tinkoff G, Blatt PM. Urgent reversal of warfarin with prothrombin complex concentrate. J Thromb Haemost. 2006;4(5): 967-970. [DOI] [PubMed] [Google Scholar]
- 7. Bruce D, Nokes T. Prothrombin complex concentrate (Beriplex P/N) in severe bleeding: experience in a large tertiary hospital. Crit Care. 2008;12(4):R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lev EI, Patel RT, Maresh KJ et al. . Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J Am Coll Cardiol. 2006;47(1):27-33. [DOI] [PubMed] [Google Scholar]
- 9. Lee PY, Chen WH, Ng W et al. . Low-dose aspirin increases aspirin resistance in patients with coronary artery disease. Am J Med. 2005;118(7):723-727. [DOI] [PubMed] [Google Scholar]
- 10. Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospec-tive, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41(6):961-965. [DOI] [PubMed] [Google Scholar]
- 11. Malinin A, Pokov A, Swaim L, Kotob M, Serebruany V. Validation of a verify now-P2Y12 (R) cartridge for monitoring platelet inhibition with clopidogrel. Methods Find Exp Clin Pharmacol. 2006;28(5):315-322. [DOI] [PubMed] [Google Scholar]
- 12. Malinin A, Pokov A, Spergling M et al. . Monitoring platelet inhibition after clopidogrel with the VerifyNow-P2Y12 rapid analyzer: the VERIfy Thrombosis risk ASsessment (VERITAS) study. Thromb Res. 2007;119(3):277-284. [DOI] [PubMed] [Google Scholar]
- 13. Chen WH, Cheng X, Lee PY et al. . Aspirin resistance and adverse clinical events in patients with coronary artery disease. Am J Med. 2007;120(7):631-635. [DOI] [PubMed] [Google Scholar]
- 14. Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ten Berg JM, Hackeng CM. High on-aspirin platelet reactivity as measured with aggregation-based, cyclooxygenase-1 inhibition sensitive platelet function tests is associated with the occurrence of atherothrombotic events. J Thromb Haemost. 2010;8(10): 2140-2148. [DOI] [PubMed] [Google Scholar]
- 15. Price MJ, Angiolillo DJ, Teirstein PS et al. . Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention a time-dependent analysis of the Gauging responsiveness with a VerifyNow P2Y12 assay: impact on Thrombosis and safety (GRAVITAS) trial. Circulation. 2011;124(10):1132-1137. [DOI] [PubMed] [Google Scholar]
- 16. Blais N, Pharand C, Lordkipanidzé M, Sia YK, Merhi Y, Diodati JG. Response to aspirin in healthy individuals–cross-comparison of light transmission aggregometry, VerifyNow system, platelet count drop, thromboelastography (TEG) and urinary 11-dehydrothromboxane B2. Thromb Haemost. 2009;102(8):404-411. [DOI] [PubMed] [Google Scholar]
- 17. Jagoda AS, Bazarian JJ, Bruns JJ Jr et al. . Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. J Emerg Nurs. 2009;35(2):e5-e40. [DOI] [PubMed] [Google Scholar]
- 18. Price MJ. Bedside evaluation of thienopyridine antiplatelet therapy. Circulation. 2009;119(19):2625-2632. [DOI] [PubMed] [Google Scholar]
- 19. Nielsen HL, Kristensen SD, Thygesen SS et al. . Aspirin response evaluated by the VerifyNow aspirin system and light transmission aggregometry. Thromb Res. 2008; 123(2):267-273. [DOI] [PubMed] [Google Scholar]
- 20. Marcucci R, Gori AM, Paniccia R et al. . Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay a 12-month follow-up. Circulation. 2009;119(2):237-242. [DOI] [PubMed] [Google Scholar]
- 21. Patti G, Nusca A, Mangiacapra F, Gatto L, D'Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (antiplatelet therapy for reduction of MYocardial damage during angioplasty-platelet reactivity predicts outcome) study. J Am Coll Cardiol. 2008;52(14):1128-1133. [DOI] [PubMed] [Google Scholar]
- 22. Michelson AD. Methods for the measurement of platelet function. Am J Cardiol. 2009;103(3 suppl):20A-26A. [DOI] [PubMed] [Google Scholar]
- 23. Briggs A, Gates JD, Kaufman RM, Calahan C, Gormley WB, Havens JM. Platelet dysfunction and platelet transfusion in traumatic brain injury. J Surg Res. 2015;193 (2):802-806. [DOI] [PubMed] [Google Scholar]
- 24. Kakouros N, Kickler T, Laws K, Rade J. Hematocrit alters VerifyNow P2Y12 assay results independently of intrinsic platelet reactivity and clopidogrel responsiveness. J Thromb Haemost. 2013;11(10):1814-1822. [DOI] [PubMed] [Google Scholar]
- 25. Hovens MM, Snoep JD, Eikenboom JC, van der Bom JG, Mertens BJ, Huisman MV. Prevalence of persistent platelet reactivity despite use of aspirin: a systematic review. Am Heart J. 2007;153(2):175-181. [DOI] [PubMed] [Google Scholar]
- 26. Snoep JD, Hovens M, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007;154 (2):221-231. [DOI] [PubMed] [Google Scholar]
- 27. Bachelani AM, Bautz JT, Sperry JL et al. . Assessment of platelet transfusion for reversal of aspirin after traumatic brain injury. Surgery. 2011;150(4):836-843. [DOI] [PubMed] [Google Scholar]
- 28. Davis PK, Musunuru H, Walsh M et al. . Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit Care. 2013;18(2): 201-208. [DOI] [PubMed] [Google Scholar]
- 29. Mega JL, Close SL, Wiviott SD et al. . Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354-362. [DOI] [PubMed] [Google Scholar]
- 30. Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. 2006;367(9510):606-617. [DOI] [PubMed] [Google Scholar]
- 31. Abaci A, Yilmaz Y, Caliskan M et al. . Effect of increasing doses of aspirin on platelet function as measured by PFA-100 in patients with diabetes. Thromb Res. 2005;116(6):465-470. [DOI] [PubMed] [Google Scholar]
- 32. Pareti F, Capitanio A, Mannucci L, Ponticelli C, Mannucci P. Acquired dysfunction due to the circulation of “exhausted” platelets. Am J Med. 1980;69(2):235-240. [DOI] [PubMed] [Google Scholar]
- 33. Rozzelle CJ, Wofford JL, Branch CL. Predictors of hospital mortality in older patients with subdural hematoma. J Am Geriatr Soc. 1995;43(3):240-244. [DOI] [PubMed] [Google Scholar]
- 34. Spektor S, Agus S, Merkin V, Constantini S. Low-dose aspirin prophylaxis and risk of intracranial hemorrhage in patients older than 60 years of age with mild or moderate head injury: a prospective study. J Neurosurg. 2003;99(4):661-665. [DOI] [PubMed] [Google Scholar]
- 35. Ivascu FA, Howells GA, Junn FS, Bair HA, Bendick PJ, Janczyk RJ. Predictors of mortality in trauma patients with intracranial hemorrhage on preinjury aspirin or clopidogrel. J Trauma. 2008;65(4):785-788. [DOI] [PubMed] [Google Scholar]
- 36. Mina AA, Knipfer JF, Park DY, Bair HA, Howells GA, Bendick PJ. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma. 2002;53(4):668-672. [DOI] [PubMed] [Google Scholar]
- 37. Ohm C, Mina A, Howells G, Bair H, Bendick P. Effects of antiplatelet agents on outcomes for elderly patients with traumatic intracranial hemorrhage. J Trauma. 2005;58(3):518-522. [DOI] [PubMed] [Google Scholar]
- 38. Chen WH, Lee PY, Ng W, Tse HF, Lau CP. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol. 2004;43(6): 1122-1126. [DOI] [PubMed] [Google Scholar]