Abstract
Metabolic Syndrome and Associated Diseases: From the Bench to the Clinic, a Society of Toxicology Contemporary Concepts in Toxicology (CCT) workshop was held on March 11, 2017. The meeting was convened to raise awareness of metabolic syndrome and its associated diseases and serve as a melting pot with scientists of multiple disciplines (eg, toxicologists, clinicians, regulators) so as to spur research and understanding of this condition. The criteria for metabolic syndrome include obesity, dyslipidemia (low high-density lipoprotein and/or elevated triglycerides), elevated blood pressure, and alterations in glucose metabolism. It can lead to a greater potential of type 2 diabetes, lipid disorders, cardiovascular disease, hepatic steatosis, and other circulatory disorders. Although there are no approved drugs specifically for this syndrome, many drugs target diseases associated with this syndrome thus potentially increasing the likelihood of drug-drug interactions. There is currently significant research focusing on understanding the key pathways that control metabolism, which would be likely targets of risk factors (eg, exposure to xenobiotics, genetics) and lifestyle factors (eg, microbiome, nutrition, and exercise) that contribute to metabolic syndrome. Understanding these pathways could also lead to the development of pharmaceutical interventions. As individuals with metabolic syndrome have signs similar to that of toxic responses (eg, oxidative stress and inflammation) and organ dysfunction, these alterations should be taken into account in drug development. With the increasing frequency of metabolic syndrome in the general population, the idea of a “normal” individual may need to be redefined. This paper reports on the substance and outcomes of this workshop.
Keywords: metabolic syndrome, diabetes, microbiome, cardiovascular disease, inflammation, mitochondria
THE SCOPE OF THE PROBLEM
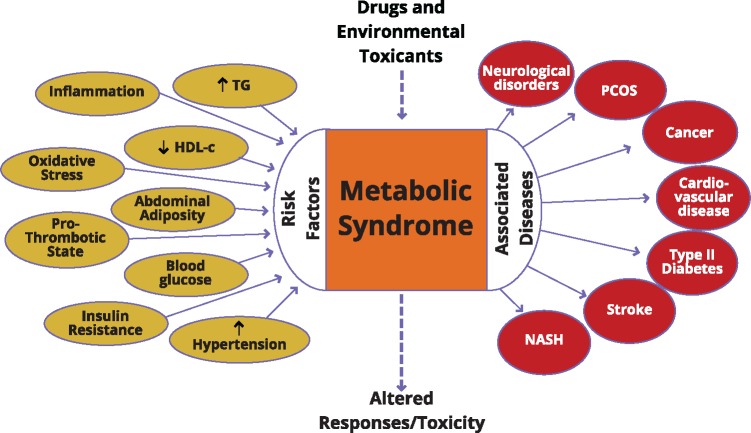
Our understanding of the science underling metabolic syndrome is evolving in this fast moving field. Dr Anna Mae Diehl (Florence McAlister Professor of Medicine, Duke University) provided the keynote lecture. Metabolic syndrome describes a constellation of metabolic abnormalities that are associated with visceral adiposity. These disorders include insulin resistance, hypertension, dyslipidemia (low high-density lipoprotein cholesterol, hypertriglyceridemia), and central obesity (Figure 1) (Tariq et al., 2016). The condition is diagnosed by the co-occurrence of three of the five aforementioned metabolic abnormalities. Pathology in various tissues is common in individuals with metabolic syndrome. Key targets for damage include the cardiovascular system, pancreas, and liver (Tariq et al., 2016). This helps to explain why cardiovascular disease, type 2 diabetes mellitus, and cirrhosis are among the leading causes of death in individuals with metabolic syndrome. Metabolic syndrome is common and its incidence has been rising for several decades, even in parts of the world where malnutrition remains common. Recent data indicate that about 25% of the adults in the United States have metabolic syndrome, and suggest that it accounts for much of the population-attributable risk for premature cardiovascular mortality (Grundy, 2008). Because diseases associated with metabolic syndrome are major causes of morbidity and mortality, identifying the root cause(s) of metabolic syndrome has been the focus of much research.
Figure 1.
Metabolic syndrome with its associated risk factors and diseases at the intersection with drug toxicity (TG, triglycerides; HDL-c, high-density lipoprotein cholesterol; PCOS, polycystic ovary syndrome; NASH, nonalcoholic steatohepatitis). Metabolic Syndrome: Toxicology’s Next Patient, Communiqué Winter 2017, Released on February 16, 2017, ©2017 Society of Toxicology. All rights reserved.
This syndrome can be driven by fat accumulation around intra-abdominal sites (possibly driven by genetic and epigenetic factors leading to predisposition of retaining fat at this site due to poor intrauterine growth), organ impairment (eg, nonalcoholic fatty liver disease), and exposure to xenobiotics. Although the causes for escalation in individual risk factors are multiple and complex, a clear recognition of the bridges that unite these risk factors to yield increased disease is lacking. Indeed, it is likely that there are pathways that are of importance to controlling metabolism that would be targets of both environmental chemicals and pharmaceutical interventions. It is known that some drugs and environmental contaminants can lead to metabolic syndrome or associated diseases as discussed later. A multidisciplinary approach to understand the underlying biological mechanisms and translate that knowledge into prevention and treatment is required.
The rise in central obesity rates in children is particularly concerning and Dr Lisa Swartz Topor (Assistant Professor, Alpert Medical School, Brown University) described the challenges. The trajectory toward weight gain begins early, as more than half of childhood obesity occurs in children who are already overweight or obese by kindergarten (Cunningham et al., 2014). Understanding the origins of childhood obesity and its complications are essential to identify areas for intervention and treatment, as well as to highlight the knowledge gaps that require attention. There are multiple factors associated with childhood obesity and some are described in the following sections.
Lifestyle
Increased portion sizes, sugar-sweetened beverage consumption, and sedentary lifestyle all contribute to childhood obesity. Rising rates of electronic device use and time spent watching television correlate with increased risk of obesity (Cespedes et al., 2014), whereas the presence of community resources that promote physical activity, such as sidewalks and playgrounds, is inversely associated with adolescent obesity (Kramer et al., 2016). Rapid weight gain during infancy is also a risk factor for childhood obesity, as weight gain in first 4 months of infancy is associated with childhood overweight and obesity and an unfavorable pattern of metabolic biomarkers (Wang et al., 2016a). Short sleep duration (<10.5 hours) at 3 years of age is associated with an increased risk of obesity at age 7 years (Reilly et al., 2005) and even one day of reduced sleep has been shown to correlate with increased caloric intake in preschool children (Mullins et al., 2017). The impact of lifestyle on metabolic syndrome will be discussed briefly in Sections Maternal Factors and Development, Microbiome, and Drug Development and Use.
Genetics
In addition to lifestyle factors, obesity is also influenced by genetics. Twin studies and adoption studies have shown that genetic factors greatly influence body mass index (BMI; Maes et al., 1997; Stunkard et al., 1990), and parental obesity is a significant risk factor for obesity in the offspring (Whitaker et al., 1997). Genome-wide association studies have identified nearly 150 genetic variants that are significantly associated with body size or obesity risk (Locke et al., 2015). However, the combined contribution of all known variants associated with body size measures is <5% so much remains unknown about the genetic factors influencing body size (Bray et al., 2016).
Maternal Factors and Development
As noted by Dr Topor, maternal obesity and weight gain before and during pregnancy are positively associated with offspring birth weight and risk of later obesity. Prepregnancy obesity is associated with increased odds of giving birth to a large-for-gestational age infant, and a 3-fold higher risk of childhood obesity. Independent of maternal prepregnancy BMI, higher maternal weight gain early in pregnancy is also associated with higher childhood BMI (Fraser et al., 2010; Gaillard et al., 2015). Associations between mother and offspring obesity may be explained by intrauterine mechanisms during pregnancy that likely involve maternal and fetal dysregulation of glucose, insulin, lipid, and amino acid metabolism. Epigenetic mechanisms are also potential mediators that link early environmental exposures during pregnancy with programmed changes in gene expression that alter offspring growth and development (Desai et al., 2015). Finally, shared environmental, lifestyle, and genetic characteristics also play a role.
Although some of the signs of metabolic syndrome and associated diseases are seen in adulthood, its roots may be planted during early life development. Dr Bouret at the Keck School of Medicine, University of Southern California spoke on the development of the neuroendocrine system as that regulates energy homeostasis. Population groups show that differences in terms of obesity and analysis of individual responses illustrate that those exposed to the same environment display unique obesogenic phenotypes. There is some genetic basis for obesity but the recently dramatic increase in the prevalence of obesity and related metabolic diseases suggest that there is much more than genetics at play. It is now recognized that the environment within the perinatal period can alter development and lead to adverse results in the offspring. Experimental and epidemiological studies have demonstrated that altered nutrition and growth during this early life can result in the onset of obesity and other metabolic diseases. The underlying mechanisms are not known but studies suggest that it may be due to the altered development of the neurons in the hypothalamus, a brain region known to control feeding and energy balance. The hypothalamus undergoes tremendous growth beginning in the fetal life and continuing through adolescence (Bouret, 2013). Because of the importance of postnatal hypothalamic development during postnatal life, animal models of postnatal metabolic programming have been largely studied. Overfeeding during the postnatal period influences the development of the hypothalamus (such as neuronal connectivity) (Plagemann, 2006). Leptin, a hormone, is a produced by adipocytes and nutrition-induced changes in this hormone during development may result in abnormal hypothalamic development and function (Bouret, 2013; Plagemann, 2006). Animals exposed to postnatal overfeedings display leptin resistance that occurs before the animals become obese suggesting that this hormonal resistance may initiate the development and maintenance of obesity (Glavas et al., 2010). A better understanding in the development of the hypothalamus will be required to develop strategies to stop or attenuate the deleterious effects of abnormal nutrition on the developing fetus and/or neonate.
Dr Michele La Merrill (Assistant Professor, University of California at Davis) used a prenatal model in mice of exposure to an environmental toxicant to study metabolic syndrome. More is discussed in Section Environmental and Drug Effects.
MECHANISMS
It is important to understand potential common mechanisms behind metabolic syndrome and its associated diseases in the hope of developing preventative and/or therapeutic strategies.
Mitochondrial Dysfunction and the Metabolic Syndrome
Mitochondria are the site of most of the energy production in eukaryotic cells and have their own circular DNA and ribosomes. In addition to generating energy, mitochondria also play an important role in many cellular tasks, such as apoptosis-programmed cell death, cellular proliferation, regulation of the cellular redox state, and heme and steroid synthesis. If mitochondrial function fails, the overall cellular function will decline and lead to subsequently cell death, organ injury, and, in the worst case, organ failure. Dr Yvonne Will (Head of Science and Technology Strategy and In Vitro Discover Toxicology, Pfizer) discussed the complex and somewhat unclear role that mitochondrial dysfunction may play as a contributor to metabolic syndrome (eg, a pathophysiological change such as insulin resistance) and associated diseases (eg, nonalcoholic fatty liver disease). Although several studies could clearly demonstrate mitochondrial impairment in insulin resistance, other studies failed to do so. A third scenario described an increase in mitochondrial function as a compensatory mechanism. The three scenarios suggest that insulin resistance can occur without mitochondrial functional changes. What seems to be clear is that the context of the dysfunction is important in terms of the model system studied (eg, species), the subpopulation under evaluation and the experimental approach (see Montgomery and Turner [2015] for a review of the three hypotheses). Mt-DNA mutations and haplotypes have been linked to insulin resistance; however, discrepancies between ethnical groups were found and might be explained by different nuclear genetic backgrounds and/or by environmental factors (Park et al., 2008; Pravenec et al., 2007).
Another intriguing hypothesis of how mitochondrial dysfunction in insulin resistance can occur is though environmental exposure. Many pesticides and herbicides that enter the food chain target mitochondria. In fact, a correlation has been found in the “corn belt” in the mid-western region of the United States and the prevalence of insulin resistance and mitochondrial dysfunction (Lim et al., 2009).
Because of the proposed role of mitochondrial dysfunction in insulin resistance, mitochondrial uncoupling, several approaches have been taken for medical intervention, such as uncoupling and the use of compounds such as berberine (AMPK activation), resveratrol (PGC-1a activation), and MitoQ (reduction of oxidative stress) (Montgomery and Turner, 2015). The role of mitochondrial dysfunction in nonalcoholic fatty liver disease has been demonstrated by numerous reports (Nassir and Ibdah, 2014) and hepatic mitochondrial function can be measured directly using Carbon-13 nuclear magnetic resonance and phosphorus-31 nuclear magnetic spectroscopy (Sunny et al., 2017).
Mitochondrial dysfunction seems to play a role in metabolic syndrome and increases as disease progresses from insulin resistance to type 2 diabetes and from nonalcoholic fatty liver disease to nonalcoholic steatohepatitis. Although some drugs have been shown to be of therapeutic value, more work is needed in hope that mitochondrial targets will be discovered that can be targeted with novel therapies.
Inflammation
Multiple presenters described the role of the immune system in metabolic disease. As reported by Dr Diehl in her keynote address, it is not clear what causes metabolic syndrome, but it is known to be a chronic inflammatory state based on evidence of increased serum levels of various proinflammatory cytokines (eg, tumor necrosis factor alpha [TNF-α] and interleukin 1 beta) and biomarkers of inflammation (eg, C-reactive protein) (Lumeng, 2013; Tornatore et al., 2012). Therefore, considerable research is being devoted to identify triggers for chronic inflammation in metabolic syndrome. Three major sites have been implicated as initiators of inflammation in the metabolic syndrome: the liver, the intestine, and adipose depots (Henao-Mejia et al., 2012; Malagon et al., 2013; Tilg and Kaser, 2011; Toubal et al., 2013). Common triggers, such as metabolic stress responses to chronic caloric excess and resultant cell death, may trigger inflammation in each of these sites (Kraja et al., 2014; Sell et al., 2012; Strowig et al., 2012). The release of inflammatory mediators from one site promotes inflammation in other tissues, thereby amplifying the chronic inflammatory state and generalized tissue dysfunction/damage (Tilg and Kaser, 2011). Improved understanding of the inflammatory triggers can help provide novel diagnostic and therapeutic targets to prevent organ damage related to the metabolic syndrome. Factors that play a role include innate immunity, overproduction of pro-inflammatory cytokines (production of TNF-α occurs in both the liver and adipose tissue in metabolic syndrome), increased exposure to intestinally derived pathogen-associated molecular signals and loss of anti-inflammatory defenses (Lumeng, 2013; Malagon et al., 2013; Tilg and Kaser, 2011). White fat is an inflammatory tissue and a secondary immune organ as discussed by Dr Rodney Dietert, Professor of Immunotoxicology, Cornell University. Unresolving and misregulated inflammation promoted by M1 macrophages drive obesity and many of its comorbid disease and conditions. Viewed from this perspective, the metabolic syndrome and its sequelae may result from deregulation of immune system activity.
Microbiome
The microbiome has gained recognition as an important player that can tip the balance between healthy and disease states. Dr Diehl noted that the gut microbiome can be altered by foods and caloric excess and lead to a defective barrier function. This can result in bacterial products entering the blood stream and causing liver inflammation. Strategies to normalize the gut microbiome include prebiotics, probiotics, and fecal transplants. Dr Robert Ratner (Professor of Medicine, Georgetown University Medical Center) described the role of the microbiome in metabolic disease. Metagenome wide association studies show an association between the fecal microbiota and the presence of obesity and type 2 diabetes (Korem et al., 2015; Qin et al., 2012). It has been demonstrated that an obese phenotype can be transferred using human fecal microbiota from a fat and lean twin into germ-free mice. Recipients of the “fat” microbiota gain weight without a concomitant change in food intake or inflammatory status, whereas the mice receiving the “thin” microbiota showed no such effects (Ridaura et al., 2013). Microbial metabolites can impact human health, as there are receptors on human cells that allow for cross-talk between microbe and host (Brown and Hazen, 2015). Bacteria in the gut can produce trimethylamine (TMA) that is converted into trimethylamine N-oxide (TMAO) through the hepatic enzyme flavin mono-oxygenase 3 (FMO3). Employing strategies that knock down or augment FMO3 expression, researchers have validated the prominent role of FMO3 in regulating the levels of TMA and TMAO (Warrier et al., 2015). Dr Jonathan Mark Brown (Associate Staff, Cleveland Clinic) spoke further on the role of gut microbial metabolites. He described some of his current mechanistic studies that further show the association of circulating TMAO with obesity, and type 2 diabetes, wherein TMAO promotes insulin resistance, promotes atherosclerosis by decreasing reverse cholesterol transport and increases forward cholesterol transport. TMAO has been shown to be proatherogenic in mice (Bennett et al., 2013), and further associations between circulating TMAO and atherosclerosis have been demonstrated in humans (Bennett et al., 2013; Koeth et al., 2013) clarifying the cardiovascular disease potential of TMAO. These studies provide strong evidence that microbe-derived metabolites, and the enzyme FMO3 may be useful potential drug targets (Brown and Hazen, 2017) for metabolic diseases such as obesity and cardiovascular diseases. An ILSI/HESI (International Life Sciences Institute/Health and Environmental Sciences Institute) Subcommittee has been formed to investigate the potential of microbial metabolites to act as biomarkers of toxicity and/or disease (http://hesiglobal.org/committees/microbiomesubcommittee/; last accessed November 4, 2017). Much of the work on the microbiome is focused on defining a normal microbiome and how its perturbation can impact human health. The work cited above suggests therapies that target the microbiome may ameliorate some of the adverse effects of the metabolic syndrome. The use of fecal microbiota transplants for metabolic syndrome and obesity is one therapy being considered with several clinical trials are underway (Marotz and Zarrinpar, 2016). As Dr Diehl noted, the precise composition of an “ideal” healthy microbiome is not yet known, so efforts to ameliorate diseases are still in its infancy.
Environmental and Drug Effects
Environmental contamination may also be implicit in disease development as described by Dr Michele La Merrill. The World Health Organization still recommends that Dichlorodiphenyltrichloroethane (DDT) for malaria control and high body burdens of DDT are found in persons living in areas with high risk of malaria (Herrera-Portugal et al., 2005) and in contemporary migrant U.S. children (Eskenazi et al., 2006, 2009). Because of its lipophilicity and persistence, DDT and especially its metabolite dichlorodiphenyldichloroethylene (DDE) are contaminants of the food supply and found in people well outside of areas using DDT for malaria vector control at levels that increase with age. Further, most adults born in the United States before the DDT ban were highly exposed to DDT during the developmental window that programs lifetime metabolic function and are now of age for heightened type 2 diabetes risk (Cohn et al., 2007; Narayan et al., 2003). Numerous meta-analyses of the association between DDT and DDE indicate that there is a positive association between these chemicals and risk of obesity, type 2 diabetes, and hypertension consistently across American, European, and Asian populations (Cano-Sancho et al., 2017; Evangelou et al., 2016; Park et al., 2016; Song et al., 2016; Tang-Peronard et al., 2011; Wang et al., 2016b). Given that these diseases are diagnosed with three components of the metabolic syndrome, eg, elevated adiposity, blood glucose, and blood pressure, it follows that DDT and/or DDE may contribute to risk of developing metabolic syndrome. An alternative, historically evoked hypothesis is that the pharmacokinetics of these lipophilic persistent organic pollutants underlie correlation with adiposity (Wolff et al., 2007) and the correlation of adiposity with other metabolic syndrome components drives their association with DDT and DDE. However, meta-analysis limited to prospective studies of obesity (Cano-Sancho et al., 2017) and type 2 diabetes (Song et al., 2016) indicates that DDE is associated with the later development of these diseases. Dr La Merrill’s laboratory has developed a mouse model of prenatal DDT exposure within the range observed in human studies to evaluate the biological plausibility of metabolic syndrome of mouse offspring at several ages up to nine months (La Merrill et al., 2014). Prenatal DDT exposure increased body fat in female mouse offspring. This plausibly resulted from impaired energy expenditure, as demonstrated by a lowered body temperature, inability to handle cold, and reduced energy use during calorimetry, and a lack of hyperphagia. The 9-month-old female offspring prenatally exposed to DDT was provided a diet high in fat for 12 weeks and demonstrated high levels of circulating insulin, alterations in blood lipid levels, reduced ability to handle glucose and changes in the metabolism of bile acids. Prenatal exposure to DDT chronically increased systolic BP in these mice (La Merrill et al., 2014) which was reversible with captopril, an angiotensin-converting enzyme inhibitor (La Merrill et al., 2016). Meta-analysis of experimental evidence indicated that DDT and DDE increased adiposity while impairing thermogenesis and lipid dynamics in several mammalian species exposed to a range of doses (Cano-Sancho et al., 2017). These findings suggest that prenatal exposure to DDT reduces heat production, alters carbohydrate, and lipid metabolism thus rendering adult female offspring more likely to develop metabolic syndrome.
Continuing on the theme of exposure and the development of metabolic syndrome, Dr Will pointed out that medications may contribute to metabolic syndrome (eg, protease inhibitors) (Anuurad et al., 2009) and to nonalcoholic fatty liver disease (eg, tamoxifen, irinotecan, corticosteroids) (Fromenty, 2013), whereas others, such as beta blockers and statins, have been linked to diabetes (https://www.diapedia.org/other-types-of-diabetes-mellitus/41040851133/drug-induced-diabetes#fnref: 3; last accessed November 4, 2017).
Drug Development and Use
Because metabolic syndrome is not a recognized disease and knowledge of shared pathways as potential drug targets is still in its infancy, the current approach is to treat each individual disease individually. In Dr Ratner’s presentation, he showed research that suggested metformin reduced the risk of metabolic syndrome in patients with elevated fasting plasma glucose concentrations, although lifestyle changes were more effective (Knowler et al., 2002). Metformin has been shown to alter the intestinal microbiota (Forslund et al., 2015), again suggesting the bacteria and viruses that populate our gut may play a significant role in metabolic syndrome and associated diseases. This opens the possibility of affecting multiple diseases by one common underlying mechanism.
Death from cardiovascular disease is common in patients with metabolic syndrome. As described by Dr Norman Stockbridge (Supervisory Medical Officer, FDA/CDER), the burden of cardiovascular disease continues to grow but there is a stable pipeline of new drugs. This area remains a challenge for several reasons including (1) some recently approved drugs have not gained traction in the marketplace and (2) precision medicine may not be viable in this field as drug development has a huge cost and subsetting this large population into individual populations with specific drugs would be prohibitively expensive.
FUTURE DIRECTIONS
The diseases associated with metabolic syndrome can be chronic, debilitating, and lethal. There is a need to find common pathways or mechanisms for new therapeutic targets. By bringing together clinicians and researchers from multiple fields, this meeting aimed to merge ideas and approaches to better understand and address the metabolic syndrome and its complications. Understanding the origins of obesity and the pathways to metabolic syndrome are essential to further our ability to prevent and treat affected patients. From early childhood and throughout life, individuals can develop metabolic syndrome. As obesity can be transmitted through generations and can lead to long-term health problems, identifying causes of childhood obesity and reducing risk factors is essential.
A nonscientific postmeeting survey was conducted. When asked if the meeting increased their knowledge on this topic, 87% agreed and the majority of attendees reported an interest in a future meeting on this topic and webinars. This hopefully suggests that there is an ongoing interest in this multifaceted field which may lead to new research and collaborations.
It is clear that much research needs to be done on the initiating events that lead to metabolic syndrome and the commonality that leads to associated diseases. At this time, each disease is treated separately leading to polypharmacy and an increased risk of adverse drug reactions. With more knowledge in hand, it may be easier to prevent and treat this fast growing syndrome that is leading to mortality at a rapid pace.
ACKNOWLEDGMENTS
The Metabolic Syndrome meeting was developed by the Scientific Liaison Coalition and was supported by the Society of Toxicology (SOT)’s CCT Conference Committee. The members of the Organizing Committee are listed alphabetically: Drs Florence Burleson (BRT, Inc.), Rodney Dietert (Cornell University), Susan Emeigh-Hart (Boehringer-Ingelheim), Kenneth L. Hastings (Hastings Toxicology Consulting LLC), Thomas Knudsen (EPA), Donna L. Mendrick (FDA/NCTR), Thaddeus Schug (NIEHS), and Lisa Swartz Topor (Brown University). We wish to thank Marcia Lawson and Clarissa Russell and the SOT staff who spent numerous hours assisting in the successful planning and implementation of this meeting. A special thank is extended to the presenters whose contributions were numerous and could not be reflected in this manuscript in their entirety. We appreciate the input of the attendees during the lunch poster session and in the survey.
FUNDING
Sponsors of the meeting included the Society of Toxicology, BRT Burleson Research Technologies, Inc., the National Institute of Environmental Health Sciences, the American College of Toxicology, Charles River, Gilead, Merck, Society of Toxicologic Pathology, and the Scientific Liaison Coalition. It was endorsed by the Endocrine Society.
REFERENCES
- Anuurad E., Semrad A., Berglund L. (2009). Human immunodeficiency virus and highly active antiretroviral therapy-associated metabolic disorders and risk factors for cardiovascular disease. Metab. Syndr. Relat. Disord. 7, 401–410.http://dx.doi.org/10.1089/met.2008.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B. J., de Aguiar Vallim T. Q., Wang Z., Shih D. M., Meng Y., Gregory J., Allayee H., Lee R., Graham M., Crooke R., et al. (2013). Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 17, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S. G. (2013). Organizational actions of metabolic hormones. Front. Neuroendocrinol. 34, 18–26.http://dx.doi.org/10.1016/j.yfrne.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M. S., Loos R. J., McCaffery J. M., Ling C., Franks P. W., Weinstock G. M., Snyder M. P., Vassy J. L., Agurs-Collins T. (2016). NIH working group report-using genomic information to guide weight management: From universal to precision treatment. Obesity (Silver Spring) 24, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Hazen S. L. (2015). The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 66, 343–359.http://dx.doi.org/10.1146/annurev-med-060513-093205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Hazen S. L. (2017). Targeting of microbe-derived metabolites to improve human health: The next frontier for drug discovery. J. Biol. Chem. 292, 8560–8568.http://dx.doi.org/10.1074/jbc.R116.765388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Sancho G., Salmon A. G., La Merrill M. A. (2017). Association between exposure to p,p′-DDT and its metabolite p,p′-DDE with obesity: Integrated systematic review and meta-analysis. Environ. Health Perspect. doi: 10.1289/EHP527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cespedes E. M., Gillman M. W., Kleinman K., Rifas-Shiman S. L., Redline S., Taveras E. M. (2014). Television viewing, bedroom television, and sleep duration from infancy to mid-childhood. Pediatrics 133, e1163–e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn B. A., Wolff M. S., Cirillo P. M., Sholtz R. I. (2007). DDT and breast cancer in young women: New data on the significance of age at exposure. Environ. Health Perspect. 115, 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham S. A., Kramer M. R., Narayan K. M. (2014). Incidence of childhood obesity in the United States. N. Engl. J. Med. 370, 1660–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M., Jellyman J. K., Ross M. G. (2015). Epigenomics, gestational programming and risk of metabolic syndrome. Int. J. Obes. (Lond.) 39, 633–641.http://dx.doi.org/10.1038/ijo.2015.13 [DOI] [PubMed] [Google Scholar]
- Eskenazi B., Chevrier J., Rosas L. G., Anderson H. A., Bornman M. S., Bouwman H., Chen A., Cohn B. A., de Jager C., Henshel D. S., et al. (2009). The Pine River statement: Human health consequences of DDT use. Environ. Health Perspect. 117, 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B., Marks A. R., Bradman A., Fenster L., Johnson C., Barr D. B., Jewell N. P. (2006). In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics 118, 233–241. [DOI] [PubMed] [Google Scholar]
- Evangelou E., Ntritsos G., Chondrogiorgi M., Kavvoura F. K., Hernandez A. F., Ntzani E. E., Tzoulaki I. (2016). Exposure to pesticides and diabetes: A systematic review and meta-analysis. Environ. Int. 91, 60–68. [DOI] [PubMed] [Google Scholar]
- Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S., Prifti E., Vieira-Silva S., Gudmundsdottir V., Krogh Pedersen H., et al. (2015). Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A., Tilling K., Macdonald-Wallis C., Sattar N., Brion M. J., Benfield L., Ness A., Deanfield J., Hingorani A., Nelson S. M., et al. (2010). Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 121, 2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromenty B. (2013). Drug-induced liver injury in obesity. J. Hepatol. 58, 824–826.http://dx.doi.org/10.1016/j.jhep.2012.12.018 [DOI] [PubMed] [Google Scholar]
- Gaillard R., Steegers E. A., Franco O. H., Hofman A., Jaddoe V. W. (2015). Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int. J. Obes. (Lond.) 39, 677–685. [DOI] [PubMed] [Google Scholar]
- Glavas M. M., Kirigiti M. A., Xiao X. Q., Enriori P. J., Fisher S. K., Evans A. E., Grayson B. E., Cowley M. A., Smith M. S., Grove K. L. (2010). Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology 151, 1598–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M. (2008). Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 28, 629–636.http://dx.doi.org/10.1161/ATVBAHA.107.151092 [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W. Z., Strowig T., Thaiss C. A., Kau A. L., Eisenbarth S. C., Jurczak M. J., et al. (2012). Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Portugal C., Ochoa H., Franco-Sanchez G., Yanez L., Diaz-Barriga F. (2005). Environmental pathways of exposure to DDT for children living in a malarious area of Chiapas, Mexico. Environ. Res. 99, 158–163. [DOI] [PubMed] [Google Scholar]
- Knowler W. C., Barrett-Connor E., Fowler S. E., Hamman R. F., Lachin J. M., Walker E. A., Nathan D. M. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth R. A., Wang Z., Levison B. S., Buffa J. A., Org E., Sheehy B. T., Britt E. B., Fu X., Wu Y., Li L., et al. (2013). Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korem T., Zeevi D., Suez J., Weinberger A., Avnit-Sagi T., Pompan-Lotan M., Matot E., Jona G., Harmelin A., Cohen N., et al. (2015). Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 349, 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraja A. T., Chasman D. I., North K. E., Reiner A. P., Yanek L. R., Kilpelainen T. O., Smith J. A., Dehghan A., Dupuis J., Johnson A. D., et al. (2014). Pleiotropic genes for metabolic syndrome and inflammation. Mol. Genet. Metab. 112, 317–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. R., Raskind I. G., Van Dyke M. E., Matthews S. A., Cook-Smith J. N. (2016). Geography of adolescent obesity in the U.S., 2007–2011. Am. J. Prev. Med. 51, 898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M., Karey E., Moshier E., Lindtner C., La Frano M. R., Newman J. W., Buettner C., Alexander B. T. (2014). Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PLoS One 9, e103337.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M. A., Sethi S., Benard L., Moshier E., Haraldsson B., Buettner C. (2016). Perinatal DDT exposure induces hypertension and cardiac hypertrophy in adult mice. Environ. Health Perspect. 124, 1722–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Ahn S. Y., Song I. C., Chung M. H., Jang H. C., Park K. S., Lee K.-U., Pak Y. K., Lee H. K., Malaga G. (2009). Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One 4, e5186.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke A. E., Kahali B., Berndt S. I., Justice A. E., Pers T. H., Day F. R., Powell C., Vedantam S., Buchkovich M. L., Yang J., et al. (2015). Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng C. N. (2013). Innate immune activation in obesity. Mol. Aspects Med. 34, 12–29.http://dx.doi.org/10.1016/j.mam.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagon M. M., Diaz-Ruiz A., Guzman-Ruiz R., Jimenez-Gomez Y., Moreno N. R., Garcia-Navarro S., Vazquez-Martinez R., Peinado J. R. (2013). Adipobiology for novel therapeutic approaches in metabolic syndrome. Curr. Vasc. Pharmacol. 11, 954–967. [DOI] [PubMed] [Google Scholar]
- Marotz C. A., Zarrinpar A. (2016). Treating obesity and metabolic syndrome with fecal microbiota transplantation. Yale J. Biol. Med. 89, 383–388. [PMC free article] [PubMed] [Google Scholar]
- Maes H. H., Neale M. C., Eaves L. J. (1997). Genetic and environmental factors in relative body weight and human adiposity. Behav. Genet. 27, 325–351.http://dx.doi.org/10.1023/A:1025635913927 [DOI] [PubMed] [Google Scholar]
- Montgomery M. K., Turner N. (2015). Mitochondrial dysfunction and insulin resistance: An update. Endocr. Connect. 4, R1–R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins E. N., Miller A. L., Cherian S. S., Lumeng J. C., Wright K. P. Jr., Kurth S., Lebourgeois M. K. (2017). Acute sleep restriction increases dietary intake in preschool-age children. J. Sleep Res. 26, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan K. M., Boyle J. P., Thompson T. J., Sorensen S. W., Williamson D. F. (2003). Lifetime risk for diabetes mellitus in the United States. JAMA 290, 1884–1890. [DOI] [PubMed] [Google Scholar]
- Nassir F., Ibdah J. A. (2014). Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15, 8713–8742.http://dx.doi.org/10.3390/ijms15058713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. S., Chan J. C., Chuang L.-M., Suzuki S., Araki E., Nanjo K., Ji L., Ng M., Nishi M., Furuta H., et al. (2008). A mitochondrial DNA variant at position 16189 is associated with type 2 diabetes mellitus in Asians. Diabetologia 51, 602–608. [DOI] [PubMed] [Google Scholar]
- Park S. H., Lim J. E., Park H., Jee S. H. (2016). Body burden of persistent organic pollutants on hypertension: A meta-analysis. Environ. Sci. Pollut. Res. Int. 23, 14284–14293.http://dx.doi.org/10.1007/s11356-016-6568-6 [DOI] [PubMed] [Google Scholar]
- Plagemann A. (2006). Perinatal nutrition and hormone-dependent programming of food intake. Horm. Res. 65, 83–89.http://dx.doi.org/10.1159/000091511 [DOI] [PubMed] [Google Scholar]
- Pravenec M., Hyakukoku M., Houstek J., Zidek V., Landa V., Mlejnek P., Miksik I., Dudova-Mothejzikova K., Pecina P., Vrbacky M., et al. (2007). Direct linkage of mitochondrial genome variation to risk factors for type 2 diabetes in conplastic strains. Genome Res. 17, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. [DOI] [PubMed] [Google Scholar]
- Reilly J. J., Armstrong J., Dorosty A. R., Emmett P. M., Ness A., Rogers I., Steer C., Sherriff A. (2005). Early life risk factors for obesity in childhood: cohort study. BMJ 330, 1357.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura V. K., Faith J. J., Rey F. E., Cheng J., Duncan A. E., Kau A. L., Griffin N. W., Lombard V., Henrissat B., Bain J. R., et al. (2013). Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell H., Habich C., Eckel J. (2012). Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 8, 709–716.http://dx.doi.org/10.1038/nrendo.2012.114 [DOI] [PubMed] [Google Scholar]
- Song Y., Chou E. L., Baecker A., You N. C., Song Y., Sun Q., Liu S. (2016). Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: A systematic review and meta-analysis. J. Diabetes 8, 516–532.http://dx.doi.org/10.1111/1753-0407.12325 [DOI] [PubMed] [Google Scholar]
- Strowig T., Henao-Mejia J., Elinav E., Flavell R. (2012). Inflammasomes in health and disease. Nature 481, 278–286. [DOI] [PubMed] [Google Scholar]
- Stunkard A. J., Harris J. R., Pedersen N. L., McClearn G. E. (1990). Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 322, 1483–1487.2336075 [Google Scholar]
- Sunny N. E., Bril F., Cusi K. (2017). Mitochondrial adaptation in nonalcoholic fatty liver disease: Novel mechanisms and treatment strategies. Trends Endocrinol. Metab. 28, 250–260.http://dx.doi.org/10.1016/j.tem.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Tang-Peronard J. L., Andersen H. R., Jensen T. K., Heitmann B. L. (2011). Endocrine-disrupting chemicals and obesity development in humans: A review. Obes. Rev. 12, 622–636. [DOI] [PubMed] [Google Scholar]
- Tariq H., Nayudu S., Akella S., Glandt M., Chilimuri S. (2016). Non-alcoholic fatty pancreatic disease: A review of literature. Gastroenterol. Res. 9, 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H., Kaser A. (2011). Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Invest. 121, 2126–2132.http://dx.doi.org/10.1172/JCI58109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore L., Thotakura A. K., Bennett J., Moretti M., Franzoso G. (2012). The nuclear factor kappa B signaling pathway: Integrating metabolism with inflammation. Trends Cell Biol. 22, 557–566.http://dx.doi.org/10.1016/j.tcb.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Toubal A., Treuter E., Clement K., Venteclef N. (2013). Genomic and epigenomic regulation of adipose tissue inflammation in obesity. Trends Endocrinol. Metab. 24, 625–634. [DOI] [PubMed] [Google Scholar]
- Wang G., Johnson S., Gong Y., Polk S., Divall S., Radovick S., Moon M., Paige D., Hong X., Caruso D., et al. (2016). Weight gain in infancy and overweight or obesity in childhood across the gestational spectrum: A prospective birth cohort study. Sci. Rep. 6, 29867.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hollis-Hansen K., Ren X., Qiu Y., Qu W. (2016b). Do environmental pollutants increase obesity risk in humans? Obes. Rev. 17, 1179–1197. [DOI] [PubMed] [Google Scholar]
- Warrier M., Shih D. M., Burrows A. C., Ferguson D., Gromovsky A. D., Brown A. L., Marshall S., McDaniel A., Schugar R. C., Wang Z., et al. (2015). The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 10, 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R. C., Wright J. A., Pepe M. S., Seidel K. D., Dietz W. H. (1997). Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 337, 869–873. [DOI] [PubMed] [Google Scholar]
- Wolff M. S., Anderson H. A., Britton J. A., Rothman N. (2007). Pharmacokinetic variability and modern epidemiology: The example of dichlorodiphenyltrichloroethane, body mass index, and birth cohort. Cancer Epidemiol. Biomarkers Prev. 16, 1925–1930. [DOI] [PubMed] [Google Scholar]